Rheological Deterioration of High Viscosity High Elasticity Asphalt (HVEA) Under the Coupling Effect UV Aging and Salt Freeze-Thaw (SFT) Cycles
Abstract
1. Introduction
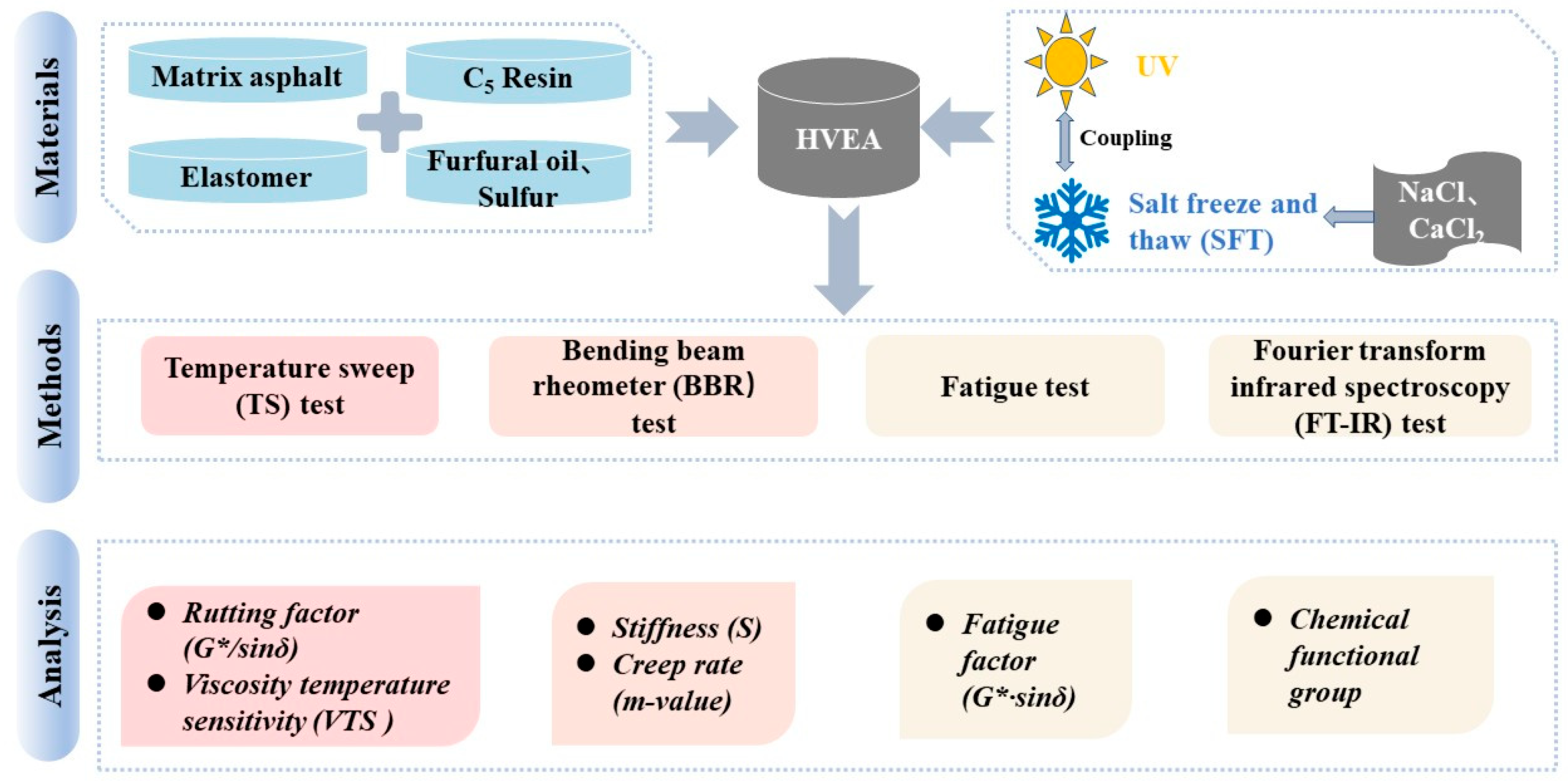
2. Materials and Methods
2.1. Raw Materials
2.2. HVEA Preparation Process
2.3. UV Aging–SFT Cycle Coupling Test Scheme Design
2.4. Characterization
2.4.1. Temperature Sweep (TS) Test
2.4.2. Bending Beam Rheometer (BBR) Test
2.4.3. Fatigue Performance Test
2.4.4. Fourier Transform Infrared Spectroscopy (FT-IR) Test
3. Results and Discussion
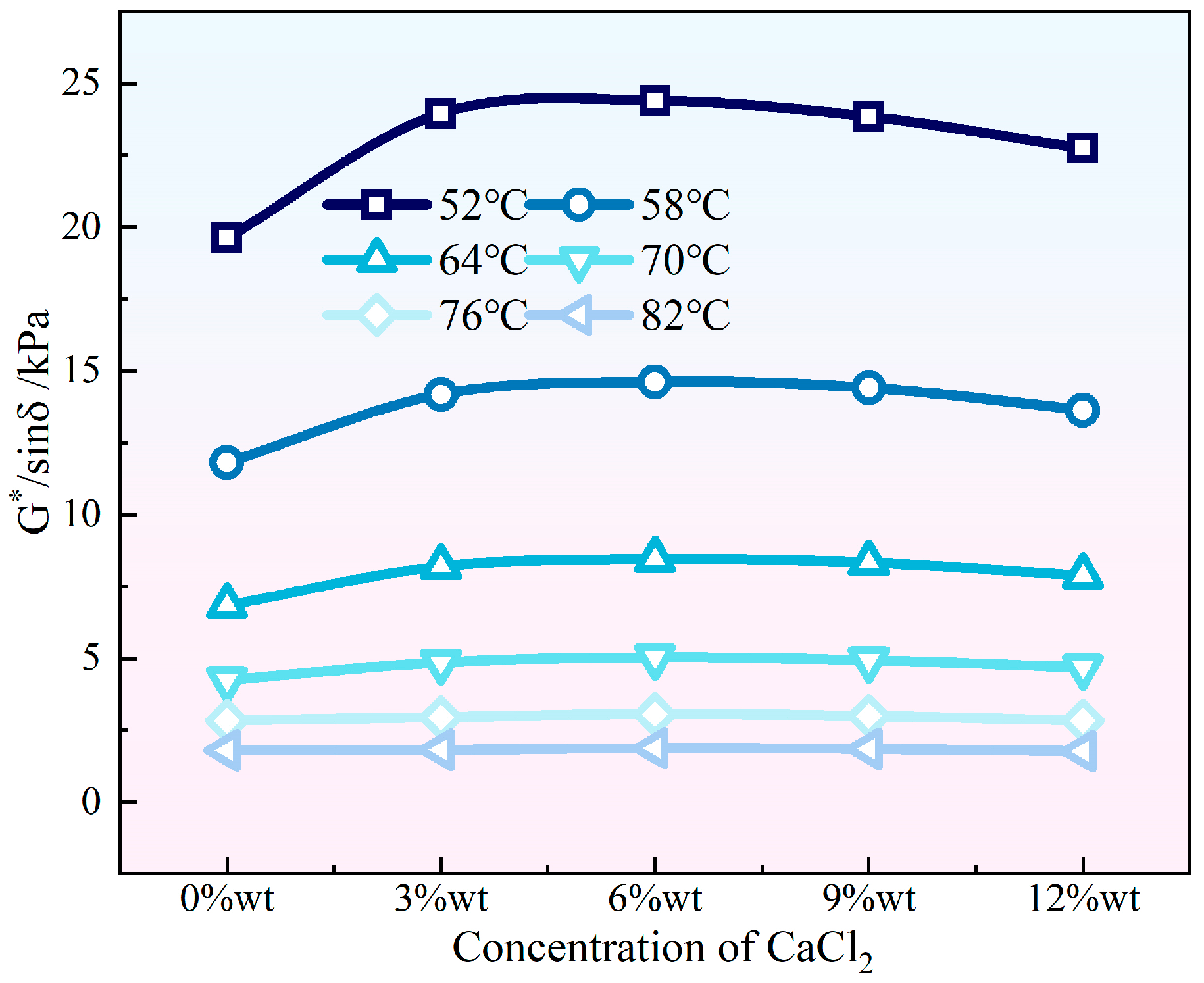
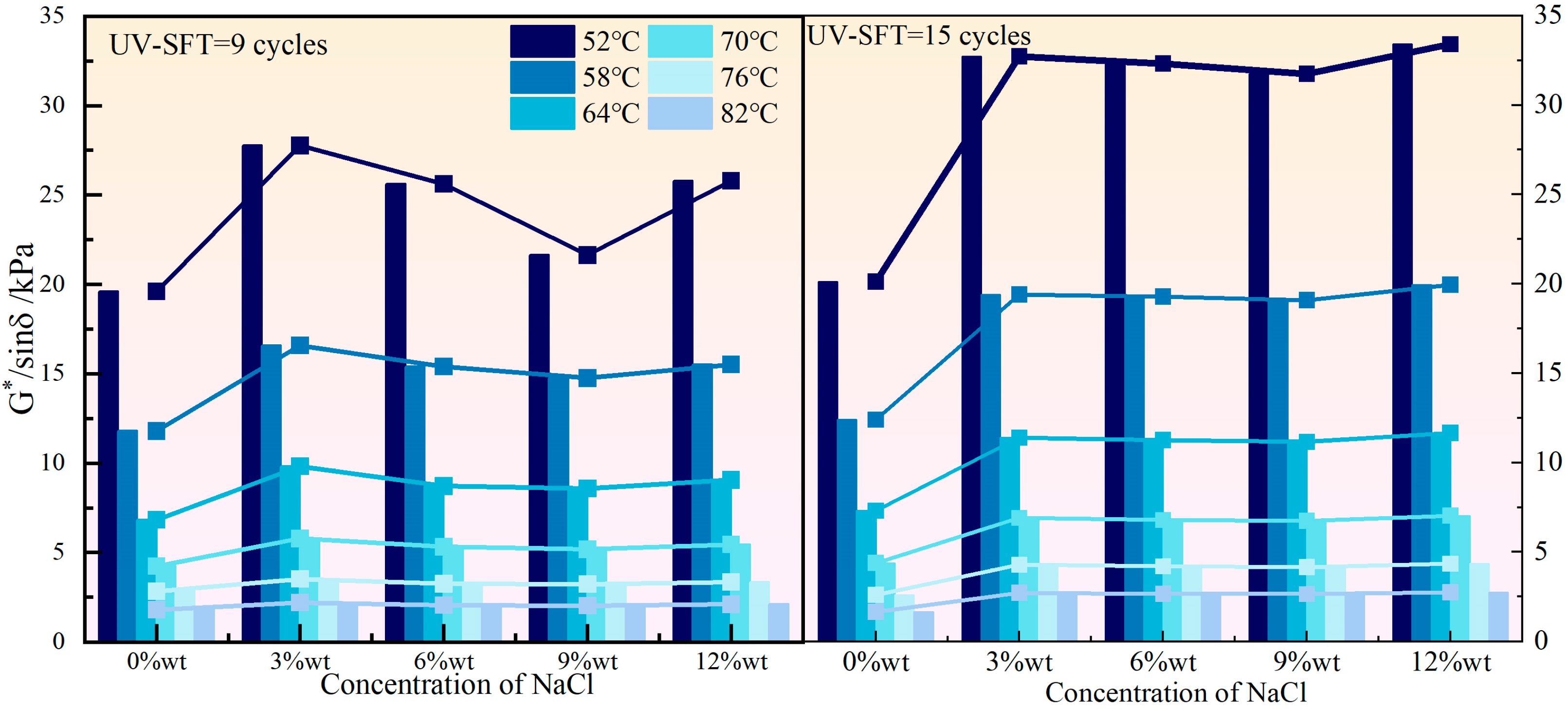
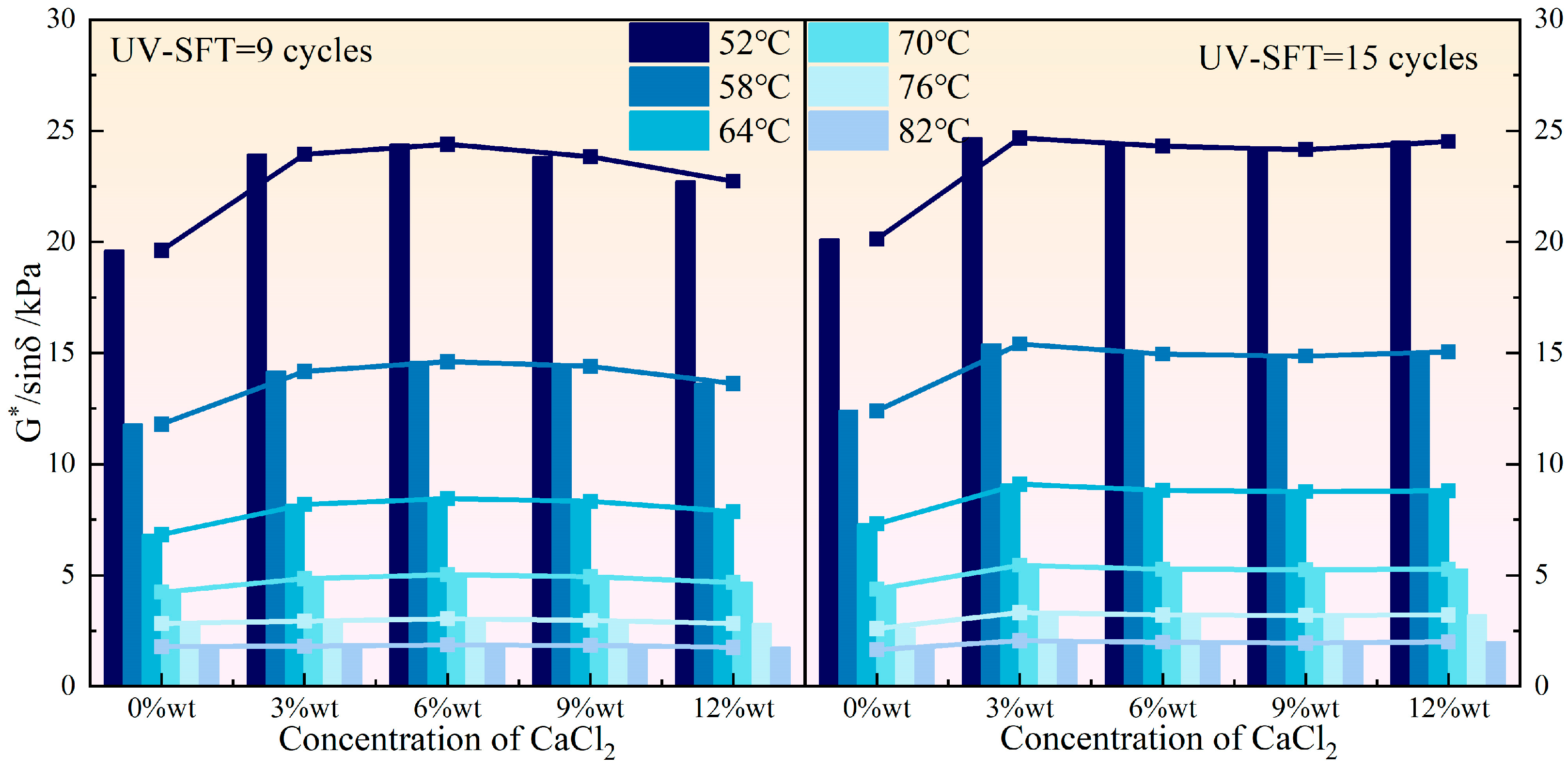
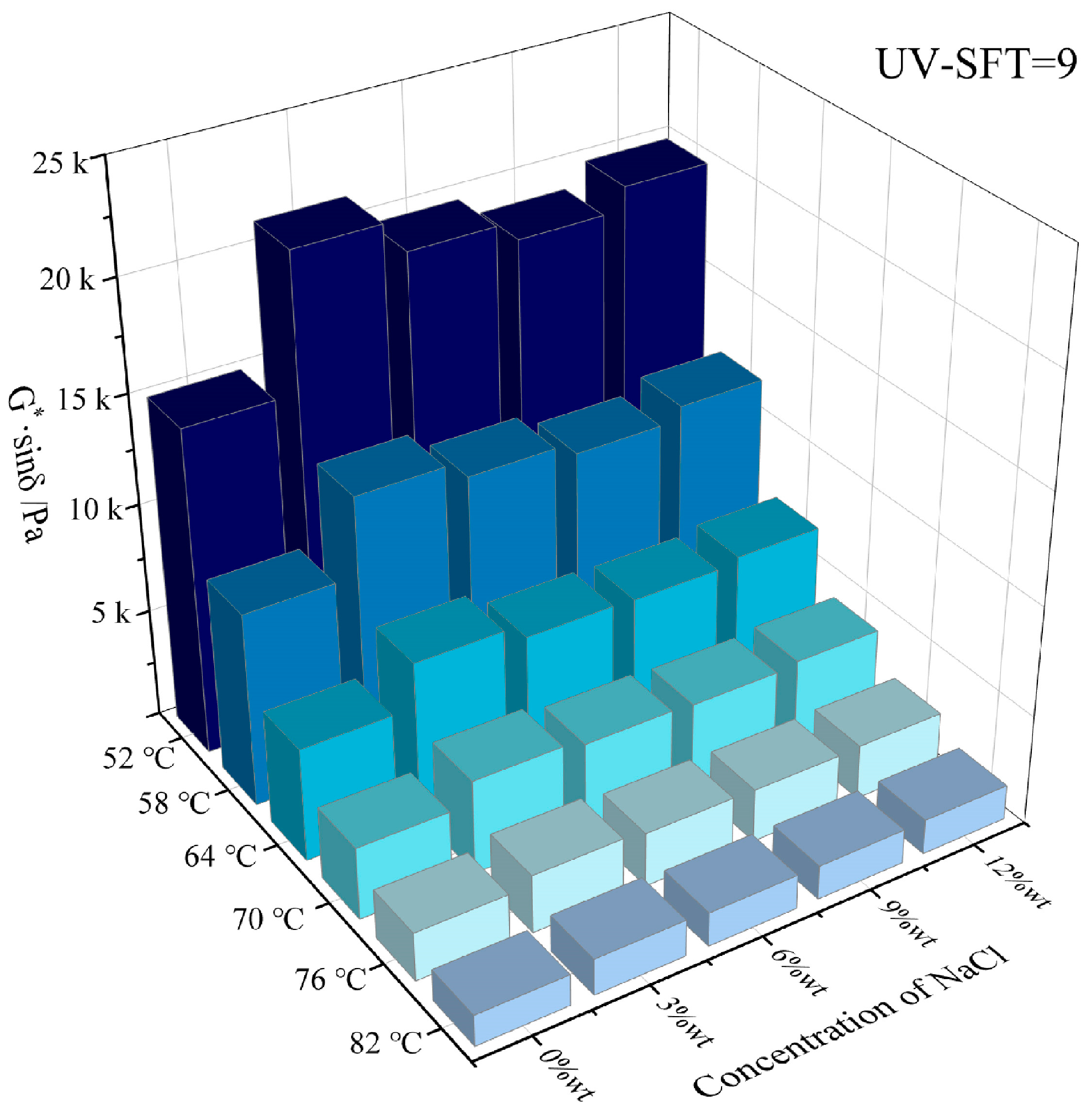
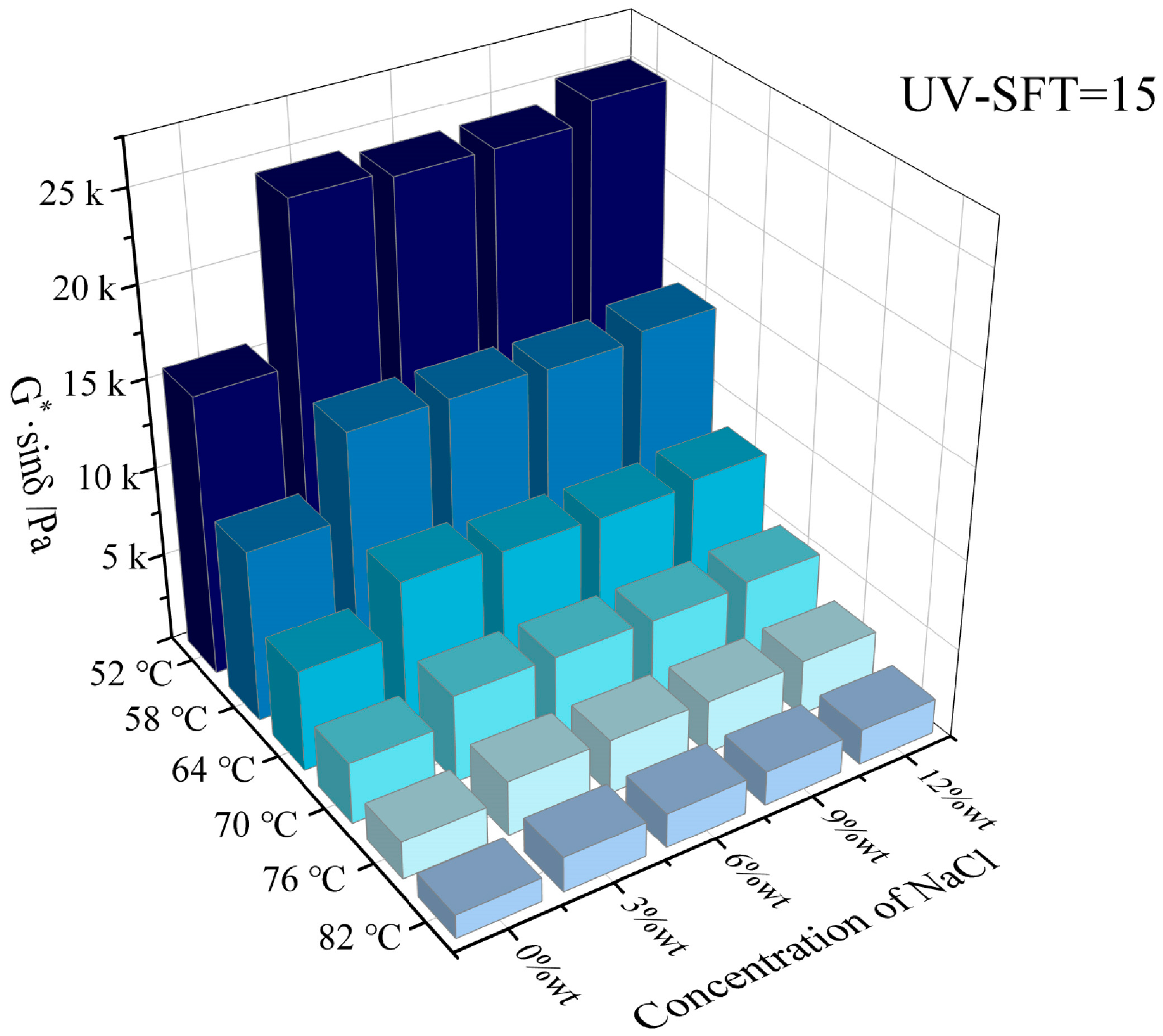
3.1. High Temperature Rheological Properties
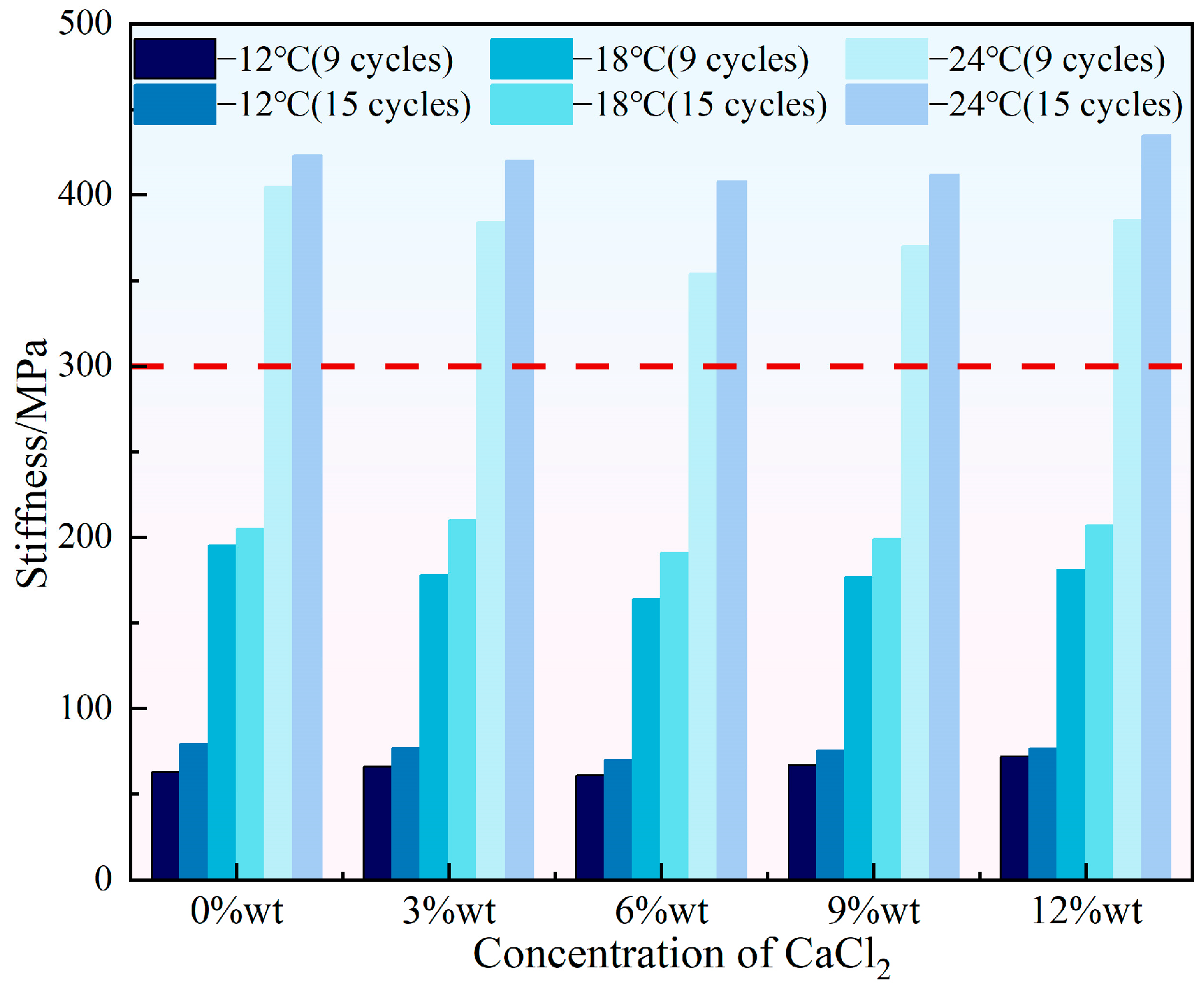
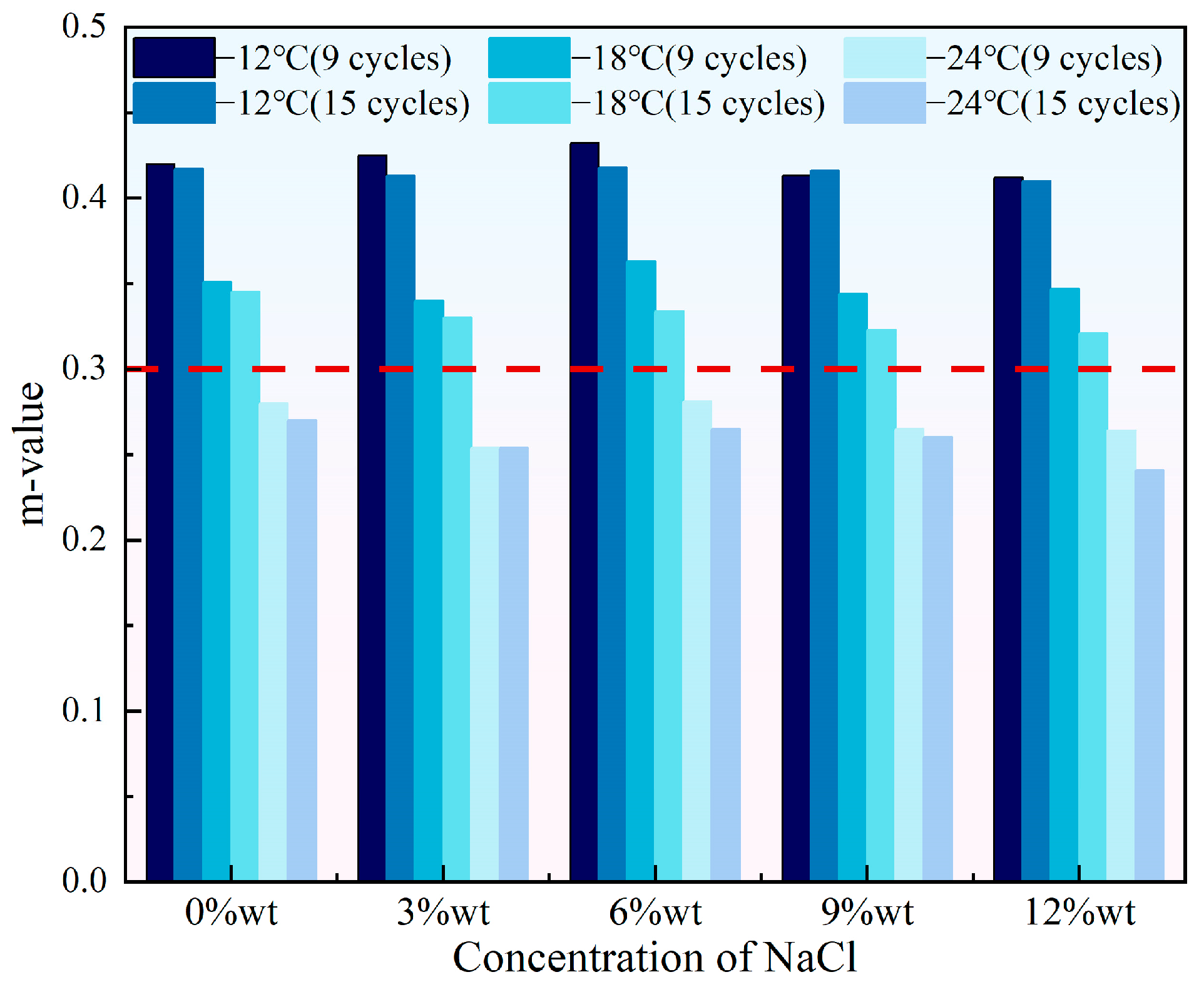
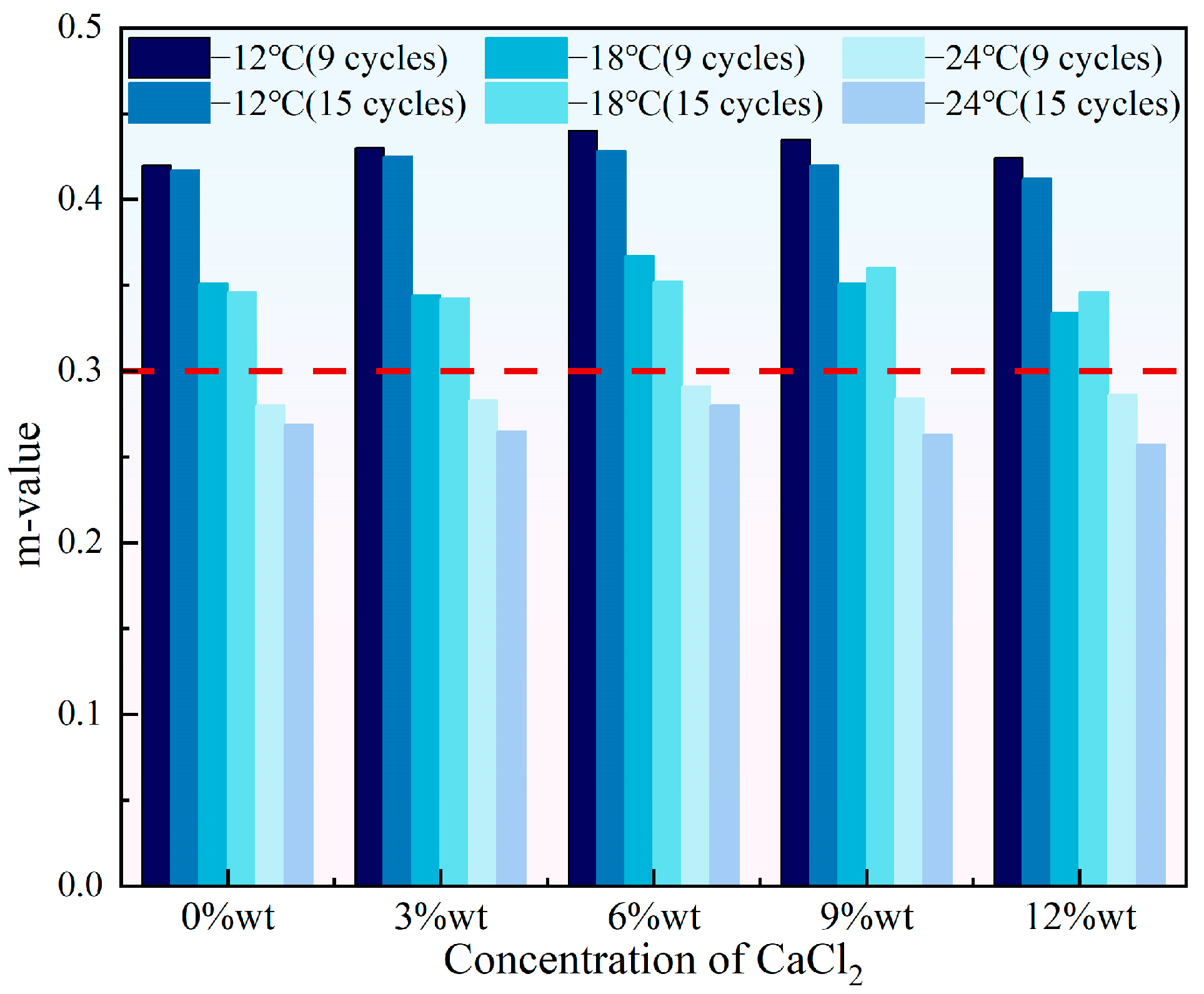
3.2. Low Temperature Rheological Properties
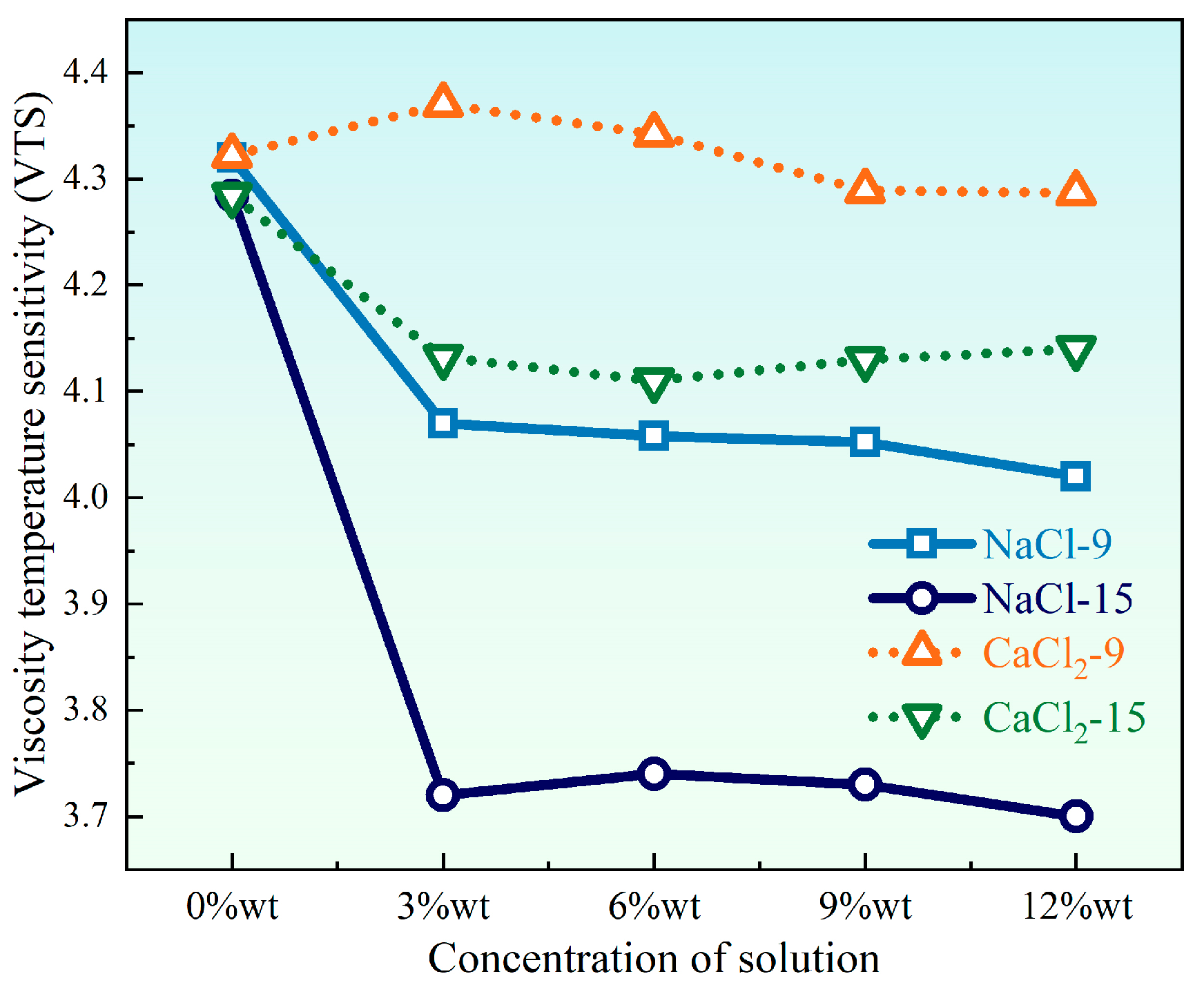
3.3. Temperature Sensitivity
3.4. Fatigue Properties
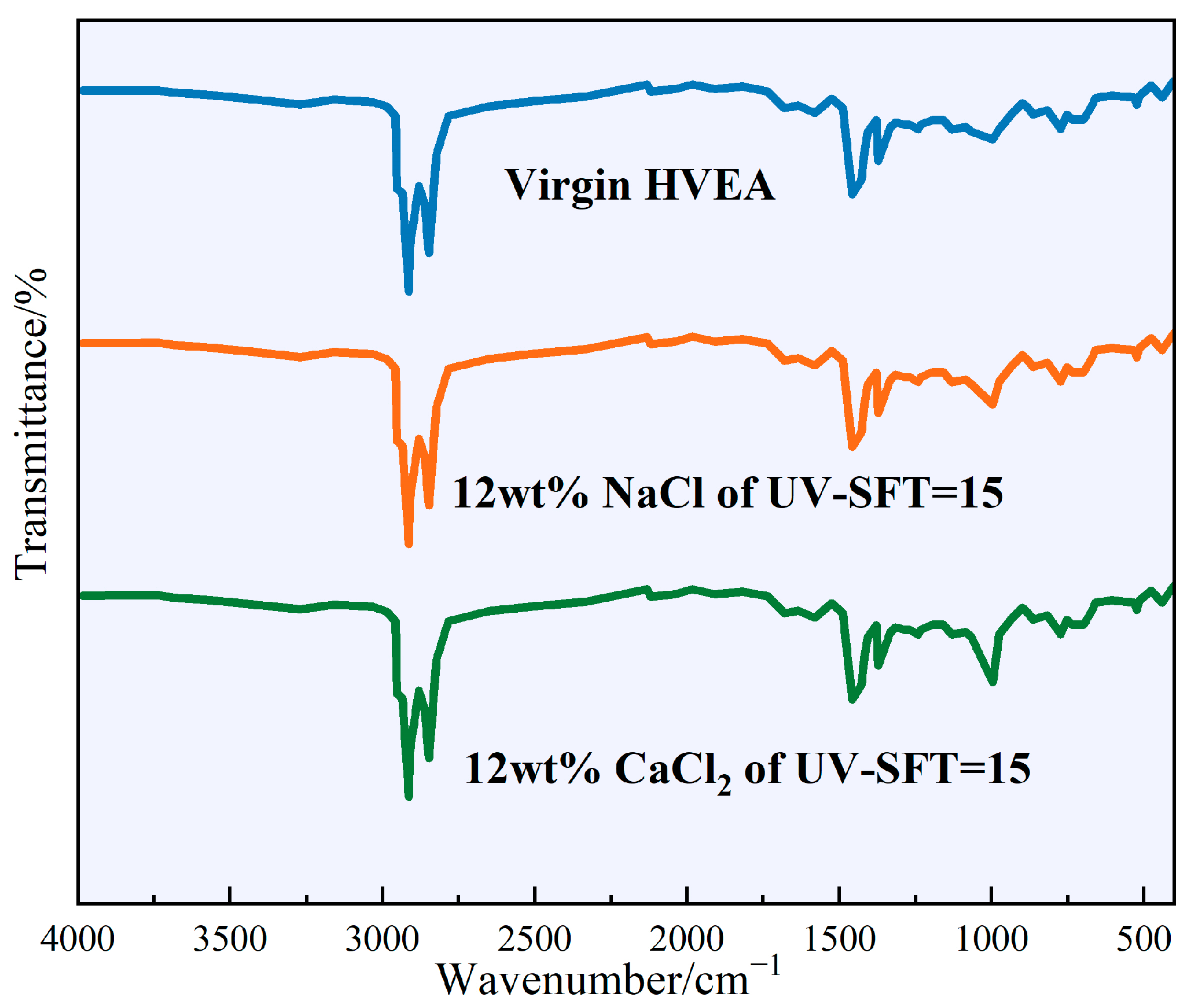
3.5. Chemical Properties
4. Conclusion
- (1)
- Both NaCl and CaCl2 solutions effectively enhanced the high-temperature rutting resistance of HVEA under the UV-SFT environment, with NaCl being the more significant enhancement in this regard. As the temperature increased, the effect of both salt solution concentrations on the high-temperature performance of HVEA diminished. After UV-SFT cycling, the G*/sinδ of HVEA increased. Moreover, the higher the number of UV-SFT cycles, the greater the increase in the G*/sinδ.
- (2)
- With the increase in the concentration of either NaCl or CaCl2, the stiffness of HVEA first decreased then increased and the m-value showed the opposite trend of first increasing then decreasing, suggesting that the addition of snow-melt salts impaired the HVEA’s low-temperature creep performance. When the salt concentration was constant, more UV-SFT aging cycles led to a higher stiffness and a lower m-value, which weakened HVEA’s low-temperature crack resistance.
- (3)
- As the number of UV-SFT cycles increased, the VTS values of HVEA showed a decreasing trend, indicating that its temperature sensitivity gradually decreased. Salt corrosion significantly weakened the temperature sensitivity of HVEA, and NaCl affected the temperature sensitivity of HVEA to a much greater extent than CaCl2.
- (4)
- The addition of snow-melt salt significantly weakened the fatigue resistance of HVEA, but the higher concentration of snow-melt salt impaired the fatigue performance of HVEA more severely. The G*·sinδ after 15 cycles of UV-SFT was significantly higher than that after 9 cycles, indicating a further weakening of the fatigue resistance by UV-SFT cycling.
- (5)
- After UV-SFT cycling, the intensity of the S=O characteristic peak was significantly higher than that of virgin HVEA, reflecting that the HVEA underwent an aging process. The enhanced intensity of the characteristic peak of C=C reflected an increased trans-olefin content, suggesting that chemical aging had occurred in HVEA, which may have increased its low-temperature brittleness and deteriorated its performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xia, L.; Su, Q.; Shan, L.; Li, F.; Cao, D.; Lu, J. Research on low-cost high-viscosity asphalt and its performance for porous asphalt pavement. Polymers 2024, 16, 1489. [Google Scholar] [CrossRef]
- Li, M.; Zeng, F.; Xu, R.; Cao, D.; Li, J. Study on compatibility and rheological properties of high-viscosity modified asphalt prepared from low-grade asphalt. Materials 2019, 12, 3776. [Google Scholar] [CrossRef]
- Luo, J.H.; Yang, Y.Z.; Huang, W.R.; Xie, C.; Chen, J.; Liu, H.; Ren, T.; Huang, X. Physical, rheological, and microsurface characteristics of high-viscosity binder modified with wma agents. Adv. Mater. Sci. Eng. 2022, 2022, 5098250. [Google Scholar] [CrossRef]
- Zhao, K.; Song, S.; Wei, Y.; Li, G.; Guo, F. Adhesion properties of recycled high-viscosity asphalt–aggregate interface under dynamic water erosion. Materials 2023, 16, 6203. [Google Scholar] [CrossRef]
- Xu, B.; Ding, R.; Yang, Z.; Sun, Y.; Zhang, J.; Lu, K.; Cao, D.; Gao, A. Investigation on performance of mineral-oil-based rejuvenating agent for aged high viscosity modified asphalt of porous asphalt pavement. J. Clean. Prod. 2023, 395, 136285. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, H.; Barbieri, D.M.; Lou, B.; Hoff, I. The classification and reutilisation of recycled asphalt pavement binder: Norwegian case study. Case Stud. Constr. Mater. 2022, 17, e01491. [Google Scholar] [CrossRef]
- Li, Y.; Feng, J.; Wu, S.; Chen, A.; Kuang, D.; Bai, T.; Gao, Y.; Zhang, J.; Li, L.; Wan, L.; et al. Review of ultraviolet ageing mechanisms and anti-ageing methods for asphalt binders. J. Road Eng. 2022, 2, 137–155. [Google Scholar] [CrossRef]
- Feng, Z.G.; Yu, J.Y.; Zhang, H.L.; Kuang, D.L. Preparation and properties of ageing resistant asphalt binder with various anti-ageing additives. Appl. Mech. Mater. 2011, 71, 1062–1067. [Google Scholar] [CrossRef]
- Yu, H.; Bai, X.; Qian, G.; Wei, H.; Gong, X.; Jin, J.; Li, Z. Impact of ultraviolet radiation on the aging properties of SBS-modified asphalt binders. Polymers 2019, 11, 1111. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Liang, M.C.; Du, X.L. Evaluation on feasibility of carbon black and hindered amine light stabilizer as UV-resistant additives of asphalt binder. J. Test. Eval. 2023, 51, 3987–4003. [Google Scholar] [CrossRef]
- Li, Y.; Wu, S.; Liu, Q.; Dai, Y.; Li, C.; Li, H.; Nie, S.; Song, W. Aging degradation of asphalt binder by narrow-band UV radiations with a range of dominant wavelengths. Constr. Build. Mater. 2019, 220, 637–650. [Google Scholar] [CrossRef]
- Sun, X.; Xu, Q.; Fang, G.; Zhu, Y.; Yuan, Z.; Chen, Q.; Yuan, J. Effect investigation of ultraviolet ageing on the rheological properties, micro-structure, and chemical composition of asphalt binder modified by modifying polymer. Adv. Mater. Sci. Eng. 2022, 2022, 7190428. [Google Scholar] [CrossRef]
- Pangarova, D.; Nikolov, A. Evaluation of the effect of different de-icing salts and freeze and thaw cycles on the performance of bituminous binders and asphalt mixtures prepared with them. In Proceedings of the International Conference on Maintenance and Rehabilitation of Pavements; Springer Nature: Cham, Switzerland, 2024; pp. 149–157. [Google Scholar]
- Zhang, K.; Luo, Y.; Xie, W.; Wu, J. Evaluation of road performance and adhesive characteristic of asphalt binder in salt erosion environment. Mater. Today Commun. 2020, 25, 101593. [Google Scholar] [CrossRef]
- Cheng, Y.; Liang, J.; Wang, W.; Wang, H.; Zhao, W.; Li, A.; Xia, W. Characteristics and evolution mechanism of creep properties for asphalt binder/mastic under salt-erosion conditions. Constr. Build. Mater. 2024, 443, 137702. [Google Scholar] [CrossRef]
- Xiong, R.; Qiao, N.; Yang, F.; Chu, C.; Li, L.; Wu, J.; Jiang, W.; Li, K.; Chen, H. Performance damage characteristics of asphalt binder suffered from the action of sulfate. Adv. Mater. Sci. Eng. 2019, 2019, 5465687. [Google Scholar] [CrossRef]
- Wei, W.; Cui, Y.; Guo, S.; Li, Z.; Wang, T.; Jia, Z.; Zhang, S. Low-temperature creep behavior and microstructural characteristics of photocatalytic N-TiO2-modified asphalt under salt freeze–thaw cycles. Constr. Build. Mater. 2025, 471, 140549. [Google Scholar] [CrossRef]
- Li, Z.; Xu, T.; Liu, S.; Zhang, W.; Gan, X. Preparation, microstructures and properties of shape memory hydrogenated epoxy resin-modified emulsified asphalt. Int. J. Pavement Eng. 2024, 25, 2316095. [Google Scholar] [CrossRef]
- Li, N.; Liu, Z.; Yin, J.; Zhang, H.; Dou, H.; Li, B. Investigation of compatibility mechanisms and diffusion behavior of polymer SBS-modified asphalt compatibilizer using molecular dynamics simulation. Materials 2025, 18, 2238. [Google Scholar] [CrossRef]
- Peng, C. Research on PAC Road Materials and Properties in Plateau Area. Master’s Thesis, Chang’an University, Xi’an, China, 2023. [Google Scholar]
- Ma, F.; Ma, X.Y.; Li, C.; Dong, W.H.; Fu, Z.; Yang, Y.F.; Hou, Y.J.; Huang, R.Z. Study on rheological properties of warm mix high viscosity asphalt mortar. Funct. Mater. 2025, 56, 8199–8204. [Google Scholar] [CrossRef]
- Ding, G.; Yu, X.; Si, J.; Mei, J.; Wang, J.; Chen, B. Influence of epoxy soybean oil modified nano-silica on the compatibility of cold-mixed epoxy asphalt. Mater. Struct. 2021, 54, 16. [Google Scholar] [CrossRef]
- Amini, N.; Latifi, H.; Hayati, P. Effects of Nano-CuO, MWCNT and SBS on the aging of asphalt binder: FTIR and XRD analyses. Jordan J. Civ. Eng. 2021, 15, 277–291. [Google Scholar]
- Ali, A.S.B.; Al Allam, A.M.; Ali, S.I.A.; Isleem, H.F.; Babalghaith, A.M.; Shaffie, E.; Khishe, M. Chemical properties of peat micro particles modified asphalt. Sci. Rep. 2024, 14, 26873. [Google Scholar] [CrossRef]
- Azarhoosh, A.; Koohmishi, M. Investigation of the rutting potential of asphalt binder and mixture modified by styrene-ethylene/propylene-styrene nanocomposite. Constr. Build. Mater. 2020, 255, 119363. [Google Scholar] [CrossRef]
- Mazalan, N.A.A.; Satar, M.K.I.M.; Mohamed, A.; Warid, M.N.M. Rheological properties of asphaltene-modified asphalt binder and mastic. Phys. Chem. Earth Parts A/B/C 2023, 131, 103422. [Google Scholar] [CrossRef]
- Jeon, I.; Lee, J.; Lee, T.; Yun, T.; Yang, S. In silico simulation study on moisture-and salt water-induced degradation of asphalt concrete mixture. Constr. Build. Mater. 2024, 417, 135229. [Google Scholar] [CrossRef]
- Albayati, A.H.; Al-ani, A.F.; Mohammed, A.M.; Al-Kheetan, M.J.; Moudhafar, M.M.; Jweihan, Y.S. Understanding the effectiveness of elastomeric and plastomeric polymers on the high-temperature performance of asphalt binders. Innov. Infrastruct. Solut. 2025, 10, 388. [Google Scholar] [CrossRef]
- Jiang, Q.; Chen, M.; Leng, B.; Wu, S.; Wu, Y.; Wang, H.; Zou, Y.; Liu, W. Investigation of adhesion and stripping properties in asphalt–aggregate systems: Impact of NaCl solubility and freeze–thaw cycles. J. Mater. Civ. Eng. 2024, 36, 04024355. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, J.; Zhao, Y.; Xie, W.; Wang, Y. Interfacial bonding property and microscopic damage mechanism of bitumen-aggregate in salt-freeze-thawing environment. Mater. Today Commun. 2023, 36, 106664. [Google Scholar] [CrossRef]
- Ogbon, W.A.; Xu, H.; Jiang, W.; Xing, C. Polymer-modified asphalt binders’ properties deterioration under the action of chloride salt. Road Mater. Pavement Des. 2023, 24, 2069–2089. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Liang, S.; Zeng, Z. Low-temperature performance grading of asphalt binder through dynamic shear rheometer (DSR) testing: A case study in China. Road Mater. Pavement Des. 2025, 1–27. [Google Scholar] [CrossRef]
- Zeng, Z.A.; Underwood, B.S.; Castorena, C. Low-temperature performance grade characterisation of asphalt binder using the dynamic shear rheometer. Int. J. Pavement Eng. 2022, 23, 811–823. [Google Scholar] [CrossRef]
- Büchner, J.; Ryś, D.; Trifunović, S.; Wistuba, M.P. Development and application of asphalt binder relaxation test in different dynamic shear rheometers. Constr. Build. Mater. 2023, 364, 129929. [Google Scholar] [CrossRef]
- Mirzaiyan, D.; Ameri, M.; Amini, A.; Sabouri, M.; Norouzi, A. Evaluation of the performance and temperature susceptibility of gilsonite-and SBS-modified asphalt binders. Constr. Build. Mater. 2019, 207, 679–692. [Google Scholar] [CrossRef]
- Arshadi, M.; Taherkhani, H. Investigating the properties of asphalt binder modified by high-and low-density polyethylene polymer and nano-silica. Road Mater. Pavement Des. 2024, 25, 838–859. [Google Scholar] [CrossRef]
- Liu, T.; Duan, W.; Zhang, J.; Li, Q.; Xu, J.; Wang, J.; Qin, Y.; Chang, R. Evaluation of the Rheological Properties of Virgin and Aged Asphalt Blends. Polymers 2022, 14, 3623. [Google Scholar] [CrossRef] [PubMed]
- Subhy, A.; Pires, G.M.; Carrión, A.J.d.B.; Presti, D.L.; Airey, G. Binder and mixture fatigue performance of plant-produced road surface course asphalt mixtures with high contents of reclaimed asphalt. Sustainability 2019, 11, 3752. [Google Scholar] [CrossRef]
- Lim, C.S.; Jang, D.S.; Yu, S.M.; Lee, J.J. Analysis of the properties of modified asphalt binder by FTIR method. Materials 2022, 15, 5743. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wen, W.; Zhou, J.; Zhou, X.; Ning, Y.; Liang, Z.; Ma, Z. Research on the interaction capability and microscopic interfacial mechanism between asphalt-binder and steel slag aggregate-filler. Coatings 2022, 12, 1871. [Google Scholar] [CrossRef]


| Item | Required Index by Standard | Measured Value | |
|---|---|---|---|
| 25 °C Penetration (0.1 mm) | 60–80 | 64 | |
| Softening point (°C) | 46.0 | 47.5 | |
| 15 °C Ductility (cm) | 100 | >100 | |
| Kinetic viscosity 60 °C (Pa·s) | 180 | 219 | |
| Flash point (°C) | 260 | 282 | |
| Wax content (%) | 2.2 | 1.4 | |
| TFOT | Loss of mass (%) | ±0.8 | 0.03 |
| Penetration ratio (%) | 65 | 67.9 | |
| Residual ductility (10 °C, cm) | 6.0 | 7.6 | |
| Indicators | Melt Index (g/10 min) | Specific Gravity (g/cm3) | Elongation (%) | Tensile Strength (MPa) |
|---|---|---|---|---|
| Measured value | 78.3 | 0.96 | 790 | 27.9 |
| Indicators | Density (kg/m3) | Flash Point (°C) | Pour Point (°C) | Kinematic Viscosity (mm2/s) | Aniline Point (°C) | Evaporation Loss (%) |
|---|---|---|---|---|---|---|
| Measured value | 896.7 | 192 | -30 | 40.12 | 93.5 | 2.2 |
| Indicators | Specific Gravity | Motion Viscosity (mm2/s at 100 °C) | Flash Point (°C) | Pour Point (°C) | Color Chromaticity | Ash (%) | Aniline Point (°C) |
|---|---|---|---|---|---|---|---|
| Measured value | 1.015 | 21 | 226 | 12 | 4.6 | 0.025 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Liu, J.; Le, Q.; Lu, Z. Rheological Deterioration of High Viscosity High Elasticity Asphalt (HVEA) Under the Coupling Effect UV Aging and Salt Freeze-Thaw (SFT) Cycles. Coatings 2025, 15, 1311. https://doi.org/10.3390/coatings15111311
Zhang B, Liu J, Le Q, Lu Z. Rheological Deterioration of High Viscosity High Elasticity Asphalt (HVEA) Under the Coupling Effect UV Aging and Salt Freeze-Thaw (SFT) Cycles. Coatings. 2025; 15(11):1311. https://doi.org/10.3390/coatings15111311
Chicago/Turabian StyleZhang, Bo, Juan Liu, Qiaoli Le, and Zhen Lu. 2025. "Rheological Deterioration of High Viscosity High Elasticity Asphalt (HVEA) Under the Coupling Effect UV Aging and Salt Freeze-Thaw (SFT) Cycles" Coatings 15, no. 11: 1311. https://doi.org/10.3390/coatings15111311
APA StyleZhang, B., Liu, J., Le, Q., & Lu, Z. (2025). Rheological Deterioration of High Viscosity High Elasticity Asphalt (HVEA) Under the Coupling Effect UV Aging and Salt Freeze-Thaw (SFT) Cycles. Coatings, 15(11), 1311. https://doi.org/10.3390/coatings15111311





