Preparation and Classification of Coatings by High-Energy Ball Milling: A Review
Abstract
1. Introduction
2. Criteria for Coating Element Screening
3. HEBM Coating Preparation Mechanisms
- (1)
- Pure Two-Phase Motion Theory
- (2)
- Three-Phase Mixed Motion Theory
- (3)
- Reniform Creep Theory
4. HEBM Coating Process System
- (1)
- Raw Material Formulation and Loading Optimization
- (2)
- Kinetics of Mechanical Alloying
- (3)
- Post-Processing and Coating Deposition
4.1. Selection and Working Mechanism of HEBM Equipment
4.2. Regulation of HEBM Process Parameters
- (1)
- Dry/Wet Milling
- (2)
- Grinding Ball Diameter
- (3)
- Milling Atmosphere
- (4)
- Ball-to-Powder Ratio (BPR)
- (5)
- Rotational Speed of Ball Mill (RPM)
- (6)
- Milling Time (MT)
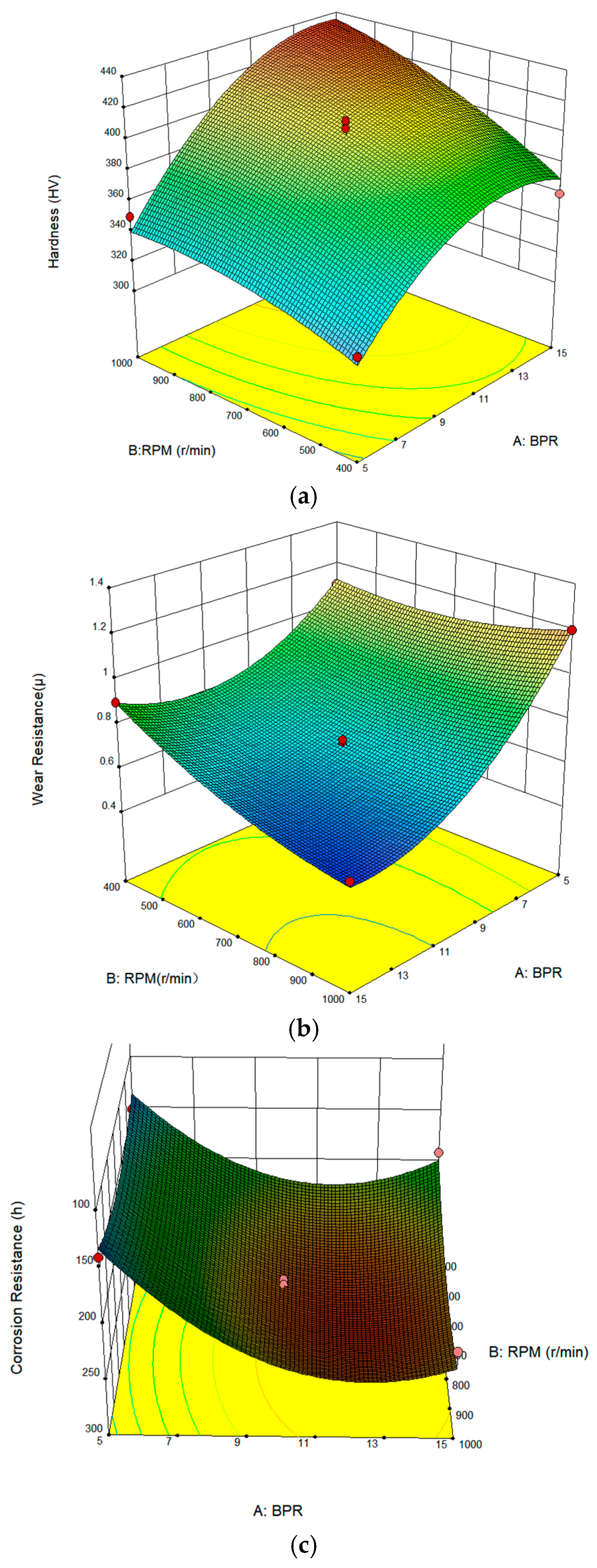
4.3. Multi-Parameter Synergistic Effects Response Surface Curve Analysis
4.4. Post-Processing and Coating Deposition Techniques
- (1)
- Sol–Gel Method
- (2)
- Encapsulation
- (3)
- Chemical Vapor Deposition (CVD)
- (4)
- Physical Vapor Deposition (PVD)
- (5)
- Laser Cladding
- (6)
- Electrochemical Deposition
- (7)
- Plasma Spraying
- (8)
- In Situ Synthesis
5. Coating Classification System and Performance Quantitative Prediction
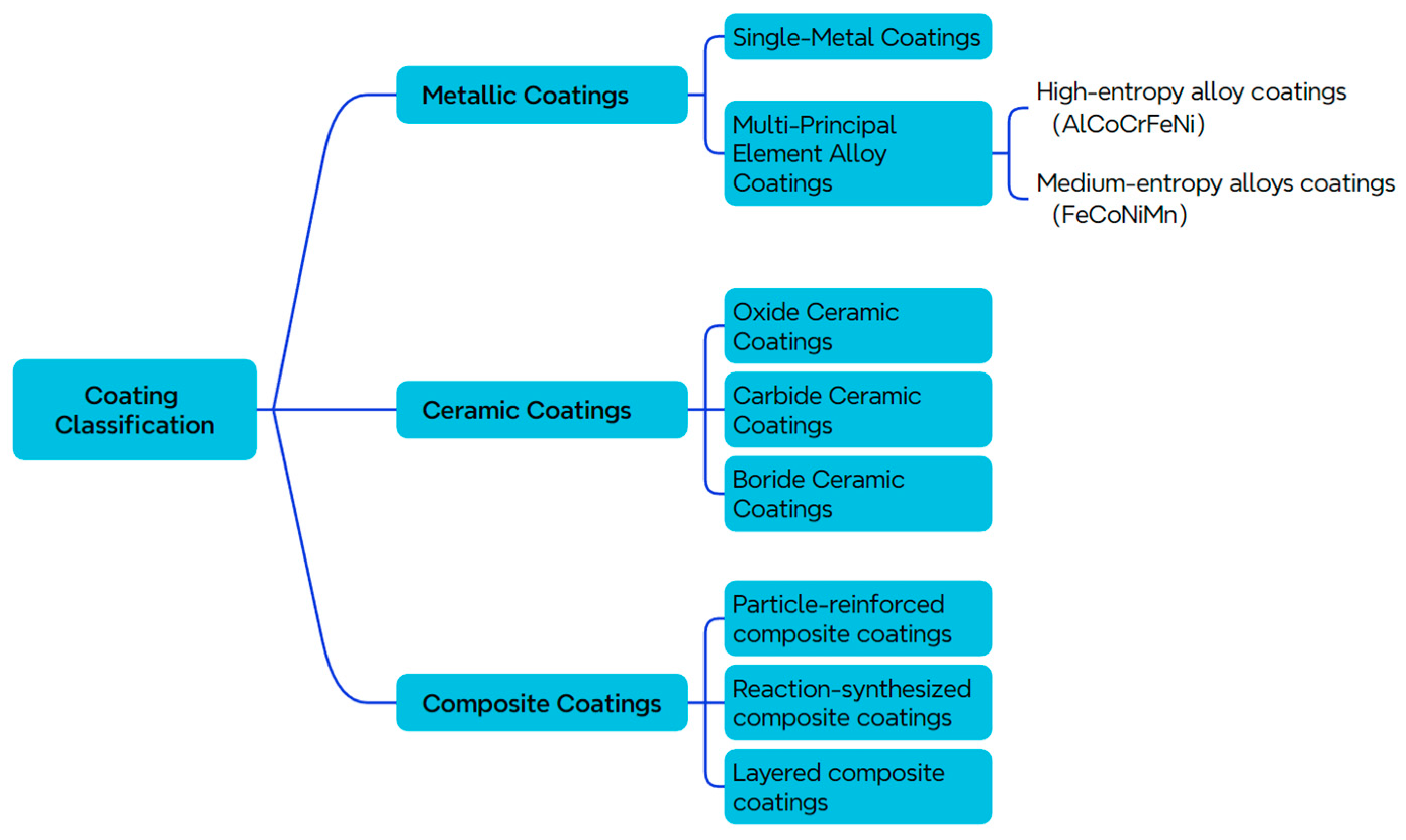
5.1. Coating Classification
- (1)
- Metallic Coatings
- (2)
- Ceramic Coatings
- (3)
- Composite Coatings
5.2. Quantitative Prediction of Coating Performance
6. Discussion and Future Perspectives
- (1)
- AI-Assisted Coating Design: Constructing a ternary knowledge graph integrating material element libraries, process parameter databases, and performance databases. Graph neural networks could mine implicit composition–process–performance relationships, potentially reducing experimental sample requirements by 50%.
- (2)
- Quantitative Analysis of Cross-Scale Mechanisms: Developing a research paradigm combining multi-physics simulation with in situ characterization. Establishing a mechanism-driven digital twin system would enable accurate prediction and active control of coating performance.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, S.; Liu, Y.; Geng, L.; Ru, Y.; Zhao, W.; Pei, Y.; Li, S. Advances in the Regulation and Interfacial Behavior of Coatings/Superalloys. Acta Met. Sin. 2023, 59, 1097–1108. [Google Scholar]
- Jiang, Y.; Liu, A.; Tang, Z.; Lu, X.; Li, F.; Hu, X.; Shi, Z. Electrorefining of aluminum in urea-imidazole chloride-aluminum chloride ionic liquids. J. Cent. South Univ. 2024, 31, 3079–3089. [Google Scholar] [CrossRef]
- Yu, Q.; Li, J.; Wei, C.; Zeng, Z.; Xu, S.; Liu, Z. Role of ball milling during Cs/X catalyst preparation and effects on catalytic performance in side-chain alkylation of toluene with methanol. Chin. J. Catal. 2020, 41, 1268–1278. [Google Scholar] [CrossRef]
- Shkodich, N.F.; Kuskov, K.V.; Sedegov, A.S.; Kovalev, I.D.; Panteleeva, A.V.; Vergunova, Y.S.; Scheck, Y.B.; Panina, E.; Stepanov, N.; Serhiienko, I.; et al. Refractory TaTiNb, TaTiNbZr, and TaTiNbZrX (X = Mo, W) high entropy alloys by combined use of high energy ball milling and spark plasma sintering: Structural characterization, mechanical properties, electrical resistivity, and thermal conductivity. J. Alloys Compd. 2022, 893, 162030. [Google Scholar] [CrossRef]
- Zhao, N.; Tao, L.; Guo, H.; Zhang, M. Microstructure and wear resistance of laser cladded Ni-based coatings with nanometer La2O3 addition. Rare Met. Mater. Eng. 2017, 46, 2092–2096. [Google Scholar]
- Sotornsak, S.; Chanlek, N.; Phromviyo, N.; Srepusharawoot, P.; Wantala, K.; Jarernboon, W.; Thongbai, P. Tailored microstructure and superior dielectric response in NbyTi1−yO2 ceramics derived from high-energy ball milling. Mater. Technol. 2025, 40, 2533939. [Google Scholar] [CrossRef]
- Han, Y.A.; Hong, J.H.; Zhang, A.R.; Guo, R.X.; Lin, K.X.; Ai, Y.J. A Review on MXene and its applications in environmental remediation. Prog. Chem. 2022, 34, 1229–1244. [Google Scholar]
- Mohery, M.; Mindil, A.; Soliman, M. Assessing the adverse impacts of biodegradable plastic bags: Chemical elements and radionuclides considerations. J. Environ. Chem. Eng. 2024, 12, 111887. [Google Scholar] [CrossRef]
- Qiu, Z.M.; Zeng, R.C.; Zhang, F.; Song, L.; Li, S.Q. Corrosion resistance of Mg–Al LDH/Mg (OH)2/silane–Cehybrid coating on magnesium alloy AZ31. Trans. Nonferrous Met. Soc. China 2020, 30, 2967–2979. [Google Scholar] [CrossRef]
- Xue, J.; Bo, J.; Wang, Y.; Feng, Q.; Chai, Z.Q.; Zou, X.J. Mechanism and Properties of Al2O3-Ru Composite Coatings Prepared by Cathode Plasma Electrolytic Deposition. Rare Met. Mater. Eng. 2024, 53, 3306–3312. [Google Scholar]
- Ma, D.Z.; Fan, Q.X.; Wang, T.G.; Zhang, C.; Yu, H.J. High Temperature Oxidation Resistance and Degradation Mechanism of Al-Si Coatings on Nickel-Based Superalloy at 1000. Rare Met. Mater. Eng. 2024, 53, 509–519. [Google Scholar]
- Zhou, L.; Luo, D.W.; An, G.S.; Ma, R. Research Progress on the Effect of Elemental Doping on the Performance of TiAlN Coating Tools. J. Mater. Eng. 2024, 52, 109–121. [Google Scholar]
- Zhao, M.; Zhou, H.; He, Y.; Gui, B.; Wang, K. Effect of Nonmetallic Vacancies on Electronic Structure and Conductivity of TiNx: First-Principles Study. Rare Met. Mater. Eng. 2024, 53, 3205–3210. [Google Scholar]
- Göl, Y.; Kılınç, B. Effect of Boron Concentration in the Fe-Cr-Mo-(B,C) Hardfacing Alloys on the Microstructure and Mechanical Properties. J. Mater. Eng. Perform. 2024, 34, 14325–14336. [Google Scholar] [CrossRef]
- Sharma, S.; Dwivedi, S.P.; Mohammed, K.A.; Kumar, A.; Awwad, F.A.; Khan, M.I.; Ismail, E.A.A. Investigation of surface hardness, thermostability, tribo-corrosion, and microstructural morphological properties of microwave-synthesized high entropy alloy FeCoNiMnCu coating claddings on steel. Sci. Rep. 2024, 14, 5160. [Google Scholar] [CrossRef]
- Singh, K.; Haq, U.I.M.; Mohan, S. Tribological behavior of novel Al2O3-La2O3 HVOF composite coatings. Tribol. Int. 2024, 193, 109427. [Google Scholar] [CrossRef]
- Feng, X.M.; Zhang, J.F.; Wang, D.; Deng, B.; Wang, J.; Xiao, B.L.; Ma, Z.Y. Effect of ball milling on densification and alloying in SiCp/Al powder metallurgy processes. Mater. Charact. 2024, 218, 114583. [Google Scholar] [CrossRef]
- Bandi, S.; Vidyasagar, D.; Adil, S.; Singh, M.K.; Basu, J.; Srivastav, A.K. Crystallite size induced bandgap tuning in WO3 derived from nanocrystalline tungsten. Scr. Mater. 2020, 176, 47–52. [Google Scholar] [CrossRef]
- Nokhrin, A.V.; Malekhonova, N.V.; Chuvil’deev, V.N.; Melekhin, N.V.; Bragov, A.M.; Filippov, A.R.; Boldin, M.S.; Lantsev, E.A.; Sakharov, N.V. Effect of high-energy ball milling time on the density and mechanical properties of W-7% Ni-3% Fe alloy. Metals 2023, 13, 1432. [Google Scholar] [CrossRef]
- Valcacer, S.M.; Silva, M.C.L.; Medeiros, I.P.M.; Gomes, U.U. Study of the effect of alumina percentage in WC-Ni-Al2O3 composite powder processed via High Energy Milling (HEM). Matéria 2021, 26, e13084. [Google Scholar]
- Wei, P.; Fang, J.; Fang, L.; Wang, K.; Lu, X.; Ren, F.Z. Novel niobium and silver toughened hydroxyapatite nanocomposites with enhanced mechanical and biological properties for load-bearing bone implants. Appl. Mater. Today 2019, 15, 531–542. [Google Scholar] [CrossRef]
- Ding, B.; Ahsan, Z.; Huang, X.; Cai, Z.F.; Ma, Y.Z.; Song, G.S.; Yang, W.D.; Wen, C. Preparation and electrochemical properties of high capacity silicon-based composites for lithium-ion batteries. Synth. Met. 2020, 261, 116324. [Google Scholar] [CrossRef]
- Wang, L.; Li, P.; Duan, R.; He, X.D. Occurrence, controlling factors and health risks of Cr6+ in groundwater in the Guanzhong Basin of China. Expo. Health 2022, 14, 239–251. [Google Scholar] [CrossRef]
- Ayoubi, P.; Ahmadi, H. Free vibration analysis of circular graphene sheet with centric circular defect based on two-phase local/nonlocal elasticity theory. Acta Mech. 2023, 234, 5425–5435. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, X.; Wang, X. Confirmation and perfection of Carcione–Leclaire three-phase theory. J. Theor. Comput. Acoust. 2022, 30, 2150002. [Google Scholar] [CrossRef]
- Lokoshchenko, A.M. Application of kinetic theory to the analysis of high-temperature creep rupture of metals under complex stress. J. Appl. Mech. Tech. Phys. 2012, 53, 599–610. [Google Scholar] [CrossRef]
- Naderi, A.; Behdad, S.; Fakher, M. Size dependent effects of two phase viscoelastic medium on damping vibrations of smart nanobeams: An efficient implementation of GDQM. Smart Mater. Struct. 2022, 31, 045007. [Google Scholar] [CrossRef]
- Robinson, D.A.; Edwards, M.A.; Liu, Y.; Ren, H.; White, H.S. Effect of viscosity on the collision dynamics and oxidation of individual Ag nanoparticles. J. Phys. Chem. C 2020, 124, 9068–9076. [Google Scholar] [CrossRef]
- Nawaz, S.; Hayat, T.; Alsaedi, A. Numerical study for peristalsis of Sisko nanomaterials with entropy generation. J. Therm. Anal. Calorim. 2020, 139, 2129–2143. [Google Scholar] [CrossRef]
- Lu, Y.; Guan, S.; Hao, L.; Yoshida, H. Review on the photocatalyst coatings of TiO2: Fabrication by mechanical coating technique and its application. Coatings 2015, 5, 425–464. [Google Scholar] [CrossRef]
- Wang, W.Q.; Wang, Z.M.; Li, Y.L.; Wang, D.; Li, M.; Chen, Q. Wear Behavior of Fe-WC/Metal Double Layer Coatings Fabricated by Resistance Seam Weld Method. Acta Met. Sin. 2018, 55, 537–546. [Google Scholar]
- Narzulloev, U.U.; Matveev, A.T.; Kaplanskaya, L.Y.; Mukanov, S.K.; Kuptsov, K.A.; Teplyakova, T.O.; Loginov, P.A.; Shchetinin, I.V.; Shtansky, D.V. Boron-doped high entropy CrFeCoNiCu alloy-based composites reinforced with oxides and borides with enhanced thermomechanical properties. J. Alloys Compd. 2025, 1036, 181778. [Google Scholar] [CrossRef]
- Wang, J.H.; Wu, C.L.; Shi, Q.N. Preparation of Nanocrystalline Ti-6Al-4V Alloy by Mechanical Alloying. Rare Met. Mater. Eng. 2017, 46, 783–789. [Google Scholar]
- Feng, X.J.; Yang, J.; Nuli, Y.N.; Wang, J.L. Synthesis and Lithium Storage Performance of Porous Silicon/Carbon Composite Material from SiCl4. Chin. J. Inorg. Chem. 2013, 29, 2289–2296. [Google Scholar]
- Liu, J.; Yang, J.; Wang, G.; Li, L.; Zhen, J. Microstructure and wear resistance of laser cladding WC reinforced Ni based composite coating on TC4 titanium alloy. Rare Met. Mater. Eng. 2022, 51, 2907–2914. [Google Scholar]
- Montiel, H.; Xu, B.; Li, J. Selective laser melting of mechanically alloyed metastable Al5Fe2 powders. J. Manuf. Sci. Eng. 2019, 141, 071008. [Google Scholar] [CrossRef]
- He, W. Classification and Installation Overview of Ball Milling Equipment. Xinjiang Nonferrous Met. 2017, 40, 88–89. [Google Scholar]
- Liu, L.; Zhang, L.; Zhu, L.; Zhang, X.; Gao, Z.; Li, H. Effect of milling treatment and additives on the morphology evolution of α-alumina from a commercial boehmite precursor. Z. Naturforschung B 2021, 76, 119–126. [Google Scholar] [CrossRef]
- Zaulychnyy, Y.; Hrubiak, A.; Karpets, M.; Gun’ko, V.; Vladymyrskyi, I.; Vashchynskyi, V.; Pedan, R.; An, T.; Guo, Y.; Vasyliev, G.; et al. Influence of the Mechanosynthesis Duration on the Structural, Electronic, and Electrochemical Characteristics of SiO2/TiO2 Nanocomposite. J. Electrochem. Soc. 2025, 172, 010525. [Google Scholar] [CrossRef]
- Kovalik, M.; Juríková, A.; Kubovčíková, M.; Mihalik, M.; Zentková, M.; Baláž, M.; Briančin, J.; Bujňáková, Z.; Vavra, M.; Lisnichuk, M.; et al. Preparation of magnetic fluids based on La0.80Ag0.15MnO3-δ nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2025, 711, 136300. [Google Scholar] [CrossRef]
- Shadab, M.; Miryala, M. Enhancing bulk MgB2 performance through optimized ball milling variables using Taguchi design approach. Ceram. Int. 2025, 51, 9647–9659. [Google Scholar] [CrossRef]
- Bańkowska-Sobczak, A.; Pryputniewicz-Flis, D.; Idźkowski, J.; Kozłowicz, Ł.; Brenk, G.; Diduszko, R.; Ostrowska, A.; Burska, D. Mechanical activation of a natural calcite for enhanced orthophosphate sorption. Desalination Water Treat. 2024, 320, 100583. [Google Scholar] [CrossRef]
- Yao, W.; Chen, Z.; Yang, J.; Zhou, F.; Zhang, T.; Dong, J.; Zhang, Y. Surface roughness and fracture cracks of Al2O3/TiO2 composite coating by wet chemical mechanical grinding with structured abrasives pad. J. Mater. Res. Technol. 2024, 33, 361–375. [Google Scholar] [CrossRef]
- Hu, G.; Luo, W.; Wu, J.; Ma, W. Study on Energy Transfer Mechanism of Grinding Balls in a Planetary Ball Milling System. Integr. Ferroelectr. 2024, 240, 1065–1079. [Google Scholar] [CrossRef]
- Celep, O.; Yazici, E.Y. Ultra fine grinding of silver plant tailings of refractory ore using vertical stirred media mill. Trans. Nonferrous Met. Soc. China 2013, 23, 3412–3420. [Google Scholar] [CrossRef]
- Yao, G.-Q. Effects of Ball Mill Rotational Speed and Grinding Ball Size on Grinding Efficiency. Chem. Miner. Process. 2015, 44, 18–20+23. [Google Scholar]
- Yu, Z.; Xiao, J.; Leng, H.; CHOU, K. Direct carbothermic reduction of ilmenite concentrates by adding high dosage of Na2CO3 in microwave field. Trans. Nonferrous Met. Soc. China 2021, 31, 1818–1827. [Google Scholar] [CrossRef]
- Salah, N.; Abdullahi, S.; Salah, Y.N.; Alshahrie, A.; Koumoto, K. Suppressing the thermal conductivity to enhance the thermoelectric performance of SnSe2 using the high-energy ball milling in a pressurised N2 atmosphere. J. Mater. Res. Technol. 2024, 31, 1067–1079. [Google Scholar] [CrossRef]
- Luo, P.; Dong, S.; Yangli, A.; Sun, S.; Xie, Z.; Zheng, Z.; Yang, W. ZrB2-TiB2 nanocomposite powder prepared by mechanical alloying. Rare Met. Mater. Eng. 2016, 45, 1381–1385. [Google Scholar]
- Yu, H.-R.; Jia, Y.-Z.; Li, D.-L.; Zhang, Q.; Ouyang, Z.-Y.; Chen, G. Effect of Ball-to-Powder Ratio on the Preparation, Microstructure, and Properties of S290 Powder High-Speed Steel. Powder Metall. Ind. 2024, 34, 77–82. [Google Scholar]
- Salleh, E.M.; Zuhailawati, H.; Ramakrishnan, S. Synthesis of biodegradable Mg-Zn alloy by mechanical alloying: Statistical prediction of elastic modulus and mass loss using fractional factorial design. Trans. Nonferrous Met. Soc. China 2018, 28, 687–699. [Google Scholar] [CrossRef]
- Sivasankaran, S.; Sivaprasad, K.; Narayanasamy, R.; Iyer, V.K. Synthesis, structure and sinterability of 6061 AA100−x–x wt.% TiO2 composites prepared by high-energy ball milling. J. Alloys Compd. 2010, 491, 712–721. [Google Scholar] [CrossRef]
- Zhang, F.-X.; Ai, X.-L.; Tong, X.-Y.; Yang, J. Effect of Ball Milling Process on the Grinding Quality of SiC Powder. Foundry Equip. Technol. 2023, 18–21. [Google Scholar] [CrossRef]
- Hussain, I.; Lee, J.E.; Jeon, S.E.; Cho, H.J.; Huh, S.-H.; Koo, B.H.; Lee, C.G. Effect of milling speed on the structural and magnetic properties of Ni70Mn30 alloy prepared by Planetary Ball Mill method. Korean J. Mater. Res. 2018, 28, 539–543. [Google Scholar] [CrossRef]
- Chen, J.-D.; Ying, Z.-J.; Xian, M.-X.; Hu, F.-W. Investigation on Rotational Speed and Grinding Efficiency of Intermittent Wet Ball Mill. Foshan Ceram. 2022, 32, 27–29. [Google Scholar]
- Wang, F.; Shi, Q.; Liu, X.; Li, B.; Tan, C.; Xie, H.; Shen, Z.; Zeng, M. Preparation of WMoTaNbV Refractory High-Entropy Alloy Spherical Powder by Mechanical Alloying-Radio Frequency Plasma Spheroidization. Rare Met. Mater. Eng. 2024, 53, 3428–3436. [Google Scholar]
- Wei, W.-Q.; Cao, G.-M.; Liu, B.-Q.; Cui, D.-W.; Wang, H.; Zhang, P. Microstructural Evolution and Mechanical Behavior of Powder Metallurgy Nb-35Ti-6Al-5Cr-8V Alloy. Rare Met. Mater. Eng. 2019, 48, 4106–4112. [Google Scholar]
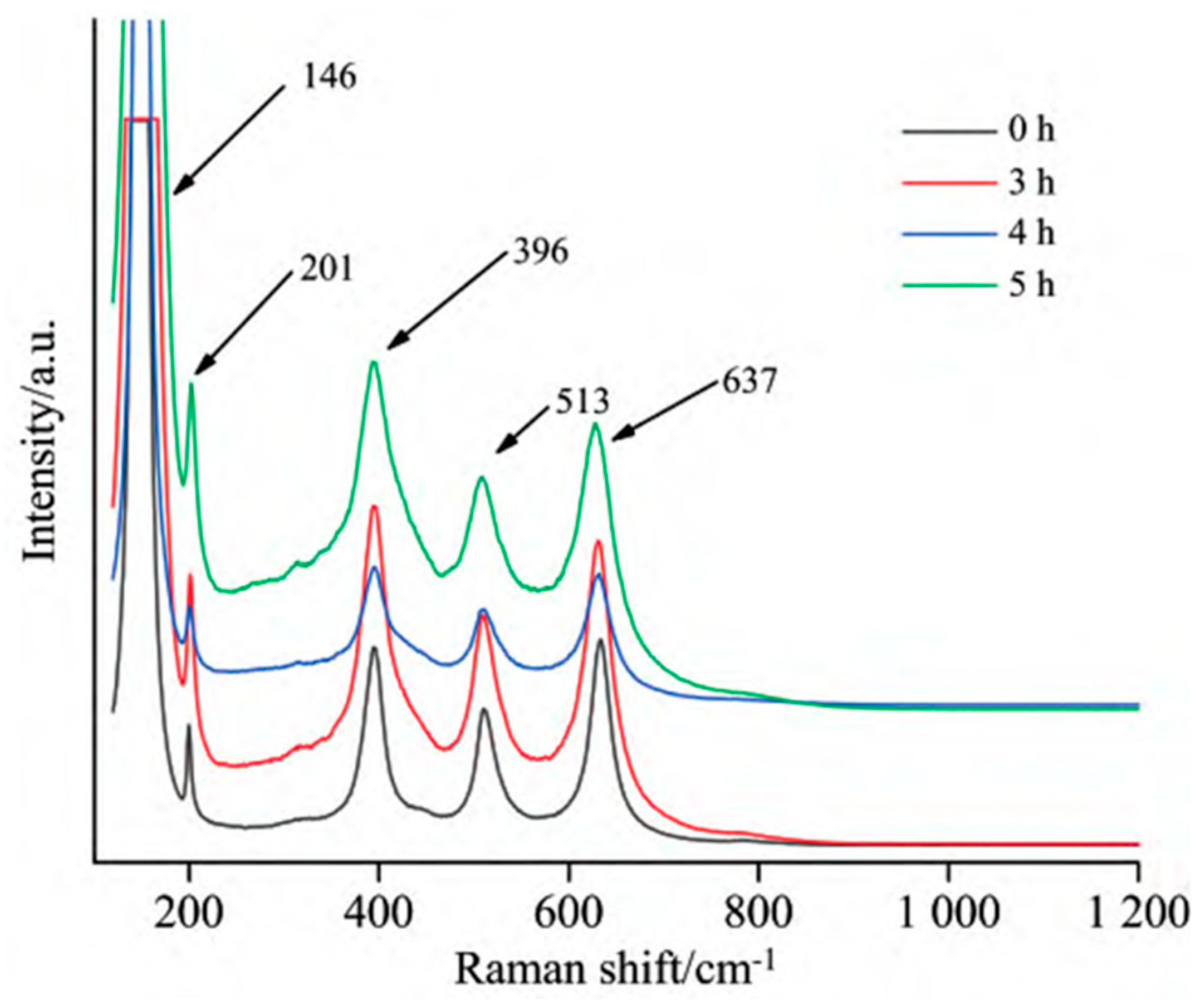
- Qi, D.L.; Cheng, J.; Sun, H.; Zhang, R.B.; Song, J.Y.; Qin, Y.L.; Li, H.D. Study on Spectral Characteristics and Photocatalytic Performance of Ball-Milled TiO2. Spectrosc. Spectr. Anal. 2022, 42, 3063–3067. [Google Scholar]
- Alluqmani, S.M.; AL-Zahrani, A.A.; Almarri, H.M.; Alabdallah, N.M. Influence of TiO2 Nanoparticles Synthesizing Techniques on Photocatalytic Degradation of Methylene Blue Dye. Pol. J. Environ. Stud. 2025, 34, 1495–1505. [Google Scholar] [CrossRef]
- Xing, L.; Hao, Y.; Rongguo, H.; Zhe, L. Effects of milling process parameters on the mechanical alloying behavior of CoCrFeNiAl0.9Nb0.1 high-entropy alloy powder. Mater. Today Commun. 2025, 48, 113458. [Google Scholar]
- Nezamdoust, S.; Seifzadeh, D.; Rajabalizadeh, Z. Application of novel sol–gel composites on magnesium alloy. J. Magnes. Alloys 2019, 7, 419–432. [Google Scholar] [CrossRef]
- Deng, H.-T.; Wang, C.-W.; Chen, S.-X.; Pan, S.-Y.; Lv, C.; Guo, M.-J.; Liu, J.-K.; Wei, G.-Z.; Zhou, X.; Li, J.-T. Improving Electrochemical Performance of High-Voltage LiCoO2 Cathode Materials via Dual Coating Method. Chin. J. Inorg. Chem. 2022, 38, 1557–1566. [Google Scholar]
- Hu, X.; Xiao, L.; Xin, Y.; Tan, X.; Huang, Q. Effects of deposition temperature on microstructures and ablative properties of SiC coatings prepared by CVD from methylsilane. Trans. Nonferrous Met. Soc. China 2023, 33, 3797–3811. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiong, J.; Guo, Z.; Liu, J.; Zhou, L.; Ye, J.; Zhao, W. Microstructures and properties of PVD TiAlN coating deposited on cermets with different Ti (C, N) grain size. J. Cent. South Univ. 2020, 27, 721–735. [Google Scholar] [CrossRef]
- Liu, R.; Jiang, X.-Y.; Ma, Z.-C.; Yang, R.; Li, Y. Temperature Field Simulation of Laser-Cladded CoCrNi-Based Alloy Coatings on TC4 Titanium Alloy Surface. Therm. Spray Technol. 2024, 16, 65–80. [Google Scholar]
- Liu, P.; Yuan, B.; Xiao, Z.W.; Xie, H.; Zhu, X.D.; Zhang, X.D. Construction and Preliminary Biological Evaluation of Bioactive HA Coatings on Porous Tantalum Scaffolds. Rare Met. Mater. Eng. 2022, 51, 225–231. [Google Scholar]
- Ma, B.X.; Xu, Y.; Xu, D.H.; Zou, B.L. Microstructure and Properties of TiB2-TiC-Co Coatings by Atmospheric Plasma Spraying. Rare Met. Mater. Eng. 2023, 52, 2791–2799. [Google Scholar]
- Yin, Q.; Yuan, X.M.; Zhang, J.T.; Feng, F.; Yang, H.G. Phase Distribution of Al2O3 Thin Films on Rare Earth- Modified Fe-Al Layer Surface. Rare Met. Mater. Eng. 2024, 53, 1549–1554. [Google Scholar]
- Cui, H.; Jiang, D. Research progress of high-entropy alloy coatings. Acta Met. Sin. 2021, 58, 17–27. [Google Scholar]
- Li, G.; Wen, Y.; Yu, Z.; Zhang, D.; Meng, Y. Microstructure and Properties of CrFeNiAlSiTix High Entropy Alloy Prepared by Laser Sintering. Rare Met. Mater. Eng. 2022, 51, 1681–1689. [Google Scholar]
- Wu, C.L.; Xu, T.Z.; Wang, Z.Y.; Zhang, C.H.; Zhang, S.; Ni, C.L.; Zhang, D.X. Laser surface alloying of FeCoCrAlNiTi high entropy alloy composite coatings reinforced with TiC on 304 stainless steel to enhance wear behavior. Ceram. Int. 2022, 48, 20690–20698. [Google Scholar] [CrossRef]
- Cao, J.; Yang, X.; Wang, S.; Zhang, H.; Yang, L.; Qiao, Y.; Li, C. Wear and corrosion resistance of laser cladding Ni60-TiC ceramic coating on 45 steel surface. Rare Met. Mater. Eng. 2020, 49, 611–617. [Google Scholar]
- Arifa, H.; Boukhachem, A.; Askri, B.; Boubaker, K.; Yumak, A.; Raouadi, K. Structural, optical and conductivity investigations on κ-Al2O3 ceramics for powder metallurgical production and sensitivity applications. Ceram. Int. 2016, 42, 2147–2157. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, Y.; Zhuang, M.; Xu, J.; Jiang, X. Study on inhibitory effect of Ag+-loaded TiO2 on the biofilm of Staphylococcus aureus. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2021, 33, 736–739. [Google Scholar] [PubMed]
- Cai, X.; Li, Y.; Jiang, P.; Ma, C.; Zhao, C.; Wang, S.; Siritanon, T. Phase evolution of Al–Al2O3–ZrO2 refractories at 1300 °C under N2 flowing. Ironmak. Steelmak. 2024, 51, 858–869. [Google Scholar] [CrossRef]
- Dercz, G.; Matuła, I.; Gurdziel, W.; Kuczera, N. Microstructure evolution of Ti/ZrO2 and Ti/Al2O3 composites prepared by powder metallurgy method. Arch. Metall. Mater. 2019, 64, 443–450. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Ramachandran, C.S. Synthesis of carbon nanotube reinforced aluminum composite powder (CNT-Al) by polymer pyrolysis chemical vapor deposition (PP-CVD) coupled high energy ball milling (HEBM) process. Diam. Relat. Mater. 2020, 104, 107748. [Google Scholar] [CrossRef]
- Tsipas, S.; Goodwin, P.; McShane, H.B.; Rawlings, R.D. Effect of high energy ball milling on titanium-hydroxyapatite powders. Powder Metall. 2003, 46, 73–77. [Google Scholar] [CrossRef]
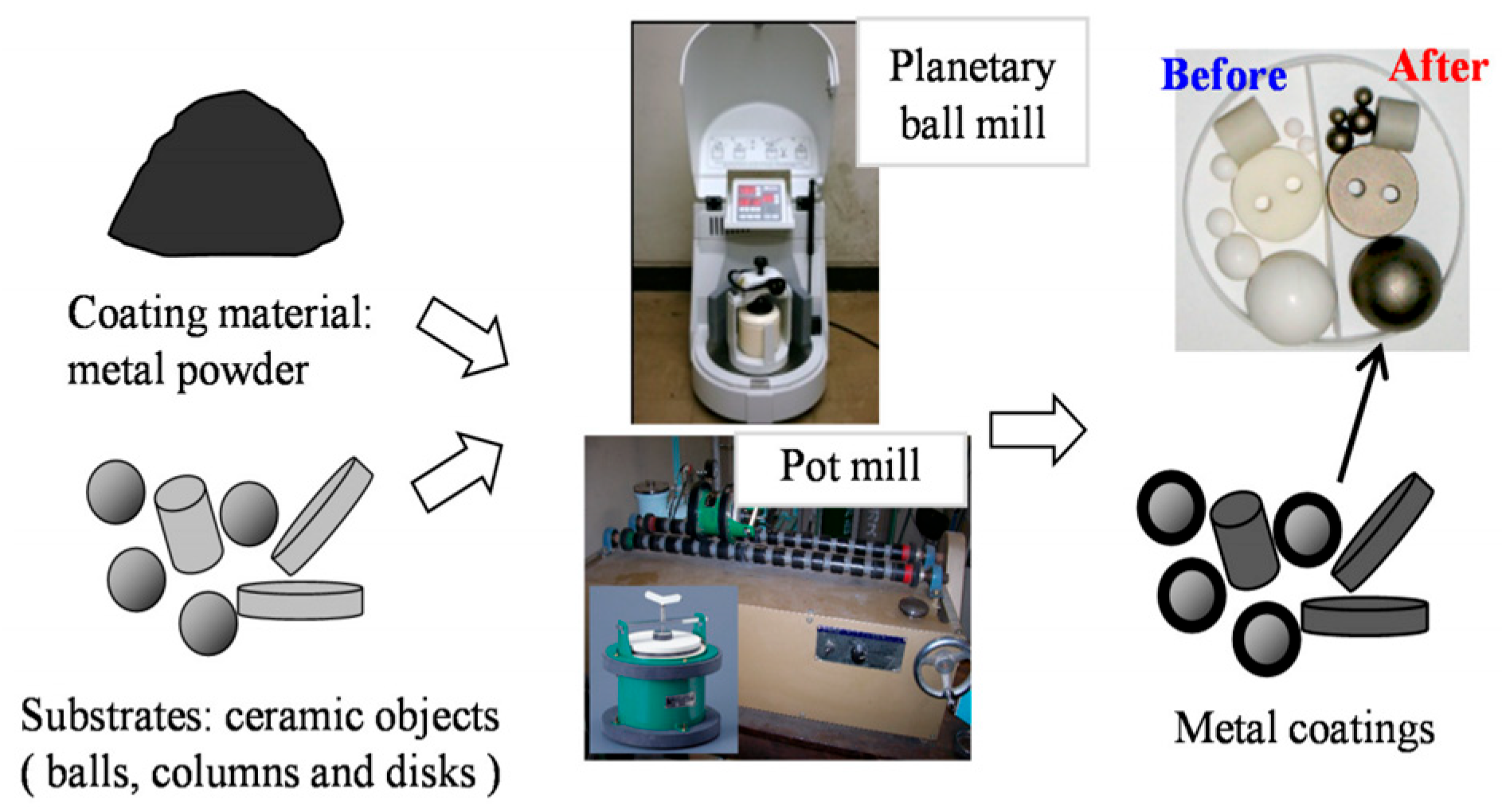
| Theory Type | Dominant Forces | Applicable Materials | Energy Efficiency | Industrial Applications | Documentary Basis |
|---|---|---|---|---|---|
| Pure Two-Phase Motion | Impact + Shear | Brittle ceramics/intermetallics | 35%–50% | Nano-ceramic powder preparation | [27] |
| Three-Phase Mixed Motion | Impact + Shear + Random Collision | Metals/alloys | 45%–60% | High-entropy alloy amorphization, nitride coatings | [28] |
| Reniform Creep | Quasi-static compression + Viscoelastic dissipation | Polymers/composites | 25%–40% | Graphene dispersion, drug nanoparticles | [29] |
| Coating Classification | Hardness (HV) | Wear Rate (mm3/N·m) | Coefficient of Friction (μ) | Salt Spray Resistance Time (h) | Bond Strength (MPa) | Highest Temperature Resistance (°C) | Rate of Oxidation (mm/year) | Thermal Conductivity (W/m·K) | Electrical Conductivity (S/m) | Biocompatible (Cell Proliferation Rate) | Thermal Shock Resistance (Number of Cycles) | Environmental Compatibility (ppm for Toxic Elements) | Documentary Basis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metallic Coatings | |||||||||||||
| Single-Metal Coatings | 70–100 | 0.6 | 0.6 | 500 | 50–80 | 200 | 0.5 | 110 | 1.2 × 107 | Not applicable | 20 | <1 | [77] |
| Multi-Principal Element Alloy Coatings | 400–1000 | 0.01–0.05 | 0.20 | 3000 | 300–500 | 800 | <0.05 | 15–25 | 1.5 × 106 | Not applicable | 100 | <1 | [38,70,71] |
| Ceramic Coatings | |||||||||||||
| Al2O3 Coatings | 1500–2200 | 0.005–0.01 | 0.35 | 4000 | 200–300 | 1200 | <0.01 | 5–8 | Non-conductive | Not applicable | 200 | <1 | [45,67,68,76] |
| TiN Coatings | 2000–2500 | 0.008 | 0.25 | 3000 | 400–600 | 600 | <0.02 | 20–30 | Non-conductive | Not applicable | 150 | <1 | [13,17] |
| SiC Coatings | 2500–3000 | 0.003 | 0.18 | 3500 | 500–700 | 1000 | <0.005 | 120–150 | Non-conductive | Not applicable | 180 | <1 | [63] |
| Composite Coatings | |||||||||||||
| Al-Al2O3-ZrO2 Coatings | 600–1000 | 0.003 | 0.40 | 2500 | 150–250 | 600 | <0.1 | 10–15 | Non-conductive | Not applicable | 80 | <1 | [75] |
| TiB2-TiC-Co Coatings | 1200–2200 | 0.001–0.005 | 0.15 | 2800 | 600–800 | 1000 | <0.01 | 25–35 | Non-conductive | Not applicable | 200 | <1 | [67] |
| HA/Ti Coatings | 800–1200 | 0.02–0.05 | 0.45 | 2000 | 100–200 | 500 | <0.10 | 5–10 | 5 × 10−6 | 50% increase | 30 | <1 | [78] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, Z.; Zhang, H.; Guo, X.; Geng, L. Preparation and Classification of Coatings by High-Energy Ball Milling: A Review. Coatings 2025, 15, 1343. https://doi.org/10.3390/coatings15111343
Qi Z, Zhang H, Guo X, Geng L. Preparation and Classification of Coatings by High-Energy Ball Milling: A Review. Coatings. 2025; 15(11):1343. https://doi.org/10.3390/coatings15111343
Chicago/Turabian StyleQi, Zhanfeng, Hengye Zhang, Xiuli Guo, and Le Geng. 2025. "Preparation and Classification of Coatings by High-Energy Ball Milling: A Review" Coatings 15, no. 11: 1343. https://doi.org/10.3390/coatings15111343
APA StyleQi, Z., Zhang, H., Guo, X., & Geng, L. (2025). Preparation and Classification of Coatings by High-Energy Ball Milling: A Review. Coatings, 15(11), 1343. https://doi.org/10.3390/coatings15111343






