Effect of Hydroxyapatite Post-Treatment on the Corrosion Resistance, Cytocompatibility and Antibacterial Properties of Copper-Containing Micro Arc Oxidation Coatings on Mg Alloy as Oral GBR Membrane Application
Abstract
1. Introduction
2. Experimental Details
2.1. Coating Preparation
2.2. Microstructural Characterization
2.3. Electrochemical Testing
2.4. Immersion Test
2.5. Cytotoxicity Testing
2.6. ALP Test
2.7. Antibacterial Testing
3. Results
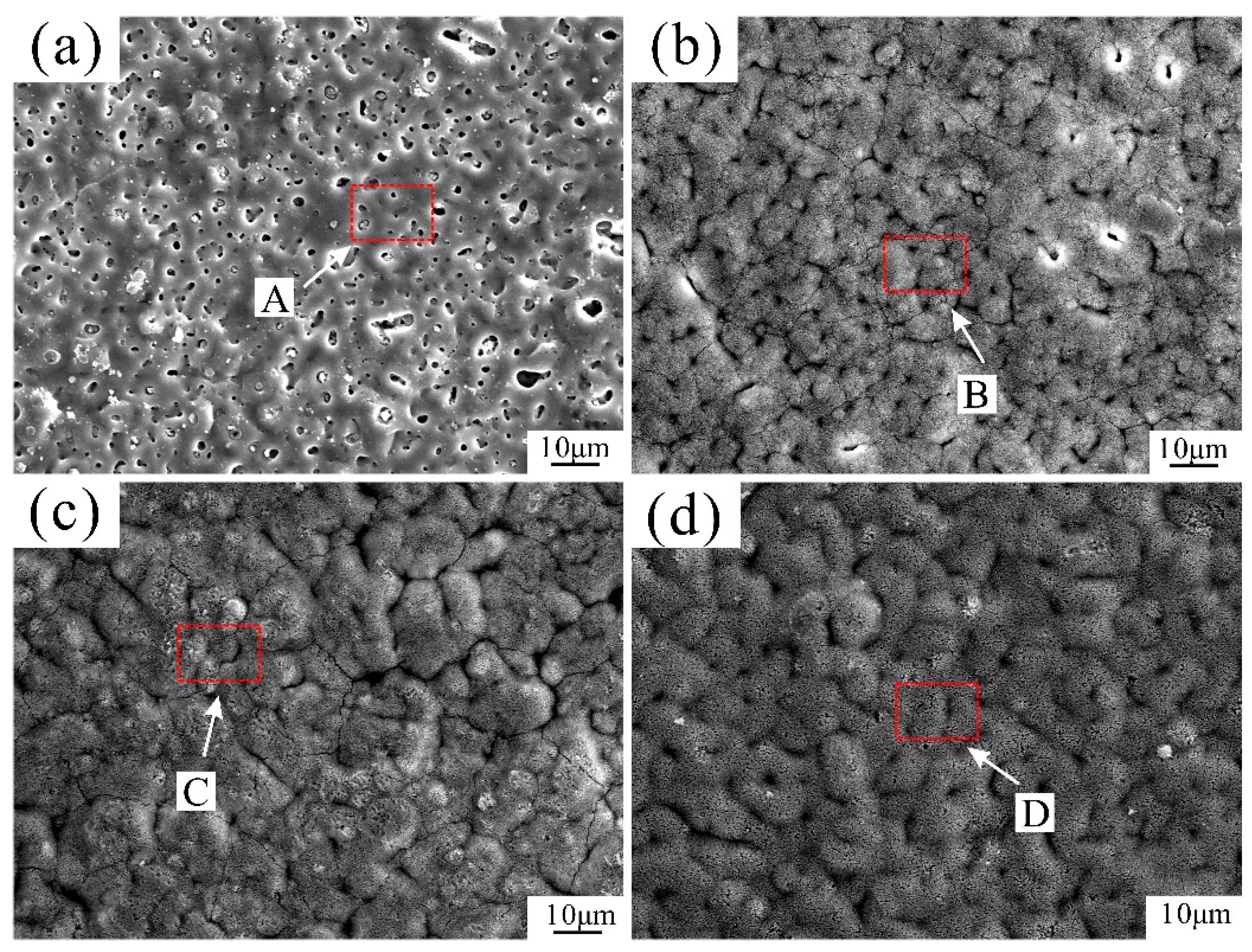
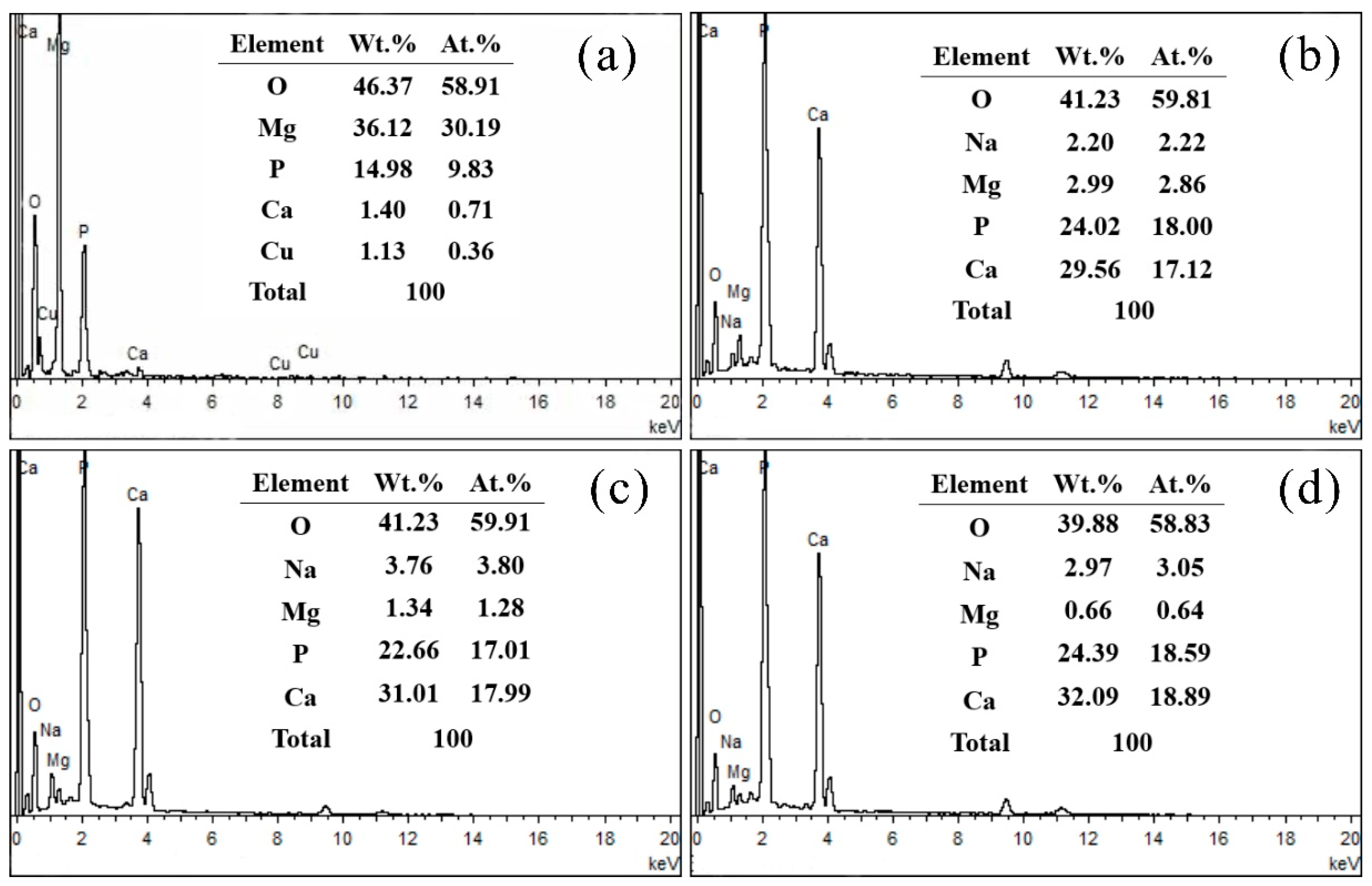
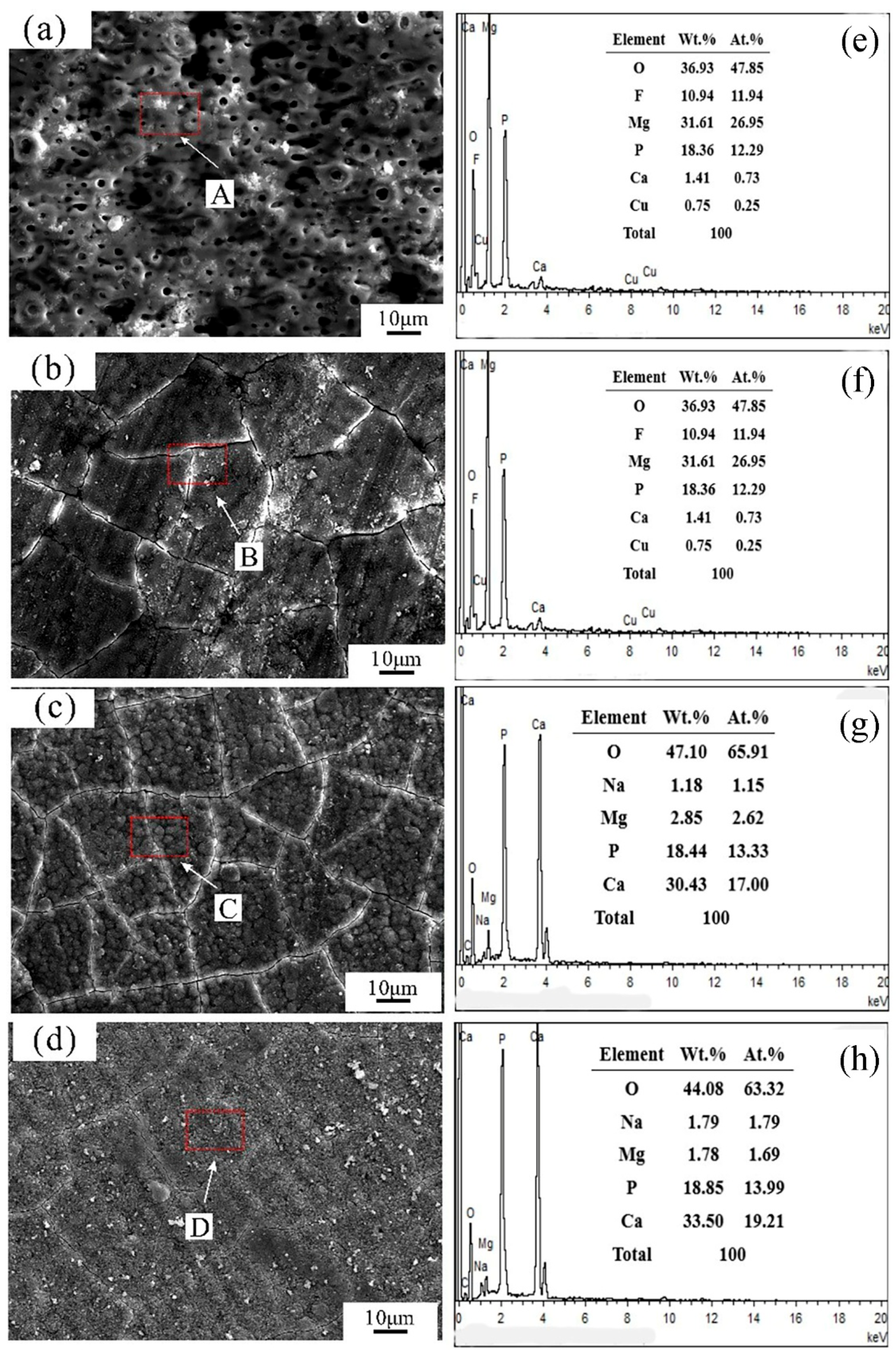
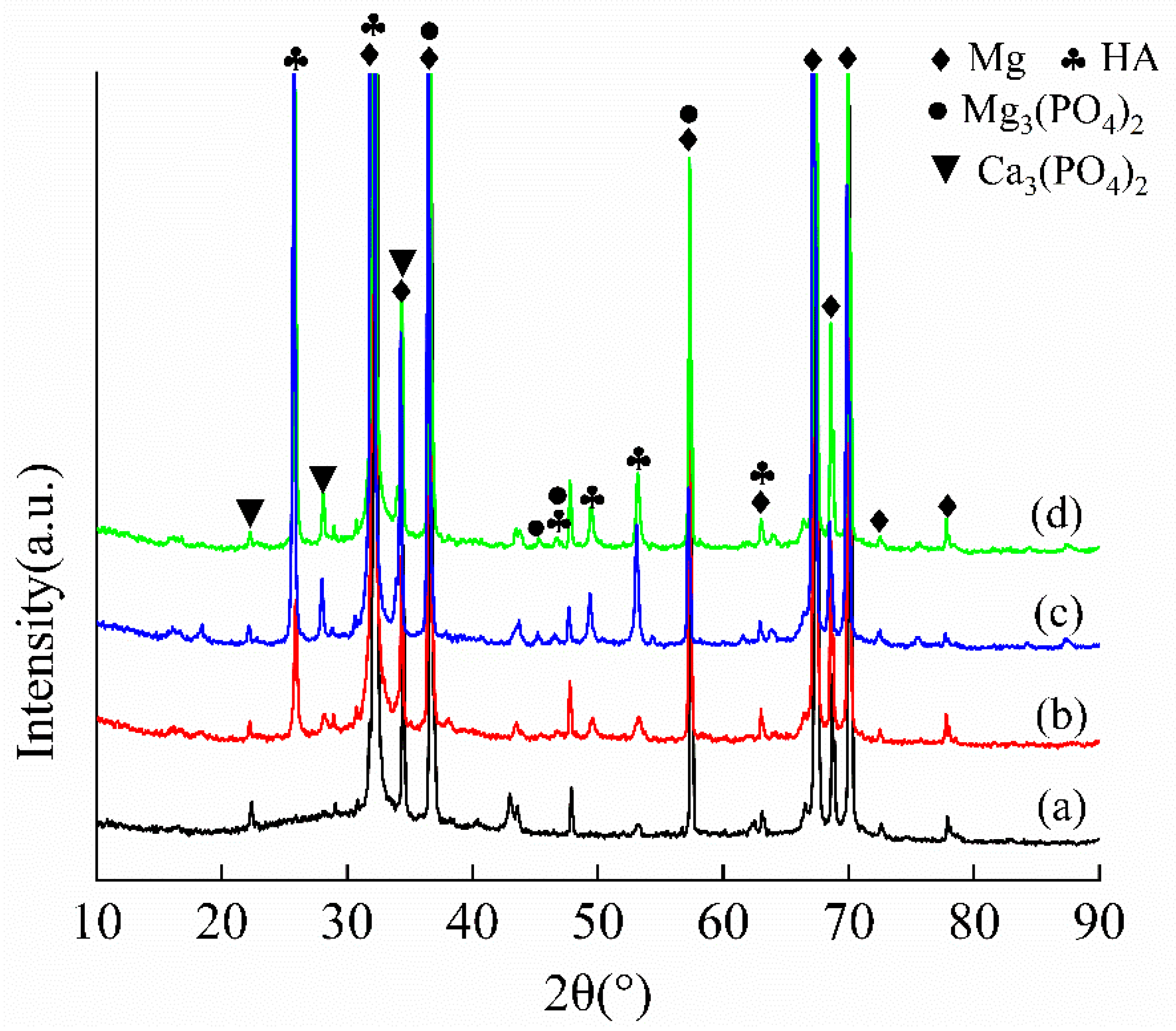
3.1. Microstructural Analysis
3.2. Electrochemical Analysis
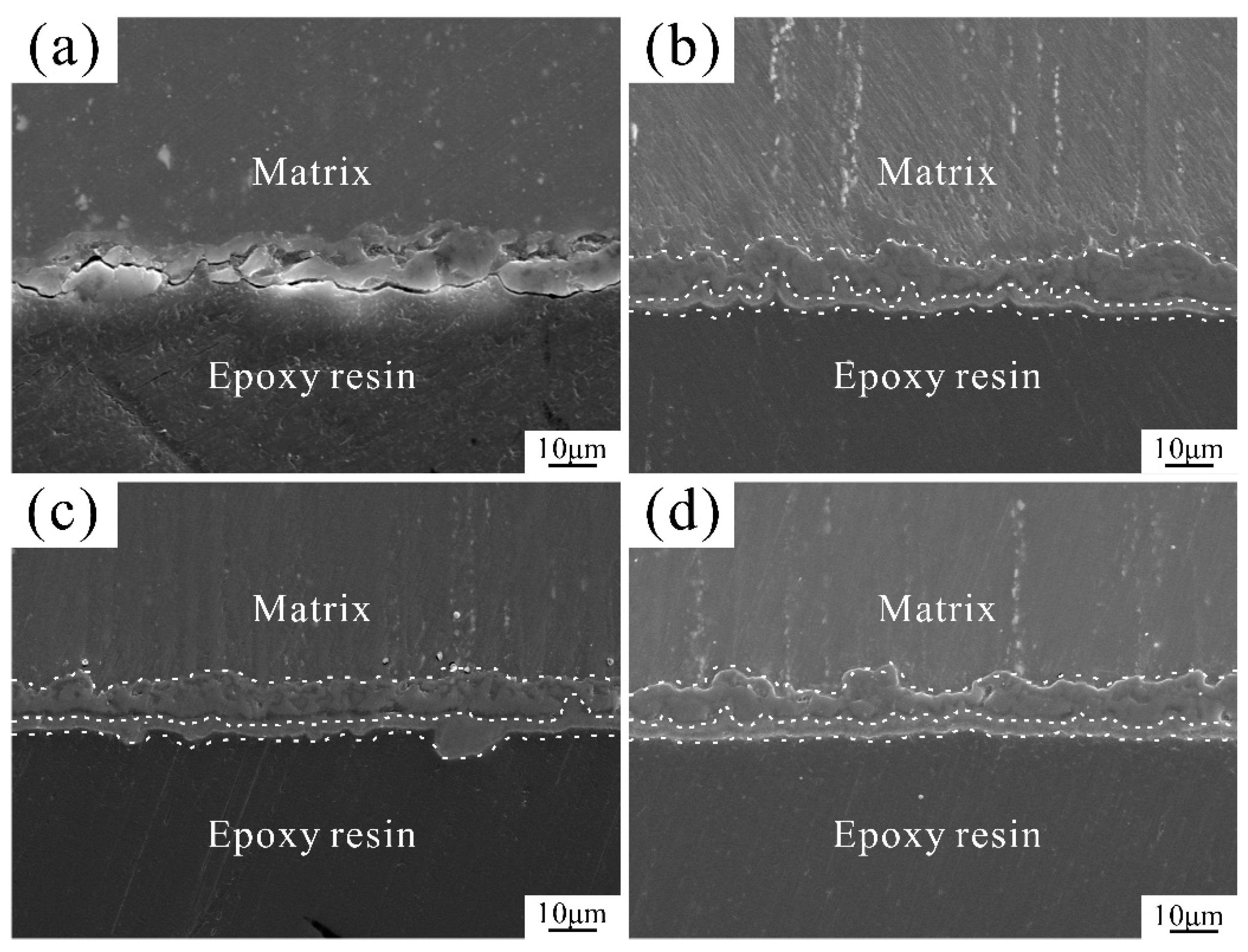
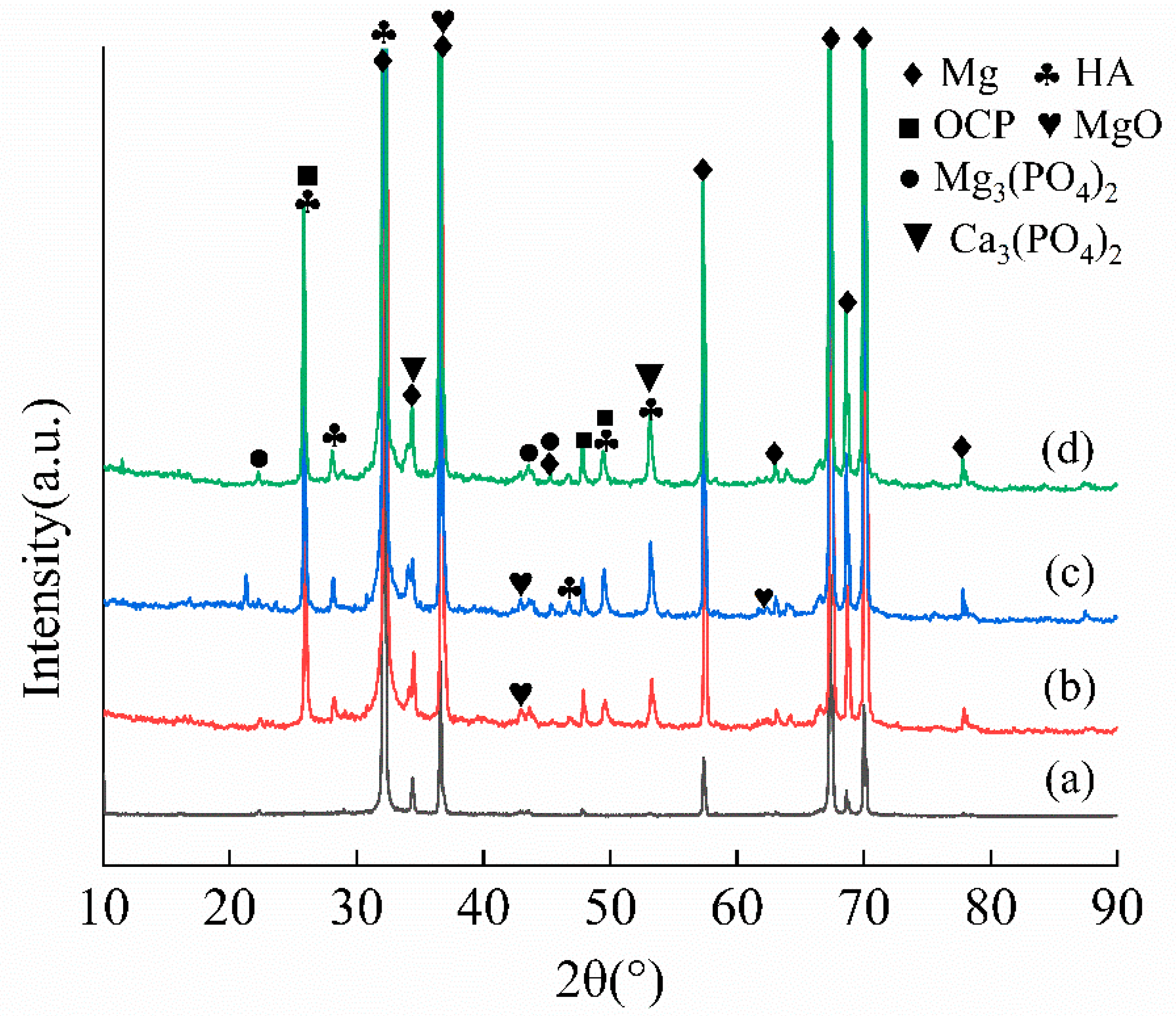
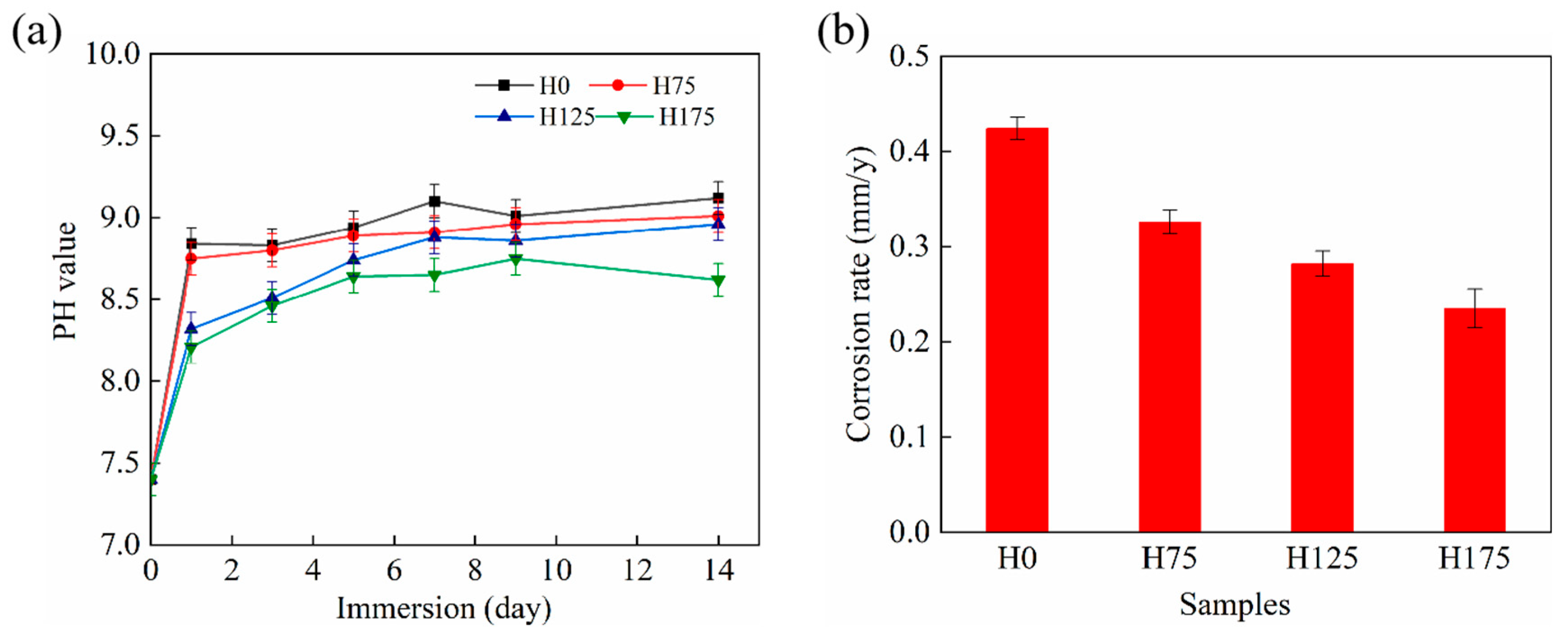
3.3. Immersion Analysis
3.4. Cytotoxicity Testing Analysis
3.5. Antibacterial Testing Analysis
4. Discussion
4.1. Effect of Hydroxyapatite Post-Treatment on the Corrosion Resistance of MAO Coating
4.2. Effect of Hydroxyapatite Post-Treatment on the Cytotoxicity and Antibacterial Performance of MAO Coating
5. Conclusions
- (1)
- Among MAO/HA coatings, H175 exhibited maximum thickness (5.0–5.5 μm) with minimal microcracking and the lowest corrosion rate.
- (2)
- The cytotoxicity assessment indicates that the composite coatings significantly improves cell compatibility compared to the bare MAO coating. Moreover, with increase in the HA concentration, the cell viability increased.
- (3)
- When the HA concentration was high, the denser HA layer acted as a barrier to retard the release of antibacterial Cu2+, leading to the decrease in the antibacterial property. The composite coating with 125 mmol/L EDTA–Ca exhibited good corrosion resistance, cytocompatibility and effective antibacterial property.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taghizadeh, E.; Negargar, S.; Nuroozi Larki, K.; Saberi Haghighi, R.; Shahoon, H. The Role of Guided Bone Regeneration in Enhancing Dental Implant Success. Galen Med. J. 2024, 13, e3681. [Google Scholar] [CrossRef]
- Kabir, H.; Munir, K.; Wen, C.; Li, Y. Recent Research and Progress of Biodegradable Zinc Alloys and Composites for Biomedical Applications: Biomechanical and Biocorrosion Perspectives. Bioact. Mater. 2021, 6, 836–879. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, Z.; Liu, S.; Chen, D.; Wang, G.; Wang, Y.; Zhang, M.; Chen, Y. Ultrasonic-Assisted Electroless Ni-P Plating on Dual Phase Mg-Li Alloy. J. Electrochem. Soc. 2014, 162, C64. [Google Scholar] [CrossRef]
- Liu, S.; Li, G.; Qi, Y.; Peng, Z.; Ye, Y.; Liang, J. Corrosion and Tribocorrosion Resistance of MAO-Based Composite Coating on AZ31 Magnesium Alloy. J. Magnes. Alloys 2022, 10, 3406–3417. [Google Scholar] [CrossRef]
- Li, C.-Y.; Gao, L.; Fan, X.-L.; Zeng, R.-C.; Chen, D.-C.; Zhi, K.-Q. In Vitro Degradation and Cytocompatibility of a Low Temperature In-Situ Grown Self-Healing Mg-Al LDH Coating on MAO-Coated Magnesium Alloy AZ31. Bioact. Mater. 2020, 5, 364–376. [Google Scholar] [CrossRef]
- Huang, Q.; Li, X.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Wu, H.; Feng, Q.; Liu, Y. The Cu-Containing TiO2 Coatings with Modulatory Effects on Macrophage Polarization and Bactericidal Capacity Prepared by Micro-Arc Oxidation on Titanium Substrates. Colloids Surf. B Biointerfaces 2018, 170, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Liu, Y.; Xi, F.; Zhang, X.; Kang, Y. Micro-Arc Oxidation (MAO) and Its Potential for Improving the Performance of Titanium Implants in Biomedical Applications. Front. Bioeng. Biotechnol. 2023, 11, 1282590. [Google Scholar] [CrossRef]
- Wang, Z.; Lan, N.; Zhang, Y.; Deng, W. Microstructure and Properties of MAO-Cu/Cu-(HEA)N Composite Coatings on Titanium Alloy. Coatings 2022, 12, 1877. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Wan, P.; Zhai, Z.; Mao, Z.; Ouyang, Z.; Yu, D.; Sun, Q.; Tan, L.; Ren, L.; et al. Biodegradable Mg-Cu Alloy Implants with Antibacterial Activity for the Treatment of Osteomyelitis: In Vitro and in Vivo Evaluations. Biomaterials 2016, 106, 250–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; Ibrahim, M.; Etim, I.P.; Tan, L.; Yang, K. In Vitro Degradation and Antibacterial Property of a Copper-Containing Micro-Arc Oxidation Coating on Mg-2Zn-1Gd-0.5Zr Alloy. Colloids Surf. B Biointerfaces 2019, 179, 77–86. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Wang, J.; Xia, X.; Lv, Y.; Cai, G.; Liu, H.; Xiao, J.; Liu, B.; Dong, Z. Microstructure, Formation Mechanism and Antifouling Property of Multi-Layered Cu-Incorporated Al2O3 Coating Fabricated through Plasma Electrolytic Oxidation. Ceram. Int. 2020, 46, 2901–2909. [Google Scholar] [CrossRef]
- Zhang, C.; Li, X.; Xiao, D.; Zhao, Q.; Chen, S.; Yang, F.; Liu, J.; Duan, K. Cu2+ Release from Polylactic Acid Coating on Titanium Reduces Bone Implant-Related Infection. J. Funct. Biomater. 2022, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-Y.; Huang, T.-Y.; Zhai, D.-J.; Wang, H.-B.; Feng, K.-Q.; Xiang, L. Study on Strontium Doped Bioactive Coatings on Titanium Alloys Surfaces by Micro-Arc Oxidation. Surf. Coat. Technol. 2022, 451, 129045. [Google Scholar] [CrossRef]
- Wang, H.; Yan, K.; Xing, H.; Chen, J.; Lu, R. Effective Removal of Cu2+ from Aqueous Solution by Synthetic Abalone Shell Hydroxyapatite Microspheres Adsorbent. Environ. Technol. Innov. 2021, 23, 101663. [Google Scholar] [CrossRef]
- Ma, J.; Xia, M.; Zhu, S.; Wang, F. A New Alendronate Doped HAP Nanomaterial for Pb2+, Cu2+ and Cd2+ Effect Absorption. J. Hazard. Mater. 2020, 400, 123143. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chen, Y.; Chen, C.; Zhong, S.; Yan, Z.; Liu, W.; Wang, Y.; Lai, X.; Zhao, Y.; Zhao, R.; et al. Preparation of HA-Containing Coating by One-Step MAO on Titanium Alloys through Synergistic Effect of Calcium Gluconate and Calcium Glycerophosphate. Surf. Coat. Technol. 2023, 466, 129655. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, S.; Wang, L.; Ji, C.; Ren, C.; Guan, S. Synthesis and Properties of a Bio-Composite Coating Formed on Magnesium Alloy by One-Step Method of Micro-Arc Oxidation. J. Alloys Compd. 2014, 590, 247–253. [Google Scholar] [CrossRef]
- Tang, H.; Tao, W.; Wang, C.; Yu, H. Fabrication of Hydroxyapatite Coatings on AZ31 Mg Alloy by Micro-Arc Oxidation Coupled with Sol–Gel Treatment. RSC Adv. 2018, 8, 12368–12375. [Google Scholar] [CrossRef]
- Abbasi, M.; Rashnavadi, M.; Gholami, M.; Molaei, S. Antibacterial Property of Hydroxyapatite Extracted from Biological Sources and Doped with Cu2+ and Ag+ by Sol-Gels Method. Sci. Rep. 2025, 15, 12101. [Google Scholar] [CrossRef]
- Li, H.; Qin, Z.; Ouyang, Y.; Zheng, B.; Wei, H.; Ou, J.; Shen, C. Hydroxyapatite/Chitosan-Metformin Composite Coating Enhances the Biocompatibility and Osteogenic Activity of AZ31 Magnesium Alloy. J. Alloys Compd. 2022, 909, 164694. [Google Scholar] [CrossRef]
- Shen, S.; Cai, S.; Li, Y.; Ling, R.; Zhang, F.; Xu, G.; Wang, F. Microwave Aqueous Synthesis of Hydroxyapatite Bilayer Coating on Magnesium Alloy for Orthopedic Application. Chem. Eng. J. 2017, 309, 278–287. [Google Scholar] [CrossRef]
- He, W.; Shao, Z.; He, J.; Zhang, Y.; Sun, M.; Jiang, Y.; Wen, Z.; Chen, F. Enhanced Anticorrosive, Antimicrobial and Biocompatible Properties of AZ91D Magnesium Alloy by MAO-Polycaprolactone-Modified ZnO Composite Coating. Surf. Coat. Technol. 2024, 494, 131484. [Google Scholar] [CrossRef]
- Feng, J.; Yuan, S.; Shi, Z.; Li, X.; Zhao, Y.; Atrens, A.; Zhao, M.-C. Micro-Arc Oxidation (MAO) Synthesized MgAl2O4 Spinel-Integrated Coatings on Mg Alloys: Multidimensional Exploration of Biodegradation, Biocompatibility, and Osseointegration. J. Mater. Res. Technol. 2025, 36, 6486–6498. [Google Scholar] [CrossRef]
- Shen, Y.; Shan, X.; Etim, I.P.; Siddiqui, M.A.; Yang, Y.; Shi, Z.; Su, X.; Chen, J. Comparative Study of the Effects of Nano ZnO and CuO on the Biodegradation, Biocompatibility, and Antibacterial Properties of Micro-Arc Oxidation Coating of Magnesium Alloy. Acta Metall. Sin. Engl. Lett. 2024, 37, 242–254. [Google Scholar] [CrossRef]
- Shan, X.; Xu, Y.; Kolawole, S.K.; Wen, L.; Qi, Z.; Xu, W.; Chen, J. Degradable Pure Magnesium Used as a Barrier Film for Oral Bone Regeneration. J. Funct. Biomater. 2022, 13, 298. [Google Scholar] [CrossRef]
- Zheng, Z.; Zhao, M.C.; Tan, L.; Zhao, Y.C.; Xie, B.; Huang, L.; Yin, D.F.; Yang, K.; Atrens, A. Biodegradation Behaviour of Hydroxyapatite-Containing Self-Sealing Micro-Arc-Oxidation Coating on Pure Mg. Surf. Eng. 2021, 37, 942–952. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, W.; Xu, T.; Li, H.; Jiang, B.; Miao, X. Preparation and Corrosion Resistance of a Self-Sealing Hydroxyapatite- MgO Coating on Magnesium Alloy by Microarc Oxidation. Ceram. Int. 2022, 48, 13676–13683. [Google Scholar] [CrossRef]
- Wang, B.; Huang, P.; Ou, C.; Li, K.; Yan, B.; Lu, W. In Vitro Corrosion and Cytocompatibility of ZK60 Magnesium Alloy Coated with Hydroxyapatite by a Simple Chemical Conversion Process for Orthopedic Applications. Int. J. Mol. Sci. 2013, 14, 23614–23628. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.-C.; Shi, Z.; Li, X.; Zhao, C.; Wang, W.; Yin, D.; Atrens, A. Insights into Nano-ZrO2 Reinforced Self-Antibacterial Ti–3Cu Composites via Laser Metal Deposition: Content-Optimized Bioactive Nano-ZrO2 Integrated for Wear Resistance, in Vitro Antibacterial and Biological Properties. J. Mater. Chem. B 2025, 13, 4353–4373. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wan, P.; Tan, L.; Zhao, M.; Qin, L.; Yang, K. Corrosion and Biological Performance of Biodegradable Magnesium Alloys Mediated by Low Copper Addition and Processing. Mater. Sci. Eng. C 2018, 93, 565–581. [Google Scholar] [CrossRef]
- Shan, Y.; Qiao, B.; Ouyang, S.; Du, C.; Zhao, L.; Wang, G.; Ye, J.; Xiong, Y.; Wei, Y.; Song, J.; et al. Biodegradable Mg-Ca/Mg-Cu Bilayer Membranes with Enhanced Mechanical, Osteogenesis and Antibacterial Performances for GBR Applications. J. Magnes. Alloys 2025, 13, 792–809. [Google Scholar] [CrossRef]
- Gao, H.; Chen, Y.; Chen, X.; Huang, L.; Yao, H.; Zhu, X.; Tang, M.; Wang, Y.; Li, X.; Xie, L. In Vitro and in Vivo Studies on Bioactive Hydroxyapatite-Coated Magnesium for Glaucoma Drainage Implant. J. Magnes. Alloys 2025, 13, 442–455. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Yang, C.; Yang, K. Release-Type Bacteriostasis of Cu-Bearing Stainless Steel against Planktonic Bacteria Served in Liquid System. Mater. Chem. Phys. 2023, 295, 127083. [Google Scholar] [CrossRef]



| Composition | NaCl | KCl | KH2PO4 | MgSO4·7H2O | NaHCO3 | CaCl2 | Na2HPO4 |
|---|---|---|---|---|---|---|---|
| Content | 8 | 0.4 | 0.06 | 0.2 | 0.35 | 0.14 | 0.13 |
| Samples | icorr (μA/cm2) | Ecorr (V vs. SCE) | Corrosion Rate Pi (mm/y) |
|---|---|---|---|
| H0 | 0.75±0.03 | −1.49 ± 0.01 | 0.384 ± 0.01 |
| H75 | 0.59±0.002 | −1.47 ± 0.02 | 0.301 ± 0.02 |
| H125 | 0.31±0.001 | −1.45 ± 0.01 | 0.152 ± 0.01 |
| H175 | 0.21±0.002 | −1.42 ± 0.02 | 0.107 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Chen, Q.; Zhang, Q.; Xu, Y.; Kolawole, S.K.; Siddiqui, M.A.; Cheng, H.; Chen, J. Effect of Hydroxyapatite Post-Treatment on the Corrosion Resistance, Cytocompatibility and Antibacterial Properties of Copper-Containing Micro Arc Oxidation Coatings on Mg Alloy as Oral GBR Membrane Application. Coatings 2025, 15, 1344. https://doi.org/10.3390/coatings15111344
Ma T, Chen Q, Zhang Q, Xu Y, Kolawole SK, Siddiqui MA, Cheng H, Chen J. Effect of Hydroxyapatite Post-Treatment on the Corrosion Resistance, Cytocompatibility and Antibacterial Properties of Copper-Containing Micro Arc Oxidation Coatings on Mg Alloy as Oral GBR Membrane Application. Coatings. 2025; 15(11):1344. https://doi.org/10.3390/coatings15111344
Chicago/Turabian StyleMa, Tingting, Qiang Chen, Qian Zhang, Yu Xu, Sharafadeen Kunle Kolawole, Muhammad Ali Siddiqui, Honghui Cheng, and Junxiu Chen. 2025. "Effect of Hydroxyapatite Post-Treatment on the Corrosion Resistance, Cytocompatibility and Antibacterial Properties of Copper-Containing Micro Arc Oxidation Coatings on Mg Alloy as Oral GBR Membrane Application" Coatings 15, no. 11: 1344. https://doi.org/10.3390/coatings15111344
APA StyleMa, T., Chen, Q., Zhang, Q., Xu, Y., Kolawole, S. K., Siddiqui, M. A., Cheng, H., & Chen, J. (2025). Effect of Hydroxyapatite Post-Treatment on the Corrosion Resistance, Cytocompatibility and Antibacterial Properties of Copper-Containing Micro Arc Oxidation Coatings on Mg Alloy as Oral GBR Membrane Application. Coatings, 15(11), 1344. https://doi.org/10.3390/coatings15111344








