Enhancing the Mechanical Robustness of Aerosol-Based Brittle Pt/C Electrodes Through Thermal Annealing
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating Solution Materials
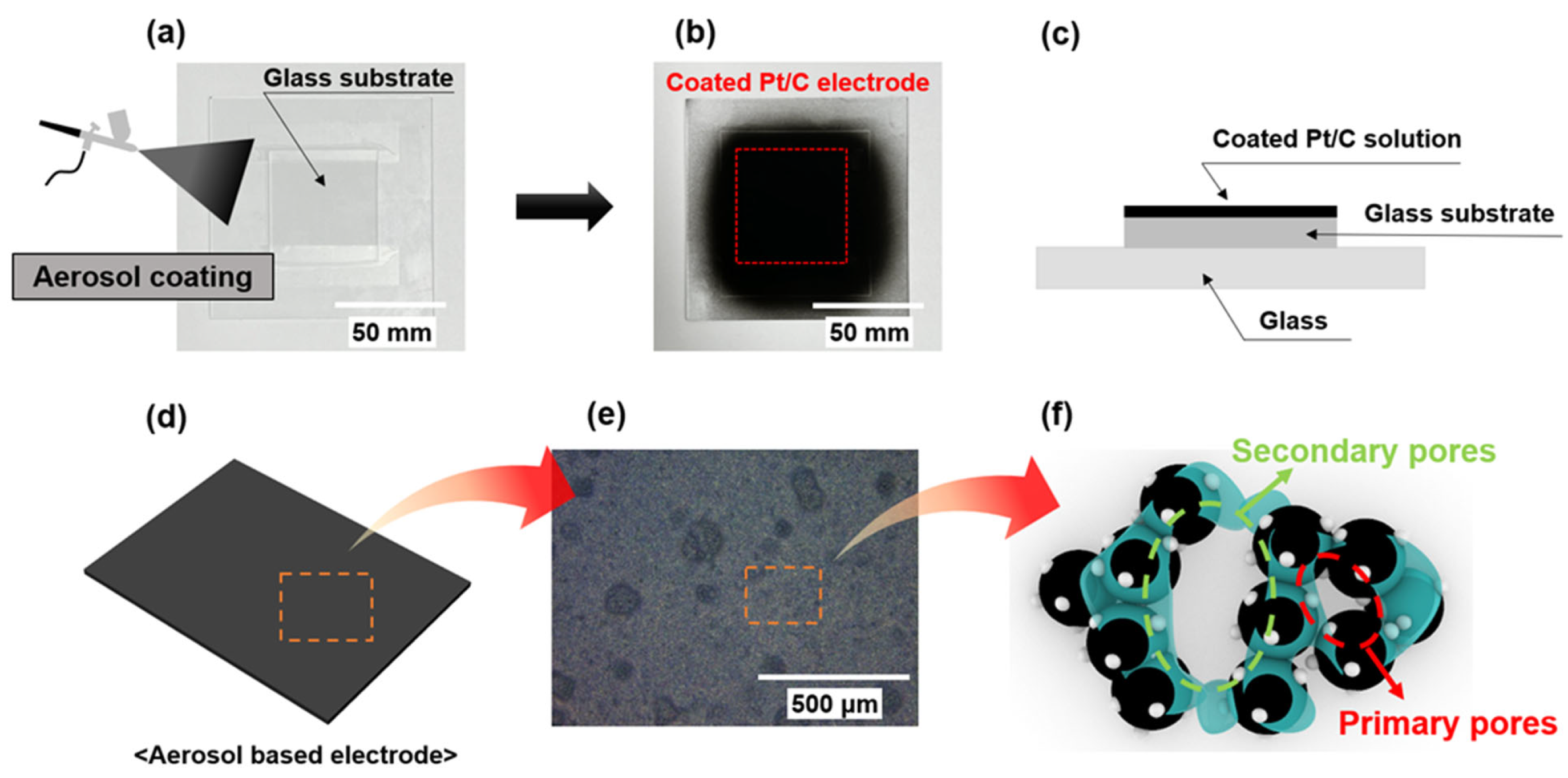
2.2. Pt/C Electrode Fabrication
2.3. Free-Standing Tensile Test Specimen Preparation
2.4. Characterization
2.5. Finite Element Simulations
3. Results and Discussion
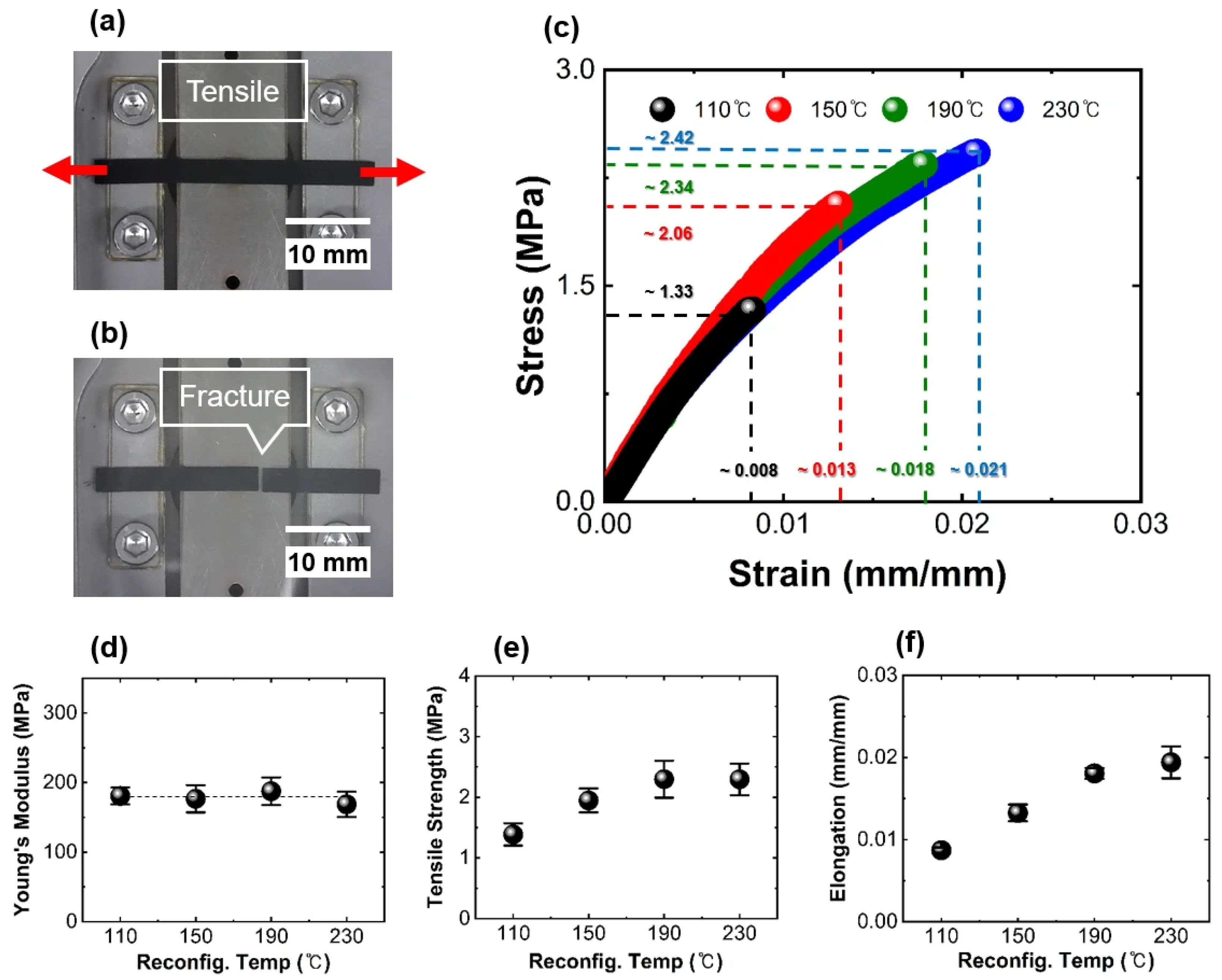
3.1. Mechanical Properties of the Aerosol-Based Electrode
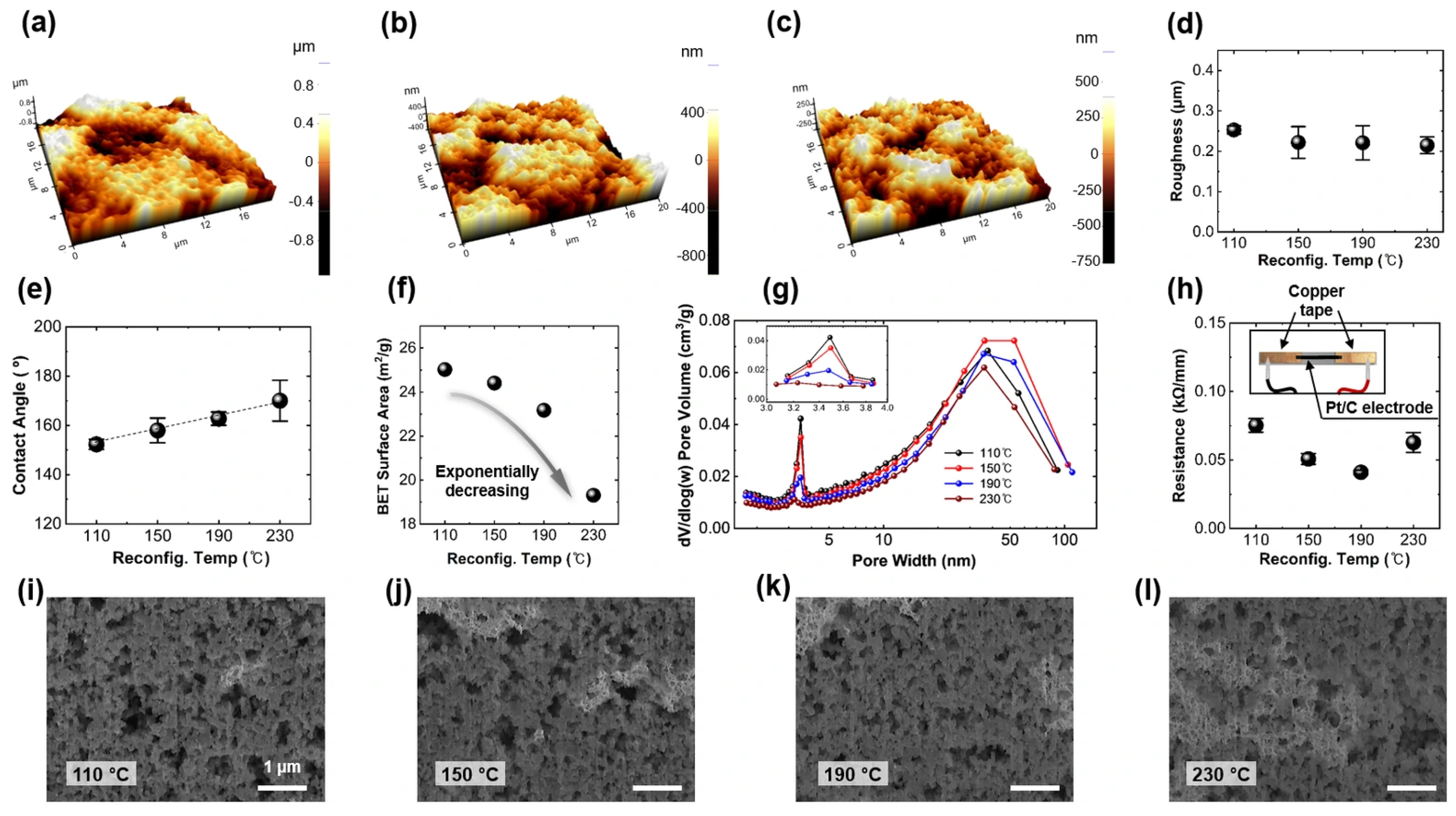
3.2. Electrode Surface Characteristics
3.3. Pore Structure Characteristics of the Pore Structure of the Electrodes
3.4. Thermally Induced Reconfiguration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett–Joyner–Halenda |
| MEA | membrane electrode assembly |
| PDMS | Polydimethylsiloxane |
| PTFE | Polytetrafluoroethylene |
| SS | stress–strain |
| Tg | glass transition temperature |
References
- Lopez-Haro, M.; Guétaz, L.; Printemps, T.; Morin, A.; Escribano, S.; Jouneau, P.H.; Bayle-Guillemaud, P.; Chandezon, F.; Gebel, G. Three-dimensional analysis of Nafion layers in fuel cell electrodes. Nat. Commun. 2014, 5, 5229. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Park, J.H.; Kabir, S.; Neyerlin, K.C.; Kariuki, N.N.; Lv, H.; Stamenkovic, V.R.; Myers, D.J.; Ulsh, M.; Mauger, S.A. Impact of catalyst ink dispersing methodology on fuel cell performance using in-situ X-ray scattering. ACS Appl. Energy Mater. 2019, 2, 6417–6427. [Google Scholar] [CrossRef]
- Mo, S.; Du, L.; Huang, Z.; Chen, J.; Zhou, Y.; Wu, P.; Meng, L.; Wang, N.; Xing, L.; Zhao, M.; et al. Recent advances on PEM fuel cells: From key materials to membrane electrode assembly. Electrochem. Energy Rev. 2023, 6, 28. [Google Scholar] [CrossRef]
- Fink, C.; Karpenko-Jereb, L.; Ashton, S. Advanced CFD analysis of an air-cooled PEM fuel cell stack predicting the loss of performance with time. Fuel Cells 2016, 16, 490–503. [Google Scholar] [CrossRef]
- Cao, X.; Guo, H.; Han, Y.; Li, M.; Shang, C.; Zhao, R.; Huang, Q.; Li, M.; Zhang, Q.; Lv, F.; et al. Sandwiching intermetallic Pt3Fe and ionomer with porous N-doped carbon layers for oxygen reduction reaction. Nat. Commun. 2025, 16, 2851. [Google Scholar] [CrossRef]
- Feng, Q.; Yuan, X.Z.; Liu, G.; Wei, B.; Zhang, Z.; Li, H.; Wang, H. A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sources 2017, 366, 33–55. [Google Scholar] [CrossRef]
- Long, C.; Fang, F.; Wang, J.; Xia, B.; Shao, C.; Yu, Y.; Wang, Y. Tailoring carbon supports for high-stability platinum-based catalysts in proton exchange membrane water electrolysis. Fuller. Nanotub. Carbon Nanostruct. 2025, 33, 1–12. [Google Scholar] [CrossRef]
- Dai, J.; Liu, D.; Yin, X.; Wen, X.; Cai, G.; Zheng, L. Anisotropic elastomer ionomer composite-based strain sensors: Achieving high sensitivity and wide detection for human motion detection and wireless transmission. ACS Sens. 2024, 9, 2156–2165. [Google Scholar] [CrossRef]
- Lee, J.W.; Yoo, Y.T. Preparation and performance of IPMC actuators with electrospun Nafion®–MWNT composite electrodes. Sens. Actuators B 2011, 159, 103–111. [Google Scholar] [CrossRef]
- Xu, B.; Wang, S.; Zhang, Z.; Ling, J.; Wu, X. Improving the torsion performance of IPMC by changing the electrode separation. Sci. Rep. 2021, 11, 7639. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xia, K.; Wang, H.; Liang, X.; Yin, Z.; Zhang, Y. Advanced carbon for flexible and wearable electronics. Adv. Mater. 2019, 31, e1801072. [Google Scholar] [CrossRef]
- Suzuki, T.; Tanaka, H.; Hayase, M.; Tsushima, S.; Hirai, S. Investigation of porous structure formation of catalyst layers for proton exchange membrane fuel cells and their effect on cell performance. Int. J. Hydrogen Energy 2016, 41, 20326–20335. [Google Scholar] [CrossRef]
- Sabharwal, M.; Pant, L.M.; Putz, A.; Susac, D.; Jankovic, J.; Secanell, M. Analysis of catalyst layer microstructures: From imaging to performance. Fuel Cells 2016, 16, 734–753. [Google Scholar] [CrossRef]
- Larbi, B.; Alimi, W.; Chouikh, R.; Guizani, A. Effect of porosity and pressure on the PEM fuel cell performance. Int. J. Hydrogen Energy 2013, 38, 8542–8549. [Google Scholar] [CrossRef]
- Ahn, K.H.; Pyo, J.B.; Song, H.; Kim, T.S. Evaluation of stress distribution in carbon-based nanoporous electrode by three-dimensional nanostructural reconstruction. Sustain. Mater. Technol. 2024, 41, e01112. [Google Scholar] [CrossRef]
- Yu, H. Optimization of Ultra-Low Loading Catalyst Layers for Pemfc and Pemw Using Reactive Spray Deposition Technology; University of Connecticut: Storrs, CT, USA, 2017; Available online: https://www.proquest.com/dissertations-theses/optimization-ultra-low-loading-catalyst-layers/docview/3073206907/se-2 (accessed on 20 August 2025).
- Fouzaï, I.; Gentil, S.; Bassetto, V.C.; Silva, W.O.; Maher, R.; Girault, H.H. Catalytic layer-membrane electrode assembly methods for optimum triple phase boundaries and fuel cell performances. J. Mater. Chem. A 2021, 9, 11096–11123. [Google Scholar] [CrossRef]
- Zhao, J.; He, X.; Wang, L.; Tian, J.; Wan, C.; Jiang, C. Addition of NH4HCO3 as pore-former in membrane electrode assembly for PEMFC. Int. J. Hydrogen Energy 2007, 32, 380–384. [Google Scholar] [CrossRef]
- Fischer, A.; Jindra, J.; Wendt, H. Porosity and catalyst utilization of thin layer cathodes in air operated PEM-fuel cells. J. Appl. Electrochem. 1998, 28, 277–282. [Google Scholar] [CrossRef]
- Tranter, T.G.; Tam, M.; Gostick, J.T. The effect of cracks on the in-plane electrical conductivity of PEFC catalyst layers. Electroanalysis 2019, 31, 619–623. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Giuliani, F.; Atkinson, A. Analyses of microstructural and elastic properties of porous SOFC cathodes based on focused ion beam tomography. J. Power Sources 2015, 273, 486–494. [Google Scholar] [CrossRef]
- Gumirova, V.; Razumovskaya, I.; Apel, P.; Bedin, S.; Naumov, A. The influence of mechanical stress micro fields around pores on the strength of elongated etched membrane. Membranes 2022, 12, 1168. [Google Scholar] [CrossRef] [PubMed]
- Zeleniakienė, D.; Kleveckas, T.; Liukaitis, J.; Fataraitė, E. The influence of porosity value and mode on soft polymer materials behaviour. Mater. Sci. 2003, 9, 201–205. [Google Scholar]
- Qiu, D.; Peng, L.; Lai, X.; Ni, M.; Lehnert, W. Mechanical failure and mitigation strategies for the membrane in a proton exchange membrane fuel cell. Renew. Sustain. Energy Rev. 2019, 113, 109289. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kim, J.W. Role of the glass transition temperature of nafion 117 membrane in the preparation of the membrane electrode assembly in a direct methanol fuel cell (DMFC). Int. J. Hydrogen Energy 2012, 37, 12580–12585. [Google Scholar] [CrossRef]
- Pyo, J.B.; Kim, J.H.; Kim, K.; Kim, T.S. Electrical resistance change in thermally reconfigured nanoporous ionomer-bound carbon films. J. Mater. Chem. A 2021, 9, 13019–13025. [Google Scholar] [CrossRef]
- Pyo, J.B.; Kim, J.H.; Kim, T.S. Highly robust nanostructured carbon films by thermal reconfiguration of ionomer binding. J. Mater. Chem. A 2020, 8, 24763–24773. [Google Scholar] [CrossRef]
- Suzuki, A.; Sen, U.; Hattori, T.; Miura, R.; Nagumo, R.; Tsuboi, H.; Hatakeyama, N.; Endou, A.; Takaba, H.; Williams, M.C.; et al. Ionomer content in the catalyst layer of polymer electrolyte membrane fuel cell (PEMFC): Effects on diffusion and performance. Int. J. Hydrogen. Energy 2011, 36, 2221–2229. [Google Scholar] [CrossRef]
- Qi, Z.; Kaufman, A. Low Pt loading high performance cathodes for PEM fuel cells. J. Power Sources 2003, 113, 37–43. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J.H.; Oh, J.G.; Jang, K.L.; Jeong, B.H.; Hong, B.K.; Kim, T.S. Mechanical behavior of free-standing fuel cell electrodes on water surface. ACS Appl. Mater. Interfaces 2016, 8, 15391–15398. [Google Scholar] [CrossRef]
- Pyo, J.B.; Kim, J.H.; Kim, T.S. Prevention of frost-driven self-fracture of ionomer-bound carbon films by controlling freezable water domain size. Carbon Energy 2025, 7, e70098. [Google Scholar] [CrossRef]
- Palma, V.; Vaiano, V.; Matarangolo, M.; Anello, G. Comparison of Pt/C electrocatalyst deposition methods for PEM fuel cells. Chem. Eng. Trans. 2018, 70, 1525–1530. [Google Scholar] [CrossRef]
- Pyo, J.B.; Lee, S.; Kim, T.S. Intrinsic swelling behavior of free-standing nanoporous ionomer-bound carbon films. Polym. Test. 2021, 100, 107241. [Google Scholar] [CrossRef]
- Ciniero, A.; Fatti, G.; Marsili, M.; Dini, D.; Righi, M.C. Defects drive the tribocharging strength of PTFE: An ab-initio study. Nano Energy 2023, 112, 108502. [Google Scholar] [CrossRef]
- Kim, J.H.; Nizami, A.; Hwangbo, Y.; Jang, B.; Lee, H.J.; Woo, C.S.; Hyun, S.; Kim, T.S. Tensile testing of ultra-thin films on water surface. Nat. Commun. 2013, 4, 2520. [Google Scholar] [CrossRef]
- Lee, T.I.; Kim, J.H.; Oh, E.S.; Kim, T.S. Direct tensile testing of free-standing ultrathin polymer films on liquid surface at high temperature. Small Methods 2025, 9, e2401291. [Google Scholar] [CrossRef]
- Ma, S.; Qin, Y.; Liu, Y.; Sun, L.; Guo, Q.; Yin, Y. Delamination evolution of PEM fuel cell membrane/CL interface under asymmetric RH cycling and CL crack location. Appl. Energy 2022, 310, 118551. [Google Scholar] [CrossRef]
- Bahrami, M.; Chen, Y.; Kumar, N.; Orfino, F.P.; Dutta, M.; Lauritzen, M.; Setzler, E.; Agapov, A.L.; Kjeang, E. Degradation and deformation patterns in reinforced fuel cell membranes subjected to chemo-mechanical stress testing. J. Electrochem. Soc. 2025, 172, 054502. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, F.; Li, B.; Dai, H.; Zheng, J.P.; Zhang, C.; Ming, P. Numerical analysis of static and dynamic heat transfer behaviors inside proton exchange membrane fuel cell. J. Power Sources 2021, 488, 229419. [Google Scholar] [CrossRef]
- Bozkurt, K.; Akyalçın, L.; Kjelstrup, S. The thermal diffusion coefficient of membrane-electrode assemblies relevant to polymer electrolyte membrane fuel cells. Int. J. Hydrogen. Energy 2023, 48, 1501–1513. [Google Scholar] [CrossRef]
- Vokoun, D.; Samal, S.; Stachiv, I. Impact of Initial Cyclic Loading on Mechanical Properties and Performance of Nafion. Sensors 2023, 23, 1488. [Google Scholar] [CrossRef]
- Uchiyama, T.; Kato, M.; Ikogi, Y.; Yoshida, T. Mechanical degradation mechanism of membrane electrode assemblies in buckling test under humidity cycles. J. Fuel Cell Sci. Technol. 2012, 9, 061005. [Google Scholar] [CrossRef]
- Paul, D.K.; Karan, K. Conductivity and wettability changes of ultrathin Nafion films subjected to thermal annealing and liquid water exposure. J. Phys. Chem. C 2014, 118, 1828–1835. [Google Scholar] [CrossRef]
- Park, Y.J.; Choi, W.Y.; Park, S.H.; Choi, H.; Choi, S.W.; Jyoung, J.Y.; Lee, E.; Park, J.L.; Ko, M.J.; Lee, K.T.; et al. Crack-engineered microporous layer for mitigating cathode flooding in polymer electrolyte fuel cells. ACS Energy Lett. 2025, 10, 3241–3248. [Google Scholar] [CrossRef]



| Materials | Parameters | |||
|---|---|---|---|---|
| (MPa) | E (MPa) | (MPa) | Reference | |
| 6.34 | 132.75 | 10.99 | Measured | |
| 6.32 | 153.0 | 8.69 | Measured | |
| 4.16 | 95.91 | 17.71 | Measured | |
| 6.33 | 150.73 | 12.21 | Measured | |
| 0.81 | 192.79 | 77.89 | Measured | |
| 1.31 | 176.67 | 69.78 | Measured | |
| 1.62 | 190.41 | 55.82 | Measured | |
| 1.54 | 186.74 | 51.19 | Measured | |
| Parameters | Materials | |||
| Electrode | Membrane | Reference | ||
| Density, | 21,450 | 1970 | [39,40] | |
| Poisson’s ratio, ν | 0.25 | 0.4 | [41,42] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, N.; Jeong, W.-Y.; Kim, J.H.; Pyo, J.-B. Enhancing the Mechanical Robustness of Aerosol-Based Brittle Pt/C Electrodes Through Thermal Annealing. Coatings 2025, 15, 1331. https://doi.org/10.3390/coatings15111331
Heo N, Jeong W-Y, Kim JH, Pyo J-B. Enhancing the Mechanical Robustness of Aerosol-Based Brittle Pt/C Electrodes Through Thermal Annealing. Coatings. 2025; 15(11):1331. https://doi.org/10.3390/coatings15111331
Chicago/Turabian StyleHeo, Nathan, Won-Yong Jeong, Ji Hun Kim, and Jae-Bum Pyo. 2025. "Enhancing the Mechanical Robustness of Aerosol-Based Brittle Pt/C Electrodes Through Thermal Annealing" Coatings 15, no. 11: 1331. https://doi.org/10.3390/coatings15111331
APA StyleHeo, N., Jeong, W.-Y., Kim, J. H., & Pyo, J.-B. (2025). Enhancing the Mechanical Robustness of Aerosol-Based Brittle Pt/C Electrodes Through Thermal Annealing. Coatings, 15(11), 1331. https://doi.org/10.3390/coatings15111331





