Preparation and Performance Study of Decanoic Acid–Stearic Acid Composite Phase-Change Ceramsite Aggregate
Abstract
1. Introduction
2. Preparation of Composite Phase-Change Ceramsite Aggregate
2.1. Raw Material
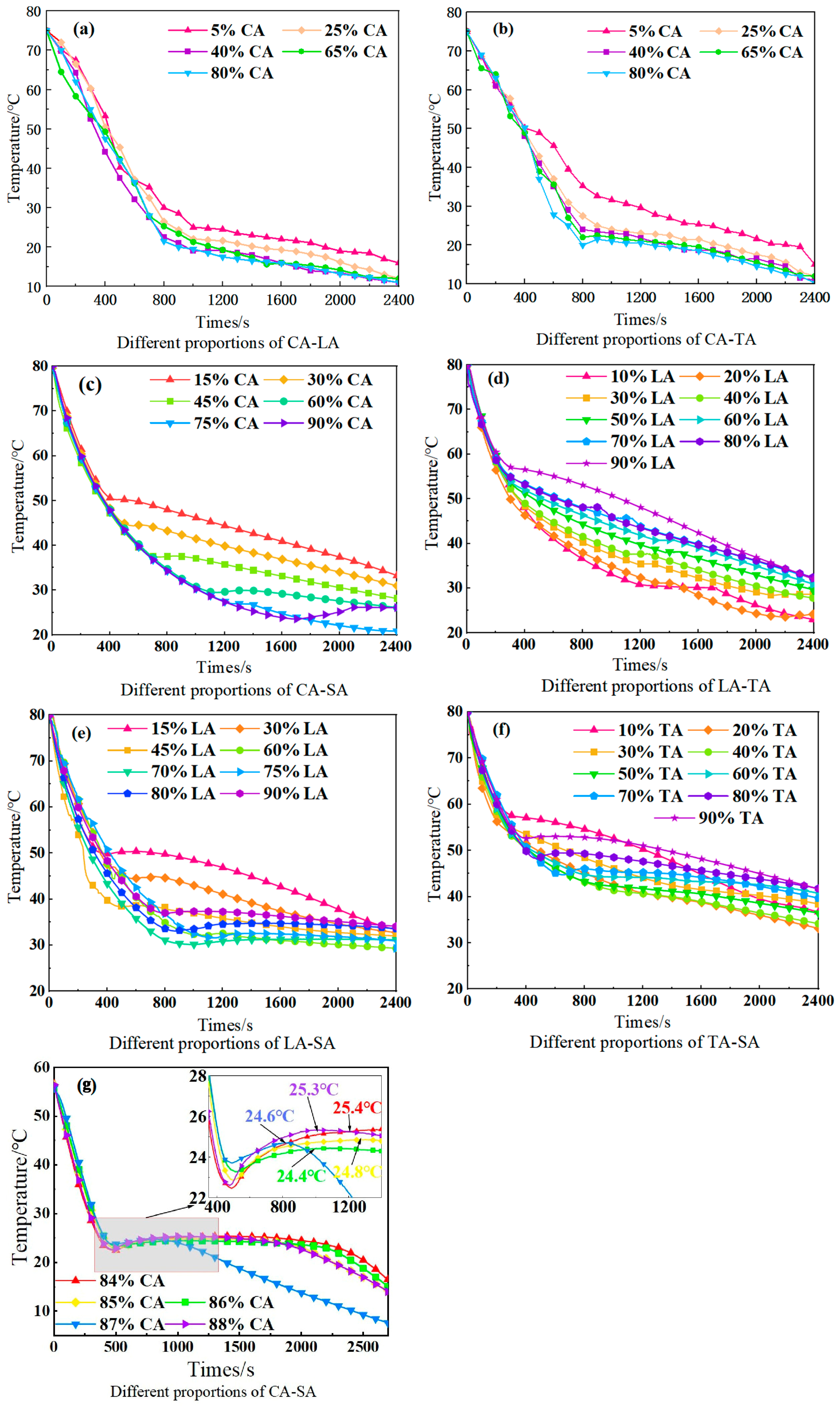
2.2. Screening of Composite Phase-Change Materials
2.3. Preparation Process of Composite Phase-Change Ceramsite Aggregate
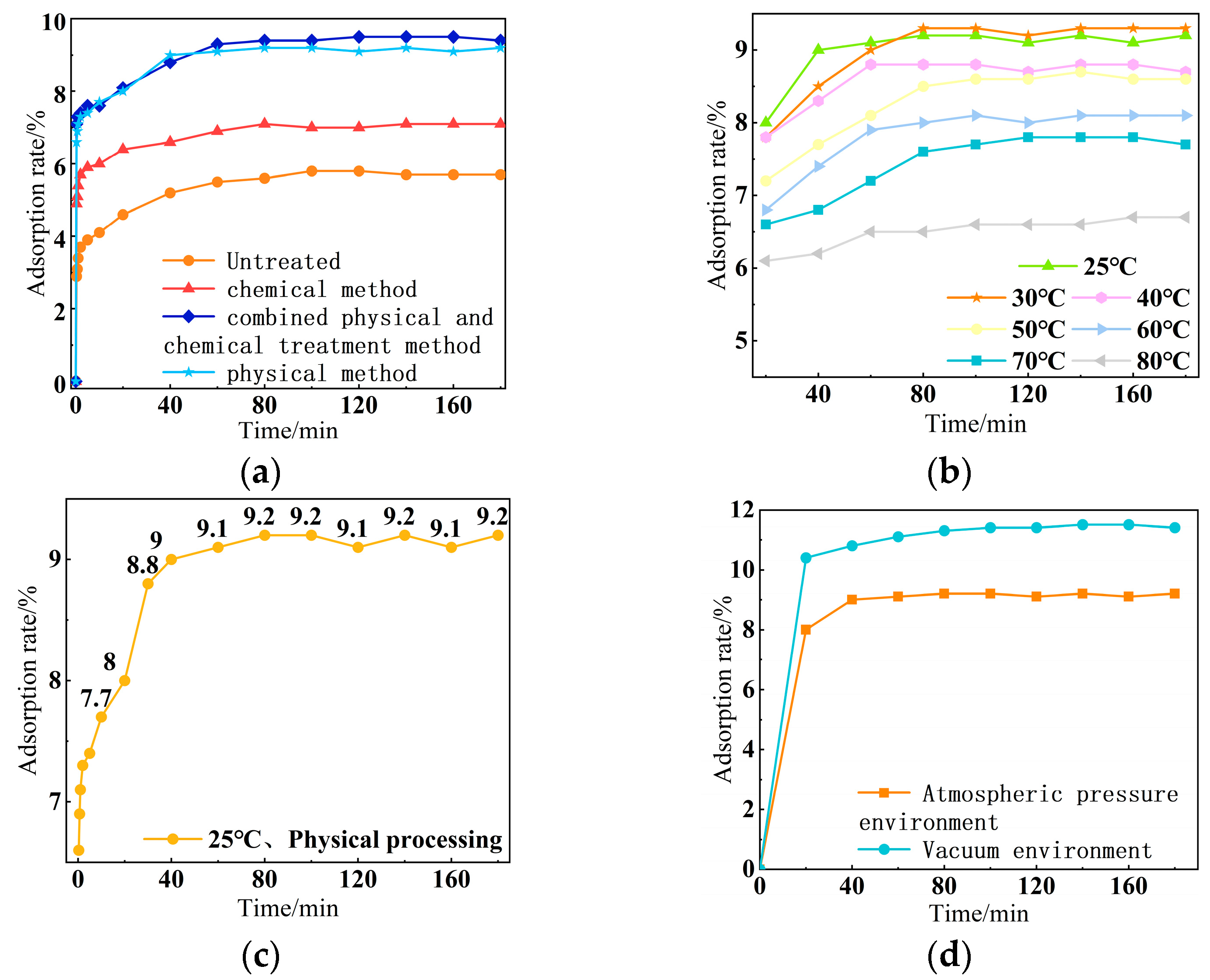
2.3.1. Adsorption Process of Composite Phase-Change Materials
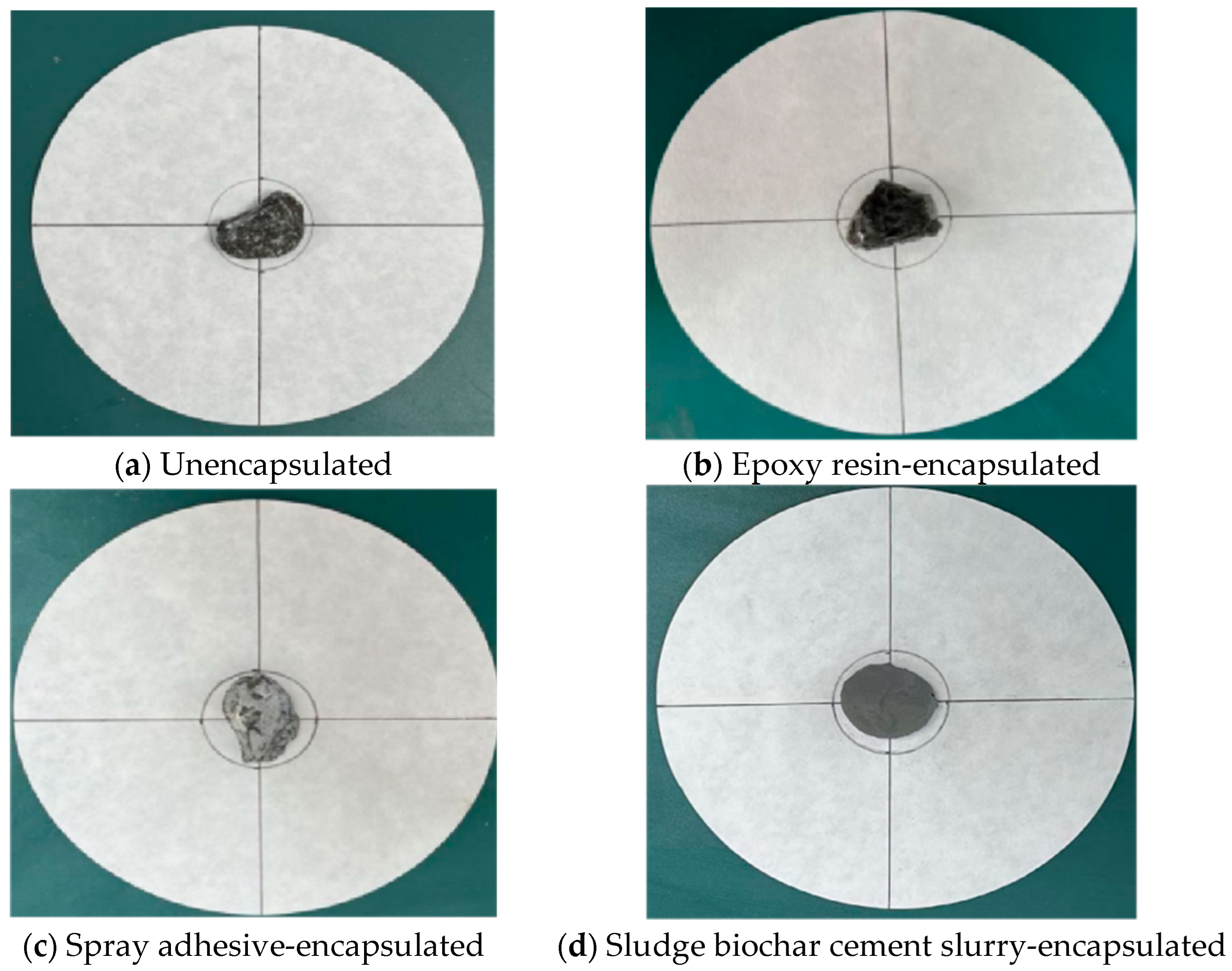
2.3.2. Packaging Process of Composite Phase-Change Materials
3. Performance of Composite Phase-Change Ceramsite Aggregate
3.1. Thermal Stability
3.1.1. Test Method
3.1.2. Results
3.2. Mixing and Crushing Rate
3.2.1. Test Method
3.2.2. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Malode, J.S.; Shetti, P.N. Thermal energy storage systems using bio-based phase change materials: A comprehensive review for building energy efficiency. J. Energy Storage 2025, 105, 114709. [Google Scholar] [CrossRef]
- Deshmukh, M.; Yadav, M. Optimizing thermal efficiency of building envelopes with sustainable composite materials. Buildings 2025, 15, 230. [Google Scholar] [CrossRef]
- Zheng, S.; Qiu, Z.; He, C.; Wang, X.; Wang, X.; Wang, Z.; Zhao, X.; Shittu, S. Research on heat transfer mechanism and performance of a novel adaptive enclosure structure based on micro-channel heat pipe. Energy 2022, 254, 124237. [Google Scholar] [CrossRef]
- Bouhezza, A.; Laouer, A.; Ismail, K.A.; Faraji, H.; Khuda, M.A.; Teggar, M.; Lino, F.A.M.; Henríquez, J.R.; Rodríguez, D. Effective techniques for performance improvement of phase change material applications: A review. J. Energy Storage 2025, 105, 114671. [Google Scholar] [CrossRef]
- Reddy, J.V.; Ghazali, F.M.; Kumarasamy, S. Advancements in phase change materials for energy-efficient building construction: A comprehensive review. J. Energy Storage 2024, 81, 110494. [Google Scholar] [CrossRef]
- Abdel-Mawla, M.A.; Hassan, M.A.; Khalil, A. Phase change materials in thermally activated building systems: A comprehensive review. Int. J. Energy Res. 2022, 46, 11676–11717. [Google Scholar] [CrossRef]
- Zhan, H.; Mahyuddin, N.; Sulaiman, R.; Khayatian, F. Phase change material (pcm) integrations into buildings in hot climates with simulation access for energy performance and thermal comfort: A review. Constr. Build. Mater. 2023, 397, 132312. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Cui, H.; Bao, X.; Tang, W.; Sang, G.; Chen, X. Cementitious composites integrated phase change materials for passive buildings: An overview. Constr. Build. Mater. 2022, 361, 129635. [Google Scholar] [CrossRef]
- Vega, M.; Marín, P.E.; Ushak, S.; Shire, S. Research trends and gaps in experimental applications of phase change materials integrated in buildings. J. Energy Storage 2024, 75, 109746. [Google Scholar] [CrossRef]
- Zhou, D.; Xiao, S.; Xiao, X.; Liu, Y. Preparation, Phase Diagrams and Characterization of Fatty Acids Binary Eutectic Mixtures for Latent Heat Thermal Energy Storage. Separations 2023, 10, 49. [Google Scholar] [CrossRef]
- Liang, X.; Fei, H.; Li, Y.; Li, P.; Du, W.; Zhou, J. Structural characteristics and properties of ca-sa-pw/eg adsorbed into porous cement-based for thermal storage. Mater. Today Commun. 2023, 37, 107046. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, W.; Fu, S.; Zhang, H. Study of a novel ceramsite-based shape-stabilized composite phase change material (pcm) for energy conservation in buildings. Constr. Build. Mater. 2020, 246, 118479. [Google Scholar] [CrossRef]
- Song, M.; Pun, W.M.; Swapnil, D.; Pan, D.; Mao, N. Thermal stability of organic binary pcms for energy storage. Energy Procedia 2017, 142, 3287–3294. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, R.; Zhang, N.; Chen, L.; Chen, D.; Li, X. Performance improvement of lauric acid-1-hexadecanol eutectic pHase change material with bio-sourced seashell powder addition for thermal energy storage in buildings. Constr. Build. Mater. 2023, 366, 130223. [Google Scholar] [CrossRef]
- Fan, Z.; Zhao, Y.; Ding, Y.; Shi, Y.; Liu, X.; Jiang, D. Fabrication and comprehensive analysis of expanded perlite impregnated with myristic acid-based pHase change materials as composite materials for building thermal management. J. Energy Storage 2022, 55, 105710. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, W.; Yang, C. Improving the adsorption of cloud concrete stone porous aggregate to phase change materials by alkali modification. Mater. Lett. 2025, 380, 137717. [Google Scholar] [CrossRef]
- Chen, R.; Li, D.; Sheng, N.; Zhu, C. Direct synthesis of porous aluminum nitride foams for enhancing heat transfer and anti-leakage performance of phase change materials. Thermochim. Acta 2024, 734, 179706. [Google Scholar] [CrossRef]
- Ghosh, D.; Ghose, J.; Datta, P.; Kumari, P.; Paul, S. Strategies for phase change material application in latent heat thermal energy storage enhancement: Status and prospect. J. Energy Storage 2022, 53, 105179. [Google Scholar] [CrossRef]
- Liu, Z.; Teng, R.; Sun, H. Application of pHase change energy storage in buildings: Classification of pHase change materials and packaging methods. Therm. Sci. 2022, 26, 4315–4332. [Google Scholar] [CrossRef]
- Huang, Y.; Stonehouse, A.; Abeykoon, C. Encapsulation methods for phase change materials—A critical review. Int. J. Heat Mass Transf. 2023, 200, 123458. [Google Scholar] [CrossRef]
- Çolak, B.A. Numerical investigation of thermal energy storage in wavy enclosures with nanoencapsulated phase change materials using deep learning. Energy 2025, 20, 135272. [Google Scholar] [CrossRef]
- Tian, Y.; Lai, Y.; Pei, W.; Qin, Z.; Li, H. Study on the physical mechanical properties and freeze-thaw resistance of artificial phase change aggregates. Constr. Build. Mater. 2022, 329, 127225. [Google Scholar] [CrossRef]







| Particle Size (cm) | Water Absorption Rate (%) | Bulk Density (kg·m−3) | Thermal Conductivity (W·m−1K−1) |
| 5–20 | 6.5 | 718 | 0.23 |
| Phase-Change Materials | Melting Enthalpy ΔHm (J/g) | Phase-Transition Temperature (°C) | Molar Mass (g/mol) |
| Decanoic acid | 151.84 | 28.83 | 172.26 |
| Lauric acid | 174.99 | 42.49 | 200.32 |
| Myristic acid | 186.68 | 52.88 | 228.38 |
| Stearic acid | 204.23 | 61.58 | 284.48 |
| Color (Hazen) | Epoxy Equivalent (g/mol) | Bonding Time (min) | Peel Strength (N/m) | Heat Resistance Temperature (°C) |
| ≤40 | 220~240 | ≤0.5 | ≤5 | ≤66 |
| Color (Hazen) | Epoxy Equivalent (g/mol) | Hydrolyzable Chlorine (%) | Inorganic Chlorine (g/kg) | Softening Point (°C) |
| ≤40 | 220~240 | ≤0.5 | ≤0.05 | 13~22 |
| Pyrolysis Temperature (°C) | Moisture Content (%) | Specification (Mesh) | Bulk Density (cm3/g) | Iodine Value (mg/g) |
| 500 | ≤3 | 200 | 0.50 | 800 |
| Composite Phase-Change Materials | Weight Ratio | Theoretical Phase-Transition Temperature (°C) | Theoretical Latent Heat of Phase Transition (J/g) |
| CA-LA | 64.3:35.7 | 17.91 | 151.21 |
| CA-TA | 75.2:24.8 | 22.53 | 152.78 |
| CA-SA | 86.7:13.3 | 26.27 | 153.29 |
| LA-TA | 61.8:39.2 | 32.57 | 171.45 |
| LA-SA | 74.2:25.8 | 37.40 | 175.57 |
| TA-SA | 61.9:38.1 | 44.75 | 185.98 |
| Composite Phase Change Materials | Theoretical Tm (°C) | Actual Tm (°C) | Actual Tc (°C) | Relative Deviation of Tm (%) |
| CA-LA | 17.91 | 18.25 | 16.89 | +1.89 |
| CA-TA | 22.53 | 23.11 | 21.57 | +2.57 |
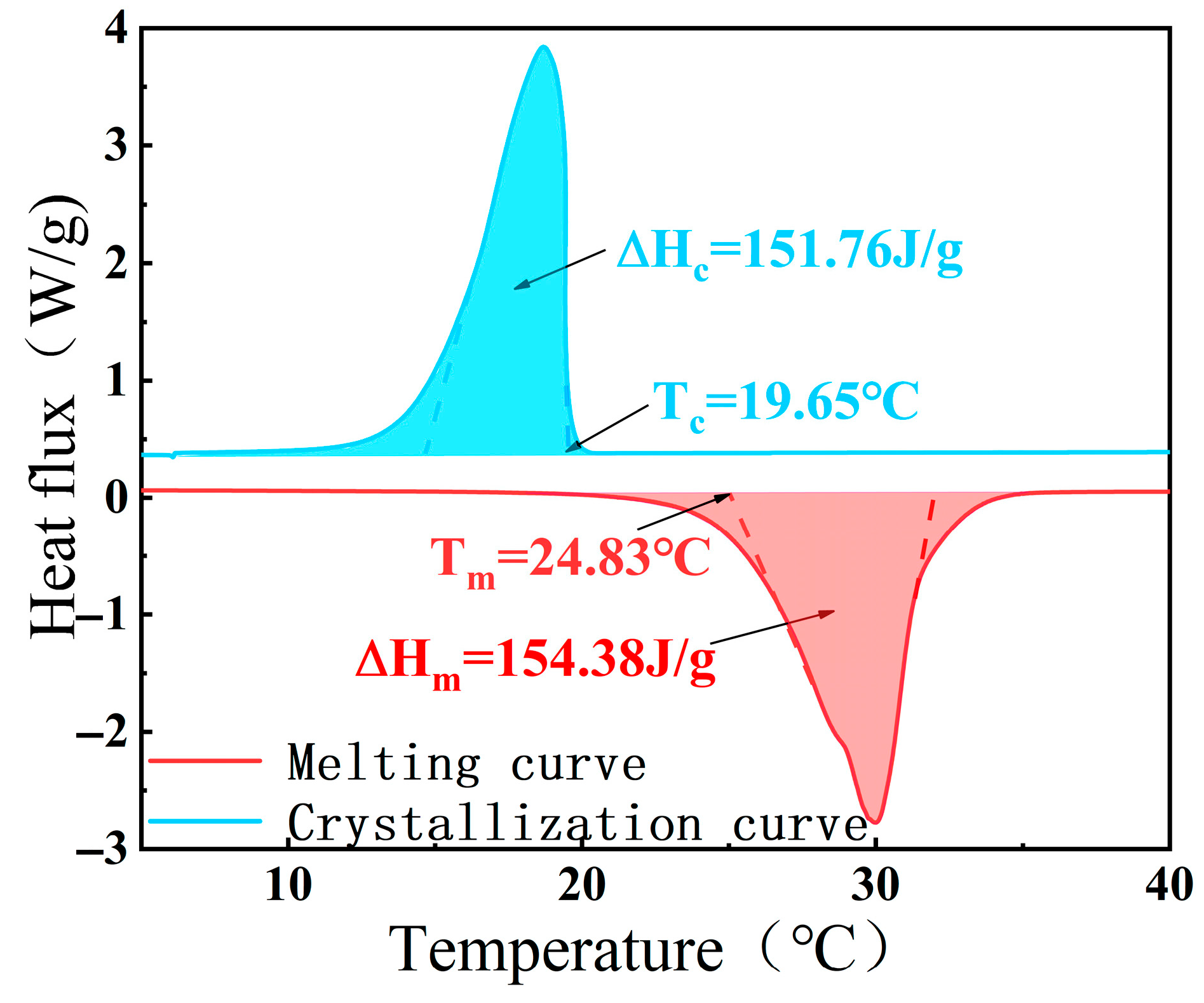
| CA-SA | 26.27 | 24.83 | 23.21 | −5.48 |
| LA-TA | 32.57 | 33.05 | 31.42 | +1.47 |
| LA-SA | 37.40 | 38.12 | 36.58 | +1.92 |
| TA-SA | 44.75 | 45.33 | 43.67 | +1.29 |
| Composite phase change materials | Theoretical ΔHm (J/g) | Actual ΔHm (J/g) | Actual ΔHc (J/g) | Relative deviation of ΔHm (%) |
| CA-LA | 151.21 | 148.63 | 146.92 | −1.71 |
| CA-TA | 152.78 | 150.35 | 148.76 | −1.60 |
| CA-SA | 153.29 | 154.38 | 152.65 | +0.71 |
| LA-TA | 171.45 | 168.92 | 167.34 | −1.48 |
| LA-SA | 175.57 | 172.86 | 171.23 | −1.54 |
| TA-SA | 185.98 | 183.25 | 181.57 | −1.47 |
| Sludge Biochar Dosage | Surface Pore Area Ratio | Surface Flatness (Standard Deviation of Gray Value) | Visual Feature Description |
| 1% | 18.7% | 0.35 | Obvious pores on the surface, distributed dispersedly |
| 3% | 5.2% | 0.12 | Dense and uniform surface, no obvious pore exposure |
| 5% | 23.4% | 0.41 | Partial aggregate exposure, strong pore connectivity |
| Sludge Biochar Dosage | 28 d Compressive Strength (MPa) | Impermeability Coefficient (m/s) | Phase-Change Material Leakage Rate (%) | Thickness of Encapsulation Layer (cm) |
| 1% | 15.3 | 3.6 × 10−9 | 8.7 | 0.4–0.6 |
| 3% | 28.6 | 1.2 × 10−9 | 1.3 | 0.3–0.5 |
| 5% | 12.5 | 4.7 × 10−9 | 12.4 | 0.2–0.4 |
| Project | Percentage of Exudation | Stability |
| Very little exudation (considered as no exudation) | φ ≤ 10 | Very stable |
| Trace | 10 < φ ≤ 15 | Stable |
| A small amount | 15 < φ ≤ 30 | Basically stable |
| Medium quantity | 30 < φ ≤ 50 | Instability |
| A large number | φ > 50 | Very unstable |
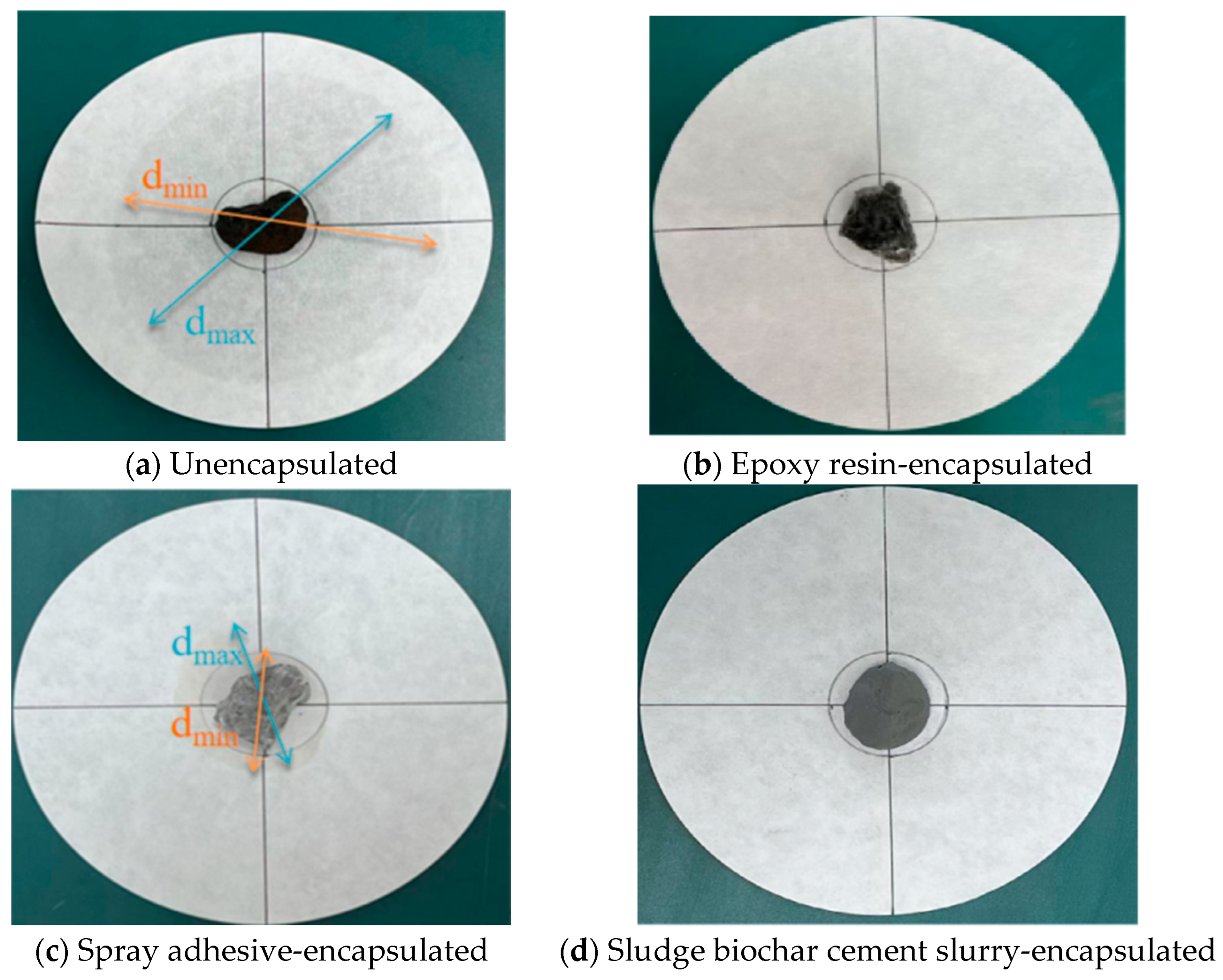
| Phase-Change Ceramsite Aggregate | No Encapsulation | Epoxy Resin Encapsulation | Spray Adhesive Packaging | Encapsulation of Sludge, Biochar, Cement Slurry |
| Maximum diameter (mm) | 96 | 0 | 52 | 0 |
| Minimum Diameter (mm) | 80 | 0 | 32 | 0 |
| Mean value (mm) | 88 | 0 | 42 | 0 |
| Percentage of exudation (%) | 193 | 0 | 40 | 0 |
| Stability evaluation | Very unstable | Very stable | Instability | Very stable |
| Aggregate Category | Epoxy Resin Encapsulation | Spray Adhesive Packaging | Encapsulation of Sludge, Biochar, Cement Slurry |
| Shell breakage rate | 0% | 0% | 7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, G.; Yuan, Q.; Li, M.; Tao, J.; Jiang, J.; Chen, D. Preparation and Performance Study of Decanoic Acid–Stearic Acid Composite Phase-Change Ceramsite Aggregate. Coatings 2025, 15, 1315. https://doi.org/10.3390/coatings15111315
Yu G, Yuan Q, Li M, Tao J, Jiang J, Chen D. Preparation and Performance Study of Decanoic Acid–Stearic Acid Composite Phase-Change Ceramsite Aggregate. Coatings. 2025; 15(11):1315. https://doi.org/10.3390/coatings15111315
Chicago/Turabian StyleYu, Gui, Qiang Yuan, Min Li, Jiaxing Tao, Jing Jiang, and De Chen. 2025. "Preparation and Performance Study of Decanoic Acid–Stearic Acid Composite Phase-Change Ceramsite Aggregate" Coatings 15, no. 11: 1315. https://doi.org/10.3390/coatings15111315
APA StyleYu, G., Yuan, Q., Li, M., Tao, J., Jiang, J., & Chen, D. (2025). Preparation and Performance Study of Decanoic Acid–Stearic Acid Composite Phase-Change Ceramsite Aggregate. Coatings, 15(11), 1315. https://doi.org/10.3390/coatings15111315






