Abstract
Surface coatings have proven highly effective in addressing the critical challenges of friction, wear, and corrosion on steel substrates, which are responsible for over 80% of mechanical failures in industrial applications. Recent research highlights that advanced coatings—such as ceramic carbides/nitrides, high-entropy alloys, and metal-matrix composites—significantly enhance hardness, wear resistance, and environmental durability through mechanisms including protective oxide film formation, solid lubrication, and microstructural refinement. Moreover, these coatings exhibit robust performance under combined tribological-corrosive (tribocorrosion) conditions, where synergistic interactions often accelerate material degradation. Key developments include multilayer and composite architectures that balance hardness with toughness, self-lubricating coatings capable of in situ lubricant release, and active or self-healing systems for sustained corrosion inhibition. Despite these advances, challenges remain in predicting coating lifetime under multifield service conditions and optimizing interfacial adhesion to prevent delamination. Future efforts should prioritize multifunctional coating designs, improved tribocorrosion models, and the integration of sustainable materials and AI-driven process optimization. This review consolidates these insights to support the development of next-generation coatings for extending the service life of steel components across demanding sectors such as marine, aerospace, and energy systems.
1. Introduction
Steel is a fundamental structural material widely utilized in pivotal industrial sectors, including machinery manufacturing, automotive engineering, and aerospace systems [1]. It is often applied in environments requiring resistance to abrasion and erosive wear, and stands as a highly promising alternative material in various application scenarios within the context of mechanical engineering [2]. In practical service environments, steel materials are highly susceptible to degradation mechanisms, primarily friction, wear, and corrosion. Among these, wear accounts for nearly 80% of mechanical component failures [3], while corrosion—especially under aggressive conditions—leads to accelerated material loss and premature structural weakening. In the marine environment, metal corrosion is a prevalent issue, as high salinity, humidity, and microbial activity accelerate the degradation process [4]. These degradation modes not only deteriorate surface properties but also severely impair functional reliability and service life, resulting in substantial economic losses and increased maintenance burdens. Conventional surface treatments such as electroplating [5] and hot-dip galvanizing [6] offer limited protection under combined mechanical and chemical attacks. Corrosion is a significant problem in all fields involving the use of metallic materials in marine environments, which arises from the interaction between environmental factors and microorganisms [7,8].
To date, a diverse array of coating materials has been utilized on metal alloy components to mitigate cavitation erosion [9]. The application of hard coatings to protect steel surfaces plays a crucial role in metal processing and various industrial sectors [10]. Moreover, iron-based coatings represent a promising option for many wear-related applications, owing to their excellent compatibility and economic efficiency [11]. The use of surface coating techniques has become a key method for improving the properties of steel substrates. Processes such as PVD, CVD, laser cladding, and plasma spraying allow the creation of customized surfaces with enhanced hardness, lubricity, and chemical stability [12,13]. Alloy-based coatings are capable of offering dual protection against mechanical wear and chemical degradation. To enhance the anti-corrosion performance of steel substrates, elements such as copper (Cu), chromium (Cr), and nickel (Ni) are widely incorporated as alloying constituents [14,15]. In recent investigations, a substantial body of work has been directed toward nitride-based coatings. Studies have indicated that nitride coatings produced via PVD can significantly boost the wear performance of high-speed tool steels [16,17].
Alloy coatings deliver synergistic protection, effectively augmenting material durability against both wear and corrosive media. Elements including copper (Cu), chromium (Cr), and nickel (Ni) are frequently introduced into steel to elevate its defense against corrosive environments. Consequently, surface coating has emerged as a pivotal strategy for advancing the comprehensive properties of steel substrates [18,19,20]. Contemporary coating designs increasingly employ composite or multilayer architectures, engineered to furnish concurrent shielding from mechanical abrasion and chemical attack [21,22,23]. For instance, HTP/epoxy nanocomposites have demonstrated a marked capacity to strengthen the anti-corrosion characteristics of steel. Such sophisticated coating systems not only prolong the operational lifespan of components, but also support lower life-cycle costs and resource usage, supporting more sustainable industrial practices [24].
Despite extensive research on wear-resistant or corrosion-resistant coatings individually, the synergistic degradation of steel components under combined mechanical and chemical attacks in real-world applications (e.g., marine engineering, chemical processing) presents a formidable challenge. However, a systematic review that critically links coating design principles to both tribological and corrosion performance, and particularly their interplays, remains scarce. This review aims to bridge this gap by not only summarizing recent advances but also by elucidating the underlying mechanisms and providing a framework for the design of next-generation multifunctional coatings.
2. Coating Preparation Methods
The performance of a coating is intrinsically linked to its preparation method. Different techniques impart unique microstructural characteristics, such as density, adhesion strength, and phase composition, which ultimately govern the tribological behavior of the coating. This section reviews the most prevalent coating deposition methods employed to enhance the wear resistance of steel substrates, focusing on their principles and applications in improving tribological performance. Studies indicate that coatings can influence the overall mechanical behavior through mechanisms such as the introduction of residual stress fields. However, the academic community has not reached a consensus regarding the dominant mechanism of this influence. Some researchers attribute performance variations to the reduction in the load-bearing cross-section caused by surface treatments, while other scholars emphasize the substrate microstructural changes induced by thermal cycles during the coating preparation process [25,26,27,28,29,30].
2.1. CVD Method
CVD is a process that utilizes gaseous precursors containing coating materials within a sealed reaction chamber. Its mechanism diagram is shown in Figure 1. CVD coatings achieve excellent adhesion through high-temperature diffusion, forming a metallurgical bond and/or strong chemical bonding at the interface. In this process, the gases react chemically under regulated temperature and pressure conditions to yield solid particles, facilitating the formation of a surface layer on the substrate [31,32].
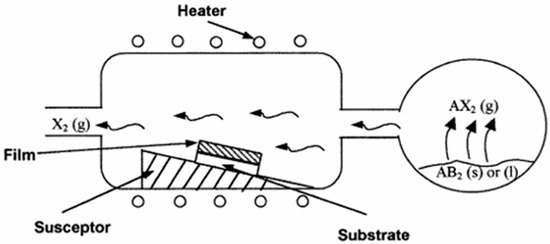
Figure 1.
CVD coating mechanism schematic [31].
The CVD technique is renowned for producing dense, high-purity coatings that significantly enhance the surface properties of steels, particularly in corrosion and high-temperature applications [33]. Similarly, the work of Raman et al. [34] highlighted the exceptional barrier properties of CVD-synthesized graphene coatings, which improved the corrosion resistance of metals like nickel and copper by up to two orders of magnitude.
Beyond corrosion protection, CVD is equally adept at creating coatings for demanding mechanical and high-temperature environments. Zhang et al. [35] exemplified this by developing aluminide coatings on ferritic and austenitic steels, offering critical insights into process parameters for achieving enhanced high-temperature wear and oxidation resistance. Furthermore, to address the critical challenge of adhesion in ultra-hard coatings, Bareiβ et al. [36] employed CVD to deposit a TiBN interlayer on steel. This interlayer was pivotal in ensuring the excellent adhesion of subsequent diamond coatings, a key requirement for wear-resistant applications where delamination is a common failure mode.
2.2. PVD Method
PVD operates by transitioning a solid coating material into a vapor phase under vacuum, which then transports and condenses onto a substrate to form a functional thin film [16,37]. This process enables the creation of coatings with exceptional characteristics, such as high hardness and low friction, on diverse substrates. The adhesion in PVD coatings relies on ion bombardment-induced interface mixing and the generation of compressive residual stresses, which enhance coating-substrate cohesion.
A defining characteristic of PVD processes is the generation of compressive residual stresses due to intense ion peening. These stresses are highly beneficial as they hinder crack initiation and propagation, thereby improving fatigue strength and wear resistance.
As illustrated in Figure 2, the operational principle of Physical Vapor Deposition (PVD) can be described in two primary methods. In magnetron sputtering (Figure 2a), the vacuum chamber is first evacuated, after which a process gas is introduced. A power supply then generates plasma, which is confined by magnetic fields, enabling energetic ions to bombard the target and dislodge atoms via sputtering. These ejected particles are subsequently transported to the substrate and deposited as a thin film. Alternatively, the evaporation method (Figure 2b) involves heating a target material within a vacuum using an evaporation source, causing atoms to evaporate and form a vapor. These vapor-phase particles are then transported to and deposited onto the substrate surface, resulting in the formation of a coherent coating. Both techniques operate under high vacuum conditions to maintain a contaminant-free environment and facilitate the transition of target material from solid to vapor phase, ultimately leading to film formation on the substrate [38].
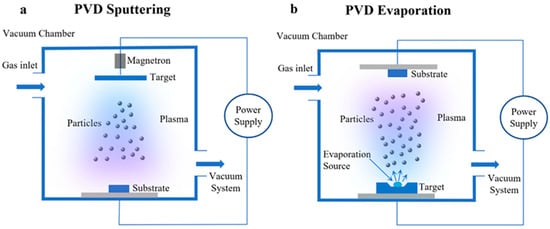
Figure 2.
Physical Vapor Deposition Process Schematic: (a) Sputtering, (b) Evaporation [38].
K. Bobzin et al. [17] employed magnetron sputtering ion plating (MSIP) with an SiC target to fabricate silicon carbide coatings, a process characterized by high deposition rates and excellent reproducibility. Their work reported the successful deposition of amorphous SiC films and further elaborated on a multilayer approach incorporating intermediate layers like TiC and TiN to enhance the adhesion of cemented carbide coatings. They emphasized that strong coating adhesion is essential to avoid failure under tribological stress conditions.
In the work of Šturm, R. et al. [39], TiN/(Ti,Al)N multilayer PVD coatings showed optimal wear resistance at room and elevated temperatures when applied to plasma-nitrided tool steel, outperforming single-layer CrN and TiN coatings. This advantage was ascribed to the multilayer’s improved adhesion strength and enhanced hardness properties.
Hyunsoo Kim et al. [40] applied a hybrid PVD process to fabricate Cr-Al-Ti-B-N coatings on AISI D2 steel, achieving a synergistic improvement in both wear and tribocorrosion resistance, demonstrating the coating’s potential for demanding applications.
Arash Fattah-alhosseini et al. [41] assessed the friction and corrosion characteristics of TiN/CrN nanostructured multilayer coatings deposited on H13 hot-work tool steel. The study integrated a diffusion layer produced by pack cementation with a cathodic arc PVD hard coating, collectively boosting the substrate’s resistance to both wear and corrosive damage.
Youn-Hoo Hwang et al. [42] assessed a tribological system comprising TiN, AlTiN, and AlTiCrN coatings in sliding contact with a stainless steel counterface, with tests conducted in air and vacuum environments. Their findings indicate that the friction and wear mechanisms are governed by distinct processes—oxide layer formation in air and surface compatibility in vacuum—offering critical guidance for the selection of coatings tailored to specific service environments. Diagrams illustrating the wear mechanisms under these two conditions are presented in Figure 3.
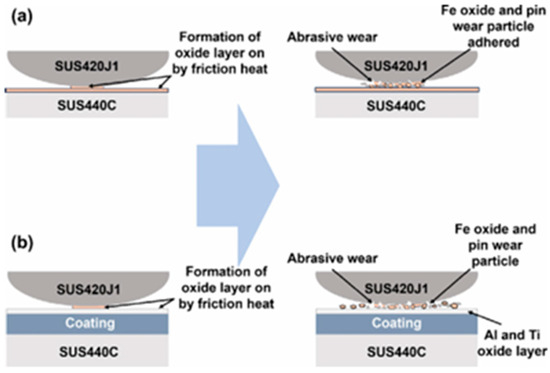
Figure 3.
Wear mechanism diagrams of materials tested in air at ambient conditions: (a) Base material (SUS440C), (b) Protective layers (TiN-series) [42].
2.3. Laser Cladding Method
Laser cladding represents a precision surface enhancement method where a high-power laser beam concurrently melts feedstock material (alloy/ceramic powder) and a shallow substrate region. Laser cladding produces a dense, metallurgically bonded interface with low dilution, resulting from the rapid melting and solidification of the substrate surface. The subsequent rapid solidification through self-quenching produces a fully dense coating characterized by coherent interfacial bonding and low dilution rate. This technique imparts significant improvements to the substrate’s functional performance, notably elevating its resistance to wear, corrosive media, and high-temperature exposure. The fundamental setup is illustrated in Figure 4 [43,44,45].
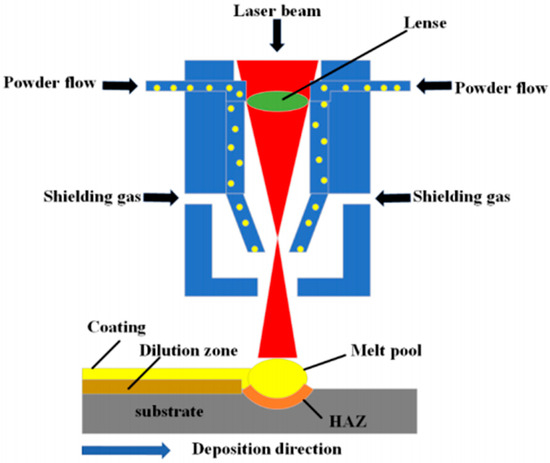
Figure 4.
Schematic representation of laser cladding technology [45].
Yang et al. [46] employed laser cladding technology to fabricate Fe–Cr alloy strengthening coatings on SS400 low-carbon steel. It was also found that wear resistance increased considerably with higher cladding overlap rates, offering an efficient solution for the surface strengthening of engineering components. Figure 5 displays a schematic representation of the employed preparation technology.

Figure 5.
Schematic diagram of the laser cladding process: (a) Schematic of laser cladding for coating. (b) Schematic of laser cladding for tensile sample [47].
In the field of laser cladding, Singh et al. dedicated their research to the laser cladding of Stellite 6 onto 13Cr-4Ni stainless steel. Their work highlighted the critical impact of energy density on coating performance: higher energy density leads to increased substrate dilution, which in turn causes a reduction in coating hardness and ultimately adversely affects its resistance to solid particle erosion and cavitation erosion. Roman Šturm et al. reported a new organic cladding pretreatment technique for preparing austenitic steel coatings using laser cladding. It was demonstrated that the applied pretreatment effectively refined the coating’s microstructure by reducing grain size and mitigating defect formation, thereby leading to a marked improvement in its tribological properties—a key factor directly influencing wear resistance [39,48,49].
Several other studies highlight the versatility of laser cladding for tribological applications:
- Qiu et al. [50] fabricated gradient composite coatings containing ZrB2 on 27SiMn steel. The 20% ZrB2 variant demonstrated the optimal tribological performance, exhibiting the lowest coefficient of friction and minimal wear volume.
- Zhang et al. [51] addressed cavitation erosion (a form of wear) in marine pumps by fabricating cobalt-based coatings on 316 stainless steel, which exhibited only 1/3 the weight loss rate of the substrate.
- HSLC was employed by Hu et al. [52] to synthesize Ni/WC composite coatings on 304 steel. The resultant coatings showed an eightfold enhancement in stable wear resistance along with a reduced coefficient of friction.
2.4. Plasma Spraying Technology
As a material deposition strategy, plasma spraying operates through a high-enthalpy thermal dynamics process: feedstock powder is superheated within a plasma jet and ballistically propelled toward a prepared substrate, where rapid quenching forms a consolidated coating. This method is characterized by its capability to build up substantial thickness, making it particularly advantageous for component refurbishment and dimensional restoration [53,54,55].
High-Velocity Oxy-Fuel (HVOF) spraying has garnered significant attention for its capability to produce coatings with exceptional density and high bonding strength. For instance, Medabalimi et al. [56] successfully deposited an SS304L stainless steel coating onto a Superfer800 substrate using the HVOF process. The resulting coating exhibited a porosity of less than 2% and a high microhardness exceeding 1160 HV, which established a solid microstructural foundation for its outstanding wear resistance. This study clearly underscores the considerable potential of HVOF technology in fabricating high-performance wear-resistant coatings [57].
Wang et al. deposited the SiAlON ceramic coating on TiO2-316 stainless steel via atmospheric pressure plasma spray deposition. Figure 6 presents a schematic diagram of the underlying deposition mechanism [58].
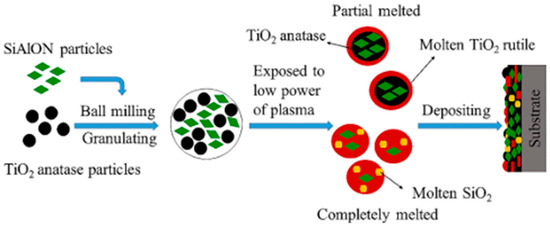
Figure 6.
Schematic diagram of phase evolution and formation mechanism of plasma-assisted coating based on SiAlON and anatase TiO2 [58].
Mansour Razavi et al. [59] fabricated Fe-TiC nanocomposite coatings in situ on CK45 steel through plasma spraying of ilmenite concentrate and carbon black. This method imparted the coating with superior mechanical properties, including significantly elevated microhardness and exceptional wear performance, thus demonstrating considerable potential for fabricating advanced nanocomposite coatings with outstanding durability.
Li et al. [60] fabricated MoAlB ceramic coatings on 316 stainless steel by employing the atmospheric plasma spraying process with a systematic variation of arc power parameters. The experimental data show that the prepared coatings exhibit superior wear resistance, with the manufacturing process of the MoAlB coatings shown in the figure.
Lakkannavar et al. [61] deposited Ni-Cr-Al-Y/Cr3C2/h-BN/Mo coatings on chromium-molybdenum steel components to evaluate their resistance to elevated-temperature degradation processes. The coatings enhanced erosion resistance by 55% at a 90° impact angle compared to the substrate, showcasing their effectiveness against solid particle wear at high temperatures.
Serkan ÖZEL [62] employed plasma spraying to fabricate Co-Mo-Cr-based coatings on stainless steel. The research found that at a current of 600 A, hard phases including Co3Mo2Si and Mo5Si3 were identified within the deposited layers, while the coating’s microhardness peaked—a crucial parameter for evaluating abrasive wear resistance.
3. Strategies for Tribological Optimization of Coatings
The ultimate goal of applying surface coatings is to control friction and wear in mechanical systems. Based on their primary function, coatings can be strategically designed into three categories: those that provide high wear resistance to extend component life, those that provide low friction to reduce energy loss, and those that are self-lubricating to operate reliably in environments where external lubrication is impossible or undesirable. This section reviews the latest developments in these three types of functional coatings for steel substrates. The Mechanism Diagram is presented in Figure 7 [63].
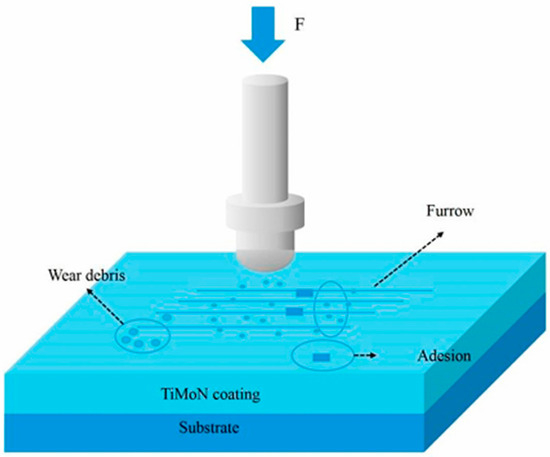
Figure 7.
Schematic diagram of friction and wear principle [63].
3.1. Wear-Resistant Coating
Wear-resistant coatings protect steel substrates through a combination of high hardness and the formation of beneficial surface layers. A direct approach to enhance hardness was exemplified by Zhang et al. [64], who developed Ni20Cr coatings using a novel plasma spray melting technique, achieving a 23.2% increase in microhardness over conventional methods, which directly translated to superior wear resistance. Similarly, Li et al. [65] applied a 304 stainless steel coating via electron beam melting, which markedly improved the wear resistance of Q345R steel, primarily through the inherent hardness of the deposited layer. The underlying friction and wear mechanism for this enhancement is presented in Figure 8 [66].
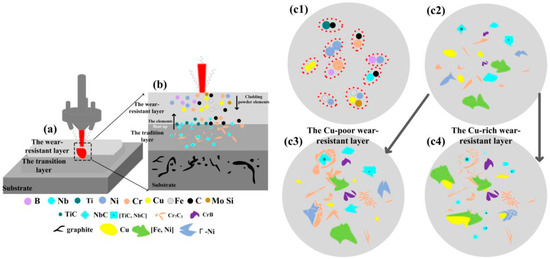
Figure 8.
Schematic of the Friction and Wear Mechanism (a): Schematic of laser cladding multi-layer structure (b) Detail of element and phase distribution in cladding layer (cladding powder elements, element floating in traditional layer) (c1–c4) Schematic of microstructural evolution in wear-resistant layers with different Cu contents (phase composition changes in Cu-poor and Cu-rich layers) [66].
The composite treatment of surface texturing and hard coatings is an effective strategy for enhancing tribological performance. Research on cold-work tool steel has demonstrated that depositing an AlCrSiN coating onto a laser-textured surface leads to a significant reduction in the wear rate, even though the coefficient of friction increases [67]. Beyond intrinsic hardness, the in situ formation of protective tribo-films during service is another key mechanism. Tian et al. [68] demonstrated this principle, revealing that high-velocity arc-sprayed FeNiCrAl coatings formed dense oxide films (Fe2O3/Fe3Al/Al2O3) on the friction surface, thereby significantly boosting wear resistance. Conversely, some applications require a tailored interface rather than maximum hardness. Davis [69] adopted this strategy by developing coatings for railway surfaces, where the enhanced durability was strategically attributed to the use of a nylon counterface coating, optimizing the tribological pair for specific rolling/sliding conditions. The development of wear-resistant coatings leverages various advanced techniques to meet specific performance demands. Utilizing a carbon-based approach, Zhao et al. [70] applied tetrahedral amorphous carbon (ta-C) coatings to protect cemented carbides in marine conditions, with the optimum coating (produced at 400 A) exhibiting a remarkably low wear rate of 3.6 × 10−6 mm3·N−1·m−1. Alternatively, a multi-element nitride strategy was adopted by Zhou et al. [71], who used multi-arc ion plating to produce an Al-Cr-N ternary coating on Cr12MoV steel, achieving notable improvements in both hardness and wear performance. Beyond vapor deposition methods, thermal spraying also offers robust solutions, as evidenced by Guney et al. [72], who utilized flame spraying to produce NiCrBSi-35W2C coatings on brake discs, resulting in significantly reduced wear and extended service life under extreme friction.
The research by Lin et al. serves as demonstration of this approach: they employed shot peening to surface-harden Q345 steel, forming a crater-like hardened layer characterized by high surface roughness (3.77 μm) and a residual compressive stress field, with the microhardness increased to 77.72 HV. On this optimized substrate, a zinc-rich epoxy coating was applied, constructing a dual-layer protection system. Tribological test results revealed the outstanding performance of this composite system, which enhanced the wear resistance of the Q345 steel substrate by 76.50% and that of the epoxy coating itself by 38.75%. The key mechanism lies in the synergistic interaction between the two layers: the shot-peened strengthening layer acts as a robust mechanical support that sustains the coating and counters its plastic deformation under load, while the epoxy coating functions as a friction buffer layer, dispersing and absorbing shear stresses during sliding. This collaborative effect collectively achieves a significant enhancement in anti-friction and anti-wear capabilities [73].
3.2. Friction-Reducing Coating
Friction-reducing coatings are designed to achieve a low coefficient of friction (COF) between contacting surfaces, thereby improving energy efficiency, reducing heat generation, and mitigating severe wear. As modern industrial manufacturing advances, mechanical components are increasingly required to operate reliably under harsher conditions while delivering enhanced performance. However, friction and wear during service remain major causes of component failure. Therefore, developing integrated materials that offer both friction-reducing and wear-resistant properties has long been a central and ongoing research challenge in the field [74,75,76,77,78].
Cao et al. [79] fabricated an AlCrN coating using multi-arc ion plating on laser shock peening micro-textured substrates. The coating effectively improved frictional stability and reduced wear loss of the specimens. Further optimization revealed that micro-textured surfaces with a 19.6% areal density and 24.72 μm depth yielded the optimal comprehensive tribological performance while maintaining wear resistance and frictional stability. Based on these findings, the team developed a synergistic anti-friction and lubrication model integrating the micro-texturing with the AlCrN coating, which is presented in Figure 9.
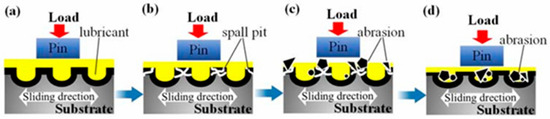
Figure 9.
Schematic of the synergistic anti-wear and lubrication process: (a) initial, (b) stable, (c) late stable, and (d) failure stages [79].
The pursuit of low-friction coatings is advancing through distinct strategies targeting the material, structural, and compositional levels. At the material level, Guo et al. [80] achieved a dramatic 90% reduction in the friction coefficient by modifying MoS2 with GO to create a synergistic dual-interface, while Raghavendra et al. [81] found that a uniform distribution of α-Al2O3 nanoparticles in electrodeposited Ni coatings was key to achieving low friction and superior wear resistance. Structurally, surface patterning has proven highly effective, as Ref. [82] demonstrated that volcanic-textured PVD coatings significantly reduce friction and adhesive wear. On the compositional front, several approaches show promise: Kim et al. [83] reported that Si-doping outperformed undoped DLC in reducing friction; Yan et al. [84] discovered that h-BN as an additive in IN625 coatings drastically lowered the friction coefficient compared to Al2O3; and Wan et al. [85] leveraged laser cladding to achieve a uniform Pb phase distribution in CuPb10Sn10 coatings, halving the friction coefficient. Tian et al. [86] further expanded this palette by using a CrCoFeNiMn high-entropy alloy coating, which concurrently enhanced hardness and reduced both wear depth and the friction coefficient.
3.3. Self-Lubricating Coating
Self-lubricating coatings are engineered to supply lubrication at the friction interface without external lubricants. The key characteristic is that components inside the coating itself in situ form a lubricating film when friction occurs, thus reducing the friction coefficient and alleviating wear in operational environments [87].
Recent research showcases a spectrum of successful strategies for achieving self-lubrication in coatings. Tan et al. [88] demonstrated that PTFE/PPS polymers can uniquely leverage thermal softening to markedly lower the friction coefficient, which drops to 0.042 (from 0.096) upon heating to 300 °C. Several studies validated the effectiveness of embedded solid lubricants: Ref. [89] incorporated Ag for wide-temperature service, Hu et al. [90] fabricated FeS coatings that offer dual lubrication and wear resistance, while Yan et al. [91] and Liu et al. [92] overcame compatibility issues with nickel-encapsulated h-BN and graphite for excellent laser-clad coatings. Architectural innovation is equally powerful, as Ren et al. [93] engineered a micro-porous PEO-PTFE composite that secures solid lubrication, and Zhao et al. [94] developed a structurally coupled coating where dedicated units actively facilitate lubricating film formation. For harsh environments, Feng et al. [95] produced a Ti–Al–(C,N) coating that sustains low friction in seawater via lubricating phases, whereas Tillmann et al. [96] devised TiAlVN coatings that intelligently form lubricious oxides at high temperatures.
To advance the tribological performance of FeAl intermetallic compounds, Chi et al. [97] designed a novel FeAl-TiB2-Cux coating with graded copper contents. As depicted in Figure 10, its lubrication mechanism at 600 °C involves the in situ generation of multiple high-temperature synergistic lubricating phases through triggered chemical reactions during friction, thereby significantly enhancing the coating’s overall tribological properties in high-temperature environments.
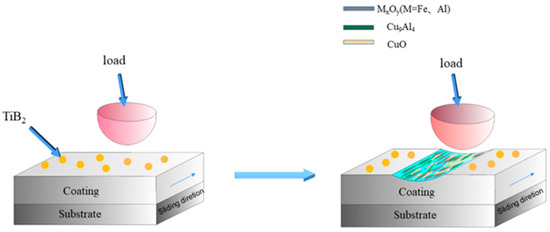
Figure 10.
Schematic illustrations of Wear Mechanisms for FeAl-TiB2-Cux Composite Coatings at 600 °C [97].
As evidenced in Table 1, the fundamental differences among wear-resistant, friction-reducing, and self-lubricating coatings arise from their distinct operational mechanisms and energy input modalities. Wear-resistant coatings primarily rely on high-energy thermal sources (e.g., laser, plasma jet) to form hard (1000–2000 HV) ceramic layers composed of carbides or oxides on the substrate surface. This process delivers exceptional abrasion resistance; however, the high thermal input often introduces residual stresses, increasing coating brittleness. Friction-reducing coatings, fabricated via medium-energy processes such as physical vapor deposition (PVD) or electrodeposition, incorporate low-shear-strength materials such as MoS2 or diamond-like carbon (DLC) at the friction interface. These coatings achieve notably low coefficients of friction (0.1–0.2), significantly improving energy efficiency. Nevertheless, their limited load-bearing capacity restricts applicability under high-contact-stress conditions. Self-lubricating coatings, typically applied using thermal spraying or laser cladding, embed solid lubricants (e.g., PTFE, Ag, h-BN) within the coating matrix. These systems exhibit a stable frictional response (μ = 0.01–0.3) over a wide thermal spectrum, enabling maintenance-free operation. An inherent limitation, however, lies in the finite service life due to gradual lubricant consumption. These three strategies reflect a clear trade-off among hardness, friction coefficient, and service life, this comparison provides a rational basis for selecting coating systems tailored to specific operational requirements.

Table 1.
Comparative overview of tribological coating strategies for steel substrates.
Polytetrafluoroethylene (PTFE) is widely regarded as an ideal solid lubricant coating material due to its excellent chemical stability, and self-lubricating properties. However, its poor wear resistance and load-bearing capacity limit its direct application in harsh operating conditions. To address this bottleneck, research has proposed a composite strategy combining laser surface texturing with PTFE coating. The effectiveness of this strategy is strongly supported by experimental evidence. For instance, research by Firuze et al. [98] found that after creating laser textures on a stainless steel substrate and coating it with a thin PTFE layer, the coating’s wear life was increased by a remarkable factor of 29 compared to the non-textured control group. The mechanism lies in the dual role of the microscale patterns generated by laser processing: on one hand, they act as “reservoirs” for the solid lubricant and “traps” for wear debris, thereby providing continuous lubrication during the friction process; on the other hand, the surface roughness generated by the textures significantly enhances the coating adhesion through a mechanical interlocking effect, effectively preventing the overall delamination of the PTFE coating. It is noteworthy that the study also indicated that laser texturing optimizes the mechanical response of the PTFE coating, making it less prone to tearing under nanoindentation scratches. Thus, the laser surface texturing strategy cleverly compensates for the intrinsic weaknesses of PTFE through interface engineering, greatly expanding its application potential as a high-performance solid lubricant coating [99,100,101].
4. Enhancing Corrosion Resistance of Surface Coatings on Steel
The electrochemical deterioration of metals under demanding service conditions critically undermines the long-term mechanical reliability and functional longevity of steel assemblies. While the preceding chapter addressed tribological protection, achieving durability in applications such as marine engineering, chemical processing, and infrastructure requires coatings to provide robust corrosion resistance. This chapter focuses on the mechanisms and strategies for designing such protective systems, which function as physical barriers, release inhibitory agents, or provide sacrificial protection. The performance is governed by the coating’s density, adhesion, chemical stability, and intrinsic inhibitory properties. This section reviews recent advances in corrosion-resistant coatings, covering barrier layers, active inhibition technologies, and performance in synergistic degradation environments. From a corrosion perspective, compressive stresses can enhance resistance to stress corrosion cracking, whereas tensile stresses, often found in thick thermally sprayed coatings, can be detrimental. Corrosion is a natural electrochemical process that leads to the degradation of engineering materials, resulting in substantial economic losses. Studies indicate that the systematic application of existing advanced protection technologies could potentially save 15% to 35% of corrosion-related costs [102,103,104].
4.1. Mechanisms of Corrosion Protection by Coatings
The fundamental strategies by which coatings protect steel substrates from corrosion can be categorized into three primary mechanisms, each employing a distinct approach to interrupt the electrochemical corrosion process.
4.1.1. Barrier Protection
The most straightforward mechanism involves the coating acting as a physically impermeable layer that isolates the steel substrate from corrosive electrolytes such as water, oxygen, and chloride ions. The effectiveness of this barrier is critically dependent on its density, its high density, minimal porosity, and excellent interfacial adhesion to suppress underfilm corrosion.
Zhang et al. [105] demonstrated the effectiveness of this approach by preparing alumina (Al2O3) coatings via plasma spraying to protect aluminum electrolysis anode steel claws. Their study revealed that the alumina coatings exhibited excellent anti-fluoride corrosion behavior in molten Na3AlF6-AlF3 salt, highlighting the role of ceramic coatings as stable barriers in extreme environments.
Bai et al. [106] developed a grafting methodology where isophorone diisocyanate (IPDI) was used to functionalize graphene oxide (GO), yielding MGO. This modified derivative was integrated with polytetrafluoroethylene (PTFE) to create a hybrid filler for enhancing waterborne polyurethane (WPU), with the overall design concept depicted in Figure 11. Composite coatings with varying MGO/WPU and MGO-PTFE/WPU ratios were fabricated. The hybrid filler system exhibited a notable synergistic effect in enhancing corrosion protection. While MGO serves as an effective physical barrier against corrosive species penetration, PTFE simultaneously improves MGO dispersion in the polymer matrix and seals interparticle gaps, collectively extending the diffusion pathway for corrosive media and strengthening the overall barrier performance.
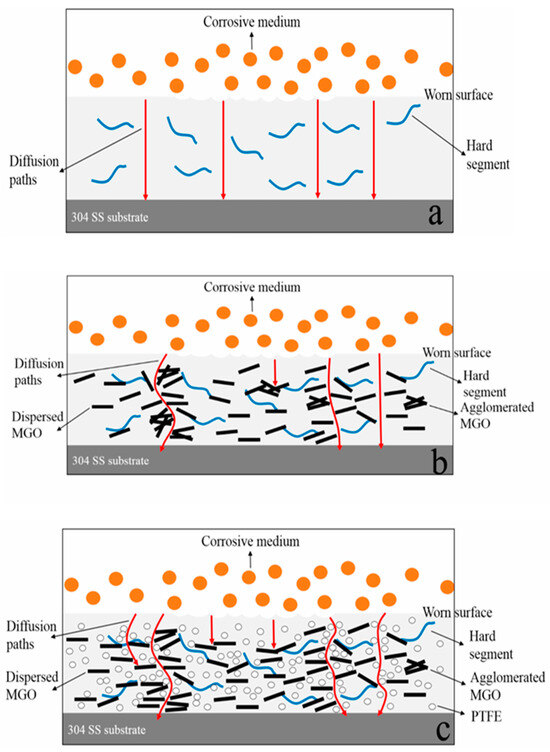
Figure 11.
Corrosion resistance mechanisms of worn neat WPU coating (a), worn MGO/WPU composite coating (b), and worn MGO-PTFE/WPU composite coating (c) [106].
4.1.2. Active Inhibition
Unlike passive barriers, active coatings provide protection through the controlled release of corrosion-inhibiting species that passivate the steel surface or neutralize corrosive agents. A key research focus is replacing traditional toxic inhibitors like chromates with environmentally friendly alternatives.
The role of LDH chloride adsorption in epoxy coating protection was examined by Cao et al. [107] and Wang et al. Figure 12 illustrates the proposed mechanisms for different LDH variants. The unmodified epoxy coating (Figure 12a) contained inherent cracks and pores that permitted corrosive species infiltration, initiating substrate corrosion. MgAl-LDH incorporation (Figure 12b) caused nanoparticle aggregation and void formation, where limited chloride adsorption was insufficient to counteract these defects. While ZnAl-LDH (Figure 12c) demonstrated superior chloride capture ability, its poor epoxy compatibility generated microcracks, yielding corrosion resistance equivalent to pure epoxy. Conversely, CaAl-LDH (Figure 12d) achieved uniform dispersion that sealed microdefects and extended diffusion trajectories, markedly inhibiting corrosion initiation. Despite moderate chloride adsorption capacity, the dominant physical barrier mechanism of CaAl-LDH substantially outweighed its ionic adsorption function in enhancing protective performance.
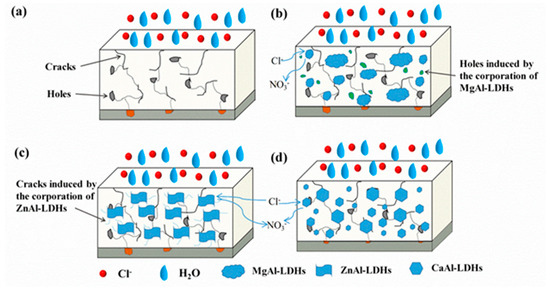
Figure 12.
Schematic of Corrosion Protection Mechanisms [107].
Basdeki et al. [4] systematically reviewed the application of lithium-based inhibitors in aluminum alloy protection, detailing their dual functionality as corrosion-inhibiting pigments in organic coatings and conversion layer formers in pretreatment. In a complementary study, Lu et al. [108] revealed a significant enhancement in the anti-corrosion properties of phytate-based surface layers on Q235 steel through rare-earth modification, contributing to the development of environmentally friendly surface pretreatment technologies.
4.1.3. Sacrificial Protection
Sacrificial coatings protect the underlying steel by functioning as a preferential anode in the electrochemical cell. These coatings, typically made of zinc or aluminum, corrode in place of the steel, thereby cathodically protecting the substrate. As shown in Figure 13, when steel is subjected to cathodic protection (CP), an alkaline environment is created, leading to the accumulation of calcareous matter on the surface, thereby reducing the requirement for cathodic protection [109].
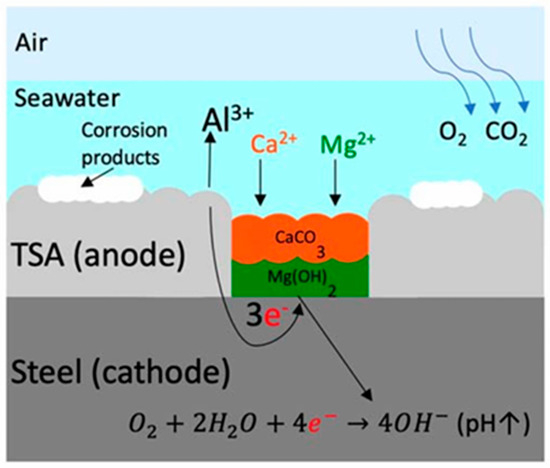
Figure 13.
Sacrificial anode protection mechanism of the Al coating [109].
In harsh marine environments, hot-dip galvanized layers provide long-term protection to the substrate through their own sustained and controlled dissolution. This protective capability is closely related to the coating thickness; even if the coating suffers localized damage, the protective effect can extend to the surrounding damaged areas. Impressed current cathodic protection is another important technique. It applies a cathodic current to steel components through an external power source, polarizing them to a protective potential. This technology is often combined with organic coatings for the protection of large-scale infrastructure such as buried pipelines and ship hulls. In the field of non-metallic coatings, phosphate conversion coatings serve as an environmentally friendly surface treatment technology, demonstrating significant advantages in the protection of low-carbon steel. By introducing trace amounts of specific additives into the phosphating bath, grain size and coverage uniformity can be effectively optimized. It is noteworthy that the introduction of calcium ions (Ca2+) significantly refines the grain size and improves the distribution uniformity of zinc phosphate crystals, thereby enhancing the coating density and overall corrosion resistance [110,111,112,113,114,115,116,117,118].
Suzuki et al. [6] explored local protection mechanisms at scratches in zinc-coated steels by introducing inhibitors into conversion coatings, a critical solution for defect-induced corrosion. Meanwhile, Sakairi et al. [119] studied the hydrogen-induced failure behavior of the same materials under varying humidity, clarifying how environmental factors govern the durability of sacrificial coating systems.
4.1.4. Self-Healing Protection
Self-healing protection represents an advanced protective mechanism that enables coatings to autonomously repair sustained damage and restore their barrier function, thereby significantly extending service life.
Fattah-alhosseini et al. [120] designed a smart MIL–53@TA/PCL coating by integrating photothermal conversion and shape memory functionalities, which collectively facilitate effective self-repair under NIR laser stimulation. The formed Fe3+–TA complex on the surface significantly enhances light absorption and thermal conversion efficiency, prompting rapid melting of the PCL matrix and subsequent scratch filling. Meanwhile, the shape memory characteristic of PCL facilitates structural recovery through chain rearrangement and scratch closure upon heating, as illustrated in Figure 14.
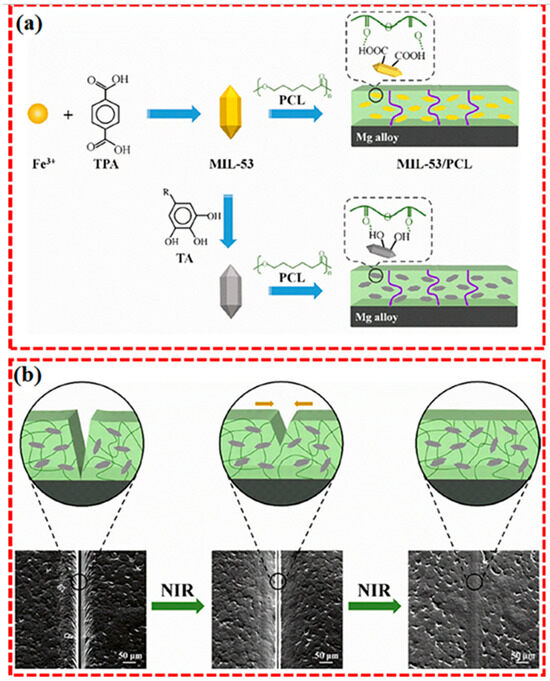
Figure 14.
Schematic of Self-Healing Coating Mechanisms (a) the process of creating MIL–53/PCL and MIL–53@TA/PCL coatings, and (b) the self–repairing mechanism of the MIL–53@TA/PCL layer [120].
4.2. Corrosion Performance of Coating Systems
The practical corrosion performance varies significantly across different coating types, each suited to specific operational environments and requirements.
4.2.1. Metallic and Alloy Coatings
Among metallic and alloy coatings, in addition to the aforementioned zinc-based coatings that primarily rely on a sacrificial anode mechanism, aluminum-silicon coatings represent another category of systems based mainly on a barrier protection mechanism. The protective effectiveness of Al-Si coatings on hot-stamped steel is closely related to their complex microstructural characteristics. Compared to zinc-based coatings with significant cathodic protection capabilities, the corrosion potential difference between the Al-Si coating and the steel substrate is relatively small (typically <30 mV), making it difficult to provide effective electrochemical protection [121]. Therefore, this coating system primarily relies on the adequate dimensional stability of the aluminum layer and the physical barrier effect created by the naturally formed Al2O3 passive film on its surface to achieve protection. This characteristic makes microcracks formed in the coating during processing or service the primary factor affecting its protective durability [121,122].
Research on atmospheric corrosion conditions shows that the degradation process of Al-Si coatings has unique mechanisms. Allély et al. [121] confirmed through VDA 233-102 cyclic corrosion tests and continuous neutral salt spray tests that Al-Si coatings and the substrate steel undergo simultaneous active corrosion. The characteristic corrosion products appeared as hydroxides and aluminosilicates after cyclic testing, while in the salt spray environment, they transformed into complex oxides of aluminum, iron, and silicon. Nicard et al. [123] examined the influence of alloying elements and observed that the addition of Zn and Mg, although improving the coating’s electrochemical protection behavior, accelerated the overall corrosion process of the coating due to promoted anodic dissolution, leading to a significant increase in the total mass loss of the specimens. A comparative evaluation by Dosdat et al. [124] further indicated that, except for specific situations such as protection at edge areas, the overall corrosion resistance of Al-Si coated hot-stamped steel is generally comparable to that of zinc-based coated steel, and the protective effects of both types of coated materials are significantly superior to those of uncoated substrates.
Albizu et al. [5] developed a rapid method for in situ monitoring of hypophosphite and phosphite concentrations in electroless plating baths, emphasizing its critical importance for ensuring the quality of Ni-P coatings; concurrently, the study by Wang et al. [125] demonstrated that introducing copper into laser-clad 316L stainless steel coatings significantly enhances corrosion resistance by reducing point defect density and stabilizing the passive layer.
To enhance the service reliability of Q345 steel welded joints in harsh acidic chloride environments, Ferkous et al. [102] successfully fabricated a Ni-Co-Fe-P quaternary alloy coating as a protective layer using electrodeposition technology. Microstructural characterization revealed that the coating exhibits a smooth and dense nanocrystalline structure, uniformly covering the welded joint area and forming an effective physical barrier. During electrochemical testing in a simulated harsh environment of 3.5% NaCl + 2% HCl solution, the coating exhibited excellent corrosion resistance, demonstrated by an extremely low corrosion current density of only 1.16 × 10−6 A/cm2, significantly lower than that of the unprotected Q345 steel substrate and its welded joint. Simultaneously, its polarization resistance (8.36 × 104 Ω·cm2), charge transfer resistance (4.27 × 103 Ω·cm2), and low-frequency impedance modulus (5.51 × 103 Ω·cm2) all exhibited orders-of-magnitude improvement. The study confirms that the dense nanocrystalline structure effectively blocks the penetration and diffusion of corrosive media, thereby significantly delaying the electrochemical corrosion process and providing an effective coating solution for the targeted protection of critical areas such as welded joints.
Among various surface modification techniques, tin-based coatings have become an indispensable protective means for steel materials, particularly in the electronics industry (e.g., connectors, lead frames, circuit boards), due to their excellent electrical conductivity, oxidation resistance, and good corrosion resistance. Their hot-dipping and electroplating processes are widely adopted owing to their cost-effectiveness and ease of control. However, this technology faces a critical challenge: the tendency to form a brittle intermetallic compound (IMC) layer at the coating/substrate interface, which significantly compromises the coating’s adhesion strength and mechanical properties. To overcome the inherent challenges of tin-based coatings, researchers have explored the incorporation of micro-alloying elements or nanoparticles to regulate the interfacial microstructure. Studies indicate that introducing particles such as Si, Zn, Cr, or Al2O3 can effectively refine and reduce the thickness of the interfacial IMC layer, resulting in a smoother and more uniform interface. For instance, specific research investigating the role of Al2O3 nanoparticles found that at a content of 0.50 wt.%, a composite coating with minimal defects and optimal corrosion resistance could be formed within the tin layer. This is attributed to the nanoparticles promoting the formation of a denser barrier layer and enhancing the overall integrity of the coating [126,127,128,129,130,131,132].
4.2.2. Inorganic and Ceramic Coatings
These coatings exhibit exceptional chemical inertness and are perfectly suited for environments with high temperatures and severe corrosivity.
Raman et al. [34] reported an engineering solution where a merely atomic-layer-thick graphene coating fabricated through CVD boosts the corrosion resistance of low-carbon steel by two orders of magnitude in chloride-containing service conditions. This study confirms that a graphene film just a single atomic layer thick can act as a highly effective protective barrier.
Sun et al. demonstrated that modified basalt flakes (MBFs) serve as a functional filler to impart enhanced corrosion resistance to the coating, primarily via a sheet-overlapping and shielding mechanism, as depicted in Figure 15 [133].
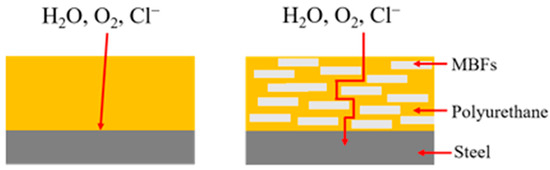
Figure 15.
Corrosion Protection Mechanism of Modified Basalt Flakes in Polymer Coating [133].
4.2.3. Organic and Polymer-Based Coatings
The protective performance of polymer coatings, despite their widespread use, is not guaranteed and is critically dependent on a holistic strategy that encompasses compositional design, interfacial integrity, and substrate preparation.
At the coating design level, the incorporation of innovative technologies like sol–gel can dramatically enhance barrier properties. For instance, the work by Watkins et al. serves as a compelling case study. They demonstrated that a simple TEOS-based sol–gel overcoat, when applied uniformly at a 10% concentration, could form a defect-free layer on polyurethane-coated steel. This dense overcoat significantly inhibited scribe creep in salt spray tests and reduced edge creep by 21%, primarily by impeding oxygen permeation. In stark contrast, a dilute (2.5%) TEOS formulation yielded a porous and ineffective coating. This comparison underscores that the efficacy of such modifications is highly sensitive to specific process parameters and the resulting microstructure [134]. Beyond the bulk coating properties, the integrity at interfaces and defects is paramount. Liu et al. [135] shed light on this aspect by employing multi-electrode arrays to characterize interfacial degradation. Their work established that at coating defects, galvanic coupling between carbon steel and copper-nickel in a 3.5% NaCl solution can dramatically accelerate corrosion, fundamentally threatening the durability of the entire coating system. This highlights a critical failure mode where the protective barrier is compromised. Furthermore, the foundational role of substrate preparation cannot be overlooked. The long-term durability of even high-performance coatings is contingent upon a properly prepared surface. This is corroborated by studies on epoxy coatings in seawater immersion, where Rajput et al. [136] identified meticulous surface preparation as a non-negotiable prerequisite for long-term protection. Similarly, Kamde et al. [137] demonstrated that the anti-corrosion capability of cement-polymer composites on steel rebars is decisively governed by the quality of the interfacial preparation. These studies collectively affirm that superior coating performance is built from the substrate up.
4.3. Synergistic Effects of Wear and Corrosion
In many real-world applications, corrosion and wear occur simultaneously, resulting in a synergistic interaction known as tribocorrosion [138]. This interplay significantly exacerbates the total material degradation beyond simple additive damage.
To significantly enhance the corrosion resistance of carbon steel, Ghada A. Alshammri et al. developed a composite coating based on Sn-4% Zn alloy incorporating Al2O3, NiO, and their hybrid nanoparticles, respectively. The coating was fabricated via a direct tinning process. The study revealed that the incorporation of nanoparticles effectively refined the intermetallic compound layer at the coating-substrate interface. The addition of Al2O3 nanoparticles alone resulted in the maximum coating thickness of 70 ± 1.8 μm, while the interfacial layer thickness was minimized (2.29 ± 0.28 μm) under this condition. Results from potentiodynamic polarization and electrochemical impedance spectroscopy demonstrated that all nanoparticles significantly improved the corrosion resistance of the Sn–Zn coating in sodium chloride solution, with the enhancement effect following the order: Al2O3/NiO mixture > NiO > Al2O3. This confirms that different nanoparticles likely generate a synergistic effect in the coating, collectively promoting the densification of the coating structure and the optimization of its protective performance [139].
Zhao et al. [140] systematically investigated the corrosion-wear performance evolution and interaction (CWI) in 8Cr4Mo4V bearing steel under varying corrosion intervals using a combined experimental approach of neutral salt spray exposure supplemented by sliding wear tests. They developed a quantitative characterization method for CWI by establishing material loss composition relationships. The results revealed a non-monotonic corrosion rate that initially increased then decreased, ultimately forming a corrosion morphology characterized by widespread general attack with embedded localized attacks. Through quantification of different material loss components illustrated in Figure 16, where total material loss (T2) comprises process loss (T1), pure wear (W1), and pure corrosion in two stages (C1, C2), the study established that the wear component (WC) includes W1 and corrosion-induced wear increment (ΔWC), while the corrosion component (CW) contains C2 and wear-induced corrosion increment (ΔCW), providing a comprehensive framework for understanding CWI mechanisms in bearing steels.
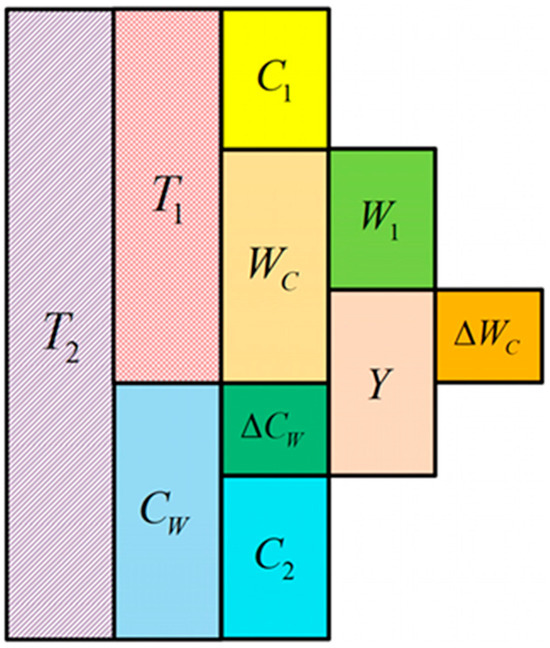
Figure 16.
Material Loss Partitioning in 8Cr4Mo4V Steel under Corrosion-Wear Synergistic Effect [140].
The study by Zhao et al. [70] ontetrahedral amorphous carbon (ta-C) coatings is highly relevant here. Although primarily deposited for wear resistance, the study evaluated their performance in a corrosive context, showing excellent tribocorrosion resistance for offshore engineering equipment. The coating fabricated at a peak current of 400 A exhibited an optimal wear rate of 3.6 × 10−6 mm3·N−1·m−1, demonstrating how a hard, dense coating can provide dual protection. Kim et al. systematically investigated the effect of nitriding pre-treatment on the tribocorrosion behavior of a PVD Cr-Al-Ti-B-N coating. They found that the coating without nitriding pre-treatment suffered from premature delamination in NaCl solution, leading to substrate exposure. This was characterized by abrupt changes in both the coefficient of friction and the open circuit potential, which subsequently accelerated material failure. In contrast, the nitriding pre-treatment significantly inhibited coating delamination by enhancing the surface hardness and coating adhesion strength, thereby maintaining coating integrity throughout the test and markedly improving the coating’s resistance to tribocorrosion [141].
The corrosion resistance of coatings is strongly dependent on their microstructural characteristics, not solely on their chemical composition. Research on laser-clad Inconel 625 coatings clearly demonstrates this point. Although all coatings exhibit similar phase constituents, their electrochemical behaviors differ significantly, the coating possessing the most positive Ecorr, the smallest Icorr, and the highest Epit demonstrates the optimal overall corrosion resistance. This superior performance is attributed to its unique microstructure: a relatively coarse grain structure along with fewer and finer Laves phase precipitates. In contrast, coatings containing a large amount of continuous, coarse Laves phase precipitates exhibit degraded resistance to both uniform and pitting corrosion, despite having finer grains. This suggests that for Inconel 625 coatings, reducing the quantity of secondary phases—which act as potential initiation sites for corrosion—is more effective in enhancing corrosion resistance than simply refining the grain size. This finding provides clear guidance for designing high-performance corrosion-resistant coatings through process-controlled microstructural engineering [142].
5. Summary and Prospects
5.1. Research Summary
In the field of coating preparation, various advanced techniques are widely employed due to their unique advantages. In the realm of preparation techniques, Chemical Vapor Deposition (CVD) has been shown to produce dense, high-purity films like graphene and aluminides, offering exceptional corrosion and high-temperature oxidation resistance for stainless steel components. Physical Vapor Deposition (PVD) excels in fabricating super-hard nitride and composite coatings, significantly extending the service life of tool steels. Laser cladding technology enables the deposition of thick, metallurgically bonded alloy coatings, dramatically improving the wear resistance of low-carbon steels or imparting specific corrosion resistance through tailored compositions. Plasma spraying stands out for preparing thick, wear-resistant multiphase ceramics and in situ synthesized composite coatings, suitable for the refurbishment and surface strengthening of large-scale steel parts.
Regarding functional performance, wear-resistant coatings (e.g., Al-Cr-N, FeNiCrAl) rely on high intrinsic derive their protective effect from high intrinsic hardness coupled with the formation of dense oxide films. Friction-reducing coatings (e.g., micro-textured AlCrN, MoS2/GO/TiO2) achieve low coefficients of friction through surface engineering and solid lubricant integration, enhancing energy efficiency in steel-based moving assemblies. Self-lubricating coatings (e.g., nickel-based graphite composites, FeS, NiCrAlYTa-Ag) ensure reliable operation in harsh environments by forming lubricating films in situ, providing a maintenance-free solution for steel components where external lubrication is impractical. For corrosion protection, coatings function through barrier, active inhibition, or sacrificial mechanisms, each offering a distinct strategy to mitigate the electrochemical degradation of steel in aggressive environments.
5.2. Future Outlook
Based on the progress summarized in this review, the development of high-performance coatings for steel substrates is set to advance in several key directions. Future research efforts will likely focus on the following interdisciplinary frontiers:
- Advanced Coating Systems and Architectures: The design of next-generation coatings will increasingly utilize multilayer, gradient, and composite architectures. These intelligent designs aim to seamlessly combine properties such as hardness, toughness, and corrosion resistance, which are often mutually exclusive in single-layer coatings. There is significant potential in hybrid processing techniques (e.g., combining PVD with laser texturing or thermal spraying) to create synergistic surface systems with tailored functionalities.
- Decoding Degradation Mechanisms under Synergistic Attacks: A paramount challenge is to fundamentally understand coating failure under combined tribological-corrosion (tribocorrosion) loads. Future work should employ in situ characterization techniques and multi-scale modeling to elucidate the real-time interaction between mechanical wear and electrochemical dissolution, particularly in complex environments involving elevated temperatures, erosion, and fatigue.
- Leveraging AI and Advanced Manufacturing: The integration of artificial intelligence and machine learning with materials science is anticipated to accelerate the discovery and optimization of coating compositions and processes. Furthermore, advances in additive manufacturing and laser processing will enable the fabrication of coatings with precisely controlled geometries and site-specific properties, opening new avenues for repairing and enhancing critical components.
- Sustainability and Life Cycle Engineering: As industries move towards greener practices, the development of environmentally friendly coating processes and materials will be crucial. Research will focus on reducing energy consumption during deposition, utilizing non-toxic elements, and designing coatings for extended service life to minimize resource use and waste throughout the entire component life cycle.
- Standardization and Performance Prediction: Establishing robust, standardized testing protocols that accurately simulate complex service conditions is essential for reliable performance comparison. Concurrently, efforts should be directed towards developing predictive models for coating service life, which will de-risk the implementation of new coating technologies in demanding industrial applications such as aerospace, marine engineering, and renewable energy systems.
5.3. Conclusions
Steel remains an indispensable engineering material, and surface coatings are a pivotal technology for mitigating its degradation under friction, wear, and corrosion. This review has synthesized the current landscape of coating technologies, from established CVD and PVD methods to laser cladding and thermal spraying, and has detailed the mechanisms by which they confer wear resistance, reduce friction, and provide corrosion protection.
Despite significant advancements, this analysis reveals critical research gaps that must be addressed to propel the field forward. Firstly, a pronounced disconnect persists between tribological and corrosion research. The synergistic degradation mechanism of tribocorrosion is often overlooked in coating design, despite its prevalence in industrial applications. Secondly, the coating–substrate interface remains a critical yet insufficiently understood frontier. The quantitative roles of deposition-induced residual stresses, interfacial toughness, and the mutual influence of microstructure on both mechanical and electrochemical properties demand systematic investigation. Thirdly, there is a notable scarcity of reliable service-life prediction models, particularly under the multi-field coupling conditions of stress, temperature, and corrosive media that mirror real-world service environments. Finally, the performance of many advanced coatings is not yet comprehensively validated across a wide spectrum of industrial substrate grades and surface conditions, limiting their widespread adoption.
Bridging these gaps defines the trajectory for future research. The development of multifunctional coating systems that are explicitly designed for tribocorrosion resistance is paramount. This necessitates a deeper integration of advanced in situ characterization and computational modeling to decode complex degradation mechanisms. Furthermore, the research community must prioritize the establishment of standardized testing protocols and the generation of open-access process-property databases to facilitate data-driven material design. By shifting the focus from optimizing single properties to designing intelligent, durable, and predictable coating systems, the next generation of surface engineering solutions will significantly extend the service life and reliability of steel components across the automotive, aerospace, marine, and energy sectors.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable. No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anitha, N.; Krishnaveni, A.; Prathipa, V.; Priya, S.S.; Jewelcy, A.L.; Anucia, T.A.A.; Parimala, S.; Jasmine, V.I.; Yuvarani, V.; Nivetha, M.V.; et al. Electrochemical studies on the corrosion resistance of mild steel in 1 M HCl solution before and after emulsion coating. Int. J. Corros. Scale Inhib. 2023, 11, 708–721. [Google Scholar] [CrossRef]
- Pokorný, P.; Kouřil, M. Predicted Corrosion Performance of Organofunctional Silane Coated Steel Reinforcement for Concrete Structures: An Overview. Buildings 2024, 14, 1756. [Google Scholar] [CrossRef]
- Coni, N.; Gipiela, M.L.; D’Oliveira, A.S.C.M.; Marcondes, P.V.P. Study of the mechanical properties of the hot dip galvanized steel and galvalume®. J. Braz. Soc. Mech. Sci. Eng. 2009, 31, 319–326. [Google Scholar] [CrossRef]
- Basdeki, M.; Apostolopoulos, C. The Effect of Shot Blasting Process on Mechanical Properties and Anti-Corrosive Behavior of Steel Reinforcement. Metals 2022, 12, 275. [Google Scholar] [CrossRef]
- Albizu, G.; Ostra, M.; Bordagaray, A.; Garcia-Arrona, R.; Vidal, M. Maintenance and control of coating baths: Rapid and simultaneous hypophosphite and phosphite determination in a Ni P deposition bath. Surf. Coat. Technol. 2024, 478, 130423. [Google Scholar] [CrossRef]
- Suzuki, Y.; Morishita, A. Influence of Corrosion Inhibitor in Chemical Conversion Coatings on Corrosion Performance in Scratches in Zinc-Coated Steels. ISIJ Int. 2019, 59, 1878–1885. [Google Scholar] [CrossRef]
- Zang, L.; Chen, Y.; Wu, Y.; Zheng, Y.; Chen, H.; You, D.; Li, L.; Li, J. Comparative tribological and friction behaviors of oil-lubricated manganese phosphate conversion coatings with different crystal sizes on AISI 52100 steel. Wear 2020, 458–459, 203427. [Google Scholar] [CrossRef]
- Apostolopoulos, C.A.; Koulouris, K. Corrosion Effect on Bond Loss between steel and concrete. In Structural Integrity and Failure; Oyguc, R., Tahmasebinia, F., Eds.; IntechOpen: London, UK, 2020. [Google Scholar]
- Lu, P.; Xu, Z.; Tian, Y.; Yang, R.; Hu, K.; Li, H.; Yin, Y.; Chen, X. Effect of Initial Surface Scratches on the Cavitation Erosion Behavior of 316L Stainless Steel Substrates and 316L Stainless Steel Coatings. Materials 2023, 16, 1392. [Google Scholar] [CrossRef]
- Lungu, M.V.; Tălpeanu, D.; Ciobanu, R.C.; Cojocaru, A.; Pătroi, D.; Marinescu, V.; Caramitu, A.R. Evaluation of Magnetron Sputtered TiAlSiN-Based Thin Films as Protective Coatings for Tool Steel Surfaces. Coatings 2024, 14, 1184. [Google Scholar] [CrossRef]
- Varis, T.; Lagerbom, J.; Suhonen, T.; Raami, L.; Terho, S.; Laurila, J.; Peura, P.; Vuoristo, P. Effect of heat treatments on the wear resistance of HVAF and HVOF sprayed tool steel coatings. Surf. Coat. Technol. 2023, 462, 129508. [Google Scholar] [CrossRef]
- Han, W.; Meng, X.M.; Zhao, J.; Zhang, J.B. Effect of heat treatment on microstructure and bending behaviour of 304 stainless steel coating by cold gas dynamic spraying. Mater. Res. Innov. 2012, 16, 109–114. [Google Scholar] [CrossRef]
- Durán, A.; Castro, Y.; Conde, A.; De, J.J. Handbook of Sol-Gel Science and Technology; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Liu, Q.; Cao, Y.; Chen, S.; Xu, X.; Yao, M.; Fang, J.; Lei, K.; Liu, G. Hot-Dip Galvanizing Process and the Influence of Metallic Elements on Composite Coatings. J. Compos. Sci. 2024, 8, 160. [Google Scholar] [CrossRef]
- Khan, H.H.; Wang, T.; Su, L.; Li, H.; Zhu, Q.; Yang, A.; Li, Z.; Wang, W.; Zhu, H. In Situ Thermal Interactions of Cu-Based Anti-Corrosion Coatings on Steel Implemented by Surface Alloying. Coatings 2024, 14, 722. [Google Scholar] [CrossRef]
- Chiadikobi, C.I.; Thornton, R.; Statharas, D.; Weston, D.P. The effects of deep cryogenic treatment on PVD-TiN coated AISI M2 high speed steel. Surf. Coat. Technol. 2024, 493, 131248. [Google Scholar] [CrossRef]
- Bobzin, K.; Kalscheuer, C.; Tayyab, M. A case study on fatigue damage in PVD coated tool steel under cyclic bending load. Surf. Coat. Technol. 2024, 478, 130505. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, K.; Dang, B.; Zhan, M.; Zhang, P.; Li, S. Anti-Corrosion and Wave-Absorbing Properties of Epoxy-Based Coatings on Q235 Steel. Coatings 2024, 14, 1315. [Google Scholar] [CrossRef]
- Sakib, M.N.; Iqba, A.A. Epoxy Based Nanocomposite Material for Automotive Application—A Short Review. Int. J. Automot. Mech. Eng. 2021, 18, 9127–9140. [Google Scholar] [CrossRef]
- Bansal, P.; Singh, G.; Sidhu, H.S. Investigation of Surface Properties and Corrosion Behavior of Plasma Sprayed HA/ZnO Coatings Prepared on AZ31 Mg Alloy. Surf. Coat. Technol. 2020, 401, 126241. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, L.; Liu, P.; Wang, S.; Ye, S.; Chen, Y. Influence of Protective Coating at High Temperature on Surface Quality of Stainless Steel. J. Iron Steel Res. Int. 2014, 21, 202–207. [Google Scholar] [CrossRef]
- Aiso, T.; Wiklund, U.; Kubota, M.; Jacobson, S. Effect of Si and Al additions to carbon steel on material transfer and coating damage mechanism in turning with CVD coated tools. Wear 2016, 368–369, 379–389. [Google Scholar] [CrossRef]
- Zhao, Q.; Geng, S.; Chen, G.; Wang, F. Initial Oxidation Behavior of Ferritic Stainless Steel Interconnect with Sputtered NiFe2 Alloy Coating. Oxid. Met. 2020, 93, 283–299. [Google Scholar] [CrossRef]
- ONgasoh, F.; Anye, V.C.; Agyei-Tuffour, B.; Oyewole, O.K.; Onwualu, P.A.; Soboyejo, W.O. Corrosion behavior of 5-hydroxytryptophan (HTP)/epoxy and clay particle-reinforced epoxy composite steel coatings. Cogent Eng. 2020, 7, 1797982. [Google Scholar] [CrossRef]
- Lehmusto, J.; Yrjas, P.; Hupa, L. Pre-oxidation as a means to increase corrosion resistance of commercial superheater steels. Oxid. Met. 2019, 91, 311. [Google Scholar] [CrossRef]
- Karimbaev, R.; Pyun, Y.S.; Maleki, E.; Unal, O.; Amanov, A. An improvement in fatigue behavior of AISI 4340 steel by shot peening and ultrasonic nanocrystal surface modification. Mater. Sci. Eng. A 2020, 791, 139752. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, H.; Seraffon, M.; Fry, A.T. Steam oxidation and mechanical performance of a ferritic–martensitic steel with slurry aluminide coating. Mater. Corros. 2020, 71, 1310–1320. [Google Scholar] [CrossRef]
- Dryepondt, S.; Zhang, Y.; Pint, B.A. Creep and corrosion testing of aluminide coatings on ferritic–martensitic substrates. Surf. Coat. Technol. 2006, 201, 3880–3884. [Google Scholar] [CrossRef]
- Chang, J.-K.; Lin, C.-S.; Wang, W.-R.; Jian, S.-Y. High temperature deformation behaviors of hot dip 55 wt% Al-Zn coated steel. Appl. Surf. Sci. 2020, 511, 145550. [Google Scholar] [CrossRef]
- Parekh, T.; Patel, P.; Sasmal, C.S.; Jamnapara, N.I. Effect of plasma processed Ti-Al coating on oxidation and tensile behavior of Ti6Al4V alloy. Surf. Coat. Technol. 2020, 394, 125704. [Google Scholar] [CrossRef]
- Gu, Y.; Xia, K.; Wu, D.; Mou, J.; Zheng, S. Technical Characteristics and Wear-Resistant Mechanism of Nano Coatings: A Review. Coatings 2020, 10, 233. [Google Scholar] [CrossRef]
- Kukla, D.; Kopec, M.; Kowalewski, Z.L.; Politis, D.J.; Jóźwiak, S.; Senderowski, C. Thermal Barrier Stability and Wear Behavior of CVD Deposited Aluminide Coatings for MAR 247 Nickel Superalloy. Materials 2020, 13, 3863. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Sawczak, M.; Dettlaff, A. Development of Microstructured Carbon Coatings by Substrate-Catalytic CVD. Coatings 2021, 11, 1403. [Google Scholar] [CrossRef]
- Raman, R.K.S.; Sanjid, A.; Banerjee, P.C.; Arya, A.K.; Parmar, R.; Amati, M.; Gregoratti, L. Remarkably corrosion resistant graphene coating on steel enabled through metallurgical tailoring. Small 2024, 20, 2302498. [Google Scholar] [CrossRef]
- Zhang, Y.; Pint, B.A.; Cooley, K.M.; Haynes, J.A. Formation of aluminide coatings on Fe-based alloys by chemical vapor deposition. Surf. Coat. Technol. 2008, 202, 3839–3849. [Google Scholar] [CrossRef]
- Bareiβ, J.C.; Hackl, G.; Popovska, N.; Rosiwal, S.M.; Singer, R.F. CVD diamond coating of steel on a CVD-TiBN interlayer. Surf. Coat. Technol. 2006, 201, 718–723. [Google Scholar] [CrossRef]
- Thelen, F.; Zehl, R.; Bürgel, J.L.; Depla, D.; Ludwig, A. A python-based approach to sputter deposition simulations in combinatorial materials science. Surf. Coat. Technol. 2025, 503, 131998. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Ramachandran, C.S.; Guo, P.; Wang, A. Microstructure and Performance of High-Velocity Oxygen-Fuel Coupled Physical Vapor Deposition (HVOF-PVD) Duplex Protective Coatings: A Review. Coatings 2022, 12, 1395. [Google Scholar] [CrossRef]
- Šturm, R.; Žnidaršič, M.; Grum, J. Crack-Growth Behavior of Laser Surface-Alloyed Low-Carbon Steel. J. Mater. Eng. Perform. 2013, 22, 2542–2549. [Google Scholar] [CrossRef]
- Kim, H.; Mandel, M.; Dalke, A.; Biermann, H.; Krüger, L. Effect of nitriding pre-treatment on the tribocorrosion behavior of physical vapor deposition-coated tool steel. Eng. Rep. 2022, 4, e12382. [Google Scholar] [CrossRef]
- Lotfi-khojasteh, E.; Sahebazamani, M.; Elmkhah, H.; Nouri, M.; Imantalab, O.; Fattah-alhosseini, A. A study of the electrochemical and tribological properties of TiN/CrN nano-layer coating deposited on carburized-H13 hot-work steel by Arc-PVD technique. J. Asian Ceram. Soc. 2020, 9, 270–282. [Google Scholar] [CrossRef]
- Hwang, Y.-H.; Seo, K.-J.; Kim, T.-H.; Min, Y.J.; Liu, Y.; Kim, D.-E. Tribological behavior of TiN, AlTiN, and AlTiCrN coatings in atmospheric and vacuum environments. Friction 2024, 12, 2611–2626. [Google Scholar] [CrossRef]
- Arulvel, S.; Winfred, R.D.D.; Akshat, J.; Jayakrishna, K.; Mridul, S. Laser processing techniques for surface property enhancement: Focus on material advancement. Surf. Interfaces 2023, 42, 103293. [Google Scholar]
- Noli, F.; Misaelides, P.; Riviere, J.P. Enhancement of the corrosion resistance of a Ti-based alloy by ion beam deposition methods. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2009, 267, 1670–1674. [Google Scholar] [CrossRef]
- Lu, K.; Zhu, J.; Guo, D.; Yang, M.; Sun, H.; Wang, Z.; Hui, X.; Wu, Y. Microstructures, Corrosion Resistance and Wear Resistance of High-Entropy Alloys Coatings with Various Compositions Prepared by Laser Cladding: A Review. Coatings 2022, 12, 1023. [Google Scholar] [CrossRef]
- Yang, Z.-Q.; Kuo, H.-M.; Chiang, H.-C.; Hung, C.-H. Enhanced wear resistance of low carbon steel by depositing iron-chromium alloy coating using high-power laser cladding. Int. J. Adv. Manuf. Technol. 2025, 137, 1133–1147. [Google Scholar] [CrossRef]
- Lu, D.; Cui, X.; Zhang, J. Microstructure and properties of high entropy alloy coating obtained by laser cladding. Sci. Rep. 2025, 15, 7357. [Google Scholar] [CrossRef]
- Jelvani, S.; Razavi, R.S.; Barekat, M.; Dehnavi, M.R.; Erfanmanesh, M. Evaluation of solidification and microstructure in laser cladding Inconel 718 superalloy. Opt. Laser Technol. 2019, 120, 105761. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, D.; Mishra, S.K.; Tiwari, S. Laser cladding of Stellite 6 on stainless steel to enhance solid particle erosion and cavitation resistance. Surf. Coat. Technol. 2014, 251, 87–97. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Y.; Shu, L.; Huang, T.; Shi, J.; Li, P. Microstructure and properties of ni-ZrB2 gradient composite coating on the 27SiMn steel surface by laser cladding. Mater. Today Commun. 2025, 43, 111657. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Ding, Q.; Duan, J.; Xu, Y.; Ji, F. Microstructure characteristics and cavitation erosion resistance of co-based coating fabricated via laser cladding. J. Laser Appl. 2025, 37, 012035. [Google Scholar] [CrossRef]
- Hu, Z.; Li, Y.; Lu, B.; Tan, N.; Cai, L.; Yong, Q. Effect of WC content on microstructure and properties of high-speed laser cladding ni-based coating. Opt. Laser Technol. 2022, 155, 108449. [Google Scholar] [CrossRef]
- Kornienko, E.E.; Lapushkina, E.J.; Kuzmin, V.I.; Vaschenko, S.P.; Gulyaev, I.P.; Kartaev, E.V.; Sergachev, D.S.; Kashapov, N.; Sharifullin, S.; Fayrushin, I. Air Plasma Sprayed Coatings of Self-Fluxing Powder Materials. J. Phys. 2014, 567, 012010. [Google Scholar] [CrossRef]
- Martin, D.T.; Rad, M.Z.; Macdonald, A.; Hussain, T. Beyond Traditional Coatings, a review on Thermal Sprayed and Smart coatings. J. Therm. Spray Tech. 2019, 28, 598–644. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Medabalimi, S.; Gudala, S.; Rokkala, U.; Hebbale, A.M.; Ramesh, M.R. Microstructure and elevated temperature wear behavior of HVOF-sprayed SS304L stainless-steel coating. Discov. Appl. Sci. 2025, 7, 428. [Google Scholar] [CrossRef]
- Gao, Y.; Bai, S.; Kou, G.; Jiang, S.; Liu, Y.; Zhang, D. Microstructure Characteristics and Elevated-Temperature Wear Mechanism of FeCoCrNiAl High-Entropy Alloy Prepared by Laser Cladding. Processes 2024, 12, 2228. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, W.; Mao, J.; Tian, L.; Li, R. Microstructure and Wear Behavior of Plasma-Sprayed TiO2–SiAlON Ceramic Coating. Coatings 2020, 10, 1268. [Google Scholar] [CrossRef]
- Firouzbakht, A.; Razavi, M.; Rahimipour, M.R. In Situ Synthesis of Fe–TiC Nanocomposite Coating on CK45 Steel From Ilmenite Concentrate by Plasma-Spray Method. J. Tribol. 2017, 139, 011302. [Google Scholar] [CrossRef]
- Li, F.; Sun, S.; Xu, Y.; Tian, L.; Wang, Y.; Xu, Z.; Li, R. Microstructure and Wear Behaviors of Plasma-Sprayed MoAlB Ceramic Coating. Coatings 2021, 11, 474. [Google Scholar] [CrossRef]
- Lakkannavar, V.; Yogesha, K.B.; Prasad, C.D.; Tiwari, A.; Vanitha, K.; Soni, P.K. Evaluation of mechanical, metallurgical, and hot corrosion-erosion behavior of plasma sprayed Ni22Cr10Al0.8Y/30%Cr3C2 /10%h-BN/10% mo composite coating. Surf. Coat. Technol. 2025, 497, 131730. [Google Scholar] [CrossRef]
- Özel, S. The effect of coating parameter on properties of plasma sprayed co based coatings. Teh. Vjesn. 2019, 26, 318–322. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Liu, G.; Xue, L.; Zhang, K. Tailoring Tribological Properties and Corrosion Resistance of Self-Lubricating Ti-Mo-N Coatings Prepared by Arc Depositions. Coatings 2025, 15, 956. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, M.; Kang, J.; Xiao, S.; Li, Y.; Fu, Z.; Wang, H. Development of the alternative technology of plasma spray melting to plasma transferred arc cladding: Microstructure and mechanical properties of Ni20Cr coatings. Surf. Coat. Technol. 2025, 496, 131613. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Guo, Q.; Zhao, G.; Li, H.; Ma, L. Research on the microstructure and properties of antimicrobial stainless steel coatings on Q345R alloy steel by electron beam surface coating. J. Mater. Res. Technol. 2024, 30, 7352–7363. [Google Scholar] [CrossRef]
- Ji, F.; Li, X.; Zhang, S.; Pang, M. Influence of Cu Content Variation on the Tribological Properties of Ni60CuMo with Sandwich-Structured Composite Coatings by Laser Cladding. Micromachines 2024, 15, 1429. [Google Scholar] [CrossRef]
- Moravčíková, J.; Moravčík, R.; Sahul, M.; Necpal, M. Influence of Laser Texturing and Coating on the Tribological Properties of the Tool Steels Properties. Machines 2024, 12, 311. [Google Scholar] [CrossRef]
- Tian, H.; Wang, C.; Guo, M.; Tang, Z.; Wei, S.; Xu, B. Study of the frictional-wear performance and abrasion resistance mechanism of a high-speed arc-sprayed FeNiCrAl coating. Surf. Coat. Technol. 2019, 370, 320–330. [Google Scholar] [CrossRef]
- Davis, C.L. Assessment of Plasma Sprayed Coatings to Modify Surface Friction for Railroad Applications. Ph.D. Thesis, Oregon Health & Science University, Portland, OR, USA, 2002. Available online: https://webofscience.clarivate.cn/wos/alldb/full-record/PQDT:65411061 (accessed on 4 April 2025).
- Zhao, W.; Xu, F.; Shi, X.; Gao, C.; Liu, Y.; Zhao, Y.; Shu, L.; Zuo, D. Effects of pulsed direct current power supply on ta-C coatings: Microstructure, mechanical properties and tribocorrosion behavior. Surf. Coat. Technol. 2025, 496, 131619. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, Q.; Luo, S.; Lin, R. Microstructure and Properties of Al-Cr-N Ternary Wear-Resistant Coatings on Cr12MoV Alloy Tool Steel by Multiarc Ion Plating. Coatings 2025, 15, 487. [Google Scholar] [CrossRef]
- Güney, B.; Mutlu, İ. Dry friction behavior of NiCrBSi-%35W2 C coated brake disks. Mater. Test. 2017, 59, 497–505. [Google Scholar] [CrossRef]
- Lin, B.; Zhang, L.; Cai, M.; Lu, J.; Yin, J.; Huang, Y.; Zhang, Y.; Fan, X.; Zhu, M. Synergistic anti-wear performance of zinc-rich epoxy coating on shot peening strengthened Q345 steel. Surf. Topogr. Metrol. Prop. 2023, 11, 045006. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Guo, Z.; Liu, X.; Qin, L.; Lu, Z. Ni20/PTFE Composite Coating Material and the Synergistic Friction Reduction and Wear Resistance Mechanism Under Multiple Working Conditions. Coatings 2025, 15, 830. [Google Scholar] [CrossRef]
- Gong, H.; Yu, C.C.; Zhang, L.; Xie, G.; Guo, D.; Luo, J.B. Intelligent lubricating materials: A review. Compos. Part B-Eng. 2020, 202, 108450. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, K.; Lin, H.; Zhang, F.; Xiong, B.; Zhang, H.; Zhang, C. Important contributions of multidimensional nanoadditives on the tribofilms: From formation mechanism to tribological behaviors. Compos. Part B-Eng. 2022, 234, 109732. [Google Scholar] [CrossRef]
- Hao, J.; Wang, B.; Ding, Y.; Yang, C.; Jiang, B.; Wang, Z.; Wang, D.; Dong, D. Influence of microstructure and stress state on service performance of TiN coatings deposited by dual-stage HIPIMS. Rare. Metal. Mat. Eng. 2024, 53, 3299–3305. [Google Scholar]
- Liu, Y.; Yu, S.; Shi, Q.; Ge, X.; Wang, W. Multilayer Coatings for Tribology: A Mini Review. Nanomaterials 2022, 12, 1388. [Google Scholar] [CrossRef]
- Cao, Y.; Bao, H.; Shi, W.; Wang, Z.; Zhang, J. Aluminum Chromium Nitride Coating on a Laser Shock Micro-Molded Surface of E690 High-Strength Steel and Its Antifriction Mechanism. Coatings 2023, 13, 1554. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Y.; Wang, N.; Xu, Y.; Zhao, Q.; Hou, Z.; Gao, G.; Kang, Y.; Zhan, H. Ultrasonic-assisted MoS2/GO/TiO2 ceramic coatings: Enhancing anti-friction performance through dual-interface optimization. Ultrason. Sonochem. 2025, 112, 107180. [Google Scholar] [CrossRef]
- Raghavendra, C.R.; Basavarajappa, S.; Sogalad, I. Adhesive strength and tribological behaviour of ni-nano-Al2O3 composite coating. Indian J. Phys. 2021, 95, 423–431. [Google Scholar] [CrossRef]
- Investigation on Friction and Wear Performance of Volcano-Shaped Textured PVD Coating-Web of Science Core Collection, (n.d.). Available online: https://webofscience.clarivate.cn/wos/woscc/full-record/WOS:000778429700001 (accessed on 10 April 2025).
- Kim, J.-H.; Kim, J.-I.; Kim, J.-W.; Kim, T.-G.; Hao, C.K.; Liew, W.Y.H.; Kim, S.-S. Frictional behavior of DLC coating on 60E1 steel under dry sliding friction. Trans. Korean Soc. Mech. Eng. A 2020, 44, 127–132. [Google Scholar] [CrossRef]
- Yan, H.; Li, L.; Hu, H.; Huang, W. Influence of Al2O3 and h-BN on Wear and Corrosion Performance of IN625 Nickel-Based Coating. Coatings 2024, 14, 1359. [Google Scholar] [CrossRef]
- Wan, L.; Cheng, M.; Fu, G.; Wei, C.; Shi, T.; Shi, S. Annular laser cladding of CuPb10Sn10 copper alloy for high-quality anti-friction coating on 42CrMo steel surface. Opt. Laser Technol. 2023, 158, 108878. [Google Scholar] [CrossRef]
- Tian, H.; Yu, Y.; Wang, X.; Chen, F.; Liu, H. Preparation of CrCoFeNiMn High-Entropy Alloy Coatings Using Gas Atomization and Laser Cladding: An Investigation of Microstructure, Mechanical Properties, and Wear Resistance. Coatings 2024, 14, 906. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Narayana, T.; Tyagi, R.; Pant, G.; Verma, P.C. Recent Developments in Self-Lubricating Thin-Film Coatings Deposited by a Sputtering Technique: A Critical Review of Their Synthesis, Properties, and Applications. Lubricants 2025, 13, 372. [Google Scholar] [CrossRef]
- Tan, S.; Luo, Y.; Yang, J.; Wang, W.; Li, X.; Jia, B.; Luo, Z.; Zhang, G. Mechanism of thermoviscoelasticity driven solid-liquid interface reducing friction for polymer alloy coating. Friction 2023, 11, 1606–1623. [Google Scholar] [CrossRef]
- Tribological Properties of NiCrAlYTa-ag Self-Lubricating Coatings at Wide Temperature Range by Detonation Spraying-Web of Science Core Collection, (n.d.). Available online: https://webofscience.clarivate.cn/wos/woscc/full-record/WOS:001012896600001 (accessed on 19 April 2025).
- Hu, J.; He, C.; Yang, X.; Li, H.; Xu, H.; Guo, N. Microstructure and Tribological Properties of Self-Lubricating FeS Coating Prepared by Chemical Bath Deposition Coating Technique. Appl. Sci. 2019, 9, 4422. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, P.; Gao, Q.; Qin, Y.; Li, R. Laser cladding ni-based alloy/nano-ni encapsulated h-BN self-lubricating composite coatings. Surf. Coat. Technol. 2017, 332, 422–427. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.; Liu, G.; Qian, Y.; Xu, Y.; Xiang, D. Surface Modification of 42CrMo Steels: A Review from Wear and Corrosion Resistance. Coatings 2024, 14, 337. [Google Scholar] [CrossRef]
- Ren, L.; Wang, T.; Chen, Z.; Li, Y.; Qian, L. Self-lubricating PEO-PTFE composite coating on titanium. Metals 2019, 9, 170. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.; Xu, B.; Lu, Y.; Zhou, C.; Wu, X.; Li, J. Fabrication and tribological properties of a self-lubricating wear-resistant coating based on structural coupling. Ceram. Int. 2019, 45, 3910–3920. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, H. Tribocorrosion Behavior of Laser Cladded Ti-Al-(C, N) Composite Coatings in Artificial Seawater. Coatings 2022, 12, 187. [Google Scholar] [CrossRef]
- Tillmann, W.; Momeni, S.; Hoffmann, F. A study of mechanical and tribological properties of self-lubricating TiAlVN coatings at elevated temperatures. Tribol. Int. 2013, 66, 324–329. [Google Scholar] [CrossRef]
- Chi, X.; Yuan, J.; Li, J.; Pan, G.; Cui, Y.; Li, X. Effect of Cu on the high-temperature wear behavior of FeAl-TiB2 coatings produced by extreme high-speed laser cladding. Appl. Surf. Sci. Adv. 2023, 17, 100439. [Google Scholar] [CrossRef]
- Soltani-Kordshuli, F.; Miller, C.; Harris, N.; Zou, M. Laser surface texturing of both thin polytetrafluoroethylene coatings and stainless steel substrates for improving tribological properties. Polym. Test. 2023, 117, 107852. [Google Scholar] [CrossRef]
- Soltani-Kordshuli, F.; Harris, N.; Zou, M. Tribological behavior of polydopamine/polytetrafluoroethylene coating on laser textured stainless steel with hilbert curves. Friction 2023, 11, 1307–1319. [Google Scholar] [CrossRef]
- Miller, C.; Choudhury, D.; Zou, M. The Effects of Surface Roughness on the Durability of Polydopamine/PTFE Solid Lubricant Coatings on NiTiNOL 60. Tribol. Trans. 2019, 62, 919–929. [Google Scholar] [CrossRef]
- Da, B.; Jiahong, L.; Yongwu, Z.; Yongguang, W. Investigation of tribological and corrosion properties in ceramic/PTFE coating. Surf. Eng. 2022, 38, 520–528. [Google Scholar] [CrossRef]
- Ferkous, H.; Delimi, A.; Kahlouche, A.; Boulechfar, C.; Djellali, S.; Belakhdar, A.; Yadav, K.K.; Ali, I.H.; Ahmad, A.; Ahn, H.-J.; et al. Electrochemical and Computational Approaches of Polymer Coating on Carbon Steel X52 in Different Soil Extracts. Polymers 2022, 14, 3288. [Google Scholar] [CrossRef]
- Pourhashem, S.; Saba, F.; Duan, J.; Rashidi, A.; Guan, F.; Nezhad, E.G.; Hou, B. Polymer/Inorganic nanocomposite coatings with superior corrosion protection performance: A review. J. Ind. Eng. Chem. 2020, 88, 29–57. [Google Scholar] [CrossRef]
- El-Sherik, A.M. Trends in Oil and Gas Corrosion Research and Technologies: Production and Transmission; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Zhang, F.; Shao, Y.; Dong, H.; Mao, P.; Zhang, W.; Tao, S. Study on the corrosion behavior of alumina coating on the surface of aluminum electrolysis anode steel claw in Na3AlF6-AlF3 molten salt. Mater. Today Commun. 2025, 44, 112033. [Google Scholar] [CrossRef]
- Bai, T.; Lv, L.; Du, W.; Fang, W.; Wang, Y. Improving the tribological and anticorrosion performance of waterborne polyurethane coating by the synergistic effect between modified graphene oxide and polytetrafluoroethylene. Nanomaterials 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Chen, K.; Zhang, X.; Zhang, B.; Fang, S.; Liang, Y.; Huang, C.; Wang, X. A Comparative Study of Chloride Adsorption Ability and Corrosion Protection Effect in Epoxy Coatings of Various Layered Double Hydroxides. Coatings 2022, 12, 1631. [Google Scholar] [CrossRef]
- Lu, Y.; Feng, H. Effect of Rare-earth Salts on Corrosion Resistance of Phytic Acid Based Conversion Coatings on Q235 Steel. Int. J. Electrochem. Sci. 2020, 15, 7968–7981. [Google Scholar] [CrossRef]
- Syrek-Gerstenkorn, B.; Paul, S.; Davenport, A.J. Sacrificial Thermally Sprayed Aluminium Coatings for Marine Environments: A Review. Coatings 2020, 10, 267. [Google Scholar] [CrossRef]
- So, S.-M.; Grandhi, S.; Kwon, E.-P.; Oh, M.-S. Effects of Si Addition on Interfacial Microstructure and Corrosion Resistance of Hot-Dip Zn–Al–Mg–Si Alloy-Coated Steel. Crystals 2024, 14, 294. [Google Scholar] [CrossRef]
- Hoque, M.A.; Yao, C.W.; Lian, I.; Zhou, J.; Jao, M.; Huang, Y.C. Enhancement of corrosion resistance of a hot-dip galvanized steel by superhydrophobic top coating. MRS Commun. 2022, 12, 415–421. [Google Scholar] [CrossRef]
- Chakraborty, A.; Ghosh, R.; Sudan, M.; Mondal, A. Improvement in hot dip galvanized coating microstructure and properties by pre-metallic deposition on steel surface: A comprehensive review. Surf. Coat. Technol. 2002, 449, 128972. [Google Scholar] [CrossRef]
- Manna, M.; Dutta, M. Improvement in galvanization and galvannealing characteristics of DP 590 steel by prior Cu or Cu–Sn flash coating. Surf. Coat. Technol. 2014, 251, 29–37. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.M.; Abd-El-Nabey, B.A.; Khamis, E.; Salman, R.M.; Rahal, H.T.; El Morr, Z. Electrochemical synthesis and corrosion behaviour of polyaniline on stainless steel in sodium hydroxide solutions. Chem. Eng. Comm. 2021, 208, 271–280. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.M.; Awad, R.; Rahal, H.T.; Moussa, D. Electrochemical behavior of composite nanoparticles on the corrosion of mild steel in different media. J. Bio. Tribo Corros. 2019, 5, 49. [Google Scholar] [CrossRef]
- Ayoola, A.A.; Fayomi, O.S.I.; Akande, I.G.; Ayeni, O.A.; Agboola, O.; Obanla, O.R.; Abatan, O.G.; Chukwuka, C.J. Inhibitive corrosion performance of the eco-friendly aloe vera in acidic media of mild and stainless steels. J. Bio. Tribo Corros. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Winkler, D.A. Predicting the performance of organic corrosion inhibitors. Metals 2017, 7, 553. [Google Scholar] [CrossRef]
- Loto, R.T.; Olowoyo, O. Corrosion inhibition properties of the combined admixture of essential oil extracts on mild steel in the presence of SO42- anions. S. Afr. J. Chem. Eng. 2018, 26, 35–41. [Google Scholar] [CrossRef]
- Sakairi, M.; Takagi, S. Effect of Surface Conditions and Relative Humidity on Hydrogen Permeation Behavior of Zinc Coated Steels during Wet and Dry Corrosion. ISIJ Int. 2016, 56, 452–458. [Google Scholar] [CrossRef]
- Fattah-alhosseini, A.; Sangarimotlagh, Z.; Karbasi, M.; Dikici, B. Enhancing corrosion resistance in Mg-based alloys through MOF-incorporated coatings: A comprehensive review. Appl. Surf. Sci. Adv. 2024, 21, 100607. [Google Scholar] [CrossRef]
- Allely, C.; Dosdat, L.; Clauzeau, O.; Ogle, K.; Volovitch, P. Anticorrosion mechanisms of aluminized steel for hot stamping. Surf. Coat. Technol. 2014, 238, 188–196. [Google Scholar] [CrossRef]
- Panossian, Z.; Mariaca, L.; Morcillo, M.; Flores, S.; Rocha, J.; Peña, J.J.; Herrera, F.; Corvo, F.; Sanchez, M.; Rincon, O.T.; et al. Steel cathodic protection afforded by zinc, aluminium and zinc/aluminium alloy coatings in the atmosphere. Surf. Coat. Technol. 2005, 190, 244–248. [Google Scholar] [CrossRef]
- Nicard, C.; Allély, C.; Volovitch, P. Effect of Zn and Mg alloying on microstructure and anticorrosion mechanisms of Al-Si based coatings for high strength steel. Corros. Sci. 2019, 146, 192–201. [Google Scholar] [CrossRef]
- Dosdat, L.; Petitjean, J.; Vietoris, T.; Clauzeau, O. Corrosion resistance of different metallic coatings on press-hardened steels for automotive. Steel Res. Int. 2011, 82, 726–733. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, T.; Wang, Q.; Zhang, Y.; Hong, M.; Chen, D.; Zhang, J. Effect of copper content on the microstructure and electrochemical corrosion behavior of laser cladding 316L stainless steel coating. Corrosion 2025, 81, 216–231. [Google Scholar] [CrossRef]
- Abdel Halim, K.S.; Ramadan, M.; Sherif, E.-S.M.; Hafez, K.M.; Subhani, T.; Fathy, N.; Alghamdi, A.S.; Khedr, M.H. Enhancement of Surface and Interface Properties of Low Carbon Steel by Hybrid ZnO and NiO Nanoparticles Reinforced Tin Coating. Crystals 2022, 12, 332. [Google Scholar] [CrossRef]
- Morita, J.; Yoshida, M. Chromium coating on partially tin precoated steel sheet. J. Appl. Electrochem. 1994, 24, 888–893. [Google Scholar] [CrossRef]
- Vitkin, A.I.; Surovtseva, V.S. New protective coating in the production of sheet metal. Metallurg 1972, 16, 286–290. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, Y.; Li, Y.; Liu, Y.; Wang, Y. Effect of Fe2O3 nanoparticles size on the properties of Sn-1.0Age0.5Cu nano-composite solders and joints. J. Alloys Compd. 2016, 662, 272–282. [Google Scholar] [CrossRef]
- Nejad, M.M.; Habibolahzadeh, A.; Yousefpour, M. Effect of Nano_Oxide Addition on Corrosion Performance of Hot Dip Zinc Coating. Prot. Met. Phys. Chem. Surf. 2016, 52, 100–103. [Google Scholar]
- Jiang, Z.H.; Han, J.P.; Li, Y. Effect of tin on corrosion resistance and formability of tin bearing ferritic stainless steel. Mater. Res. Innov. 2014, 18, 9–11. [Google Scholar] [CrossRef]
- Dey, P.P.; Modak, P.; Ghosh, A.; Chakrabarti, D.; Banerjeem, P.S.; Ghosh, M. Investigation of phase evolution of Al–Si–Mg coating on hot dipped interstitial-free steel. Results Mater. 2020, 6, 100078. [Google Scholar] [CrossRef]
- Sun, K.; Cai, W.; He, X.; Chen, H.; Chen, K.; Jiang, T.; Li, W.; Zhao, Y. Preparation and Properties of Silane Coupling Agent Modified Basalt Flake Polyurethane Anti-Corrosion Coatings. Coatings 2023, 13, 2022. [Google Scholar] [CrossRef]
- Watkins, E.; Griffiths, C.M.; Richards, C.A.J.; Potts, S.-J.; Batchelor, C.; Barker, P.; Searle, J.; Jewell, E. Improving the Corrosion Performance of Organically Coated Steel Using a Sol–Gel Overcoat. Materials 2024, 17, 1075. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Z.; Zhang, L.; Li, C.; Ding, R.; Zhao, X.; Zhang, P.; Wang, B.; Cui, H. Studies of corrosion behaviors of a carbon steel/copper-nickel alloy couple under epoxy coating with artificial defect in 3.5 wt.% NaCl solution using the WBE and EIS techniques. Prog. Org. Coat. 2020, 148, 105909. [Google Scholar] [CrossRef]
- Rajput, A.; Ak, M.; Kim, S.J.; Noh, S.H.; Park, J.H.; Paik, J.K. Effects of the surface preparation on the life of epoxy coating in steel ship plates: An experimental study. Ships Offshore Struct. 2019, 14, 199–206. [Google Scholar] [CrossRef]
- Kamde, D.K.; Pillai, R.G. Effect of surface preparation on corrosion of steel rebars coated with cement-polymer-composites (CPC) and embedded in concrete. Constr. Build. Mater. 2020, 237, 117616. [Google Scholar] [CrossRef]
- Hoque, M.A.; Yao, C.-W.; Khanal, M.; Lian, I. Tribocorrosion Behavior of Micro/Nanoscale Surface Coatings. Sensors 2022, 22, 9974. [Google Scholar] [CrossRef] [PubMed]
- Alshammri, G.A.; Fathy, N.; Al-Shomar, S.M.; Alshammari, A.H.; Sherif, E.-S.M.; Ramadan, M. Effect of Al2O3 and NiO Nanoparticle Additions on the Structure and Corrosion Behavior of Sn—4% Zn Alloy Coating Carbon Steel. Sustainability 2023, 15, 2511. [Google Scholar] [CrossRef]
- Zhao, C.; Ying, L.; Nie, C.; Zhu, T.; Tang, R.; Liu, R. Investigation of the Corrosion–Wear Interaction Behavior of 8Cr4Mo4V Bearing Steel at Various Corrosion Intervals. Coatings 2024, 14, 1245. [Google Scholar] [CrossRef]
- de Matos Macedo, M.; Tercini, M.B.; Antunes, R.A.; de Oliveira, M.C.L. Exploring the Interplay between Tribocorrosion and Surface Chemistry of the ASTM F139 Surgical Stainless Steel in Phosphate-Buffered Saline Solution. Materials 2024, 17, 2295. [Google Scholar] [CrossRef]
- Guo, L.; Cui, L.; Xiao, F.; Xu, B.; Wu, Z.; Cao, Y.; Wei, Z. Influence of process parameters on surface properties and corrosion resistance of Inconel 625 coating prepared by laser cladding. Int. J. Electrochem. Sci. 2023, 18, 100213. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).