1. Introduction
Nanocrystalline Cu films exhibit excellent physical and mechanical properties, and therefore have been extensively and critically applied in modern industry as well as high-tech fields such as microelectronics and micromechanics [
1,
2]. However, pure Cu films possess relatively low strength. Under most service conditions, their hardness and wear resistance fail to meet the requirements for normal use. Alloying by adding alloying elements to pure Cu films is a common method currently used to enhance the strength of Cu-based films. Existing studies [
3] have shown that in Cu-Ti thin films fabricated via the sputtering method, due to the extensive solid solution of Ti atoms, the grain size of Cu is significantly reduced to the nanoscale. The hardness of the thin film correspondingly increases from 1.7 GPa of pure copper to approximately 5 GPa when the Ti content is 6.5 at.%. Similar results, including increased solid solubility, grain nanocrystallization, and a significant improvement in hardness, have also been obtained in studies on other binary systems [
4,
5,
6,
7,
8,
9], such as Cu-Cr [
4], Cu-Ta [
5], Cu-Ag [
6] and so on [
7,
8,
9].
In addition, nanocrystalline pure Cu films have low structural stability and are prone to grain growth. Adding alloying elements has been demonstrated as a significant and dependable approach for enhancing the stability of copper alloys. Some alloy nanocrystalline materials have demonstrated much better high-temperature performance than pure metals, and they can maintain stability over a relatively wide temperature range [
10,
11,
12,
13,
14,
15,
16]. For instance, Rajagopalan et al. [
13] investigated the influence of Ta addition on the stability of nanocrystalline Cu alloys. The findings indicated that the stability of Cu-Ta nanocrystalline alloys was remarkably higher than that of nanocrystalline pure Cu. Although nanocrystalline Cu alloy films have higher strength and structural stability compared with nanocrystalline pure Cu films, the addition of alloying elements also complicates the study of their strengthening mechanisms. This is because the addition of alloying elements simultaneously causes multiple structural changes, such as in the size of grains, the content of solute, and the characteristic of grain boundary. The strengthening effects induced by these various structural factors overlap, making it difficult to distinguish them from one another.
In this study, nanocrystalline Cu-Zr alloy was selected as the research object, and magnetron sputtering technology was selected as the preparation process for alloy systems. The Cu-Zr alloy system is selected in light of the subsequent considerations. In the equilibrium state, the solid solubility of Zr in Cu is extremely limited, only about 0.12 at.% at 1000 °C. In addition, the atomic radii of Cu (0.128 nm) and Zr (0.160 nm) differ greatly. Therefore, grain boundary segregation is prone to occur in Cu-Zr alloys, which improves the stability of the thin film. The magnetron sputtering process offers advantages including high deposition rate, dense and uniform films, excellent film adhesion, low working pressure, low-temperature deposition, and good process reproducibility, making it suitable for coating a wide range of materials with high-quality and uniformity [
17]. It is characterized by high dispersion, high kinetic energy and high non-equilibrium. These characteristics force Zr atoms, which are difficult to dissolve into the Cu lattice in the equilibrium state, to remain. This forms a supersaturated solid solution and provides experimental guarantee for the conduct of this study. Based on the above considerations, a series of Cu-Zr nanocrystalline alloy films with varying Zr contents were fabricated via magnetron co-sputtering technology. The effect of Zr content on the microstructure and mechanical properties of the alloy films was studied, and the corresponding strengthening mechanism was revealed. Furthermore, the stability of the Cu-Zr nanocrystalline alloy films was investigated via annealing experiments conducted at various temperatures.
2. Materials and Methods
Cu-Zr films were fabricated through the co-sputtering approach employing a SPC-350 multi-target magnetron sputtering system (Anelva, Tokyo, Japan). A metallic Cu target (99.99% purity, Φ76 mm) was mounted on a DC cathode. A Ti target (99.999% purity) was controlled by an independent RF cathode.
Monocrystalline silicon wafers were ultrasonically cleaned. Then, they were installed on a rotatable substrate holder inside the vacuum chamber. The distance between the targets and the substrate was set to 50 mm. Upon the reduction in the base pressure within the vacuum chamber to a level below 5 × 10−4 Pa, high-purity argon (Ar) with a purity of 99.999% was introduced. The pressure of Ar was maintained at 0.6 Pa. During the sputtering process, the power of the direct-current (DC) Cu target was fixed at 0.4 A. The power of the radio-frequency (RF) Zr target was regulated within the range of 0–120 W. The substrate holder was rotated at a high speed of 30 r/min. Through these operations, Cu-Zr nanocrystalline alloy films with varying Zr contents were obtained. During the film deposition process, no heating was applied to the substrate, and no negative bias voltage was imposed. The thickness of all experimental Cu-Zr films was controlled to be approximately 2 μm.
The stability of Cu-Zr alloy films was investigated via annealing experiments. To prevent thin film oxidation, the thin film samples were first placed in quartz tubes for vacuum pumping and sealing. Subsequently, the quartz tubes were placed in a vacuum annealing furnace. The vacuum degree inside the furnace was pumped to 10−1 Pa. After that, the samples were subjected to high-temperature annealing at 100 °C, 200 °C, and 300 °C, respectively. During the annealing process, the heating rate of the vacuum furnace was set to 10 °C/min. The holding time was 1 h. After the annealing was completed, the alloy films were cooled to room temperature along with the furnace.
The chemical compositions of Cu and Zr in the experimental films were analyzed using an OXFORDINCA X-ray energy dispersive spectroscopy (EDS) instrument (Oxford, Oxford, UK). The analysis was conducted via area scanning, with a scanning area of approximately 2 mm × 2 mm. The phase composition and microstructure of the alloy films were characterized by X-ray diffraction (XRD) and transmission electron microscope (TEM), respectively. That is, a D/max-2550/PC XRD instrument was used for phase composition characterization (Rigaku, Tokyo, Japan), and a JEM-2100F field emission TEM was employed for microstructure characterization (JEOL, Tokyo, Japan).
The mechanical properties of Cu-Zr nanocrystalline alloy films were measured via nanoindentation employing a Step 300-NTH3 instrument (Anton Paar, Graz, Austria). A force of 10 mN was loaded to ensure the indentation depth did not exceed the total film thickness. The loading and unloading durations were set to 30 s, with a holding time of 10 s. In order to minimize measurement errors, each alloy sample underwent 10 repeated measurements. The Oliver-Pharr method [
18] was employed to analyze the load–displacement curves to determine the mechanical properties of Cu-Zr alloy films.
3. Results
3.1. Microstructure and Mechanical Properties of Alloy Films
Figure 1 shows the variation in the total Zr content in the Cu-Zr alloy thin films in relation to the Zr target power. The total Zr content of the alloy films was measured by EDS. As is evident from the figure, the Zr content in the alloy thin films exhibits an almost linear upward trend with the increases in the Zr target power. When the Zr target power reaches 120 W, the total Zr content increases to approximately 4.1 at.%.
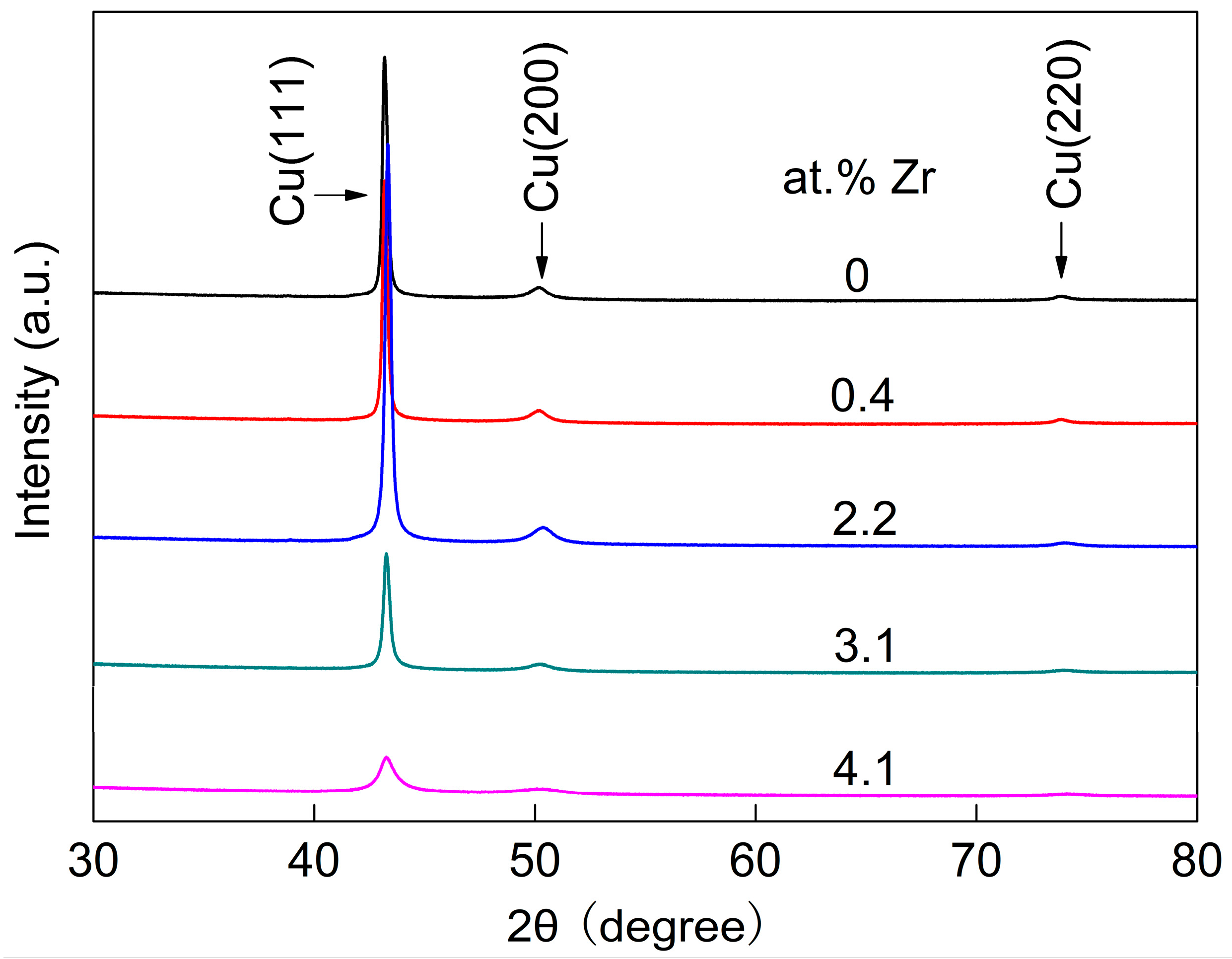
Figure 2 presents the XRD patterns of Cu-Zr alloy films with different Zr contents. In the figure, the pure Cu film, as well as the films containing 0.4 at.% Zr and 2.2 at.% Zr, exhibit sharp Cu (111) diffraction peaks. Weak Cu (200) and Cu (220) diffraction peaks are also present in these films. With the increase in Zr content, the Cu (111) diffraction peak of the films gradually broadens, while the Cu (200) diffraction peak weakens gradually. This phenomenon indicates that the grain size of Cu-Zr alloy films gradually decreases. When the content of Zr element increases to 4.1 at.%, the Cu (200) diffraction peak of the alloy film becomes extremely weak. It is also observable that the intensity of the diffraction peak of the Cu (111) crystal plane exhibits a significant decline.
The result indicates that the grain size of the film has become extremely refined.
To further confirm the grain size of Cu-Zr alloy films, the Scherrer equation was employed to calculate the grain size of each alloy film. The outcomes are presented in
Figure 3.
Figure 3a shows that the pure Cu film exhibits a grain size of approximately 50 nm. while increasing Zr content continuously reduces the grain size of Cu-Zr alloy films, reaching ~14 nm at 4.1 at.% Zr. In addition, it can be further observed from the XRD patterns that with the increase in Zr content, the peak positions of all diffraction peaks in the alloy films shift significantly toward the small-angle direction. This phenomenon indicates that as the Zr content increases, the number of Zr atoms dissolved in the crystal lattice of the alloy film increases gradually. The lattice distortion of the Cu film caused by the dissolution of Zr atoms (characterized by a relatively larger atomic radius) leads to the shift in diffraction peaks of the alloy film toward small angles.
Figure 3b shows the relationship between interplanar spacing and Zr content in Cu-Zr alloy thin films. As shown in the figure, the interplanar spacing of the pure Cu film is 0.2086 nm. As the Zr content increases, the interplanar spacing of Cu-Zr alloy films exhibits a continuous increase. Upon the increment of the Zr content to 4.1 atomic percent, the interplanar spacing of the Cu-Zr alloy film expands to 0.2094 nm. The dotted line in the figure denotes the fitting curve and its corresponding expression derived from the experimental results.
Figure 4 shows the TEM images of alloy films. From the bright-field TEM images (
Figure 4a–c), the pure Cu film has a grain size of approximately 50 nm. As Zr is added, the film’s grain size reduces step by step—reaching ~30 nm at 2.2 at.% Zr and further decreasing to around 20 nm when Zr content reaches 4.1 at.%. Furthermore, the selected area electron diffraction (SAED) is used for further microstructure characterization. The SAED patterns of the films inserted in the upper right corner of the bright-field TEM images indicate that the SAED of Cu-Zr alloy films consists of only a set of polycrystalline diffraction rings with a face-centered cubic (fcc) structure. The diffraction rings in the SAED pattern become continuous and gradually broaden with the increase in Zr content.
The characterization results of TEM are consistent with those obtained by XRD.
Figure 4d–f present the high-resolution TEM (HRTEM) images of Cu-Zr alloy films with different Zr contents. These images also show that the grain size of the alloy films decreases gradually with the increase in Zr content. The interplanar spacing of each alloy film can be measured from the fast Fourier transform (FFT) patterns (inset in the top-right corner). As indicated by the results, as the content of Zr element increases, the interplanar spacing of the alloy films increases gradually from 0.2088 nm (for the pure Cu film) to 0.2096 nm (for the Cu-4.1 at.% Zr film). This value is slightly higher than the calculated result from the XRD, but the changing trend is consistent.
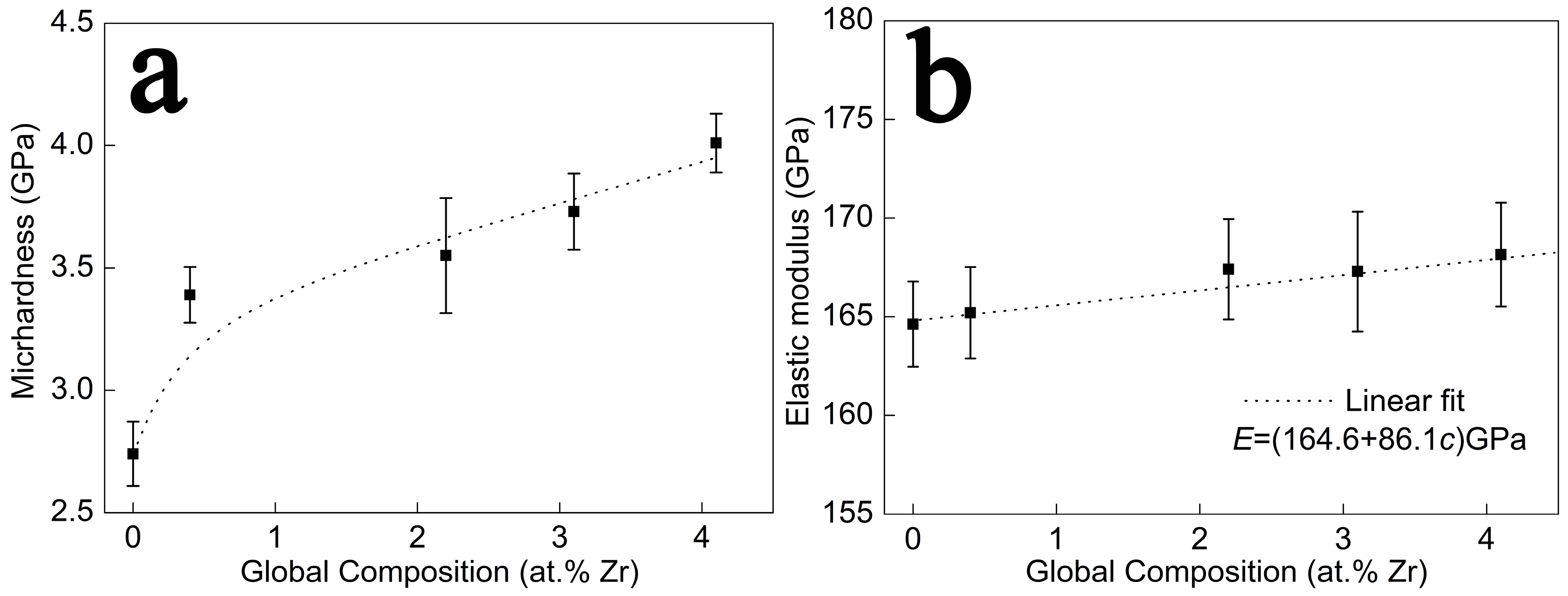
Figure 5 shows the mechanical properties of Cu-Zr alloy films. As is observable from
Figure 5a, the hardness of Cu-Zr alloy films exhibits a two-stage variation with the increase in Zr content. As the Zr content rises from 0 to 0.4 at.%, the hardness of the film experiences a rapid increase from 2.7 GPa to 3.4 GPa. With a further increase in Zr content, the hardness of the Cu-Zr alloy film still increases, but the increasing rate slows down. Finally, when the Zr content reaches 4.1 at.%, the hardness of the film reaches a maximum value of 4.0 GPa.
Figure 5b presents the variation trend of the elastic modulus of Cu-Zr alloy films in relation to the Zr content. The elastic modulus of the alloy films increases gradually as the Zr content increases. The dashed lines in the figure represent the fitting curve and its expression for the variation trend of the film’s elastic modulus, respectively.
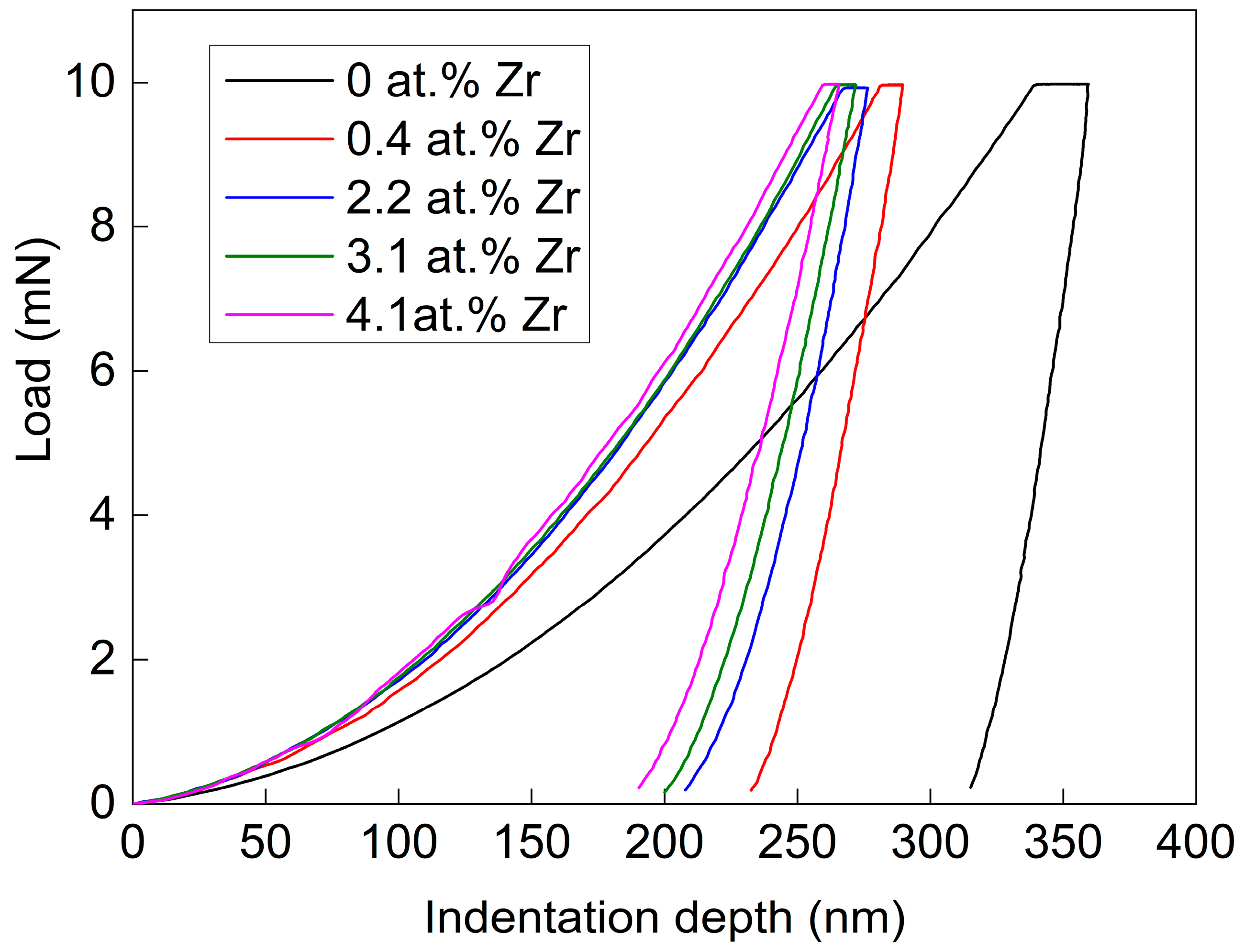
Figure 6 shows the displacement-load curves of the Cu-Zr alloy films. As shown in this figure, the pure Cu film has the deepest maximum indentation depth, approximately 350 nm, while the maximum indentation depths of other Cu-Zr alloy thin films do not exceed 300 nm. Moreover, with the increase in Zr content, the maximum indentation depth decreases gradually. This also means that the hardness gradually increases. The result also verifies the variation trend of the thin films’ hardness in
Figure 5.
3.2. Stability of Alloy Films
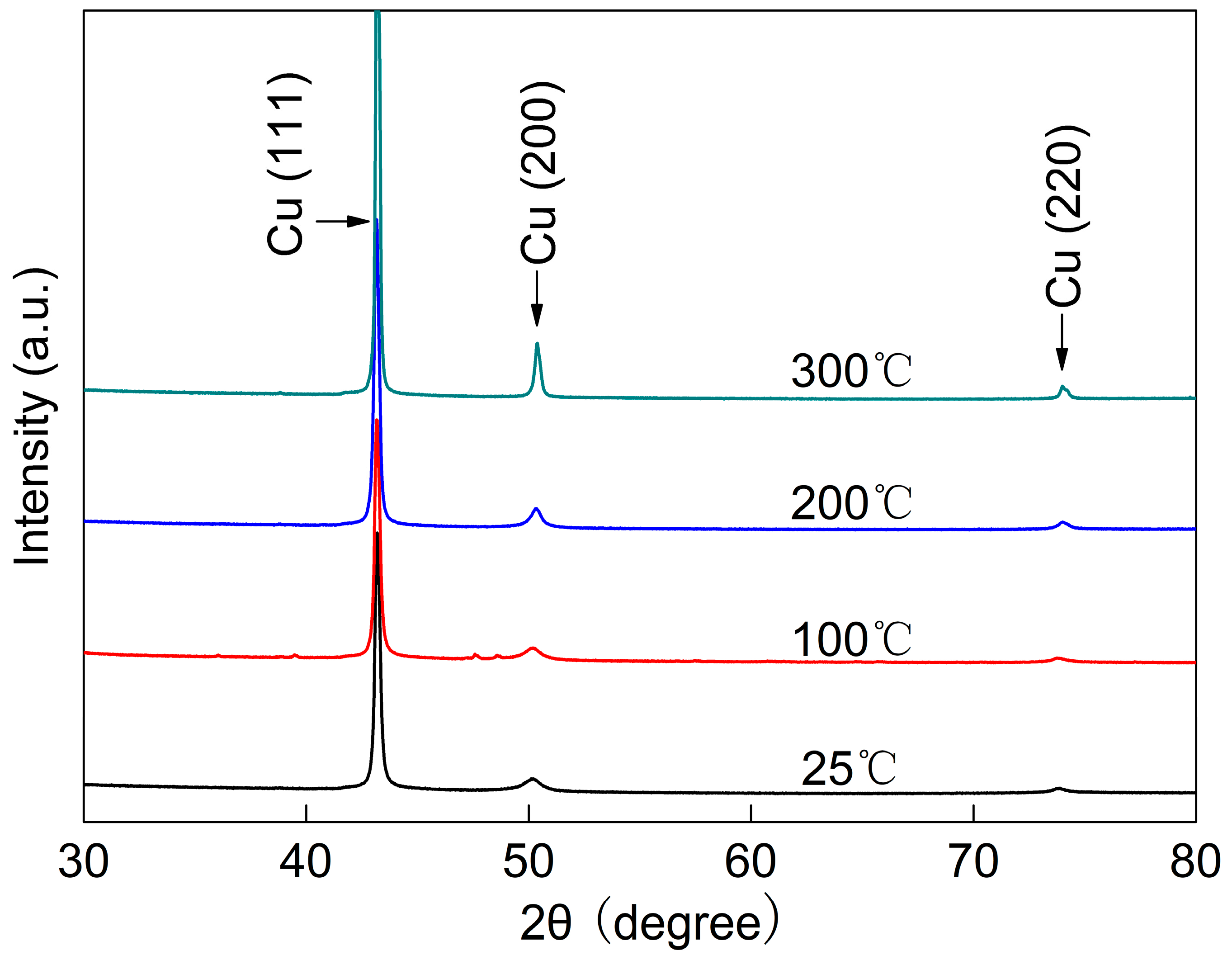
Figure 7 shows the XRD patterns of the pure Cu film after annealing at different temperatures. As can be seen from the figure, the film exhibits a strong Cu (111) texture, while the Cu (200) and Cu (220) diffraction peaks are relatively weak. For the pure Cu film, the diffraction peaks increase in intensity even after annealing at 100 °C. This indicates that grain growth of the film may have occurred during annealing at 100 °C. As the annealing temperature is further elevated, the intensity of the film’s diffraction peaks increases further. When the annealing temperature reaches 300 °C, the intensity of the diffraction peaks of the pure Cu film increases remarkably, accompanied by sharpening of the peaks. This suggests that the grains of the film may have grown obviously at this point.
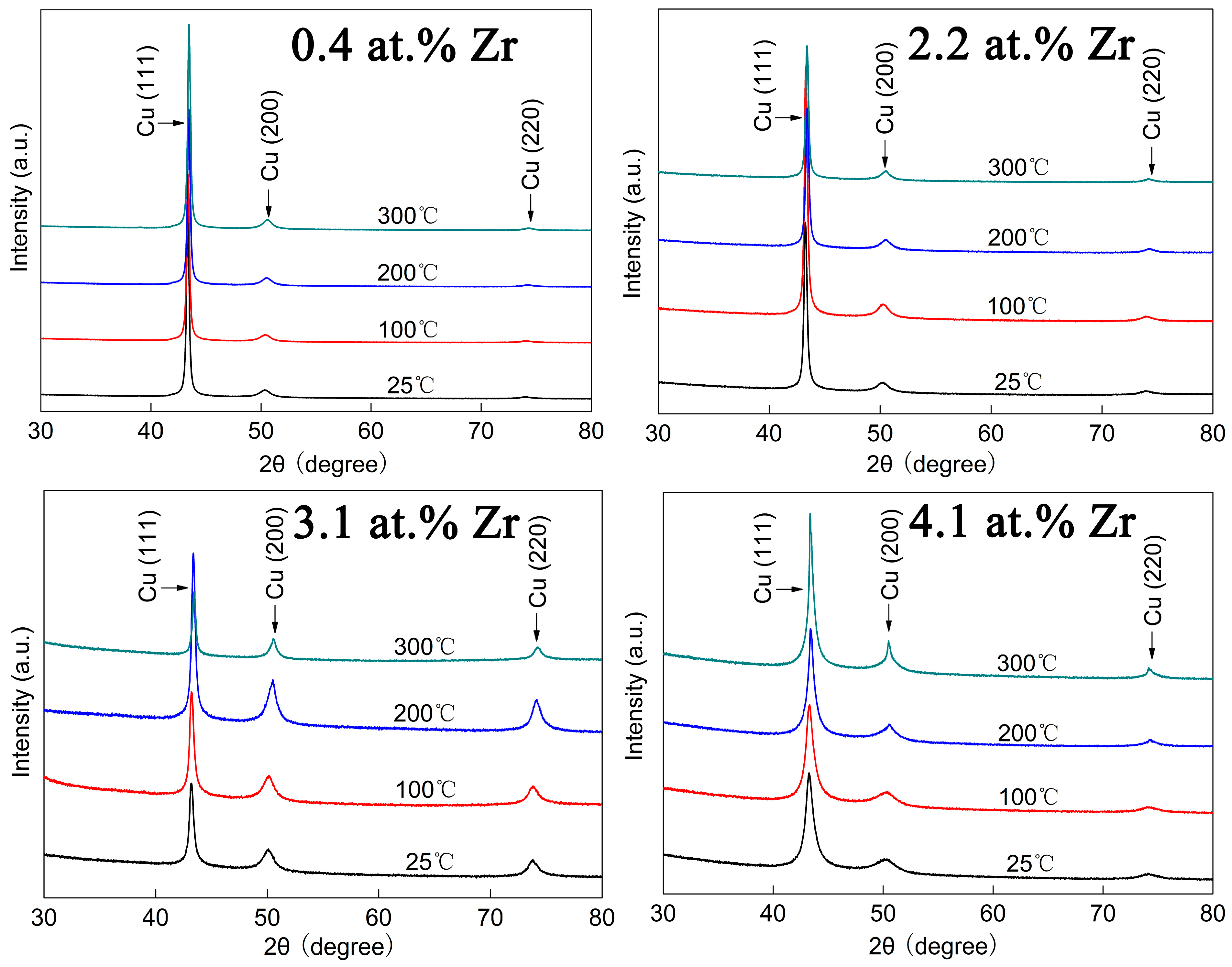
Figure 8 shows the XRD patterns of Cu-Zr alloy films with different Zr contents after annealing at various temperatures. As is evident from the figure, the alloy films exhibit three diffraction peaks, namely Cu (111), Cu (200) and Cu (220). Among these peaks, the Cu (111) diffraction peak is relatively strong, while the Cu (200) and Cu (220) diffraction peaks are relatively weak. This indicates that the films have a distinct Cu (111) orientation. Different from the annealing results of the pure Cu film shown in
Figure 7, the diffraction peaks of the Cu-Zr alloy films show almost no change after annealing at 100 °C, 200 °C, and 300 °C. This demonstrates that the Cu-Zr alloy films have good stability at these temperatures. The Cu-Zr alloy films were stabilized by Zr addition.
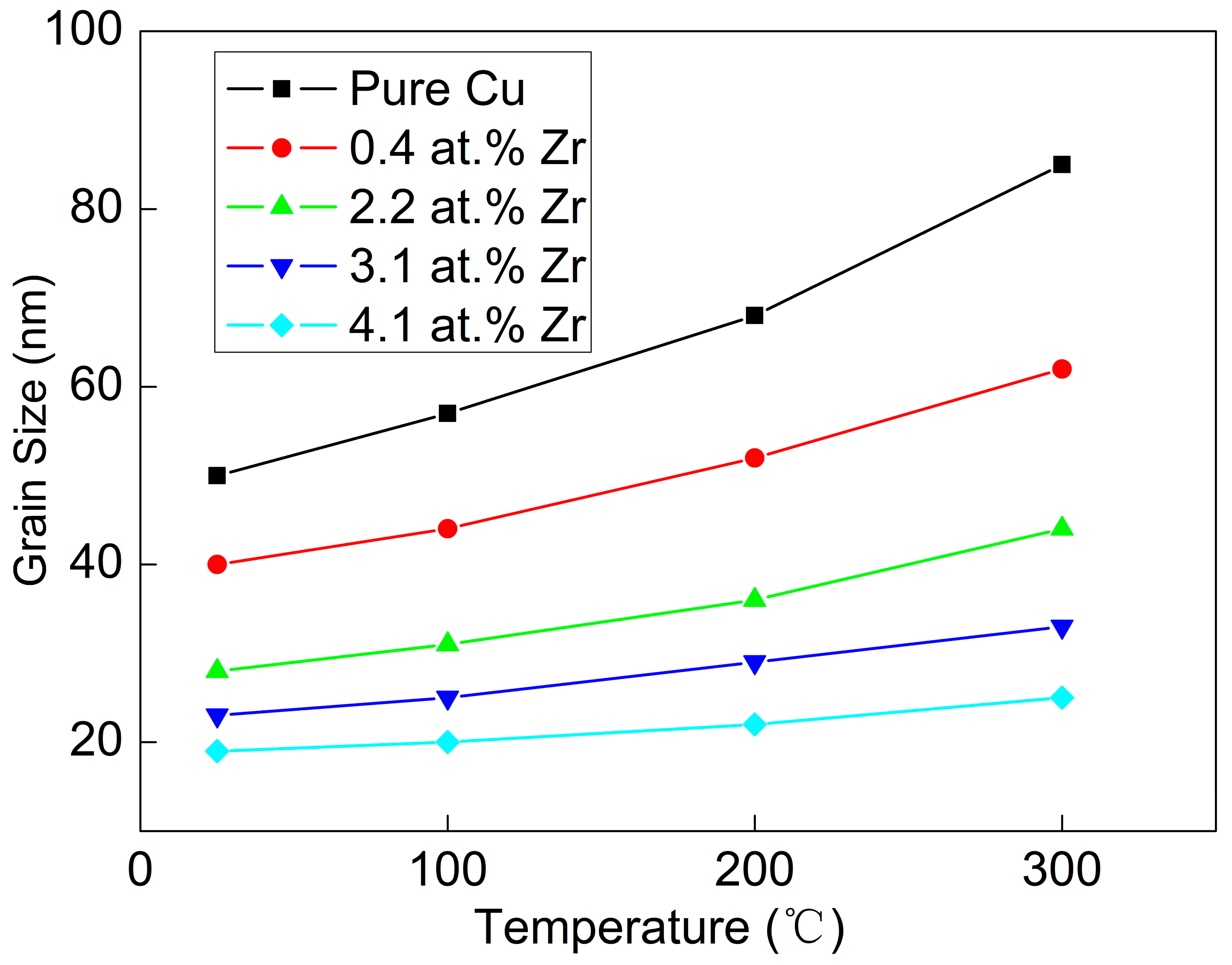
Figure 9 presents the grain sizes of various Cu-Zr alloy films after annealing. These values were calculated using the Scherrer equation, based on the XRD patterns of the annealed Cu-Zr alloy films. As can be seen from the figure, with the increase in annealing temperature, the grain size of all Cu-Zr alloy films shows a tendency to grow. Among them, the grain growth of the pure Cu film is the most obvious. After annealing at 100 °C, the grain size of the pure Cu film increases from 50 nm (as-deposited state) to approximately 60 nm. When the annealing temperature rises to 300 °C, the grain size of the pure Cu film increases rapidly to about 85 nm. The findings fully demonstrate that the pure Cu film has low structural stability, and grain coarsening occurs even at a relatively low annealing temperature. In contrast, with the increase in annealing temperature, the grain size of Cu-Zr alloy films (formed by adding Zr element) also increases slightly. However, the increase amplitude is much smaller than that of the pure Cu film. Moreover, with the increase in Zr content, this grain growth tendency becomes increasingly weaker. For the Cu-4.1 at.% Zr alloy film, its grain size remains almost unchanged. This result demonstrates that the addition of Zr element significantly improves the structural stability of Cu-Zr alloy films.
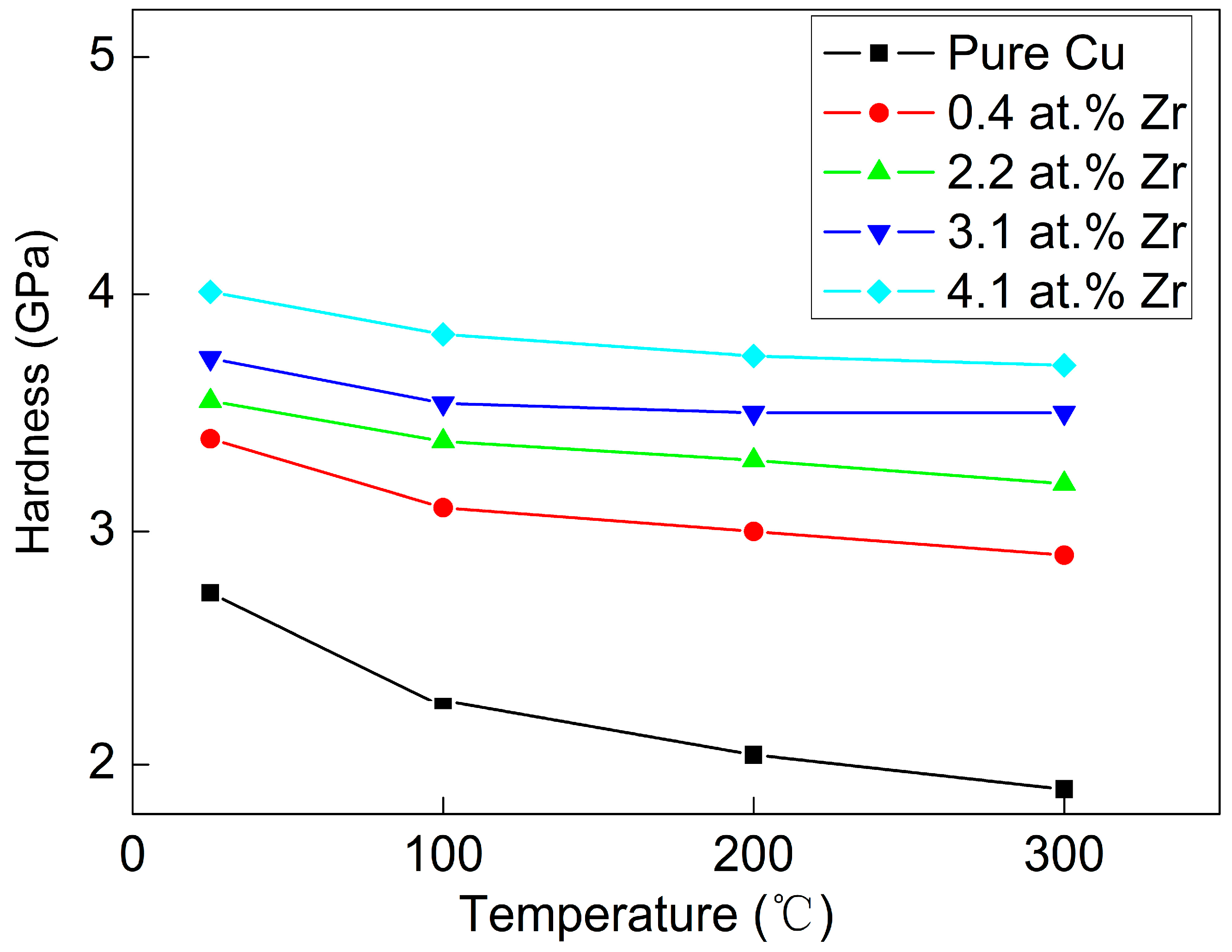
Figure 10 shows the hardness variation in various Cu-Zr alloy films after annealing. As can be seen from the figure, with the increase in annealing temperature, the hardness of all Cu-Zr alloy films decreases to different degrees. Among them, the hardness of the pure Cu film decreases most significantly. After annealing at 100 °C, its hardness decreases from 2.7 GPa (as-deposited state) to less than 2.2 GPa. When the annealing temperature rises to 300 °C, the hardness of the pure Cu film further decreases to 1.8 GPa. Combined with the grain sizes of various Cu-Zr alloy films after annealing at different temperatures (shown in
Figure 9), the significant hardness decrease in the pure Cu film with the increase in annealing temperature stems from two aspects. First, the grains of the pure Cu film grow rapidly after annealing. Grain coarsening weakens the grain refinement strengthening effect, thereby leading to a decrease in the film’s hardness. Second, the as-deposited film contains a large amount of residual stress. Annealing releases the residual stress in the as-deposited film, which also results in a decrease in the film’s hardness. The superposition of these two effects causes the hardness of the pure Cu film to decrease significantly with increasing annealing temperature. The addition of Zr element can greatly improve the stability of Cu-Zr alloy films. Their grain size no longer grows with the increase in annealing temperature. Therefore, although the hardness of Cu-Zr alloy films also decreases slightly with increasing annealing temperature, the decrease amplitude is small. Moreover, it can be seen from
Figure 10 that the higher the Zr content, the less obvious this decreasing trend becomes.
4. Discussion
4.1. Strengthening Mechanism of Alloy Films
Based on the XRD and TEM characterization, the results indicate that the Cu-Zr alloy films with varying Zr contents in this study exhibit a solid solution structure where Zr is dissolved in Cu. No secondary phases of compounds or simple substances are formed. Therefore, the strengthening of the alloy film mainly originates from three factors:
One aspect is the grain refinement strengthening effect (
) induced by grain refinement. The hardness increment induced by grain refinement can be calculated via the Hall-Petch equation. In this work, the hardness increment of the film is defined as the difference between the hardness of the Cu alloy film and that of the pure Cu film. Thus,
can be calculated as:
where
and
represent the hardness of Cu-Zr alloy films and pure Cu film, respectively;
and
are constants,
is usually between hardness and strength, here
is taken as 3.0,
= 3.478 GPa/nm
−1/2 (for Cu alloy) [
19];
and
are the grain sizes of Cu-Zr alloy films and pure Cu film, respectively.
The other aspect is the solid solution strengthening effect (
) caused by the dissolution of Zr solute atoms in the Cu lattice. The lattice distortion resulting from this dissolution hinders the movement of dislocations in the alloy, thereby generating the solid solution strengthening effect (
). The value of
can be calculated using the following Fleischer model formula [
20]:
where
is a constant related to dislocation type, here
= 3 (for screw dislocation);
represents the content of solute atoms;
is the shear modulus of the alloy;
and
are the lattice mismatch coefficient and the modulus mismatch coefficient, respectively, where
,
can be obtained as follows:
where
. The slopes of the black dashed lines in
Figure 3b and
Figure 5b are equal to the values of
and
, respectively.
Finally, Rupert et al.’s research [
21] suggests that when the grain size of the alloy decreases to the nanoscale, the grain boundaries with nanoscale spacing will also exert a pinning effect on dislocation movement, thereby resulting in nanocrystalline solution pinning strengthening (
).
where
is the interaction parameter related to
,
and
as follows:
Therefore, the total hardness increment (
) of the Cu-Zr alloy film can be expressed as:
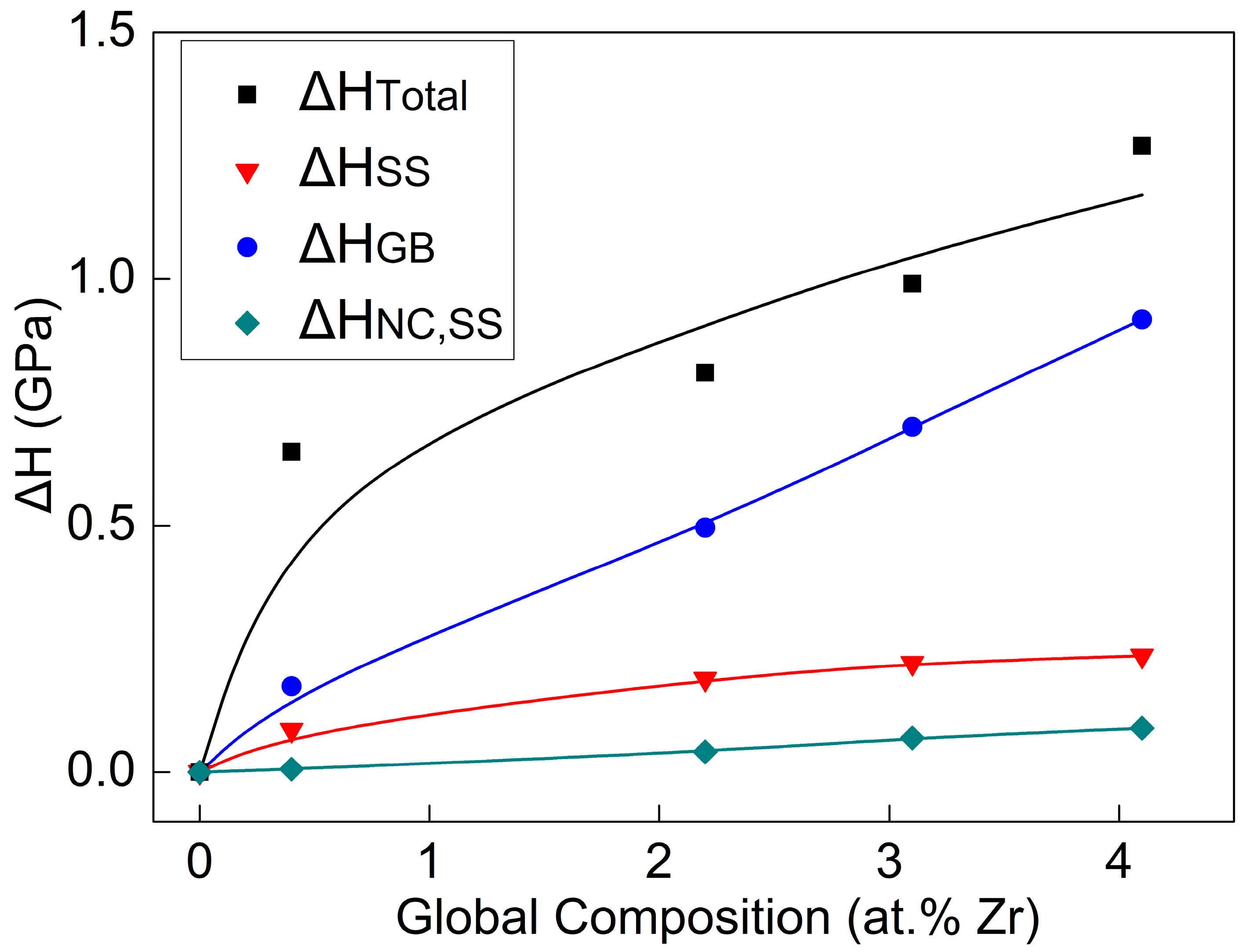
Figure 11 presents a schematic illustration of the variation laws of
,
,
and
of the Cu-Zr alloy film calculated in accordance with the aforementioned formula with the alloy content. The data points in the figure represent the measured or calculated hardness increment values of each Cu-Zr alloy thin film, while the curves are the fitting curves corresponding to the data points. It is evident that the increase in the hardness of the alloy film mainly comes from the strengthening effect of grain refinement, with the contribution of
accounting for approximately 70%. The second is the contribution of
(approximately 20%), while the effect of nanocrystalline solute atom pinning strengthening (
) is the smallest, less than 10%.
4.2. Structural Stability of Alloy Films
The phase diagram of Cu-Zr binary alloy shows that the solid solubility of Zr atoms in the Cu lattice is very small (<0.5 at.%) under equilibrium conditions. However, during magnetron sputtering, the deposited particles lose energy rapidly after reaching the substrate. Owing to the constraints of kinetic conditions, Zr solute atoms are compelled to persist in a supersaturated state within the Cu lattice, resulting in the formation of a supersaturated solid solution structure. Meanwhile, owing to thermodynamic instability, the incorporated Zr atoms are more prone to segregate at grain boundaries, consequently lowering the system’s energy. Therefore, grain boundary segregation may occur in the Cu-Zr nanocrystalline alloy films. Based on the following formula, the atomic content at the grain boundaries (
) can be calculated:
where
is the total content of alloy elements in the film;
and
are the volume fractions of nanograins and grain boundaries in the film, respectively. The
can be obtained by the following formula [
22]:
The
represents the width of the grain boundary, the value of
is assumed as 2 nm in this work, while
in the formula represents the grain size of alloy films.
refers to the solute content within grains. In accordance with the well-known empirical electron theory of solids and molecules (EET) proposed by Ruihuang Yu in 1978 [
23], which is widely employed to forecast structure and properties of materials and guide alloy design from an electronic perspective [
24,
25], the solute content within grains and the interplanar spacing have the following relationship:
The
in the formula represents the lattice constant of the alloy; while
is the alloy content within grains;
and
are the single-bond radii and covalent electron numbers of solute atoms and solvent atoms in the alloy, respectively;
is a constant that corresponds to the type of crystal structure (for FCC structure,
=
);
is also a constant, here
= 0.071.
By substituting
calculated by Formula (7), and
,
calculated by Formula (6) into Formula (5), the atomic content at grain boundaries () can be obtained.
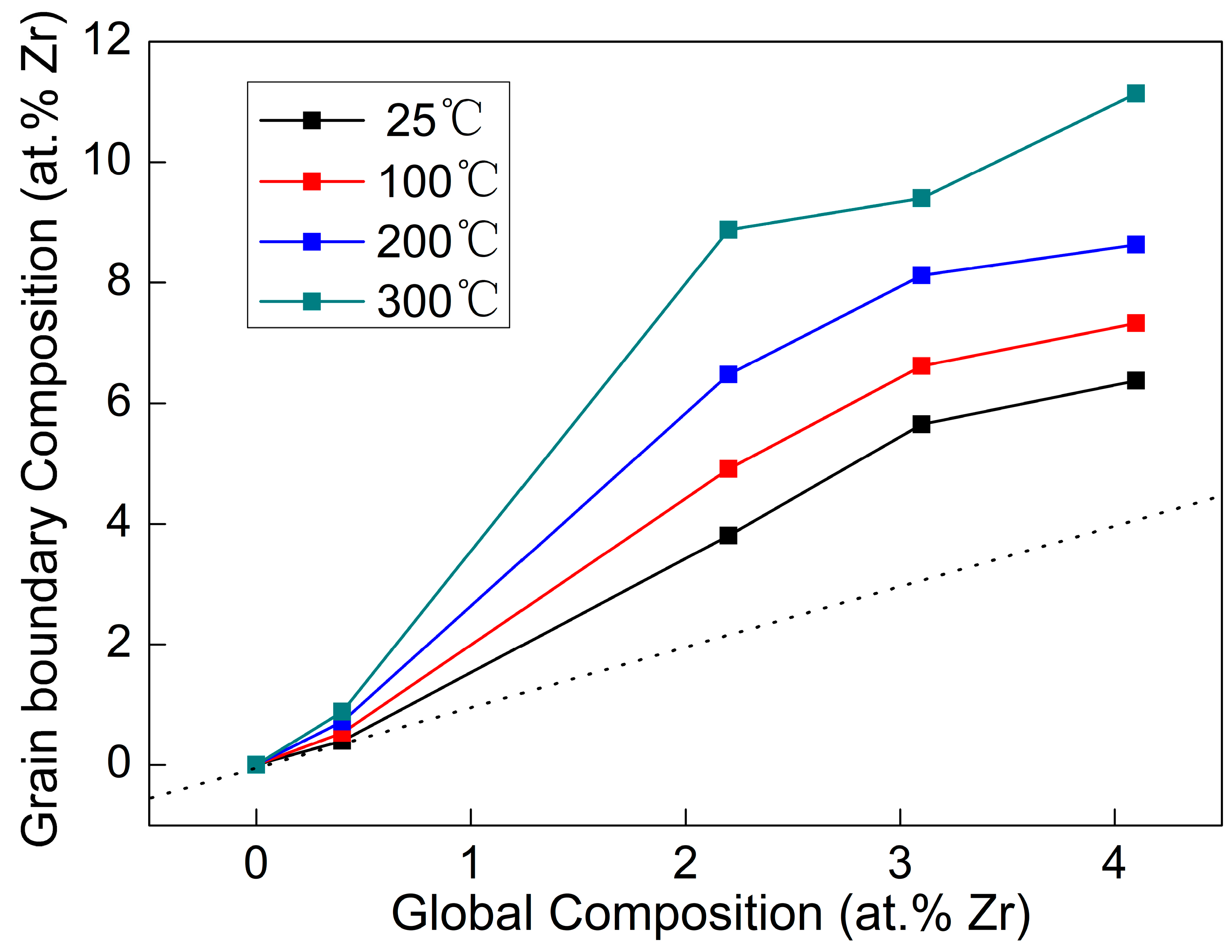
Figure 12 illustrates the relationship between the content of Zr atoms at grain boundaries and the total content in Cu-Zr alloy films under varying annealing temperatures. The dashed line in the figure is a curve formed by points where the grain boundary alloy content equals the total content. All data points in the figure lie above this curve, indicating that the content at grain boundaries is higher than the total content and that grain boundary segregation occurs in the films. Moreover, the higher the annealing temperature, the higher the content of Zr atoms at grain boundaries, which indicates a more obvious grain boundary segregation phenomenon in the alloy films. This is because increasing the temperature raises the system energy of the alloy films, enabling more dissolved Zr atoms to have higher kinetic energy to break free from the constraints of the Cu lattice and migrate to grain boundaries. These Zr atoms segregated at grain boundaries can exert a pinning effect on the grain boundaries, thereby improving the structural stability of the Cu-Zr alloy films. In addition, although this grain boundary segregation phenomenon usually contributes to the strengthening of alloy thin films, the results in
Figure 10 show that with the increase in annealing temperature, the hardness of all Cu-Zr alloy thin films decreases to varying degrees. This indicates that during the annealing process, the strengthening effect caused by grain boundary segregation is weaker than the softening effect caused by thin film stress relaxation and grain coarsening. Therefore, residual stress relaxation and grain coarsening are the dominant mechanisms for the property changes in the alloy thin films during annealing.
5. Conclusions
Nanocrystalline alloys have garnered extensive attention on account of their enhanced stability and superior properties, while their strengthening mechanisms are more complex. In this study, a series of nanocrystalline Cu-Zr alloy films with different Zr contents were prepared via magnetron co-sputtering technology. The influence of Zr content on the microstructure, mechanical properties and stability of the alloy films was investigated, and the corresponding strengthening and stability mechanisms were revealed. The results show that part of the added Zr atoms are dissolved in the Cu lattice to form a solid solution structure of Zr in Cu, while the other part segregates at the grain boundaries. The addition of Zr significantly refines the grain size of the alloy films. As the Zr content increases, the hardness of the alloy films exhibits a gradual upward trend, and the structural stability of the films is remarkably improved.
The key highlights of this paper are as follows: it distinguishes the roles of three main factors in film strengthening-grain refinement strengthening effect (), solid solution strengthening effect () and nanocrystalline solute atom pinning strengthening effect (). The proportion of their respective contributions are quantitatively calculated. Among these, grain refinement strengthening plays the most significant role, accounting for over 70% of the alloy film strengthening, followed by solid solution strengthening (accounting for approximately 20%), while nanocrystalline solute atom pinning strengthening has the weakest effect (accounting for less than 10%). Additionally, the improved stability of Cu-Zr alloy thin films stems from the grain boundary segregation of Zr atoms. The Zr atoms enriched at grain boundaries are capable of pinning the grain boundaries and impeding their migration, thereby enhancing the structural stability of the alloy thin films. The research results potentially contribute to the understanding of alloying effect in nanocrystalline alloy films and provide theoretical guidance for their further applications.
Author Contributions
Conceptualization, Y.W., H.S. and X.Q.; methodology, Y.W. and X.Q.; validation, Y.J. and S.Y.; formal analysis, Y.W. and X.Q.; investigation, Y.W. and X.Q.; writing—original draft preparation, Y.W.; writing—review and editing, H.S., X.Q. and N.Z.; visualization, Y.W. and X.Q.; supervision, H.S. and N.Z.; project administration, X.Q.; funding acquisition, Y.W. and X.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 52002240 and 51401121), Shanghai Engineering Research Center of Hot Manufacturing (18DZ2253400) and Shanghai Collaborative Innovation Center of Manufacturing Technology for Heavy Casting and Forging.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pellicer, E.; Varea, A.; Pané, S.; Nelson, B.J.; Menéndez, E.; Estrader, M.; Suriñach, S.; Baró, M.D.; Nogués, J.; Sort, J. Nanocrystalline electroplated Cu–Ni: Metallic thin films with enhanced mechanical properties and tunable magnetic behavior. Adv. Funct. Mater. 2010, 20, 983–991. [Google Scholar] [CrossRef]
- Li, G.; Yang, Y.; Gou, B.; Zhang, J.; Li, J.; Wang, Y.; Cao, L.; Liu, G.; Ding, X.; Sun, J. Stabilizing defective coherent twin boundaries for strong and stable nanocrystalline nanotwinned Cu. Acta Mater. 2022, 241, 118368. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Zhao, J.T.; Li, X.G.; Wang, Y.Q.; Wu, K.; Liu, G.; Sun, J. Alloying effects on the microstructure and mechanical properties of nanocrystalline Cu-based alloyed thin films: Miscible Cu-Ti vs immiscible Cu-Mo. Acta Mater. 2018, 143, 55–66. [Google Scholar] [CrossRef]
- Li, X.G.; Cao, L.F.; Zhang, J.Y.; Li, J.; Zhao, J.T.; Feng, X.B.; Wang, Y.Q.; Wu, K.; Zhang, P.; Liu, G.; et al. Tuning the microstructure and mechanical properties of magnetron sputtered Cu-Cr thin films: The optimal Cr addition. Acta Mater. 2018, 151, 87–99. [Google Scholar] [CrossRef]
- Qin, W.; Fu, L.C.; Xie, T.L.; Zhu, J.J.; Yang, W.L.; Li, D.Y.; Zhou, L.P. Abnormal hardness behavior of Cu-Ta films prepared by magnetron sputtering. J. Alloys Compd. 2017, 708, 1033–1037. [Google Scholar] [CrossRef]
- Agrotis, S.; Sener, M.E.; Hagger, O.S.J.; Handoko, A.D.; Caruana, D.J. One-Step synthesis of nanosized Cu-Ag films using atmospheric pressure plasma jet. Appl. Mater. Today 2024, 39, 102286. [Google Scholar] [CrossRef]
- Xie, T.L.; Zhu, J.J.; Fu, L.C.; Zhang, R.L.; Li, N.; Yang, M.Z.; Wang, J.L.; Qin, W.; Yang, W.L.; Li, D.Y.; et al. The evolution of hardness in Cu-W alloy thin films. Mater. Sci. Eng. A 2018, 729, 170–177. [Google Scholar] [CrossRef]
- Souli, I.; Gruber, G.C.; Terziyska, V.L.; Zechner, J.; Mitterer, C. Thermal stability of immiscible sputter-deposited Cu-Mo thin films. J. Alloys Compd. 2019, 783, 208–218. [Google Scholar] [CrossRef]
- Zhadko, M.; Benediktová, A.; Čerstvý, R.; Houška, J.; Čapek, J.; Kolenatý, D.; Minár, J.; Baroch, P.; Zeman, P. Unveiling effects of Zr alloying on structure and properties of nanocrystalline Cu-Zr films. Mater. Des. 2025, 253, 113949. [Google Scholar] [CrossRef]
- Peng, H.R.; Huo, W.T.; Zhang, W.; Tang, Y.; Zhang, S.; Huang, L.K.; Hou, H.Y.; Ding, Z.G.; Liu, F. Correlation between stabilizing and strengthening effects due to grain boundary segregation in iron-based alloys: Theoretical models and first-principles calculations. Acta Mater. 2023, 251, 118899. [Google Scholar] [CrossRef]
- Zhou, X.; Li, X.Y.; Lu, K. Enhanced thermal stability of nanograined metals below a critical grain size. Science 2018, 360, 526–530. [Google Scholar] [CrossRef]
- Atwater, M.A.; Scattergood, R.O.; Koch, C.C. The stabilization of nanocrystalline copper by zirconium. Mater. Sci. Eng. A 2013, 559, 250–256. [Google Scholar] [CrossRef]
- Rajagopalan, M.; Darling, K.; Turnage, S.; Koju, R.K.; Hornbuckle, B.; Mishin, Y.; Solanki, K.N. Microstructural evolution in a nanocrystalline Cu-Ta alloy: A combined in-situ TEM and atomistic study. Mater. Des. 2017, 113, 178–185. [Google Scholar] [CrossRef]
- Liang, N.; Zhao, Y. A review on thermal stability of nanostructured materials. J. Alloys Compd. 2023, 938, 168528. [Google Scholar] [CrossRef]
- Kotan, H.; Darling, K.A.; Saber, M.; Koch, C.C.; Scattergood, R.O. Effect of zirconium on grain growth and mechanical prop-erties of a ball-milled nanocrystalline FeNi alloy. J. Alloys Compd. 2013, 551, 621–629. [Google Scholar] [CrossRef]
- Devaraj, A.; Wang, W.; Vemuri, R.; Kovarik, L.; Jiang, X.; Bowden, M.; Trelewicz, J.R.; Mathaudhu, S.; Rohatgi, A. Grain boundary segregation and intermetallic precipitation in coarsening resistant nanocrystalline aluminum alloys. Acta Mater. 2019, 165, 698–708. [Google Scholar] [CrossRef]
- Kelly, P.J.; Arnell, R.D. Magnetron sputtering: A review of recent developments and applications. Vacuum 2000, 56, 159–172. [Google Scholar] [CrossRef]
- Pharr, G.M.; Oliver, W.C. Measurement of thin film mechanical properties using nanoindentation. MRS Bull. 1992, 17, 28–33. [Google Scholar] [CrossRef]
- Zhao, J.T.; Zhang, J.Y.; Cao, L.F.; Wang, Y.Q.; Zhang, P.; Wu, K.; Liu, G.; Sun, J. Zr alloying effect on the microstructure evolution and plastic deformation of nanostructured Cu thin films. Acta Mater. 2017, 132, 550–564. [Google Scholar] [CrossRef]
- Fleischer, R.L. Solid-solution hardening. In The Strengthening of Metals; Reinhold Publishing Corp.: New York, NY, USA, 1964; p. 93. [Google Scholar]
- Rupert, T.J.; Trenkle, J.C.; Schuh, C.A. Enhanced solid solution effects on the strength of nanocrystalline alloys. Acta Mater. 2011, 59, 1619–1631. [Google Scholar] [CrossRef]
- Chookajorn, T.; Murdoch, H.A.; Schuh, C.A. Design of stable nanocrystalline alloys. Science 2012, 337, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.H. The empirical electron theory of solids and molecules. Chin. Sci. Bull. 1978, 23, 217–224. [Google Scholar] [CrossRef]
- Guo, J.B.; Guo, Z.Z.; Zhang, W.; Han, H.; Xu, C.Y. The application of EET in high-entropy alloy. J. Phys. Conf. Ser. 2023, 2478, 122081. [Google Scholar] [CrossRef]
- Fu, B.Q.; Liu, W.; Li, Z.L. Calculation of the surface energy of bcc-metals with the empirical electron theory. Appl. Surf. Sci. 2009, 255, 8511–8519. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).