Intelligent Sensing and Responsive Separators for Lithium Batteries Using Functional Materials and Coatings for Safety Enhancement
Abstract
1. Introduction

2. Materials and Techniques for Intelligent Separators
2.1. Materials and Coatings for Intelligent Separators
2.1.1. Intelligent Sensing
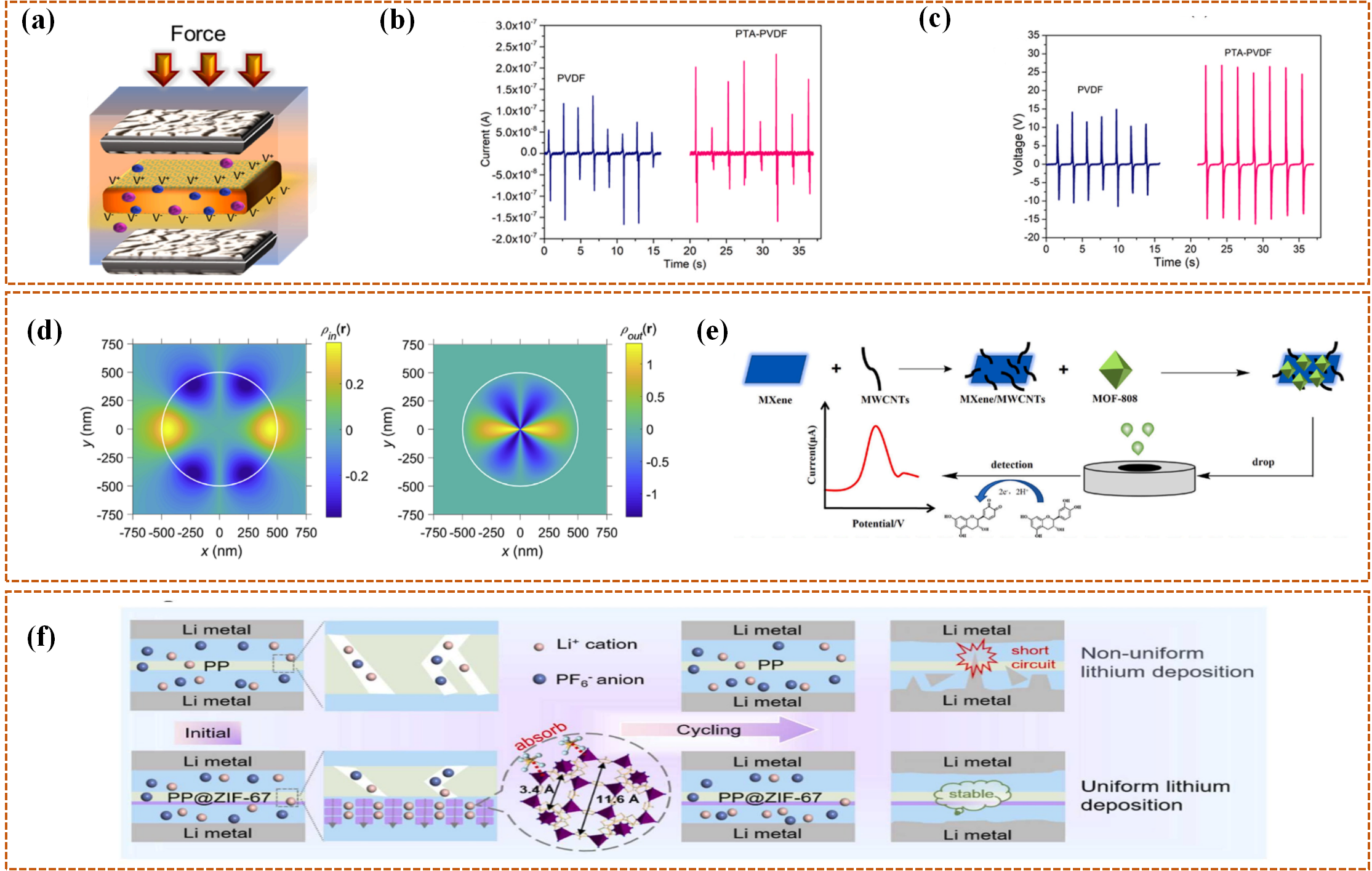
2.1.2. Materials for Intelligent Response
2.2. Fabrication Techniques for Intelligent Separators
2.2.1. Electrospinning
2.2.2. Atomic Layer Deposition (ALD)

2.2.3. Synergistic Application of Layer-by-Layer Self-Assembly (LbL), Spraying, and Coating Techniques
3. Intelligent Sensing of Separators
3.1. Temperature Sensing Separator
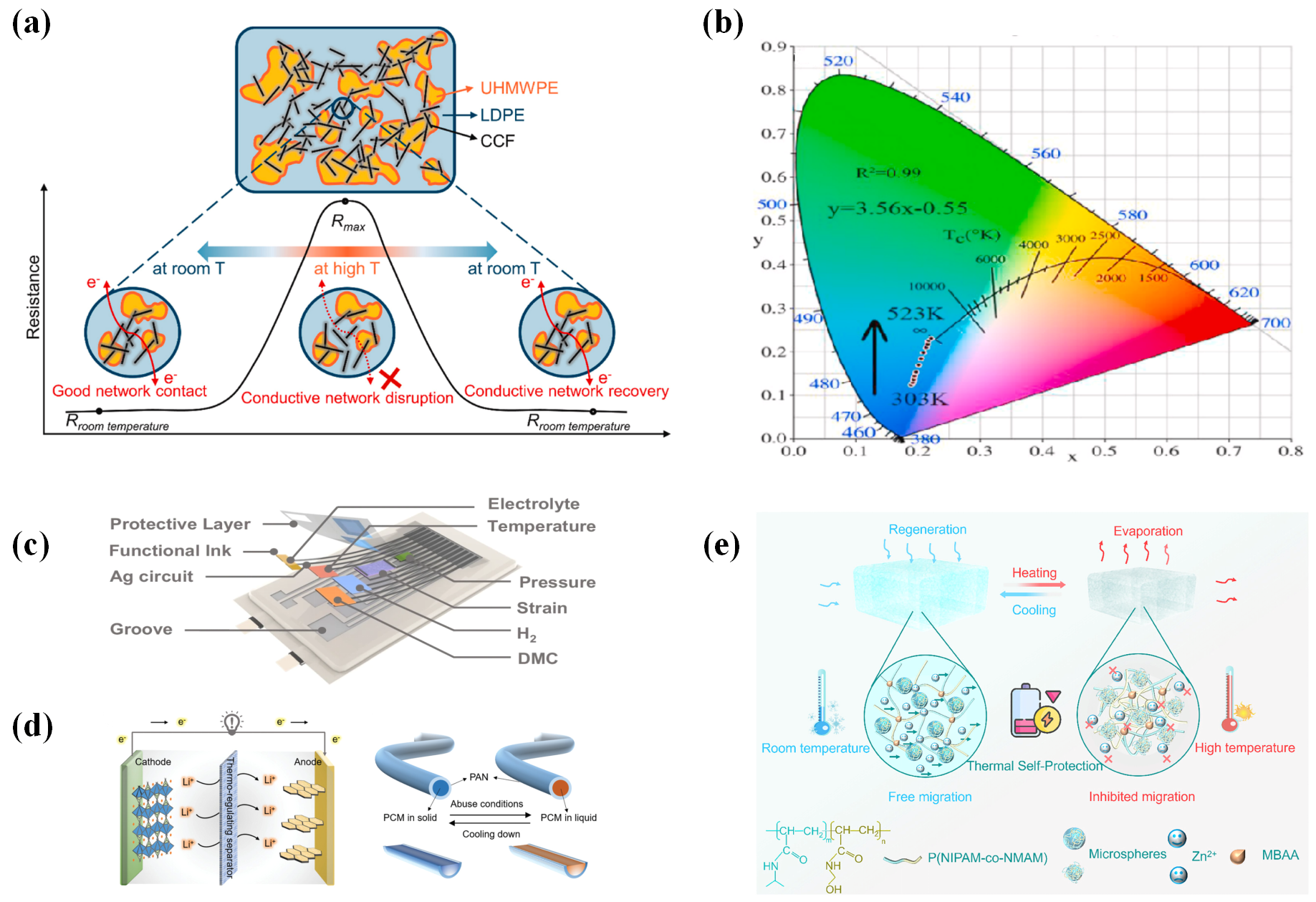
3.2. Mechanical Pressure/Strain Sensing Separator
3.3. Chemical Substance Sensing Separator
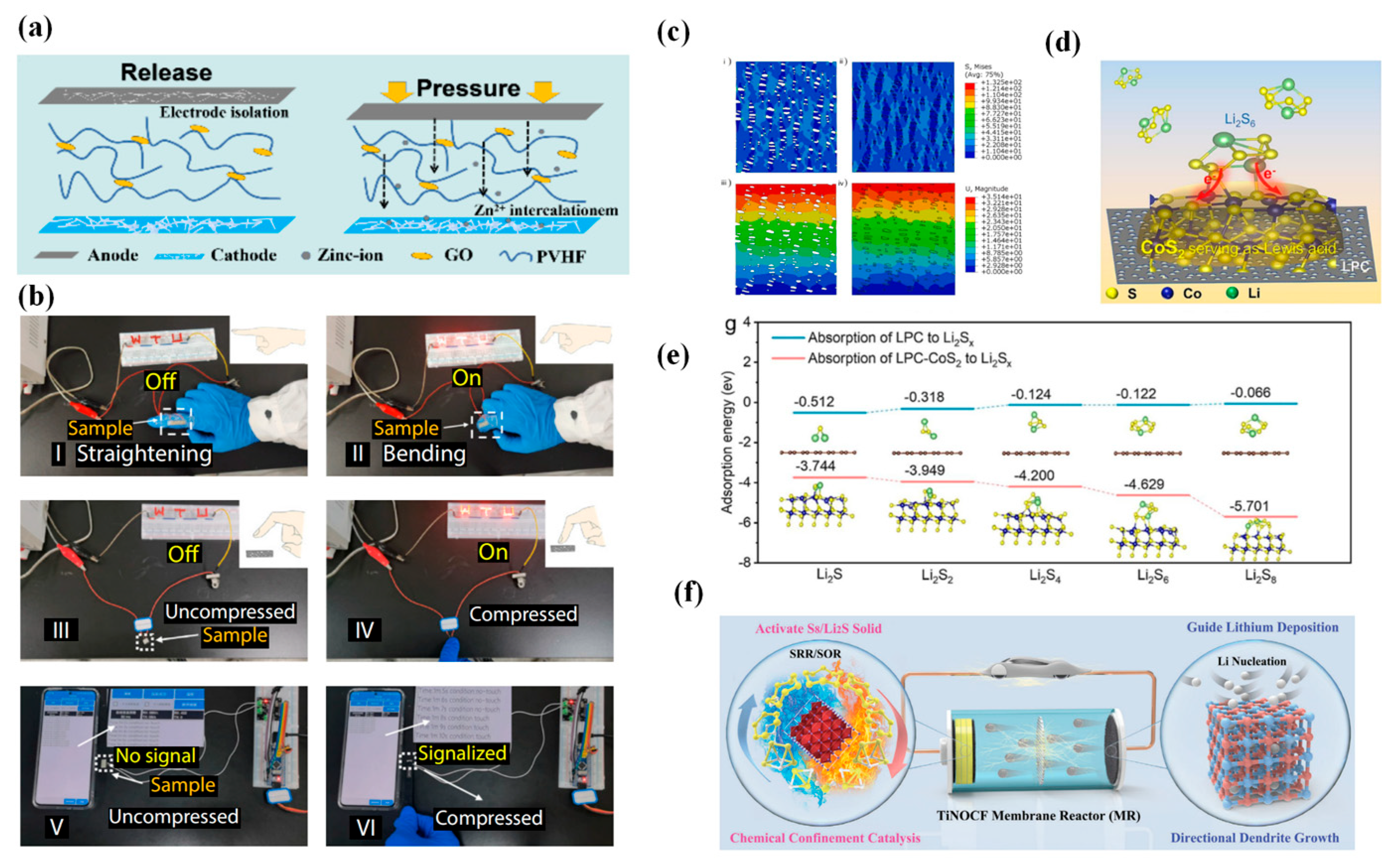
3.4. Preliminary Exploration of Ion Flow Distribution Sensing
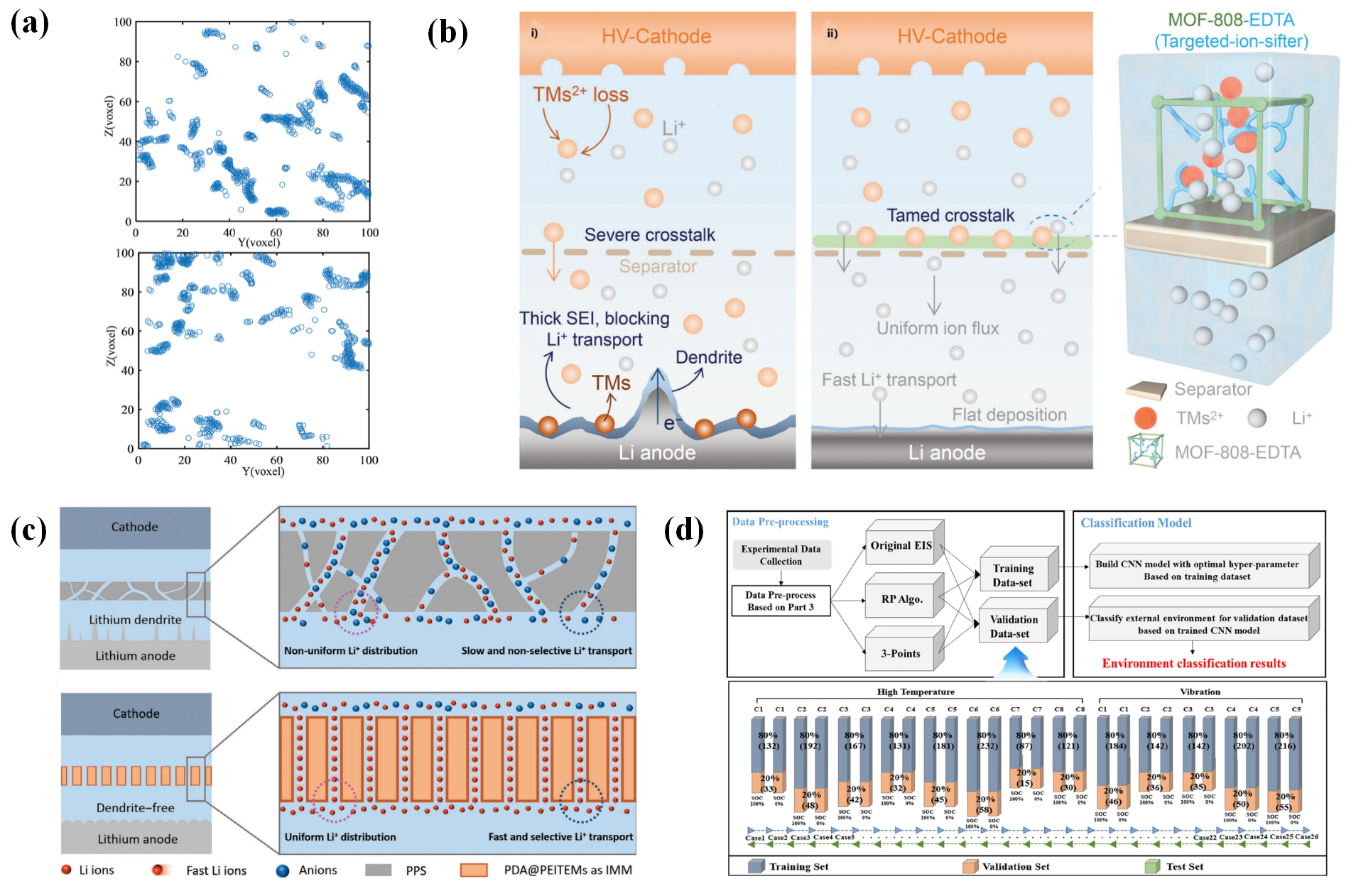
4. Intelligent Response of Separators
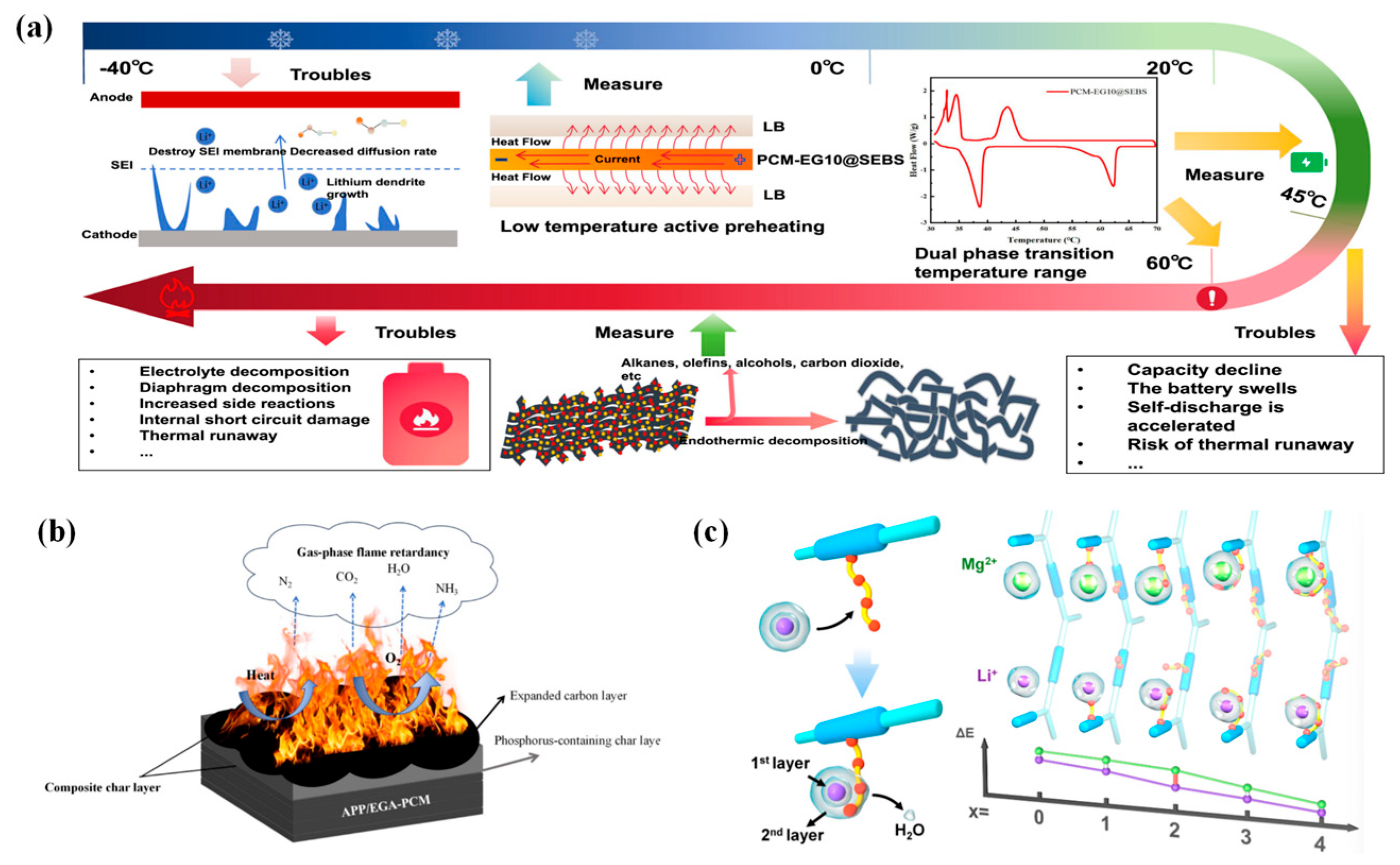
4.1. Thermally Responsive Intelligent Separators
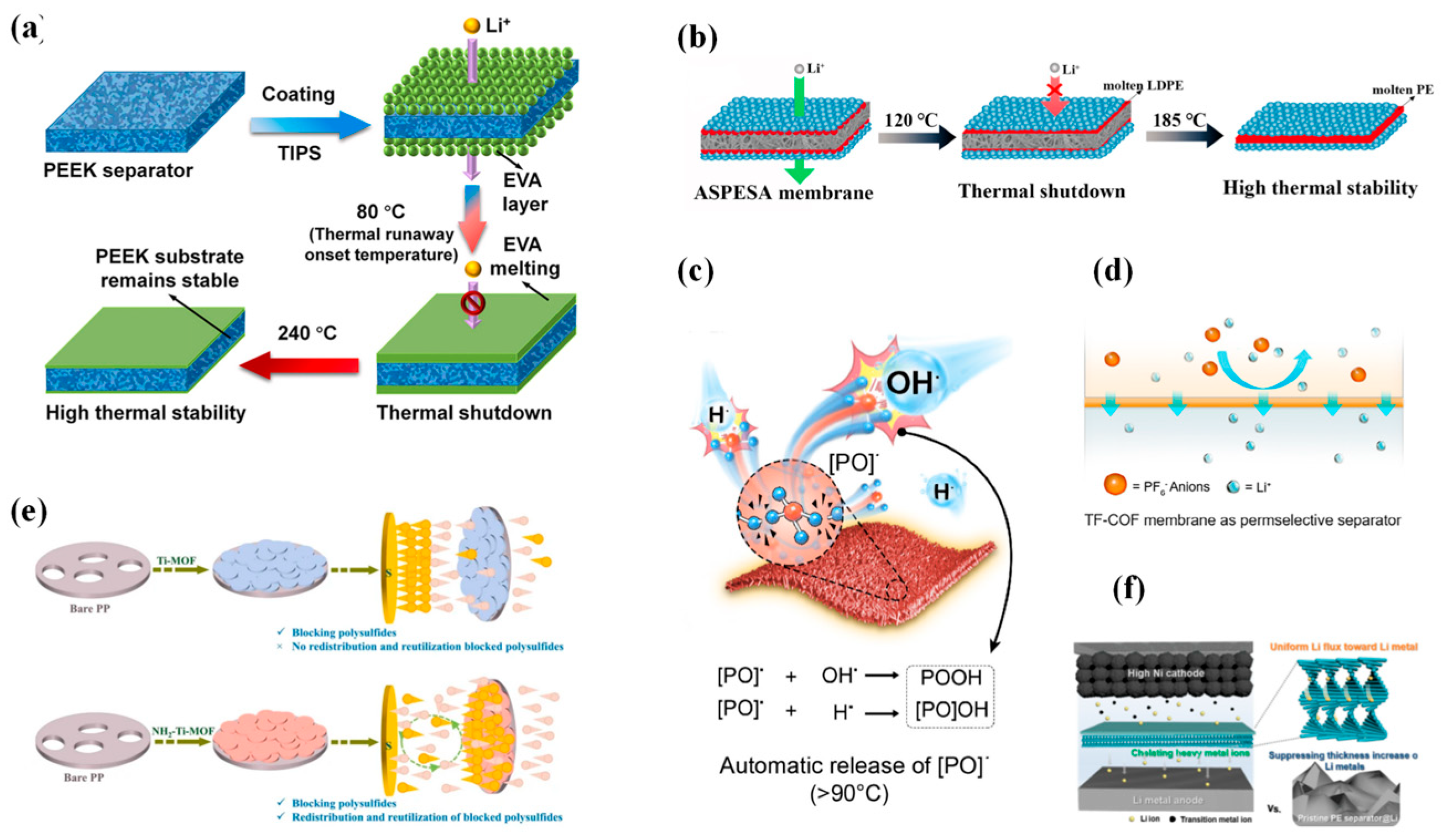
4.2. Electrochemically Responsive Intelligent Separators
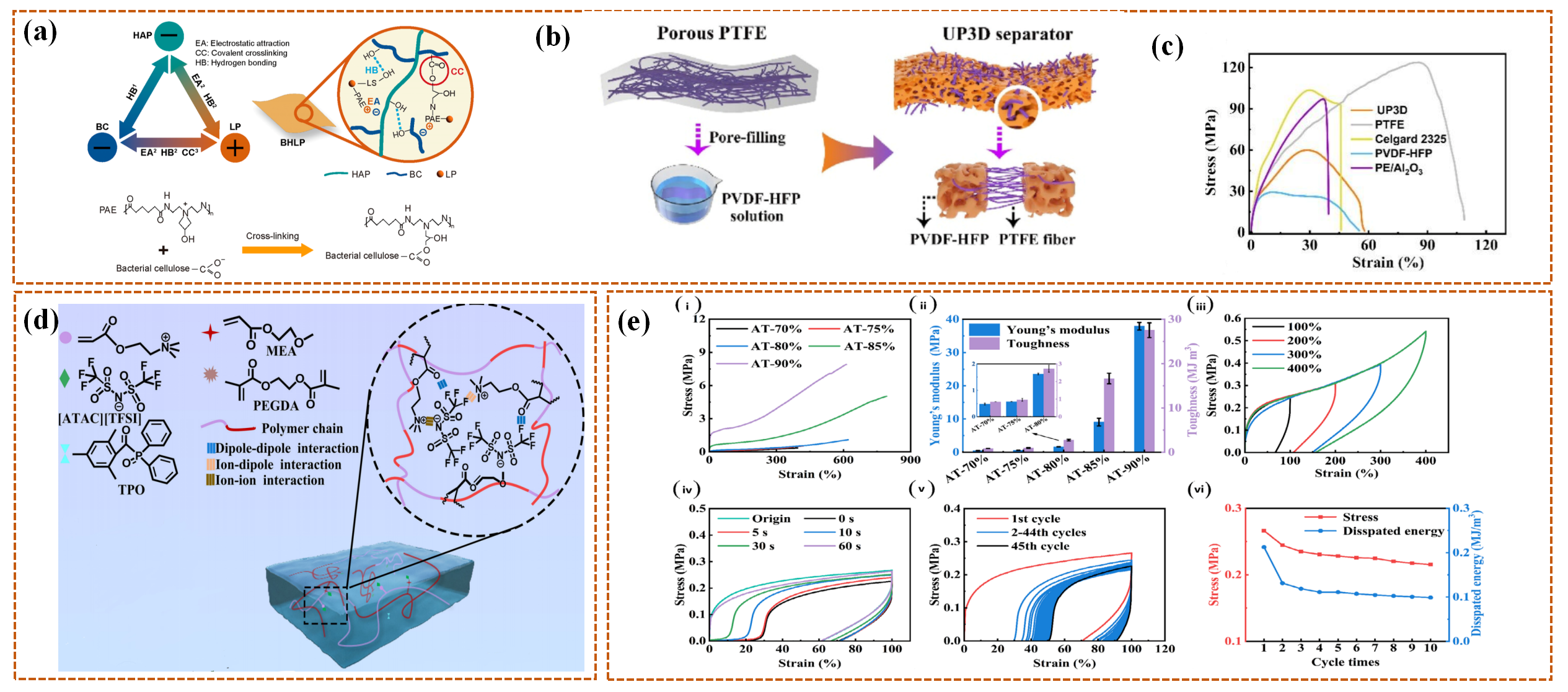
4.3. Mechanical Response Intelligent Separators
5. Multifunctional Integration of Separators
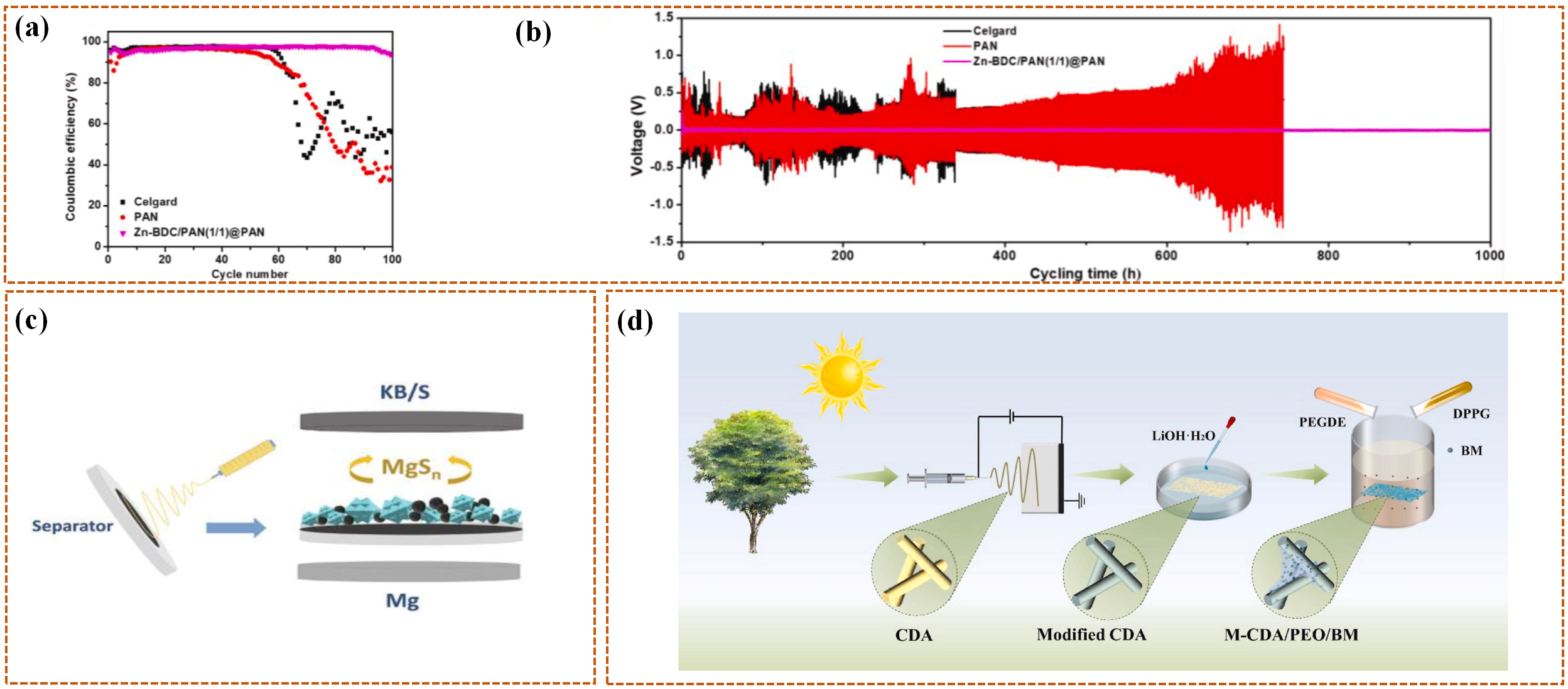
5.1. Integrated “Sensing-Response” Separators
5.2. Intelligent Separators with Multilayer Structure Design
6. Challenges and Future Prospects
6.1. Challenges
6.2. Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thackeray, M.M.; Amine, K. Layered Li–Ni–Mn–Co oxide cathodes. Nat. Energy 2021, 6, 933. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Wei, Z.; Yu, L.; Lu, S.; Zhao, Y. Reversibly thermo-responsive materials applied in lithium batteries. Energy Storage Mater. 2023, 61, 102901. [Google Scholar] [CrossRef]
- Chen, Z.; Jia, L.; Yin, L.; Dang, C.; Ren, H.; Zhang, Z. A Review on Thermal Management of Li-ion Battery: From Small-Scale Battery Module to Large-Scale Electrochemical Energy Storage Power Station. J. Therm. Sci. 2024, 34, 1–23. [Google Scholar] [CrossRef]
- Du, C.; Zhao, Z.; Liu, H.; Song, F.; Chen, L.; Cheng, Y.; Guo, Z. The Status of Representative Anode Materials for Lithium-Ion Batteries. Chem. Rec. 2023, 23, e202300004. [Google Scholar] [CrossRef]
- Khan, F.M.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N.K. Design and optimization of lithium-ion battery as an efficient energy storage device for electric vehicles: A comprehensive review. J. Energy Storage 2023, 71, 108033. [Google Scholar] [CrossRef]
- Cao, Y.; Zuo, P.; Lou, S.; Sun, Z.; Li, Q.; Huo, H.; Ma, Y.; Du, C.; Gao, Y.; Yin, G. A quasi-solid-state Li–S battery with high energy density, superior stability and safety. J. Mater. Chem. A 2019, 7, 6533–6542. [Google Scholar] [CrossRef]
- Xing, W. Development of a High Energy Density, Long Cycle Life and Safety Enhanced Li-S Battery. ECS Meet. Abstr. 2020, 97, 121. [Google Scholar] [CrossRef]
- Zhu, Z.; Xue, M.; Wu, X.; Zheng, J.; Wang, Q.; Zhang, C.; Geng, Z. Construction of solid-state lithium batteries with high energy density and high safety by in-situ polymerization. Chem. Eng. J. 2025, 514, 163402. [Google Scholar] [CrossRef]
- Kong, L.; Li, Y.; Feng, W. Strategies to Solve Lithium Battery Thermal Runaway: From Mechanism to Modification. Electrochem. Energy Rev. 2021, 4, 633–679. [Google Scholar] [CrossRef]
- Kim, H.J.; Umirov, N.; Park, J.-S.; Lim, J.-H.; Zhu, J.; Kim, S.-S.; Myung, S.-T. Lithium dendritic growth inhibitor enabling high capacity, dendrite-free, and high current operation for rechargeable lithium batteries. Energy Storage Mater. 2022, 46, 76–89. [Google Scholar] [CrossRef]
- Du, H.; Wang, Y.; Kang, Y.; Zhao, Y.; Tian, Y.; Wang, X.; Tan, Y.; Liang, Z.; Wozny, J.; Li, T.; et al. Side Reactions/Changes in Lithium-Ion Batteries: Mechanisms and Strategies for Creating Safer and Better Batteries. Adv. Mater. 2024, 36, 2401482. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Z.; Wang, Z.; Liu, P.; Liu, Z.; Lin, N. Timely Thermal Runaway Prognosis for Battery Systems in Real-World Electric Vehicles Based on Temperature Abnormality. IEEE J. Emerg. Sel. Top. Power Electron. 2022, 11, 120–130. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Li, Y.; Sun, J.; Zhao, Y.; Zhang, J.; Jin, C.; Xu, C.; Feng, X.; Wang, H. Tracing the patterns of thermal runaway in LFP lithium-ion batteries. J. Energy Storage 2025, 125, 117007. [Google Scholar] [CrossRef]
- Li, Y.; Hua, Y.; Cai, S.; Zhou, R.; Wang, M.; Wei, Z.; Zhao, Y. Separator’s contribution to the ion transport in lithium batteries. Energy Storage Mater. 2025, 82, 104636. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kumar, A.; Park, H.; Kim, H.; Ahmed, M.S.; Saleque, A.M.; Vikram, M.P.; Saidur, R.; Ma, Y.; Hwang, J.-Y. Composite separators for internal thermal management in rechargeable lithium batteries: A review. J. Energy Storage 2023, 73, 108873. [Google Scholar] [CrossRef]
- Fan, Z.; Chen, X.; Shi, J.; Nie, H.; Zhang, X.; Zhou, X.; Xie, X.; Xue, Z. Functionalized Separators Boosting Electrochemical Performances for Lithium Batteries. Nano-Micro Lett. 2025, 17, 128. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, N.; Guo, Z.; Wang, M.; Qiu, Y.; Tian, D.; Guan, B.; Fan, L.; Zhang, N. Constructing Multi-functional Janus Separator toward Highly Stable lithium Batteries. Energy Storage Mater. 2020, 28, 153–159. [Google Scholar] [CrossRef]
- Kim, K.W.; Lee, J.H.; Shin, S.; Kim, I.; Yun, T.G.; Hwang, B.; Choi, S.-J.; Jung, J.-W.; Yoon, K.R. Thermally Stable Al2O3/PTFE Composite Separator for High-Safety Lithium-Ion Batteries. ACS Omega 2025, 10, 39669–39679. [Google Scholar] [CrossRef]
- Lin, G.; Bai, Z.; Liu, C.; Liu, S.; Han, M.; Huang, Y.; Liu, X. Mechanically robust, nonflammable and surface cross-linking composite membranes with high wettability for dendrite-proof and high-safety lithium-ion batteries. J. Membr. Sci. 2022, 647, 120262. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Q.-S.; Zhu, G.-R.; Wu, G.; Wang, X.-L.; Wang, Y.-Z. Cooperation of metal-organic coordination complex separator and wide-temperature-range electrolyte enables safe and high-performance lithium batteries. J. Energy Chem. 2024, 99, 692–702. [Google Scholar] [CrossRef]
- Ugata, Y.; Motoki, C.; Nishikawa, S.; Yabuuchi, N. Improved reversibility of lithium deposition and stripping with high areal capacity under practical conditions through enhanced wettability of the polyolefin separator to highly concentrated electrolytes. Energy Adv. 2023, 2, 503–507. [Google Scholar] [CrossRef]
- Vinci, V.; Flachard, D.; Henke, H.; Bouchet, R.; Drockenmuller, E. Enhancing the Performances of Lithium Batteries through Functionalization of Porous Polyolefin Separators with Cross-Linked Single-Ion Polymer Electrolytes. ACS Appl. Mater. Interfaces 2025, 17, 25742–25753. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Han, J.; Gu, J.; Feng, Y.; Liu, G.; Yuan, H. Co-pyrolysis of polyolefin separators and active materials from lithium batteries: Thermal conversion performance, interaction behaviors, and products. Fuel 2025, 404, 136198. [Google Scholar] [CrossRef]
- Narla, A.; Fu, W.; Kulaksizoglu, A.; Kume, A.; Johnson, B.R.; Raman, A.S.; Wang, F.; Magasinski, A.; Kim, D.; Kousa, M.; et al. Nanodiamond-Enhanced Nanofiber Separators for High-Energy Lithium-Ion Batteries. ACS Appl. Mater. Interfaces 2023, 15, 32678–32686. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Hussain, A.; Raza, W.; Cai, X.; Liu, D.; Shen, J. Review on current development of polybenzimidazole membrane for lithium battery. J. Energy Chem. 2024, 91, 579–608. [Google Scholar] [CrossRef]
- Su, M.; Huang, G.; Wang, S.; Wang, Y.; Wang, H. High safety separators for rechargeable lithium batteries. Sci. China Chem. 2021, 64, 1131–1156. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Meng, G.; Zhang, J. Function-directed design of battery separators based on microporous polyolefin membranes. J. Mater. Chem. A 2022, 10, 14137–14170. [Google Scholar] [CrossRef]
- Cheng, H.; Tan, R.; Li, J.; Huang, J.; Song, W. Coatings on Lithium Battery Separators: A Strategy to Inhibit Lithium Dendrites Growth. Molecules 2023, 28, 7788. [Google Scholar] [CrossRef]
- Li, Z.; Liao, C.; Han, L.; Cao, Y.; Huang, J.; Kan, Y.; Hu, Y. A flame-retardant, dendrite-inhibiting sandwich separator prepared by electrospinning for high-performance and safety lithium batteries. Polym. Adv. Technol. 2024, 35, e6384. [Google Scholar] [CrossRef]
- Hyun, D.-E.; Jung, Y.-J.; Kim, T.-W.; Koo, S.-M.; Park, C.; Kim, H.S.; Kim, S.; Shin, W.H.; Lee, D.-W.; Oh, J.-M. Multi-functional Al2O3-coated separators for high-performance lithium-ion batteries: Critical effects of particle shape. Ceram. Int. 2023, 49, 30147–30155. [Google Scholar] [CrossRef]
- Song, Y.H.; Wu, K.J.; Zhang, T.W.; Lu, L.L.; Guan, Y.; Zhou, F.; Wang, X.X.; Yin, Y.C.; Tan, Y.H.; Li, F.; et al. A Nacre-Inspired Separator Coating for Impact-Tolerant Lithium Batteries. Adv. Mater. 2019, 31, e1905711. [Google Scholar] [CrossRef] [PubMed]
- Fang, D.; Wang, Y.; Liu, X.; Yu, J.; Qian, C.; Chen, S.; Wang, X.; Zhang, S. Spider-Web-Inspired Nanocomposite-Modified Separator: Structural and Chemical Cooperativity Inhibiting the Shuttle Effect in Li–S Batteries. ACS Nano 2019, 13, 1563–1573. [Google Scholar] [CrossRef]
- Jiang, L.-L.; Deng, Y.-Z.; Luo, T.; Xie, R.; Ju, X.-J.; Wang, W.; Pan, D.-W.; Liu, Z.; Chu, L.-Y. A smart membrane with negative thermo-responsiveness in battery electrolyte solution. J. Membr. Sci. 2023, 692, 122266. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, Y.; Meng, T.; Yu, L.; Wang, S.; Hu, X. Thermal-triggered fire-extinguishing separators by phase change materials for high-safety lithium-ion batteries. Energy Storage Mater. 2022, 47, 445–452. [Google Scholar] [CrossRef]
- Yang, Y.; Bai, Z.; Liu, S.; Zhu, Y.; Zheng, J.; Chen, G.; Huang, B. Self-Protecting Aqueous Lithium-Ion Batteries. Small 2022, 18, e2203035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Hou, T.; Han, G.; Yu, Y.; Xu, H.; Huang, Y. Smart batteries: Materials, monitoring, and artificial intelligence. Chem. Soc. Rev. 2025, 54, 10006–10139. [Google Scholar] [CrossRef]
- Dang, Q.; Ling, X.; Zhang, Q.; Song, S.; Hu, X. Flexible Integrated Sensor Array with Long Cyclic Life and High Measuring Accuracy for Real-Time Monitoring of Internal Temperature and Pressure of Lithium Battery. Adv. Mater. Technol. 2025, 10, 2500152. [Google Scholar] [CrossRef]
- Peng, X.; Han, J.; Zhang, Q.; Xiang, Y.; Hu, X. Real-time mechanical and thermal monitoring of lithium batteries with PVDF-TrFE thin films integrated within the battery. Sens. Actuators A Phys. 2022, 338, 113484. [Google Scholar] [CrossRef]
- Wahl, M.S.; Lamb, J.; Sundby, E.; Thomas, P.J.; Hjelme, D.R.; Burheim, O.S. Towards in-situ State of Health Monitoring of Lithium-Ion Batteries Using Internal Fiber-Optic Sensors. ECS Meet. Abstr. 2022, MA2022-01, 2166. [Google Scholar] [CrossRef]
- Gu, J.; Feng, Y.; Wei, X.; Zhang, C.; Peng, T.; Lu, L.; Wei, Z.; Zhao, Y. Flexible fibrous separator asymmetrically coated by silica and MXene for high performance lithium batteries with enhanced safety. J. Power Sources 2023, 581, 233515. [Google Scholar] [CrossRef]
- Huang, Z.; Farahmandjou, M.; Marlton, F.; Guo, X.; Gao, H.; Sun, B.; Wang, G. Surface and structure engineering of MXenes for rechargeable batteries beyond lithium. J. Mater. 2023, 10, 253–268. [Google Scholar] [CrossRef]
- Gu, Y.; Du, J.; Ein-Eli, Y.; Hyun, W.J. Boosting rate capability and cycling stability of lithium-ion batteries with high-mass-loading electrodes via printable graphene on separators. J. Power Sources 2025, 645, 237210. [Google Scholar] [CrossRef]
- Yu, L.; Chen, X.; Yao, N.; Gao, Y.C.; Yuan, Y.H.; Gao, Y.B.; Tang, C.; Zhang, Q. Advanced carbon as emerging energy materials in lithium batteries: A theoretical perspective. InfoMat 2025, 7, e12653. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, Q.S.; Long, M.C.; Zhu, G.R.; Wu, G.; Wang, X.L.; Wang, Y.Z. Nano-Interfacial Supramolecular Adhesion of Metal–Organic Framework-Based Separator Enables High-Safety and Wide-Temperature-Range Lithium Batteries. Small 2024, 20, e2400980. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, T.; Zhang, W.; Gou, B.; Li, F.; Wang, H.; Deng, X.; Li, D.; Xu, H.; Huang, Y. Anion-repulsive polyoxometalate@MOF-modified separators for dendrite-free and high-rate lithium batteries. Interdiscip. Mater. 2024, 4, 190–200. [Google Scholar] [CrossRef]
- Zhong, J.; Tong, Y.; Guo, L.; Zhang, A.; Xu, Q.; Qin, Y. Cationic Covalent Organic Framework-Modified Polypropylene Separator for High-Performance Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2024, 16, 56106–56115. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Z.; Feng, Y.; Cai, S.; Gao, H.; Wei, Z.; Zhao, Y. Cellulose-based separators for lithium batteries: Source, preparation and performance. Chem. Eng. J. 2023, 471, 144593. [Google Scholar] [CrossRef]
- Liang, T.; Cheng, D.; Chen, J.; Wu, X.; Xiong, H.; Yu, S.; Zhang, Z.; Liu, H.; Liu, S.; Song, X. Evolution from passive to active components in lithium metal and lithium-ion batteries separators. Mater. Today Energy 2024, 45, 101684. [Google Scholar] [CrossRef]
- Tong, B.; Li, X. Towards separator safety of lithium-ion batteries: A review. Mater. Chem. Front. 2023, 8, 309–340. [Google Scholar] [CrossRef]
- Hu, F.; Ye, Z.; Pickel, A.D.; Tenhaeff, W.E. Operando characterization of lithium battery internal temperatures via upconverting nanoparticle thermometry. Nanoscale 2025, 17, 14597–14606. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, W.; Ma, Y.; Yao, Y.; Yu, T.; Zhang, W.; Guo, H.; Duan, X.; Yan, R.; Xu, D.; et al. A flexible integrated temperature-pressure sensor for wearable detection of thermal runaway in lithium batteries. Appl. Energy 2024, 381, 125191. [Google Scholar] [CrossRef]
- Qin, S.; Wang, Y.; Wu, X.; Zhang, X.; Zhu, Y.; Yu, N.; Zhang, Y.; Wu, Y. Nylon-Based Composite Gel Membrane Fabricated via Sequential Layer-By-Layer Electrospinning for Rechargeable Lithium Batteries with High Performance. Polymers 2020, 12, 1572. [Google Scholar] [CrossRef]
- Su, Y.; Hao, J.; Liu, X.; Yang, Y. Progress of Atomic Layer Deposition and Molecular Layer Deposition in the Development of All-Solid-State Lithium Batteries. Batter. Supercaps 2022, 6, e202200359. [Google Scholar] [CrossRef]
- Sun, H.; Dai, H.; Zhang, G.; Sun, S. Interface engineering of inorganic solid-state lithium batteries via atomic and molecular layer deposition. InfoMat 2025, 7, e12650. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, H.; Liu, K.; Dong, N.; Jia, N.; Liu, B.; Lin, D.; Zhao, Z.; Tian, G.; Qi, S.; et al. Separator Engineering Regulating Reversible Li Electrodeposition Enabled by Lithiophilic Polyimide@Ag Coating Layer for Dendrite-Free Lithium Metal Batteries. Small 2025, 21, e2504870. [Google Scholar] [CrossRef] [PubMed]
- Cortada-Torbellino, M.; Elvira, D.G.; El Aroudi, A.; Valderrama-Blavi, H. Review of Lithium-Ion Battery Internal Changes Due to Mechanical Loading. Batteries 2024, 10, 258. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, C.; Zhao, T.; Kuang, Y.; Yin, B.; Yuan, R.; Song, L. Review—Online Monitoring of Internal Temperature in Lithium-Ion Batteries. J. Electrochem. Soc. 2023, 170, 057517. [Google Scholar] [CrossRef]
- Rodrigues-Marinho, T.; Pace, G.; Tubio, C.R.; Lanceros-Méndez, S.; Costa, P. Poly(vinylidene fluoride) Copolymers for Hybrid Piezoelectric and Triboelectric Energy Harvesting. ACS Appl. Energy Mater. 2024, 7, 10454–10465. [Google Scholar] [CrossRef]
- Sun, W.; Gao, C.; Liu, H.; Zhang, Y.; Guo, Z.; Lu, C.; Qiao, H.; Yang, Z.; Jin, A.; Chen, J.; et al. Scaffold-Based Poly(Vinylidene Fluoride) and Its Copolymers: Materials, Fabrication Methods, Applications, and Perspectives. ACS Biomater. Sci. Eng. 2024, 10, 2805–2826. [Google Scholar] [CrossRef]
- Manoharan, S.; Pazhamalai, P.; Mariappan, V.K.; Murugesan, K.; Subramanian, S.; Krishnamoorthy, K.; Kim, S.-J. Proton conducting solid electrolyte-piezoelectric PVDF hybrids: Novel bifunctional separator for self-charging supercapacitor power cell. Nano Energy 2021, 83, 105753. [Google Scholar] [CrossRef]
- Tan, J.; Wang, Y.; Wang, Z.; He, X.; Liu, Y.; Wang, B.; Katsnelson, M.I.; Yuan, S. Large out-of-plane piezoelectricity of oxygen functionalized MXenes for ultrathin piezoelectric cantilevers and diaphragms. Nano Energy 2019, 65, 104058. [Google Scholar] [CrossRef]
- Chen, J.; Xue, W.; Shi, Y.; Liu, W.; Ma, R. MXene/MWCNTs-COOH/MOF-808-based electrochemical sensor for the detection of Catechin. Microchem. J. 2025, 215, 114339. [Google Scholar] [CrossRef]
- Han, G.; Hu, Q.; Xia, Y.; Gao, K.; Wang, Y.; Yao, J. Stabilizing dendrite-free lithium metal batteries via an anionphilic ZIF-67 modified separator. J. Alloys Compd. 2025, 1036, 181713. [Google Scholar] [CrossRef]
- Koo, W.-T.; Jang, J.-S.; Kim, I.-D. Metal-Organic Frameworks for Chemiresistive Sensors. Chem 2019, 5, 1938–1963. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, J.; Gao, Y.; Chen, Y.; Li, H.; Liao, Y. Polyphenylene sulfide woven membrane modified by ZIF-8 for alkaline electrolytic cell diaphragm. J. Power Sources 2025, 652, 237534. [Google Scholar] [CrossRef]
- Xia, X.; Xiao, Y.; Xie, W.; Liu, J.; Yu, Y.; Ren, Y.; Chen, J.; Yang, B.; Zhang, J.; Yang, Z.; et al. A sulfonate ligand hybrid ZIF-8 modified separator achieved high-performance Li–S batteries. J. Mater. Chem. A 2025, 13, 30632–30641. [Google Scholar] [CrossRef]
- Xu, G.; Huang, L.; Lu, C.; Zhou, X.; Cui, G. Revealing the multilevel thermal safety of lithium batteries. Energy Storage Mater. 2020, 31, 72–86. [Google Scholar] [CrossRef]
- Zeng, Y.; Liu, F.; Zhang, Q.; Cheng, D.; Xu, Y.; Chi, S.-S.; Xu, X.; Wang, C.; Wang, J.; Xu, K.; et al. A thermoresponsive electrolyte additive for high-energy, long-cycling, and safe lithium batteries. Joule 2025, 9, 102100. [Google Scholar] [CrossRef]
- Fernandez, V.V.A.; Aguilar, J.; Soltero, J.F.A.; Moscoso-Sánchez, F.J.; Sánchez-Díaz, J.C.; Hernandez, E.; Bautista, F.; Puig, J.E. Thermoresponsive poly(N-isopropylacrylamide) nanogels/poly(acrylamide) nanostructured hydrogels. J. Macromol. Sci. Part A 2016, 53, 152–159. [Google Scholar] [CrossRef]
- Li, X.; Lu, T.; Liu, W.; Lv, W.; Lv, S. Ultra-wide-temperature-range thermal self-responsive phase-change materials for lithium battery thermal management. Cell Rep. Phys. Sci. 2025, 6, 102617. [Google Scholar] [CrossRef]
- Shen, L.; Hu, Y.; Zhang, X.; Chen, D.; Xu, C.; Zhou, R.; Hao, M.; Jiang, J. Research on flame-retardant phase change materials in thermal management of lithium iron phosphate batteries. J. Energy Storage 2025, 137, 118515. [Google Scholar] [CrossRef]
- Meng, Q.-W.; Zhu, X.; Xian, W.; Wang, S.; Zhang, Z.; Zheng, L.; Dai, Z.; Yin, H.; Ma, S.; Sun, Q. Enhancing ion selectivity by tuning solvation abilities of covalent-organic-framework membranes. Proc. Natl. Acad. Sci. USA 2024, 121, e2316716121. [Google Scholar] [CrossRef]
- Elizalde, F.; Trano, S.; Ayestarán, J.; de Pariza, X.L.; Aguirresarobe, R.; Francia, C.; Mecerreyes, D.; Sardon, H.; Bella, F. A Light-Mediated, 3D-Printable, and Self-Healable Polymer Electrolyte for Lithium Batteries. Adv. Funct. Mater. 2024, 35, 2419034. [Google Scholar] [CrossRef]
- Zerrin, T.; Kurban, M.; Dickson, M.M.; Ozkan, M.; Ozkan, C.S. Suppression of the Shuttle Effect in Li–S Batteries via Magnetron Sputtered TiO2 Thin Film at the Electrode–Electrolyte Interface. ACS Appl. Energy Mater. 2020, 3, 1515–1529. [Google Scholar] [CrossRef]
- Kong, D.; Guo, W.; Zhao, Y.; Zhao, Y. Electrospun Multiscale Structured Nanofibers for Lithium-Based Batteries. Adv. Energy Mater. 2024, 15, 2403983. [Google Scholar] [CrossRef]
- Ju, Y.; Liu, H.; Chen, Y.; Sheng, J.; Zhai, Y.; Dong, B.; Cheng, R.; Zhou, Y.; Li, L. An ultrathin Zn-BDC MOF nanosheets functionalized polyacrylonitrile composite separator with anion immobilization and Li+ redistribution for dendrite-free Li metal battery. Compos. Commun. 2022, 37, 101449. [Google Scholar] [CrossRef]
- Ji, Y.; Liu-Théato, X.; Xiu, Y.; Indris, S.; Njel, C.; Maibach, J.; Ehrenberg, H.; Fichtner, M.; Zhao-Karger, Z. Polyoxometalate Modified Separator for Performance Enhancement of Magnesium–Sulfur Batteries. Adv. Funct. Mater. 2021, 31, 2100868. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.; Liang, J.; Chen, Q.; Huang, J. Environmentally friendly separators based on cellulose diacetate-based crosslinked networks for lithium-ion batteries. Polymer 2023, 290, 126564. [Google Scholar] [CrossRef]
- Shen, X.; Li, C.; Shi, C.; Yang, C.; Deng, L.; Zhang, W.; Peng, L.; Dai, J.; Wu, D.; Zhang, P.; et al. Core-Shell Structured Ceramic Nonwoven Separators by Atomic Layer Deposition for Safe Lithium-Ion Batteries. Appl. Surf. Sci. 2018, 441, 165–173. [Google Scholar] [CrossRef]
- Prasada Rao, R.; Adams, S. Performance enhancement of lithium-polysulphide batteries by atomic layer deposition of lithium tantalate on sulphide solid electrolytes. Solid State Ion. 2018, 323, 97–104. [Google Scholar] [CrossRef]
- Pei, H.; Yang, C.; Wang, P.; Lin, J.; Yin, L.; Zhou, X.; Xie, X.; Ye, Y. Efficient thermal management of lithium-sulfur batteries by highly thermally conductive LBL-assembled composite separators. Electrochim. Acta 2021, 407, 139807. [Google Scholar] [CrossRef]
- Li, Z.; Peng, M.; Zhou, X.; Shin, K.; Tunmee, S.; Zhang, X.; Xie, C.; Saitoh, H.; Zheng, Y.; Zhou, Z.; et al. In Situ Chemical Lithiation Transforms Diamond-Like Carbon into an Ultrastrong Ion Conductor for Dendrite-Free Lithium-Metal Anodes. Adv. Mater. 2021, 33, 2100793. [Google Scholar] [CrossRef]
- Shi, Q.X.; Yang, C.Y.; Pei, H.J.; Chang, C.; Guan, X.; Chen, F.Y.; Xie, X.L.; Ye, Y.S. Layer-by-layer self-assembled covalent triazine framework/electrical conductive polymer functional separator for Li-S battery. Chem. Eng. J. 2020, 404, 127044. [Google Scholar] [CrossRef]
- Cheng, Z.; Pan, H.; Chen, J.; Meng, X.; Wang, R. Separator Modified by Cobalt-Embedded Carbon Nanosheets Enabling Chemisorption and Catalytic Effects of Polysulfides for High-Energy-Density Lithium-Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1901609. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Lin, D.; Pei, A.; Cui, Y. Materials for lithium-ion battery safety. Sci. Adv. 2018, 4, eaas9820. [Google Scholar] [CrossRef]
- He, Z.; Li, Z.; Xiang, Q.; Yan, W.; Zhang, H. Electrical properties of Y/Mg modified NiO simple oxides for negative temperature coefficient thermistors. Int. J. Appl. Ceram. Technol. 2018, 16, 160–169. [Google Scholar] [CrossRef]
- Lenz, T.; Sharifi Dehsari, H.; Asadi, K.; Blom, P.W.M.; Groen, W.A.; de Leeuw, D.M. Thin film thermistor with positive temperature coefficient of resistance based on phase separated blends of ferroelectric and semiconducting polymers. Appl. Phys. Lett. 2016, 109, 133302. [Google Scholar] [CrossRef]
- Bendi, R.; Bhavanasi, V.; Parida, K.; Nguyen, V.C.; Sumboja, A.; Tsukagoshi, K.; Lee, P.S. Self-powered graphene thermistor. Nano Energy 2016, 26, 586–594. [Google Scholar] [CrossRef]
- Okutani, C.; Yokota, T.; Someya, T. Ultrathin Fiber-Mesh Polymer Thermistors. Adv. Sci. 2022, 9, e2202312. [Google Scholar] [CrossRef]
- Le, K.; Sang, C.; Luo, Q.; Li, H.; Fang, Y.; Ai, X. Cathode with a temperature-switchable interlayer for thermally self-regulating smart lithium-ion batteries. eTransportation 2025, 26, 100483. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, N.; Zhu, M.; Lv, W.; Ouyang, R.; Shao, B. Photoluminescence and ratiometric fluorescence temperature sensing abilities of zincate phosphors. J. Lumin. 2020, 228, 117600. [Google Scholar] [CrossRef]
- Zheng, Y.; Meana, Y.; Mazza, M.M.A.; Baker, J.D.; Minnett, P.J.; Raymo, F.M. Fluorescence Switching for Temperature Sensing in Water. J. Am. Chem. Soc. 2022, 144, 4759–4763. [Google Scholar] [CrossRef]
- Sun, N.; Rong, Q.; Wu, J.; Huang, L.; Passerini, S.; Li, H.; Wang, H.; Chen, J.; Huang, Y.; Liu, Z.; et al. Fully printable integrated multifunctional sensor arrays for intelligent lithium-ion batteries. Nat. Commun. 2025, 16, 7361. [Google Scholar] [CrossRef]
- Liu, Z.; Hu, Q.; Guo, S.; Yu, L.; Hu, X. Thermoregulating Separators Based on Phase-Change Materials for Safe Lithium-Ion Batteries. Adv. Mater. 2021, 33, 2008088. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Chen, P.; Ding, Y.; Liu, Y.; Gao, C. Smart Quasi-Solid-State Electrolytes with the “Dual Insurance” Mechanism for Thermal Safety and Autonomous Operation in Flexible Energy Storage Devices. Adv. Energy Mater. 2025, 15, 2500591. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, L.; Peng, M.; Shen, D.; Zhu, C.; Qian, S.; Liu, J.; Cao, Y.; Yan, C.; Zhou, J.; et al. Developing Thermoregulatory Hydrogel Electrolyte to Overcome Thermal Runaway in Zinc-Ion Batteries. Adv. Funct. Mater. 2022, 32, 2206653. [Google Scholar] [CrossRef]
- Lei, Y.; Pei, J.; Xue, P.; Ma, L.; Liu, Z.; Long, R. Impacts of separator on lithium dendrite growth and morphology under non-isothermal configurations. J. Power Sources 2024, 629, 236034. [Google Scholar] [CrossRef]
- Yu, W.-J.; Wang, J.; Liu, F.; Cao, Y.; Deng, B.; Li, J.; Xie, H.; Zhang, J.; Tong, H.; Liang, C. Self-supporting heteroatomic S/N co-doped carbon scaffold for robust lithium metal anodes. Carbon 2025, 236, 120106. [Google Scholar] [CrossRef]
- Zhang, Q.; Lei, D.; Liu, N.; Liu, Z.; Ren, Z.; Yin, J.; Jia, P.; Lu, W.; Gao, Y. A Zinc-Ion Battery-Type Self-Powered Pressure Sensor with Long Service Life. Adv. Mater. 2022, 34, e2205369. [Google Scholar] [CrossRef]
- Jiang, C.-Y.; Li, H.-R.; Peng, Y.; Zhang, L.-Y.; Fan, W.-H.; Wang, L.-X.; Jiang, M.; Cai, Z.-Q.; Dong, L.-J. Dual-responsive Flexible Dielectric Switching Composites for Overheating Warning and Small Deformation Monitoring. Chin. J. Polym. Sci. 2025, 43, 1885–1893. [Google Scholar] [CrossRef]
- Xie, W.; Wu, L.; Liu, W.; Dang, Y.; Tang, A.; Luo, Y. Modelling electrolyte-immersed tensile property of polypropylene separator for lithium-ion battery. Mech. Mater. 2021, 152, 103667. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Y.; Yang, L.; Karouta, F.; Sun, K. Electron-Induced Perpendicular Graphene Sheets Embedded Porous Carbon Film for Flexible Touch Sensors. Nano-Micro Lett. 2020, 12, 136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Xiao, P.; Liang, Y.; Wang, Q.; Liu, D.; Yang, Q.; Chen, J.; Nie, Y.; Kuo, S.W.; Chen, T. Bionic Adaptive Thin-Membranes Sensory System Based on Microspring Effect for High-Sensitive Airflow Perception and Noncontact Manipulation. Adv. Funct. Mater. 2021, 31, 2105323. [Google Scholar] [CrossRef]
- Wu, N.; Wang, M.; Shadike, Z.; Hu, Z.; Hu, Y.; Gao, Y. Suppressing Interfacial Side Reactions of Anode-Free Lithium Batteries by an Organic Salt Monolayer. Small 2023, 19, e2303952. [Google Scholar] [CrossRef]
- Zhong, Q.; Gao, L.; Li, W.; Zhao, J.; Garg, A.; Panda, B. A novel preheating systems for columnar lithium batteries for below zero degrees celsius environment based on topology optimization. Int. Commun. Heat Mass Transf. 2024, 158, 107789. [Google Scholar] [CrossRef]
- Wang, X.; Zou, N.; Qiao, Y.; Ren, G.; Wang, Q.; Zhou, T.; Chen, Y. Spatially structured lamellar porous Carbon-CoS2 separator for Enhanced adsorption and conversion of lithium polysulfides in High-Performance Li-S batteries. Chem. Eng. J. 2025, 515, 163529. [Google Scholar] [CrossRef]
- Ji, L.; Yang, D.; Xue, J.; Jia, M.; Wu, T.; Zhuang, Q.; Zhang, Y.; Liu, J.; Zhang, Y. Flexible Titanium Nitride-Based Membrane Reactor for S8/Li2S and Dendrite Regulation in Lithium-Sulfur Batteries. Adv. Energy Mater. 2025, 15, 2404738. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, P.; Tang, M.; Sun, S.; Min, H.; Han, J.; Shen, X.; Yang, H.; Chao, D.; Wang, J. A high-throughput screening permeability separator with high catalytic conversion kinetics for Li–S batteries. J. Mater. Chem. A 2022, 10, 22080–22092. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, S.; Dai, S.; Liu, D.; Wang, X.; Tang, W.; Guo, X.; Duan, J.; Luo, W.; Yang, B.; et al. Ultrasensitive Detection of Electrolyte Leakage from Lithium-Ion Batteries by Ionically Conductive Metal-Organic Frameworks. Matter 2020, 3, 904–919. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Y.; Li, L.; Wang, X.; Liu, D.; Zhang, J.; Dai, S.; Hao, D.; Yang, B.; Sun, Q.; et al. Sensitive sensors based on bilayer organic field-effect transistors for detecting lithium-ion battery electrolyte leakage. Sci. China Mater. 2022, 65, 1187–1194. [Google Scholar] [CrossRef]
- Jia, H.; Zeng, C.; Lim, H.S.; Simmons, A.; Zhang, Y.; Weber, M.H.; Engelhard, M.H.; Gao, P.; Niu, C.; Xu, Z.; et al. Important Role of Ion Flux Regulated by Separators in Lithium Metal Batteries. Adv. Mater. 2023, 36, e2311312. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhong, B.; Zhang, R.; Yu, J.; Huang, Z.; Huang, Y.; Wu, Z.; Fan, Y.; Tian, J.; Xie, W.; et al. Taming Active-Ion Crosstalk by Targeted Ion Sifter Toward High-Voltage Lithium Metal Batteries. Adv. Energy Mater. 2023, 13, 2302295. [Google Scholar] [CrossRef]
- Zhang, Q.; Hou, B.; Wu, X.; Li, X.; Guo, Z.; Liu, J.; Cao, D.; Huang, X.; Duan, J.; Mo, D.; et al. An Ion-Management Membrane with Vertically Aligned Uniform Electronegative Nanochannels Toward Dendrite-Free Lithium Metal Anodes. Adv. Energy Mater. 2024, 14, 2401377. [Google Scholar] [CrossRef]
- Han, D.; Kwon, S.; Lee, M.; Kim, J.; Yoo, K. Electrochemical impedance spectroscopy image transformation-based convolutional neural network for diagnosis of external environment classification affecting abnormal aging of Li-ion batteries. Appl. Energy 2023, 345, 121336. [Google Scholar] [CrossRef]
- Li, L.; Zhou, M.; Xiong, R.; Wang, X.; Shen, G.; Sun, S.; Chen, Y.; Huang, T.; Zhou, H.; Zhang, Y. High-safety poly(ethylene-co-vinyl acetate)/poly(ether ether ketone)/poly(ethylene-co-vinyl acetate) composite separator with the thermal shutdown feature for lithium-ion battery. J. Membr. Sci. 2023, 687, 122059. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, C.; Dong, F.; Xie, H.; Sun, L. Multilayer polyethylene separator with enhanced thermal properties for safe lithium-ion batteries. Particuology 2024, 91, 29–37. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, L.; Zhang, X.-D.; Wang, Y.-H.; Yang, C.; Liu, X.; Wang, W.-P.; Zhang, Y.; Li, X.-T.; Li, G.; et al. A smart risk-responding polymer membrane for safer batteries. Sci. Adv. 2023, 9, eade5802. [Google Scholar] [CrossRef]
- Lin, G.; Liu, C.; Bai, Z.; Liu, S.; Han, M.; Huang, Y.; Liu, X. Heat-resistant poly (arylene ether nitriles) separator for high-safety lithium batteries via dual-functional modification. J. Phys. Conf. Ser. 2022, 2338, 012031. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Li, W.; You, C.; Zhu, X.; Wei, J.; Fang, Z.; Wang, H.; Huang, S.; Bai, S. Permselective Covalent Organic Framework Membrane as Self-Extinguishing Separator for High-Safety Lithium-Ion Battery. Angew. Chem. Int. Ed. 2025, e202512591. [Google Scholar] [CrossRef]
- Kang, X.; He, T.; Dang, H.; Li, X.; Wang, Y.; Zhu, F.; Ran, F. Designing Amino Functionalized Titanium-Organic Framework on Separators Toward Sieving and Redistribution of Polysulfides in Lithium-Sulfur Batteries. Nano-Micro Lett. 2025, 17, 277. [Google Scholar] [CrossRef]
- Seo, J.-Y.; Lee, Y.-H.; Kim, J.-H.; Hong, Y.-K.; Chen, W.; Lee, Y.-G.; Lee, S.-Y. Electrode-customized separator membranes based on self-assembled chiral nematic liquid crystalline cellulose nanocrystals as a natural material strategy for sustainable Li-metal batteries. Energy Storage Mater. 2022, 50, 783–791. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Y.; Liu, G.; Yu, Z.; Sun, S.; Fu, Y.; Chen, D.; Cai, W.; Cui, J.; Wang, G.; et al. BaTiO3/PVDF-TrFE skeleton separators with superior ion conductivity and mechanical property integrated for flexible seconds self-charging supercapacitors. J. Power Sources 2022, 528, 231213. [Google Scholar] [CrossRef]
- Zhu, G.; Wu, Q.; Zhang, X.; Bao, Y.; Zhang, X.; Shi, Z.; Zhang, Y.; Ma, L. Designing metal sulfide-based cathodes and separators for suppressing polysulfide shuttling in lithium-sulfur batteries. Nano Res. 2023, 17, 2574–2591. [Google Scholar] [CrossRef]
- Sun, H.; Liu, Z.; Sang, X.; Li, R.; Li, P.; Hu, X. Mechanically Robust and Safe Separators Based on Inorganic Nanofibers for Lithium-Ion Batteries. ACS Appl. Energy Mater. 2024, 7, 5537–5547. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Y.; Feng, C.; He, Y.; Zhou, S.; Yuan, B.; Dong, Y.; Xie, H.; Zeng, G.; Han, J.; et al. Unified throughout-pore microstructure enables ultrahigh separator porosity for robust high-flux lithium batteries. Electron 2023, 1, e1. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Liu, J.; Zhang, M.; Fu, L.; Lin, B.; Xu, C.; Huang, B. All-Solid-State Hydrophobic Ionic-Conductor with Large Strain Self-Recovery and High Toughness for Multi-Scenario Sensing Applications. Adv. Funct. Mater. 2025, e22963. [Google Scholar] [CrossRef]
- Li, B.; Cai, C.; Liu, Y.; Wang, F.; Yang, B.; Li, Q.; Zhang, P.; Deng, B.; Hou, P.; Liu, W. Ultrasensitive mechanical/thermal response of a P(VDF-TrFE) sensor with a tailored network interconnection interface. Nat. Commun. 2023, 14, 4000. [Google Scholar] [CrossRef]
- Yu, X.; Ji, Y.; Shen, X.; Le, X. Self-Powered Pressure–Temperature Bimodal Sensing Based on the Piezo-Pyroelectric Effect for Robotic Perception. Sensors 2024, 24, 2773. [Google Scholar] [CrossRef]
- Zheng, A.; Shen, C.; Yan, K.; Gong, M.; Dong, C.; Yu, Y.; Zhou, C.; Pi, Y.; Xu, X. In Situ Polymerization of Ultra-Thin and Defect-Free Polyamide Layer for Effectively Impeding the Polysulfides Shuttle. Small 2024, 20, e2405159. [Google Scholar] [CrossRef]
- Feng, C.; Wang, X.; Zeng, G.; Chen, D.; Lv, W.; Han, Y.; Jian, X.; Dou, S.X.; Xiong, J.; He, W. Heat-Resistant Trilayer Separators for High-Performance Lithium-Ion Batteries. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2020, 14, 1900504. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, H.; Wang, B.; Yang, S.; Wu, B.; Zhou, Y.; Li, H.; Wei, Z.; Zhao, Y. Microfiber/Nanofiber/Attapulgite Multilayer Separator with a Pore-Size Gradient for High-Performance and Safe Lithium-Ion Batteries. Molecules 2024, 29, 3277. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-T.; Yang, J.; Liang, H.-Q.; Pi, J.-K.; Zhang, C.; Xu, Z.-K. Sandwich-structured composite separators with an anisotropic pore architecture for highly safe Li-ion batteries. Compos. Commun. 2018, 8, 46–51. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Wang, Z.; Zhang, Y.; Bin, D.; Lu, H. Intelligent Sensing and Responsive Separators for Lithium Batteries Using Functional Materials and Coatings for Safety Enhancement. Coatings 2025, 15, 1325. https://doi.org/10.3390/coatings15111325
Tang J, Wang Z, Zhang Y, Bin D, Lu H. Intelligent Sensing and Responsive Separators for Lithium Batteries Using Functional Materials and Coatings for Safety Enhancement. Coatings. 2025; 15(11):1325. https://doi.org/10.3390/coatings15111325
Chicago/Turabian StyleTang, Junbing, Zhiyan Wang, Yongzheng Zhang, Duan Bin, and Hongbin Lu. 2025. "Intelligent Sensing and Responsive Separators for Lithium Batteries Using Functional Materials and Coatings for Safety Enhancement" Coatings 15, no. 11: 1325. https://doi.org/10.3390/coatings15111325
APA StyleTang, J., Wang, Z., Zhang, Y., Bin, D., & Lu, H. (2025). Intelligent Sensing and Responsive Separators for Lithium Batteries Using Functional Materials and Coatings for Safety Enhancement. Coatings, 15(11), 1325. https://doi.org/10.3390/coatings15111325





