Gellan Gum-Based Edible Coatings Enriched with Scenedesmus spp. Extract to Enhance the Postharvest Quality and Shelf Life of Mangoes
Abstract
1. Introduction
2. Materials and Methods
2.1. Fruit Material
2.2. Preparation and Application of the Composite Coating
2.3. Weight Loss and Soluble Solids
2.4. Determination of the Titratable Acidity
2.5. Determination of Color Change (ΔE)
2.6. Malondialdehyde Content
2.7. Determination of the Antioxidant Activity
2.8. Rate of Respiration and Ethylene Production
2.9. Hydrogen Peroxide (H2O2) Content
2.10. Statistical Analysis
3. Results and Discussion
3.1. Quality Characteristics of the Analyzed Mangoes
3.2. Malondialdehyde Content
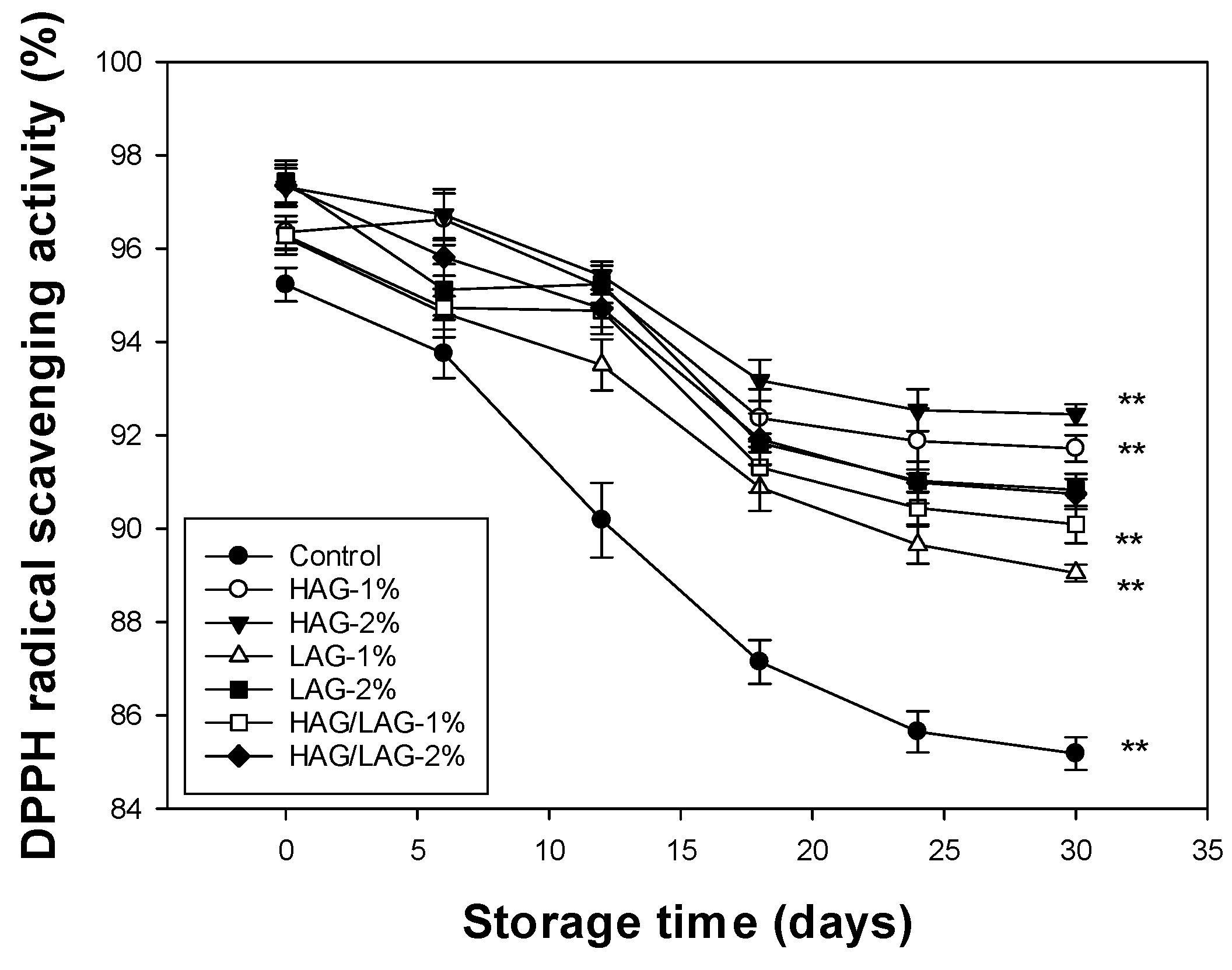
3.3. Determination of the Antioxidant Activity
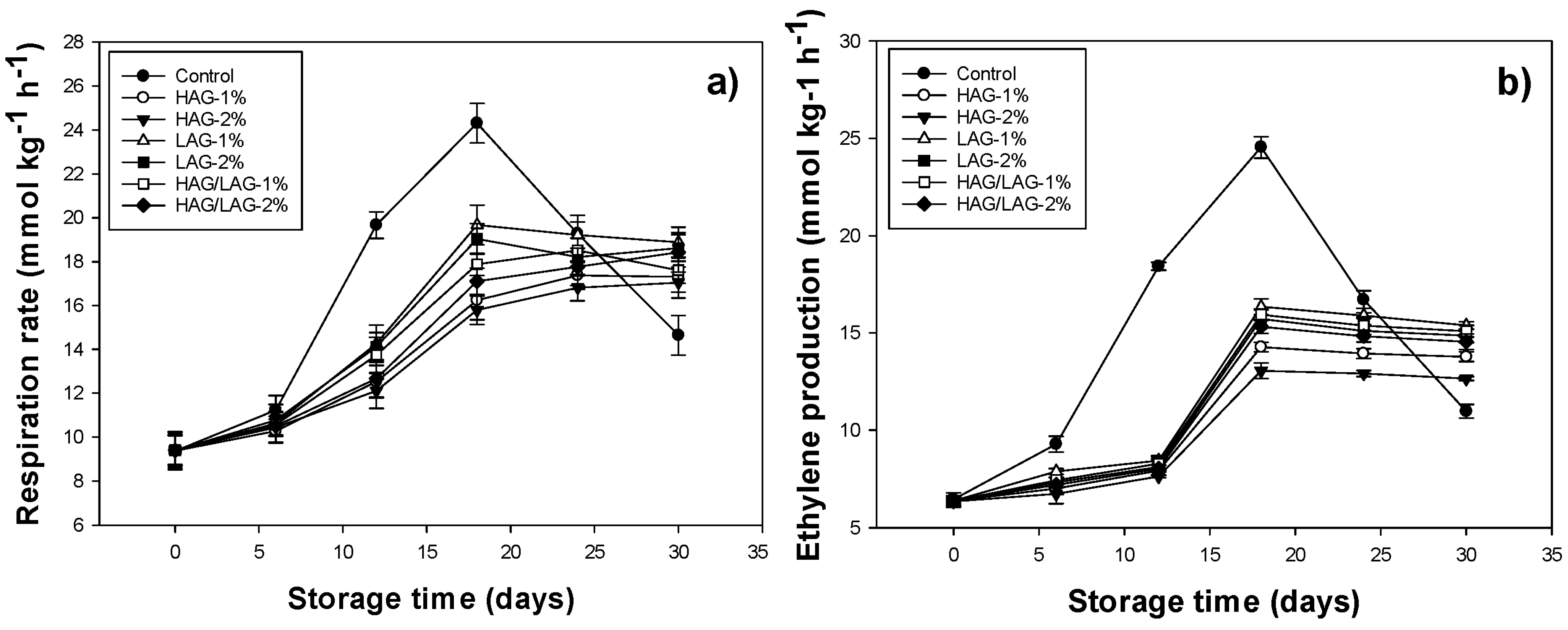
3.4. Rate of Respiration and Ethylene Production
3.5. Hydrogen Peroxide (H2O2) Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dai, L.; Wang, X.; Mao, X.; He, L.; Li, C.; Zhang, J.; Chen, Y. Recent Advances in Starch-Based Coatings for the Postharvest Preservation of Fruits and Vegetables. Carbohydr. Polym. 2024, 328, 121736. [Google Scholar] [CrossRef]
- Mumuni, S.; Aleer, M.J. Zero Hunger by 2030—Are We on Track? Climate Variability and Change Impacts on Food Security in Africa. Cogent Food Agric. 2022, 9, 2171830. [Google Scholar] [CrossRef]
- Tharanathan, R.N.; Yashoda, H.M.; Prabha, T.N. Mango (Mangifera indica L.), “The King of Fruits”—An Overview. Food Rev. Int. 2006, 22, 95–123. [Google Scholar] [CrossRef]
- Ribeiro, S.M.R.; Schieber, A. Bioactive Compounds in Mango (Mangifera indica L.). In Bioactive Foods in Promoting Health; Elsevier: Amsterdam, The Netherlands, 2010; pp. 507–523. [Google Scholar] [CrossRef]
- Liu, S.; Huang, H.; Huber, D.J.; Pan, Y.; Shi, X.; Zhang, Z. Delay of Ripening and Softening in ‘Guifei’ Mango Fruit by Postharvest Application of Melatonin. Postharvest Biol. Technol. 2020, 163, 111136. [Google Scholar] [CrossRef]
- Sousa, F.F.; Junior, J.S.P.; Oliveira, K.T.; Rodrigues, E.C.; Andrade, J.P.; Mattiuz, B.H. Conservation of ‘Palmer’ Mango with an Edible Coating of Hydroxypropyl Methylcellulose and Beeswax. Food Chem. 2021, 346, 128925. [Google Scholar] [CrossRef]
- Xu, B.; Wu, S. Preservation of Mango Fruit Quality Using Fucoidan Coatings. LWT 2021, 143, 111150. [Google Scholar] [CrossRef]
- Šuput, D.Z.; Lazić, V.L.; Popović, S.Z.; Hromiš, N.M. Edible Films and Coatings: Sources, Properties and Application. Food Feed Res. 2015, 42, 11–22. [Google Scholar] [CrossRef]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible Films and Coatings: Structures, Active Functions and Trends in Their Use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Khaliq, G. Effects of Edible Coatings Enriched with Calcium Chloride on Physiological, Biochemical and Quality Responses of Mango (Mangifera indica L. cv. Choke Anan) Fruit during Cold Storage. Ph.D. Thesis, Universiti Putra Malaysia, Serdang, Selangor, Malaysia, 2015. [Google Scholar]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent Advances on Polysaccharides, Lipids and Protein-Based Edible Films and Coatings: A Review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Bremenkamp, I.; Sousa Gallagher, M.J. Edible Coatings for Ready-to-Eat Products: Critical Review of Recent Studies, Sustainable Packaging Perspectives, Challenges and Emerging Trends. Polymers 2025, 17, 376. [Google Scholar] [CrossRef]
- Rajaei Lak, H.; Bazargani-Gilani, B.; Karami, M. Different Coating Application Methods: Zein-Based Edible Coating Containing Heracleum persicum Essential Oil for Shelf-Life Enhancement of Whey-Less Cheese. Food Sci. Nutr. 2024, 12, 5990–6010. [Google Scholar] [CrossRef] [PubMed]
- González-Cuello, R.; Fuentes, L.G.; Castellanos, H.M.; Hernández-Fernández, J.; Ortega-Toro, R. Composite Coatings with Liposomes of Melissa officinalis Extract for Extending Tomato Shelf Life. J. Compos. Sci. 2024, 8, 283. [Google Scholar] [CrossRef]
- Danalache, F.; Carvalho, C.Y.; Alves, V.D.; Moldão-Martins, M.; Mata, P. Optimisation of gellan gum edible coating for ready-to-eat mango (Mangifera indica L.) bars. Int. J. Biol. Macromol. 2016, 84, 43–53. [Google Scholar] [CrossRef]
- Wang, M.; Wei, Z.; Zhang, Z. Antimicrobial Edible Films for Food Preservation: Recent Advances and Future Trends. Food Bioprocess Technol. 2024, 17, 1391–1411. [Google Scholar] [CrossRef]
- Molaei Moqbeli, M.; Abdollahi, F.; Rastegar, S.; Jafari, L. Bio-based edible coatings for mango preservation: Investigating the effects of moringa leaf extract and chitosan on shelf life and fruit quality. S. Afr. J. Bot. 2025, 187, 97–107. [Google Scholar] [CrossRef]
- Barros de Medeiros, V.P.; da Costa, W.K.A.; da Silva, R.T.; Pimentel, T.C.; Magnani, M. Microalgae as Source of Functional Ingredients in New-Generation Foods: Challenges, Technological Effects, Biological Activity, and Regulatory Issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 4929–4950. [Google Scholar] [CrossRef]
- Shirai, M.A.; Baú, T.R.; Zanela, J.; Pimentel, T.C. Microalgae as an Innovative Active Ingredient for Edible Films and Coatings for Food Applications. Algal Res. 2025, 86, 103959. [Google Scholar] [CrossRef]
- Ahamed, A.A.P.; Rasheed, M.U.; Noorani, K.P.M.; Reehana, N.; Santhoshkumar, S.; Mohamed Imran, Y.M.; Alharbi, N.S.; Arunachalam, C.; Alharbi, S.A.; Akbarsha, M.A.; et al. In Vitro Antibacterial Activity of MGDG-Palmitoyl from Oscillatoria acuminata NTAPC05 against Extended-Spectrum β-Lactamase Producers. J. Antibiot. 2017, 70, 754–762. [Google Scholar] [CrossRef]
- Martha, S.T.; José, L.P.D.; Claudia, O.M. Influence of Composite Edible Coating of Pectin, Glycerol, and Oregano Essential Oil on Postharvest Deterioration of Mango Fruit. Food Sci. Nutr. 2024, 12, 10646–10654. [Google Scholar] [CrossRef]
- Fitch-Vargas, P.R.; Aguilar-Palazuelos, E.; Ruiz-Armenta, X.A.; Delgado-Nieblas, C.I.; Barraza-Elenes, C.; Calderón-Castro, A. Development of Edible Films Based on Reactive Extrusion Succinylated Corn Starch for the Preservation of Mango (Mangifera indica L. cv. Kent). J. Food Meas. Charact. 2024, 18, 2345–2358. [Google Scholar] [CrossRef]
- Almeida, M.F.; Silva, G.L.; Gondim, G.D.; Alves, C.E.F.; Silva, M.C.; Mendes, B.D.B.; Veloso, C.M. Maintenance of Postharvest Quality of “Palmer” Mango Coated with Biodegradable Coatings Based on Cassava Starch and Emulsion of Lemongrass Essential Oil. Int. J. Biol. Macromol. 2024, 277, 134323. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Velikova, V.; Loreto, F. On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ. 2005, 28, 318–327. [Google Scholar] [CrossRef]
- Lufu, R.; Ambaw, A.; Opara, U. Water loss of fresh fruit: Influencing pre-harvest, harvest and postharvest factors. Sci. Hortic. 2020, 272, 109519. [Google Scholar] [CrossRef]
- Yu, K.; Zhou, L.; Xu, J.; Jiang, F.; Zhong, Z.; Zou, L.; Liu, W. Carboxymethyl cellulose-based water barrier coating regulated postharvest quality and ROS metabolism of pakchoi (Brassica chinensis L.). Postharvest Biol. Technol. 2022, 185, 111804. [Google Scholar] [CrossRef]
- Hasan, M.U.; Singh, Z.; Shah, H.M.S.; Kaur, J.; Woodward, A. Water loss: A postharvest quality marker in apple storage. Food Bioprocess Technol. 2024, 17, 2155–2180. [Google Scholar] [CrossRef]
- Hassan, M.; Ali, S. Carboxymethyl cellulose coating delays quality deterioration in harvested table grapes during cold and ambient storage conditions. Prog. Org. Coat. 2025, 200, 109031. [Google Scholar] [CrossRef]
- Amarante, C.; Banks, N.H. Postharvest physiology and quality of coated fruits and vegetables. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; Volume 26, pp. 161–238. [Google Scholar] [CrossRef]
- Pang, X.; Huang, Y.; Xiao, N.; Wang, Q.; Feng, B.; Shad, M.A. Effect of EVA film and chitosan coating on quality and physicochemical characteristics of mango fruit during postharvest storage. Food Chem. X 2024, 21, 101169. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, M.; Wang, W.; Xia, Y.; Wu, G.; Deng, H.; Lin, Q. Konjac glucomannan/carboxylated cellulose nanofiber-based edible coating with tannic acid maintains quality and prolongs shelf-life of mango fruit. Food Chem. 2025, 478, 143750. [Google Scholar] [CrossRef]
- Díaz-Mula, H.M.; Serrano, M.; Valero, D. Alginate coatings preserve fruit quality and bioactive compounds during storage of sweet cherry fruit. Food Bioprocess Technol. 2012, 5, 2990–2997. [Google Scholar] [CrossRef]
- Khaliq, G.; Mohamed, M.T.M.; Ghazali, H.M.; Ding, P.; Ali, A. Influence of gum arabic coating enriched with calcium chloride on physiological, biochemical and quality responses of mango (Mangifera indica L.) fruit stored under low temperature stress. Postharvest Biol. Technol. 2016, 111, 362–369. [Google Scholar] [CrossRef]
- Dong, F.; Wang, X. Guar gum and ginseng extract coatings maintain the quality of sweet cherry. LWT Food Sci. Technol. 2018, 89, 117–122. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Guo, W.; Dai, L.; Chen, W. Modification of cell wall polysaccharide during ripening of Chinese bayberry fruit. Sci. Hortic. 2013, 160, 155–162. [Google Scholar] [CrossRef]
- Burdon, J.; Pidakala, P.; Martin, P.; Billing, D.; Boldingh, H. Fruit maturation and the soluble solids harvest index for ‘Hayward’ kiwifruit. Sci. Hortic. 2016, 213, 193–198. [Google Scholar] [CrossRef]
- Razzaq, K.; Singh, Z.; Khan, A.S.; Khan, S.A.K.U.; Ullah, S. Role of 1-MCP in regulating ‘Kensington Pride’ mango fruit softening and ripening. Plant Growth Regul. 2016, 78, 401–411. [Google Scholar] [CrossRef]
- Su, L.; Diretto, G.; Purgatto, E.; Danoun, S.; Zouine, M.; Li, Z.; Roustan, J.P.; Bouzayen, M.; Giuliano, G.; Chervin, C. Carotenoid accumulation during tomato fruit ripening is modulated by the auxin-ethylene balance. BMC Plant Biol. 2015, 15, 114. [Google Scholar] [CrossRef]
- Sudheeran, P.K.; Love, C.; Feygenberg, O.; Maurer, D.; Ovadia, R.; Oren-Shamir, M.; Alkan, N. Induction of red skin and improvement of fruit quality in ‘Kent’, ‘Shelly’ and ‘Maya’ mangoes by preharvest spraying of prohydrojasmon at the orchard. Postharvest Biol. Technol. 2019, 149, 18–26. [Google Scholar] [CrossRef]
- Lo’ay, A.A.; Taher, M.A. Influence of edible coatings chitosan/PVP blending with salicylic acid on biochemical fruit skin browning incidence and shelf life of guava fruits cv. ‘Banati’. Sci. Hortic. 2018, 235, 424–436. [Google Scholar] [CrossRef]
- Saberi, B.; Golding, J.B.; Marques, J.R.; Pristijono, P.; Chockchaisawasdee, S.; Scarlett, C.J.; Stathopoulos, C.E. Application of biocomposite edible coatings based on pea starch and guar gum on quality, storability and shelf life of ‘Valencia’ oranges. Postharvest Biol. Technol. 2018, 137, 9–20. [Google Scholar] [CrossRef]
- Mirshekari, A.; Madani, B.; Yahia, E.M.; Golding, J.B.; Vand, S.H. Postharvest melatonin treatment reduces chilling injury in sapota fruit. J. Sci. Food Agric. 2020, 100, 1897–1903. [Google Scholar] [CrossRef]
- Xiao, J.; Gu, C.; Zhu, D.; Huang, Y.; Luo, Y.; Zhou, Q. Development and characterization of an edible chitosan/zein-cinnamaldehyde nano-cellulose composite film and its effects on mango quality during storage. LWT 2021, 140, 110809. [Google Scholar] [CrossRef]
- Andrade, P.B.; Barbosa, M.; Matos, R.P.; Lopes, G.; Vinholes, J.; Mouga, T.; Valentão, P. Valuable compounds in macroalgae extracts. Food Chem. 2013, 138, 1819–1828. [Google Scholar] [CrossRef]
- Ansari, M.A.; Anurag, A.; Fatima, Z.; Hameed, S. Natural phenolic compounds: A potential antifungal agent. Microb. Pathog. 2013, 56, 189–195. [Google Scholar]
- Subbuvel, M.; Kavan, P. Preparation and characterization of polylactic acid/fenugreek essential oil/curcumin composite films for food packaging applications. Int. J. Biol. Macromol. 2022, 194, 470–483. [Google Scholar] [CrossRef] [PubMed]
- González-Cuello, R.; Hernández-Fernández, J.; Ortega-Toro, R. Composite coating enriched with lemon peel extract for enhancing the postharvest quality of cherry tomatoes. Coatings 2025, 15, 810. [Google Scholar] [CrossRef]
- González, R.; Ramos, G.; Cruz, A.; Salazar, A. Rheological characterization and activation energy values of binary mixtures of gellan. Eur. Food Res. Technol. 2012, 234, 305–313. [Google Scholar] [CrossRef]
- Fagundes, C.; Palou, L.; Monteiro, A.R.; Pérez-Gago, M.B. Effect of antifungal hydroxypropyl methylcellulose-beeswax edible coatings on gray mold development and quality attributes of cold-stored cherry tomato fruit. Postharvest Biol. Technol. 2014, 92, 1–8. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Development and application of rice starch-based edible coating to improve the postharvest storage potential and quality of plum fruit (Prunus salicina). Sci. Hortic. 2018, 237, 59–66. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Khan, A.S.; Nawaz, A.; Ejaz, S.; Khaliq, G.; Iqbal, S.; Ullah, S.; Rehman, R.N.U.; Ali, M.M. Carboxymethyl cellulose coating delays ripening of harvested mango fruits by regulating softening enzymes activities. Food Chem. 2022, 380, 131804. [Google Scholar] [CrossRef]
- Fagundes, C.; Moraes, K.; Pérez-Gago, M.; Palou, L.; Maraschin, M.; Monteiro, A. Effect of active modified atmosphere and cold storage on the postharvest quality of cherry tomatoes. Postharvest Biol. Technol. 2015, 109, 73–81. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Hu, M.; Pan, Y.; Xu, X.; Zhang, Z. Delay of ripening and senescence in mango fruit by 6-benzylaminopurine is associated with inhibition of ethylene biosynthesis and membrane lipid catabolism. Postharvest Biol. Technol. 2022, 185, 111797. [Google Scholar] [CrossRef]
- Gull, S.; Ejaz, S.; Ali, S.; Ali, M.M.; Sardar, H.; Azam, M.; Deng, H.; Yousef, A.F.; Alrefaei, A.F.; Almutairi, M.H. Xanthan gum-based edible coating effectively preserves postharvest quality of ‘Gola’ guava fruits by regulating physiological and biochemical processes. BMC Plant Biol. 2024, 24, 450. [Google Scholar] [CrossRef]
- Naveed, F.; Nawaz, A.; Ali, S.; Ejaz, S. Xanthan gum coating delays ripening and softening of jujube fruit by reducing oxidative stress and suppressing cell wall polysaccharides disassembly. Postharvest Biol. Technol. 2024, 209, 112689. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Boonyaritthongchai, P.; Buanong, M.; Supapvanich, S.; Wongs-Aree, C. Chitosan- and κ-carrageenan-based composite coating on dragon fruit (Hylocereus undatus) pretreated with plant growth regulators maintains bract chlorophyll and fruit edibility. Sci. Hortic. 2021, 281, 109916. [Google Scholar] [CrossRef]
- Qasim, M.; Ali, S.; Javed, H.U.; Naz, S.; Khan, A.S.; Hassan, M.; Rehman, R.N.U. Xanthan gum coating delays ripening of harvested mango (Mangifera indica L.) fruit by suppressing chlorophyll catabolism and cell wall polysaccharides degradation. Postharvest Biol. Technol. 2025, 228, 113635. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Cuello, R.; Hernández-Fernández, J.; Ortega-Toro, R. Gellan Gum-Based Edible Coatings Enriched with Scenedesmus spp. Extract to Enhance the Postharvest Quality and Shelf Life of Mangoes. Coatings 2025, 15, 1333. https://doi.org/10.3390/coatings15111333
González-Cuello R, Hernández-Fernández J, Ortega-Toro R. Gellan Gum-Based Edible Coatings Enriched with Scenedesmus spp. Extract to Enhance the Postharvest Quality and Shelf Life of Mangoes. Coatings. 2025; 15(11):1333. https://doi.org/10.3390/coatings15111333
Chicago/Turabian StyleGonzález-Cuello, Rafael, Joaquín Hernández-Fernández, and Rodrigo Ortega-Toro. 2025. "Gellan Gum-Based Edible Coatings Enriched with Scenedesmus spp. Extract to Enhance the Postharvest Quality and Shelf Life of Mangoes" Coatings 15, no. 11: 1333. https://doi.org/10.3390/coatings15111333
APA StyleGonzález-Cuello, R., Hernández-Fernández, J., & Ortega-Toro, R. (2025). Gellan Gum-Based Edible Coatings Enriched with Scenedesmus spp. Extract to Enhance the Postharvest Quality and Shelf Life of Mangoes. Coatings, 15(11), 1333. https://doi.org/10.3390/coatings15111333










