Improved Mechanical Performance and Green Corrosion Inhibition of Copper Matrix Composites Reinforced with Crassostrea Madrasensis via Powder Metallurgy and Allium sativum Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Materials
2.2. Preparation of Powder
2.3. Characterization Studies
Thermal Analysis
2.4. Preparation of Composite
2.4.1. Powder Compaction
2.4.2. Metal Sintering
2.5. Mechanical Testing
2.6. Corrosion Testing
2.6.1. Preparation of Corrosion Inhibitor
2.6.2. Corrosion Studies
3. Experimental Results and Discussion
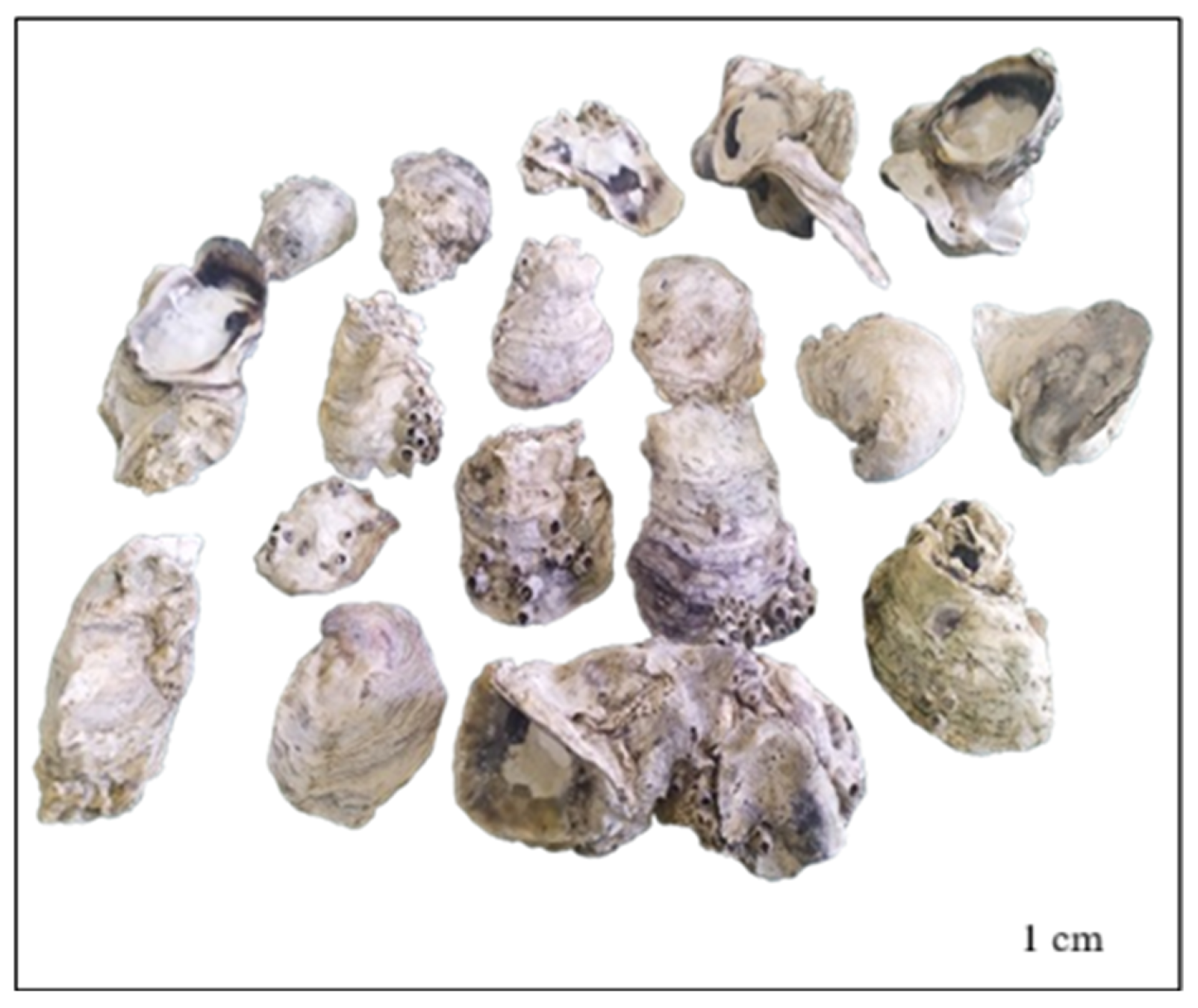
3.1. Crassostrea Madrasensis Seashell
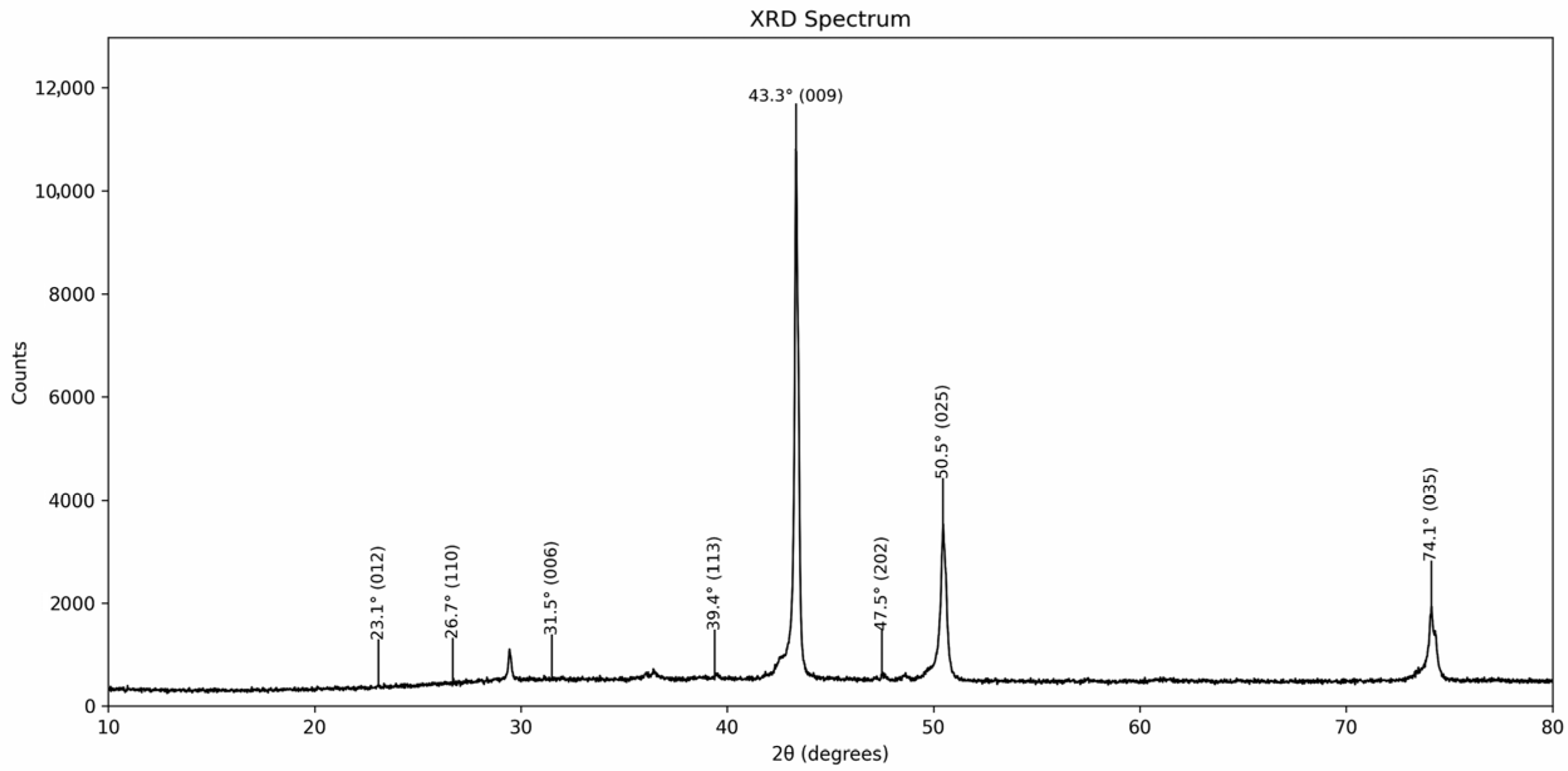
3.1.1. X-Ray Diffraction (XRD)
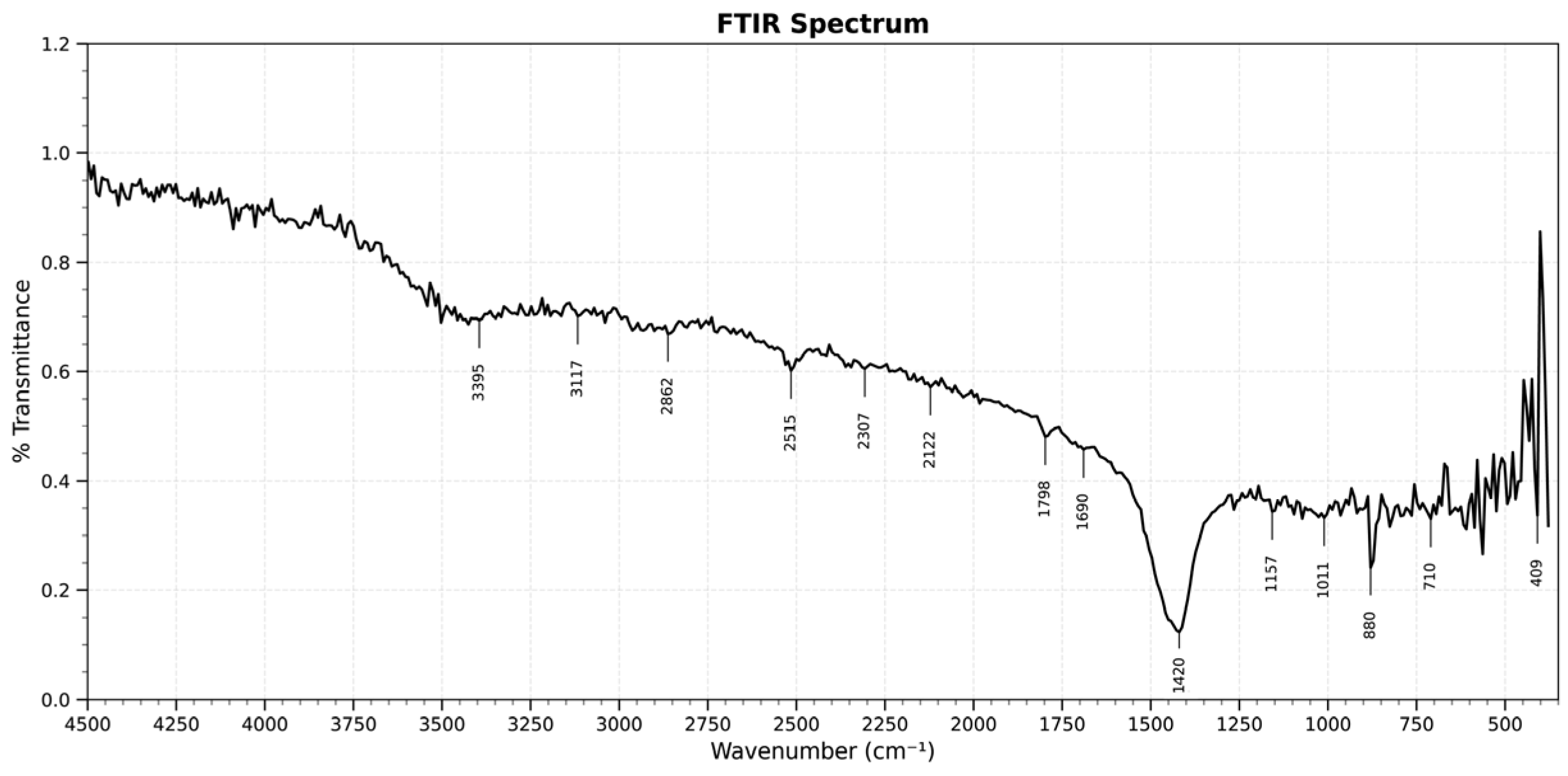
3.1.2. FTIR
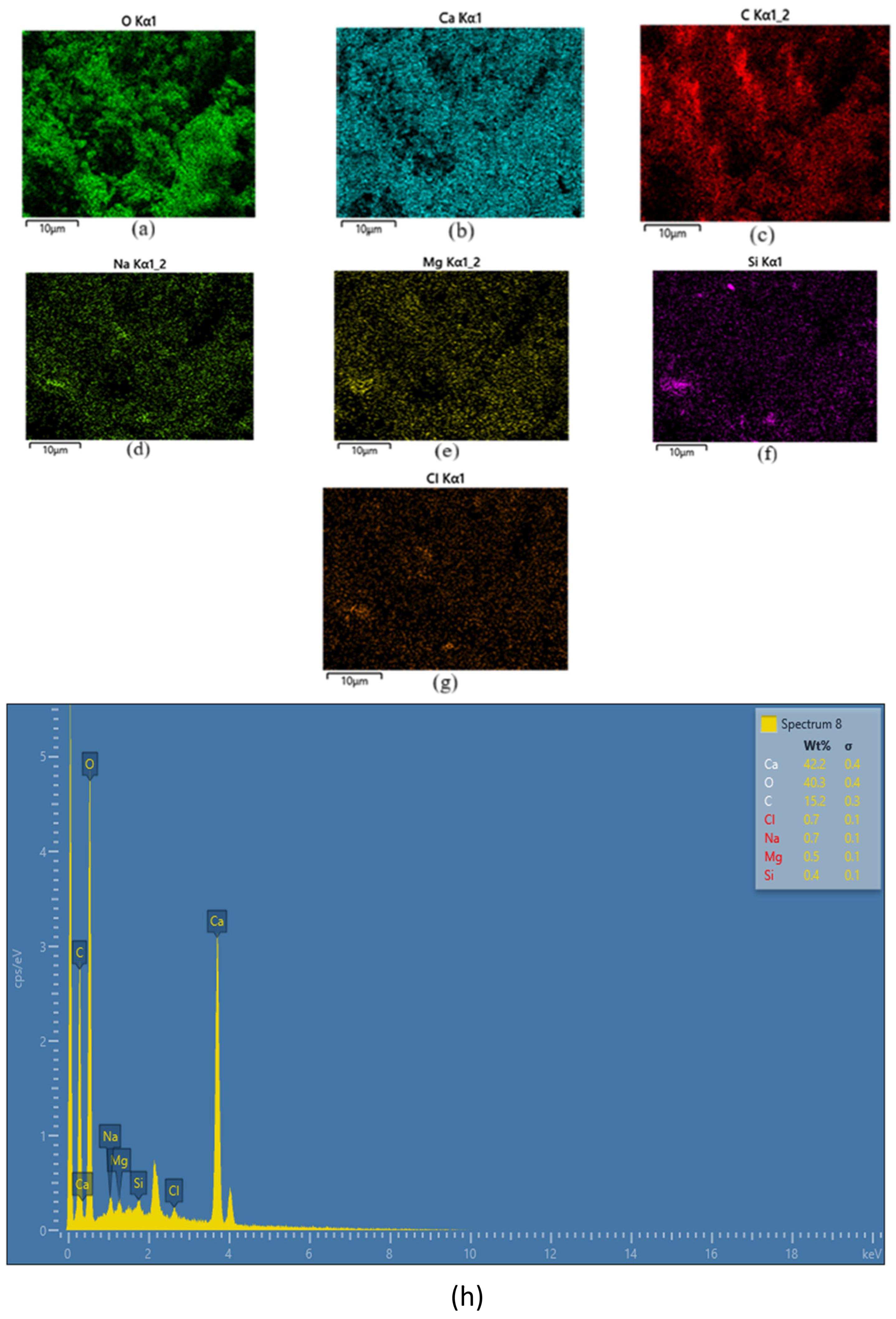
3.1.3. FESEM and EDX Analysis
3.2. Crassostrea Madrasensis Seashell Reinforced Copper Matrix Composite
3.2.1. X-Ray Diffraction (XRD)
3.2.2. FTIR
3.2.3. Thermal Analysis
3.2.4. Optical Microstructure (OM) Characterization
3.2.5. Density and Porosity Characteristics
3.2.6. Mechanical Properties
Effect of Crassostrea Madrasensis Seashell Powder Addition on HARDNESS
Effect of Crassostrea Madrasensis Seashell Powder Addition on Compressive Strength
3.2.7. Corrosion Studies: Electrochemical Corrosion Testing Techniques
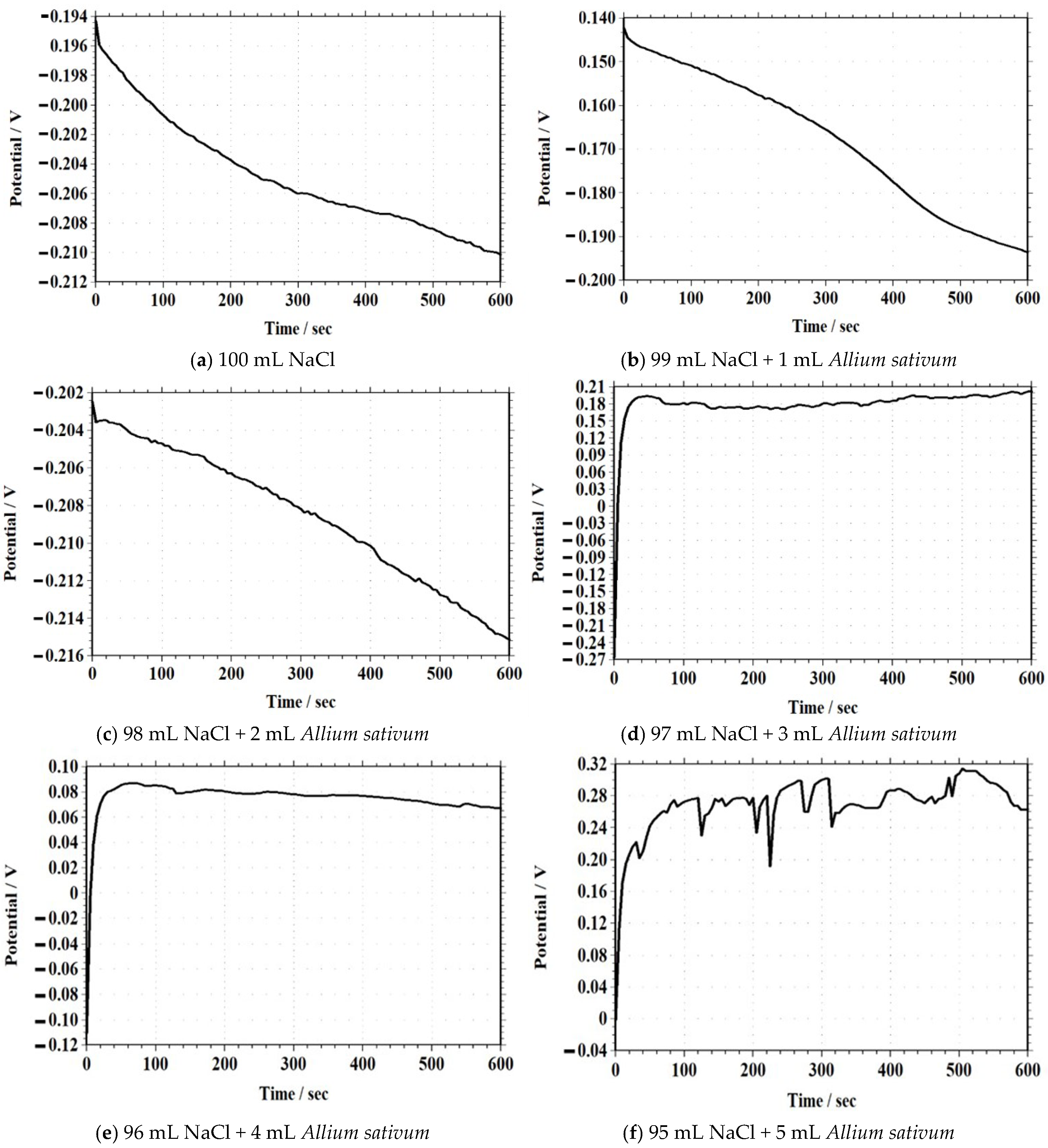
Open-Circuit Potential–Time (OCPT)
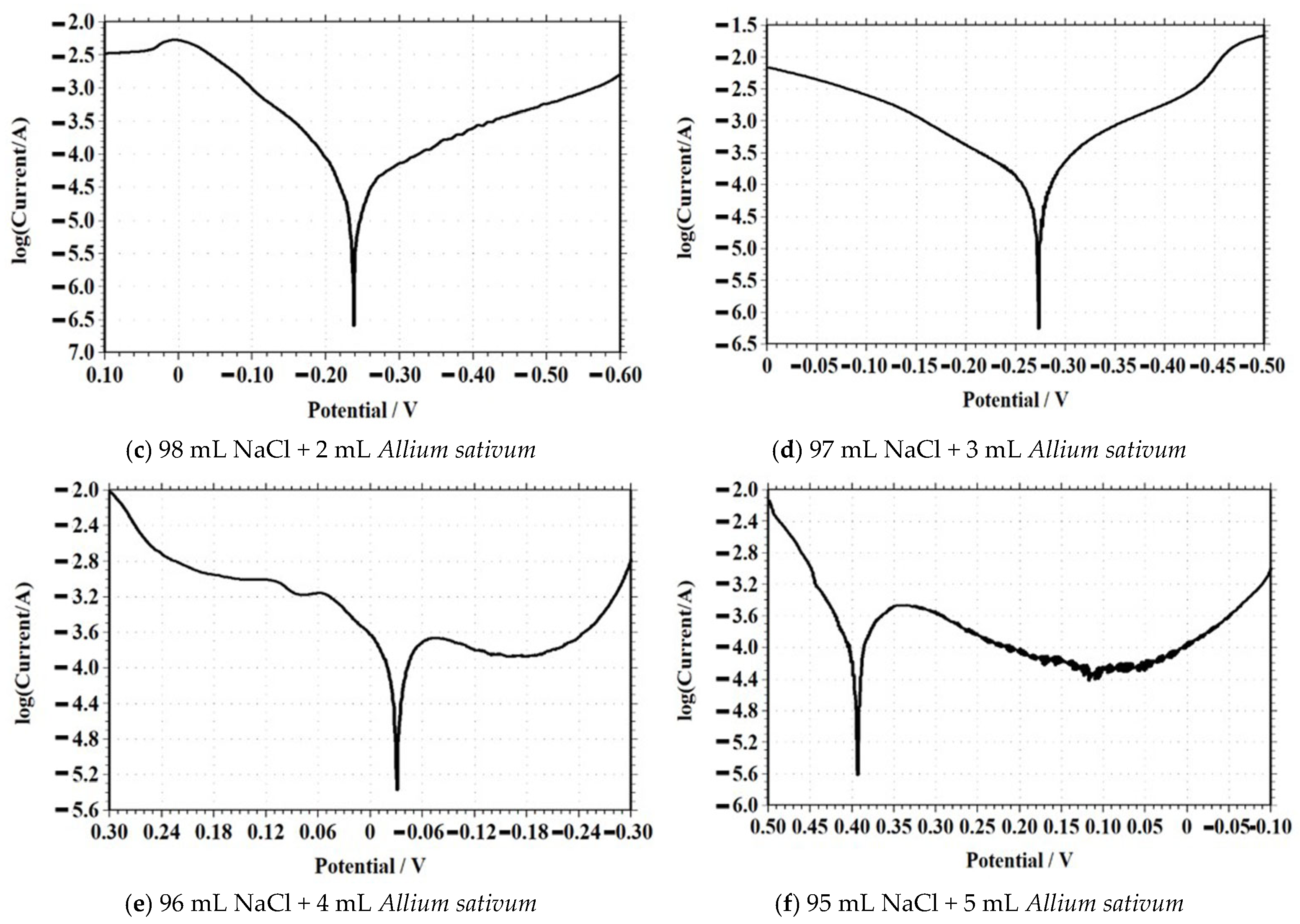
Tafel Polarization
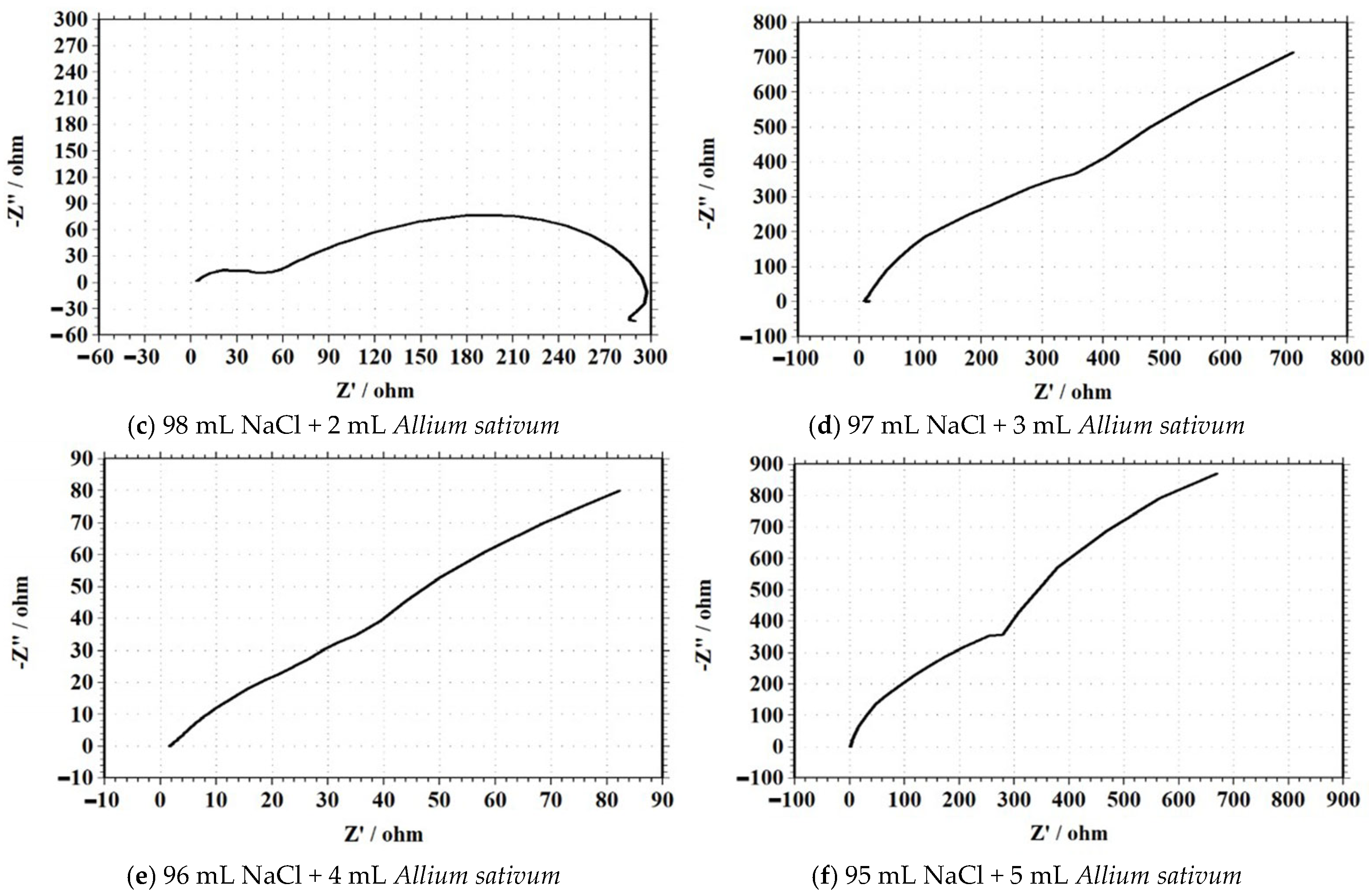
Electrochemical Impedance Spectroscopy (EIS)
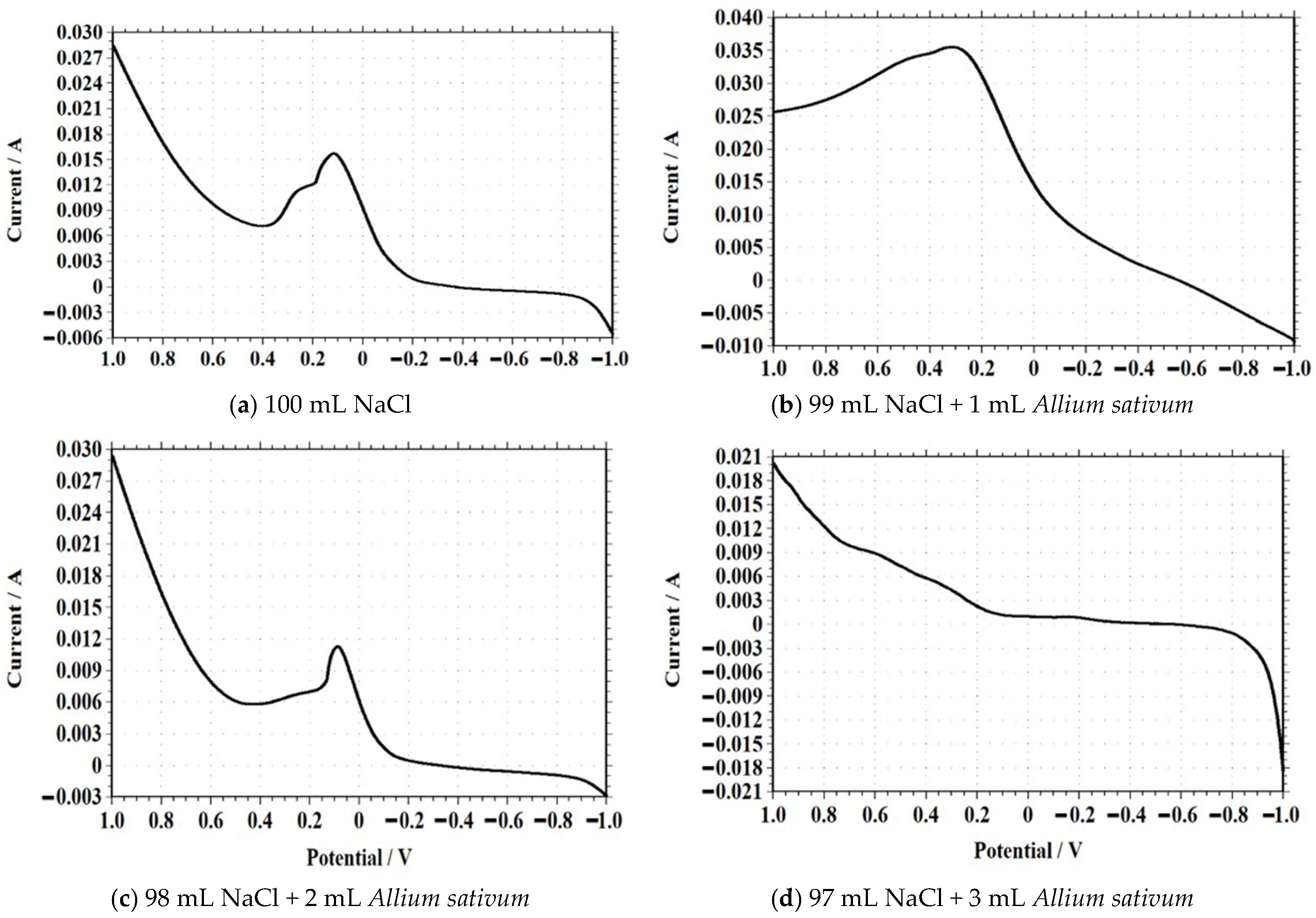
Linear Sweep Voltammetry (LSV)
3.2.8. Chronocoulometry (CC)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sridhar, M.M.J.; Ravichandran, M.; Meignanamoorthy, M. Effect of Silicon Carbide on Microstructural, Mechanical and Corrosion Behavior of Electrolytic Copper Matrix Composite Produced by the Powder Metallurgy Route. Silicon 2022, 14, 5877–5886. [Google Scholar] [CrossRef]
- Simić, M.; Ružić, J.; Božić, D.; Stašić, J. The Effect of Ball-Powder Ratio on The Mechanical and Structural Properties of CuZrB Composite Materials Fabricated by Powder Metallurgy. Adv. Funct. Mater. 2023, 1, 19–24. [Google Scholar] [CrossRef]
- Kalidas, N.; Gopal, P.M.; Kavimani, V. Development and Characterization of Silicon Nitride and Synthesized Graphene Oxide Reinforced Cu/GO/xSi3N4 Composites. Silicon 2025, 17, 141–153. [Google Scholar] [CrossRef]
- Ankur; Bharti, A.; Prasad, D.; Kumar, N.; Kuldeep, K.S. A Re-investigation: Effect of various parameters on mechanical properties of copper matrix composite fabricated by powder metallurgy. Mater. Today Proc. 2021, 45, 4595–4600. [Google Scholar] [CrossRef]
- Taha, M.A.; El-zaidia, M.M.; Zaki, M.Z.; Abomostafa, H.M. Influence of Nano-Hybrid Reinforcements on the Improvement Strength, Thermal Expansion and Wear Properties of Cu–SiC–Fly Ash Nanocomposites Prepared by Powder Metallurgy. ECS J. Solid State Sci. Technol. 2023, 12, 33011. [Google Scholar] [CrossRef]
- Vijay Ponraj, N.; Azhagurajan, A.; Vettivel, S.C.; Shajan, X.S.; Nabhiraj, P.Y.; Sivapragash, M. Graphene nanosheet as reinforcement agent in copper matrix composite by using powder metallurgy method. Surf. Interfaces 2017, 6, 190–196. [Google Scholar] [CrossRef]
- Yan, Y.M.; Kou, S.; Yang, H.; Shu, S.; Qiu, F.; Jiang, Q.; Zhang, L. Ceramic particles reinforced copper matrix composites manufactured by advanced powder metallurgy: Preparation, performance, and mechanisms. Int. J. Extrem. Manuf. 2023, 5, 32006. [Google Scholar] [CrossRef]
- Kumar, N.; Bharti, A.; Dixit, M.; Nigam, A. Effect of Powder Metallurgy Process and its Parameters on the Mechanical and Electrical Properties of Copper-Based Materials: Literature Review. Compos. Mater. 2023, 59, 401–410. [Google Scholar] [CrossRef]
- Dixit, M.; Srivastava, R.K. Effect of compaction pressure on microstructure, density and hardness of Copper prepared by Powder Metallurgy route. IOP Conf. Ser. Mater. Sci. Eng. 2018, 377, 12209. [Google Scholar] [CrossRef]
- Venkatesh, V.S.S.; Rao, R.N.; Patnaik, L. Effect of Spark Plasma Sintering Temperature on Phase Evaluation and Mechanical Behaviour of cu- 4 Wt% SiC Composite. Silicon 2023, 15, 6439–6449. [Google Scholar] [CrossRef]
- Silva, T.H.; Mesquita-Guimarães, J.; Henriques, B.; Silva, F.S.; Fredel, M.C. The Potential Use of Oyster Shell Waste in New Value-Added By-Product. Resources 2019, 8, 13. [Google Scholar] [CrossRef]
- Magnabosco, G.; Giuri, D.; Di Bisceglie, A.P.; Scarpino, F.; Fermani, S.; Tomasini, C.; Falini, G. New Material Perspective for Waste Seashells by Covalent Functionalization. ACS Sustain. Chem. Eng. 2021, 9, 6203–6208. [Google Scholar] [CrossRef]
- Zhu, Y.; Chen, D.; Yu, X.; Liu, R.; Liao, Y. Properties of Cementitious Materials Utilizing Seashells as Aggregate or Cement: Prospects and Challenges. Materials 2024, 17, 1222. [Google Scholar] [CrossRef] [PubMed]
- Bamigboye, G.O.; Nworgu, A.T.; Odetoyan, A.O.; Kareem, M.; Enabulele, D.O.; Bassey, D.E. Sustainable use of seashells as binder in concrete production: Prospect and challenges. J. Build. Eng. 2021, 34, 101864. [Google Scholar] [CrossRef]
- Sankar, M.; Devaneyan, S.P.; Pushpanathan, D.P.; Myszka, D. Microstructural Characterization and Mechanical Behavior of Copper Matrix Composites Reinforced by B4C and Sea Shell Powder. J. Cast. Mater. Eng. 2018, 2, 24. [Google Scholar] [CrossRef]
- Srirama, D.; Jayanthi, P.N.V. Experimental investigation of lime produced from oyster shell waste as a potential soil stabilizing material: A sustainable approach for lime replacement. Prog. Eng. Sci. 2025, 2, 100111. [Google Scholar] [CrossRef]
- Pruncu, C.I.; Vladescu, A.; Hynes, N.R.J.; Sankaranarayanan, R. Surface Investigation of Physella Acuta Snail Shell Particle Reinforced Aluminium Matrix Composites. Coatings 2022, 12, 794. [Google Scholar] [CrossRef]
- Lu, J.; Cong, X.; Li, Y.; Hao, Y.; Wang, C. Scalable recycling of oyster shells into high purity calcite powders by the mechanochemical and hydrothermal treatments. J. Clean. Prod. 2018, 172, 1978–1985. [Google Scholar] [CrossRef]
- Gbadeyan, O.J.; Adali, S.; Bright, G.; Sithole, B.; Onwubu, S. Optimization of Milling Procedures for Synthesizing Nano-CaCO3 from Achatina fulica Shell through Mechanochemical Techniques. J. Nanomater. 2020, 2020, 4370172. [Google Scholar] [CrossRef]
- Chen, J.; Bao, S.; Li, J.; Yu, B.; Li, K.; Yang, X.; Zuo, K.; Gao, T.; Xie, G. Powder metallurgy process enables production of high-strength conductive Cu-based composites reinforced by Cu50Zr43Al7 metallic glass. Intermetallics 2023, 163, 108062. [Google Scholar] [CrossRef]
- Sundar, G.; Hynes, N.R.J. Corrosion issues in metal matrix composites & Bi-metals. AIP Conf. Proc. 2019, 2142, 70007. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, K.; Niu, J.; Sun, M.; Dai, G.; Sun, Z.; Chang, H. Effect of reinforcement content on microstructures and mechanical properties of graphene nanoflakes-reinforced titanium alloy matrix composites. J. Mater. Res. Technol. 2021, 15, 6871–6882. [Google Scholar] [CrossRef]
- Vidyuk, T.M.; Ukhina, A.V.; Gavrilov, A.I.; Shikalov, V.S.; Anisimov, A.G.; Lomovsky, O.I.; Dudina, D.V. Synthesis of Tungsten Carbides in a Copper Matrix by Spark Plasma Sintering: Microstructure Formation Mechanisms and Properties of the Consolidated Materials. Materials 2023, 16, 5385. [Google Scholar] [CrossRef] [PubMed]
- Usca, Ü.A.; Şap, S.; Uzun, M.; Giasin, K.; Pimenov, D.Y. Evaluation of Mechanical and Tribological Aspect of Self-Lubricating Cu-6Gr Composites Reinforced with SiC–WC Hybrid Particles. Nanomaterials 2022, 12, 2154. [Google Scholar] [CrossRef] [PubMed]
- Samad, A.; Ansari, S.; Arif, S.; Muaz, M.; Khan, A.U.; Muhammad, Z.; Bashiri, A.H.; Zakri, W. Tribological performance of Cu-silica sand hybrid composite reinforced with graphene nanoparticles by electric resistance sintering: Experimental studies and modeling. Eng. Sci. Technol. Int. J. 2024, 54, 101713. [Google Scholar] [CrossRef]
- Hynes, N.R.J.; Vignesh, N.J.; Barile, C.; Velu, P.S.; Baskaran, T.; Jappes, J.T.W.; Al-Khashman, O.A.; Brykov, M.; Ene, A. Green Corrosion Inhibition on Carbon-Fibre-Reinforced Aluminium Laminate in NaCl Using Aerva Lanata Flower Extract. Polymers 2022, 14, 1700. [Google Scholar] [CrossRef]
- Hynes, N.R.J.; Selvaraj, R.M.; Mohamed, T.; Mukesh, A.M.; Olfa, K.; Nikolova, M.P. Aerva lanata flowers extract as green corrosion inhibitor of low-carbon steel in HCl solution: An in vitro study. Chem. Pap. 2021, 75, 1165–1174. [Google Scholar] [CrossRef]
- Darweesh, M.A.; Emam, S.M.; Wahba, A.M.; Ayad, M.I.; El-Nahass, M.N.; Abd-Elhamied, A.S.; Hammad, W.A. Onion Peel Extract/Copper Oxide Nanoparticles as Corrosion Inhibitors for Carbon Steel in Hydrochloric Acid: Extraction, Characterization, Electrochemical Study, and Theoretical Explorations. Results Chem. 2024, 9, 101626. [Google Scholar] [CrossRef]
- Devikala, S.; Kamaraj, P.; Arthanareeswari, M.; Patel, M.B. Green corrosion inhibition of mild steel by aqueous Allium sativum extract in 3.5% NaCl. Mater. Today Proc. 2019, 14, 580–589. [Google Scholar] [CrossRef]
- Hajsafari, N.; Razaghi, Z.; Tabaian, S.H. Electrochemical study and molecular dynamics (MD) simulation of aluminum in the presence of garlic extract as a green inhibitor. J. Mol. Liq. 2021, 336, 116386. [Google Scholar] [CrossRef]
- Bhavyasree, P.G.; Xavier, T.S. Green synthesis of Copper Oxide/Carbon nanocomposites using the leaf extract of Adhatoda vasica Nees, their characterization and antimicrobial activity. Heliyon 2020, 6, e03323. [Google Scholar] [CrossRef] [PubMed]
- Guma, T.N.; Aremo, J.O. A Review of Up-To-Date Research Knowledge on the Corrosion Inhibiting Capability of Garlic (Allicin sativum) for Steel Materials. Int. J. Adv. Eng. Manag. 2025, 7, 104–117. [Google Scholar] [CrossRef]
- Parthipan, P.; Elumalai, P.; Narenkumar, J.; Machuca, L.L.; Murugan, K.; Karthikeyan, O.P.; Rajasekar, A. Allium sativum (garlic extract) as a green corrosion inhibitor with biocidal properties for the control of MIC in carbon steel and stainless steel in oilfield environments. Int. Biodeterior. Biodegrad. 2018, 132, 66–73. [Google Scholar] [CrossRef]
- Cáceres, L.; Frez, Y.; Galleguillos, F.; Soliz, A.; Gómez-Silva, B.; Borquez, J. Aqueous Dried Extract of Skytanthus acutus Meyen as Corrosion Inhibitor of Carbon Steel in Neutral Chloride Solutions. Metals 2021, 11, 1992. [Google Scholar] [CrossRef]
- Pérez, F.A.N. Electrochemical Analysis of Corrosion Resistance of Manganese-Coated Annealed Steel: Chronoamperometric and Voltammetric Study. AppliedChem 2024, 4, 367–383. [Google Scholar] [CrossRef]
- Rossi, R.; Pant, D.; Logan, B.E. Chronoamperometry and linear sweep voltammetry reveals the adverse impact of high carbonate buffer concentrations on anode performance in microbial fuel cells. J. Power Sources 2020, 476, 228715. [Google Scholar] [CrossRef]
- Nan, T.; Yang, J.; Chen, B. Electrochemical mechanism of tin membrane electrodeposition under ultrasonic waves. Ultrason. Sonochem. 2018, 42, 731–737. [Google Scholar] [CrossRef]
- Wang, F.; Cui, Y.O.; Sang, J.; Zhang, H.; Zhu, H. Cross-linked of poly (biphenyl pyridine) and poly(styrene-b-(ethylene-co-butylene)-b-styrene) grafted with double cations for anion exchange membrane. Electrochim. Acta 2022, 405, 139770. [Google Scholar] [CrossRef]
- Salcı, A.; Yüksel, H.; Solmaz, R. Experimental studies on the corrosion inhibition performance of 2-(2-aminophenyl) benzimidazole for mild steel protection in HCl solution. J. Taiwan Inst. Chem. Eng. 2022, 134, 104349. [Google Scholar] [CrossRef]
- Annaraj, J.P.; Bose, N.; Hynes, N.R.J. A review on mechanical and tribological properties of sintered copper matrix composites. AIP Conf. Proc. 2019, 2142, 70027. [Google Scholar] [CrossRef]











| Element | O | Ca | C | Cl | Na | Mg | Si |
|---|---|---|---|---|---|---|---|
| wt.% | 42.0 | 38.1 | 17.4 | 0.9 | 0.7 | 0.6 | 0.3 |
| Element | wt.% | ±(σ) | Atomic % | Observations |
|---|---|---|---|---|
| Ca | 42.2 | 0.4 | 22.8 | CaCO3 primary component |
| O | 40.3 | 0.4 | 55.9 | Oxygen bonded to carbonate group |
| C | 15.2 | 0.3 | 20.6 | Possibly from carbonate coating |
| Cl | 0.7 | 0.1 | 0.2 | Trace impurity (residual salt) |
| Na | 0.7 | 0.1 | 0.3 | Trace impurity (biogenic origin) |
| Mg | 0.5 | 0.1 | 0.2 | Minor substitution in Ca lattice |
| Si | 0.4 | 0.1 | 0.1 | Potential contamination |
| Total | 100.0 | — | 100.0 | — |
| Composition | Hardness (HV) | Average ± SD (HV) | Improvement (%) |
|---|---|---|---|
| 100% Pure Cu | 57, 54, 51 | 54.0 ± 3.0 | – |
| 88% Cu + 12% Seashell | 53, 59, 56 | 56.0 ± 3.0 | 3.7 |
| Sample | Garlic Extract (mL) | Initial OCPT (V) | Final OCPT (V) | OCPT Shift (V) |
|---|---|---|---|---|
| 100 mL NaCl (Control) | 0 | −0.194 | −0.212 | −0.018 |
| 99 mL NaCl + 1 mL Garlic | 1 | −0.196 | −0.210 | −0.014 |
| 98 mL NaCl + 2 mL Garlic | 2 | −0.198 | −0.206 | −0.008 |
| 97 mL NaCl + 3 mL Garlic | 3 | −0.199 | −0.205 | −0.006 |
| 96 mL NaCl + 4 mL Garlic | 4 | −0.200 | −0.202 | −0.002 |
| 95 mL NaCl + 5 mL Garlic | 5 | −0.202 | −0.200 | +0.002 |
| Volume of Allium sativum Extract (mL) | Rate of Corrosion (mpy) | Inhibition Efficiency (%) |
|---|---|---|
| 100 mL NaCl (Control) | 0.0339 | 0 |
| 99 mL NaCl + 1 mL Garlic | 0.0285 | 16 |
| 98 mL NaCl + 2 mL Garlic | 0.0202 | 40 |
| 97 mL NaCl + 3 mL Garlic | 0.0145 | 57 |
| 96 mL NaCl + 4 mL Garlic | 0.0101 | 70 |
| 95 mL NaCl + 5 mL Garlic | 0.0028 | 92 |
| S.No | Allium sativum (mL) | NaCl (mL) | Total Volume (mL) | Inhibition Zone (mm) | % Activity vs. Max (15 mm) |
|---|---|---|---|---|---|
| 1. | 0 | 100 | 100 | 0 | 0% |
| 2. | 1 | 99 | 100 | 9 | 46.7% |
| 3. | 2 | 98 | 100 | 11 | 60% |
| 4. | 3 | 97 | 100 | 13 | 73.3% |
| 5. | 4 | 96 | 100 | 14 | 86.7% |
| 6. | 5 | 95 | 100 | 15 | 100% |
| Solution Composition (NaCl + Allium sativum) | Peak Current (A) | Peak Potential (V) | Observation |
|---|---|---|---|
| 100 mL NaCl (Blank) | 0.028 | ~0.20 | Baseline oxidation peaks |
| 99 mL NaCl + 1 mL Extract | 0.036 | ~0.25 | Mild increase in current |
| 98 mL NaCl + 2 mL Extract | 0.030 | ~0.15 | Suppressed current, early peak |
| 97 mL NaCl + 3 mL Extract | 0.021 | ~0.10 | Lower current, stable profile |
| 96 mL NaCl + 4 mL Extract | 0.16 | ~0.05 | Steady inhibition pattern |
| 95 mL NaCl + 5 mL Extract | 0.16 | ~0.05 | Most effective inhibition observed |
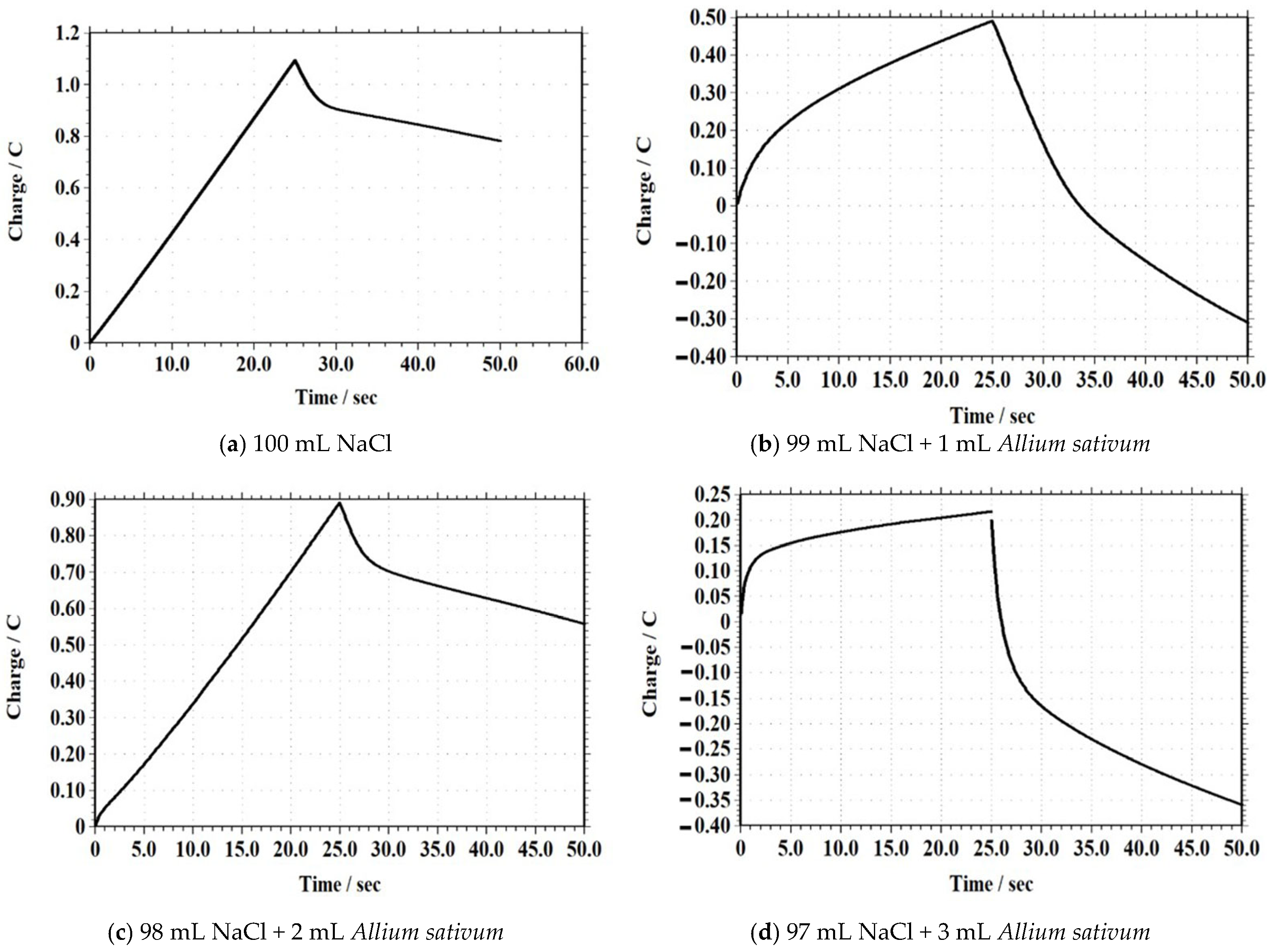
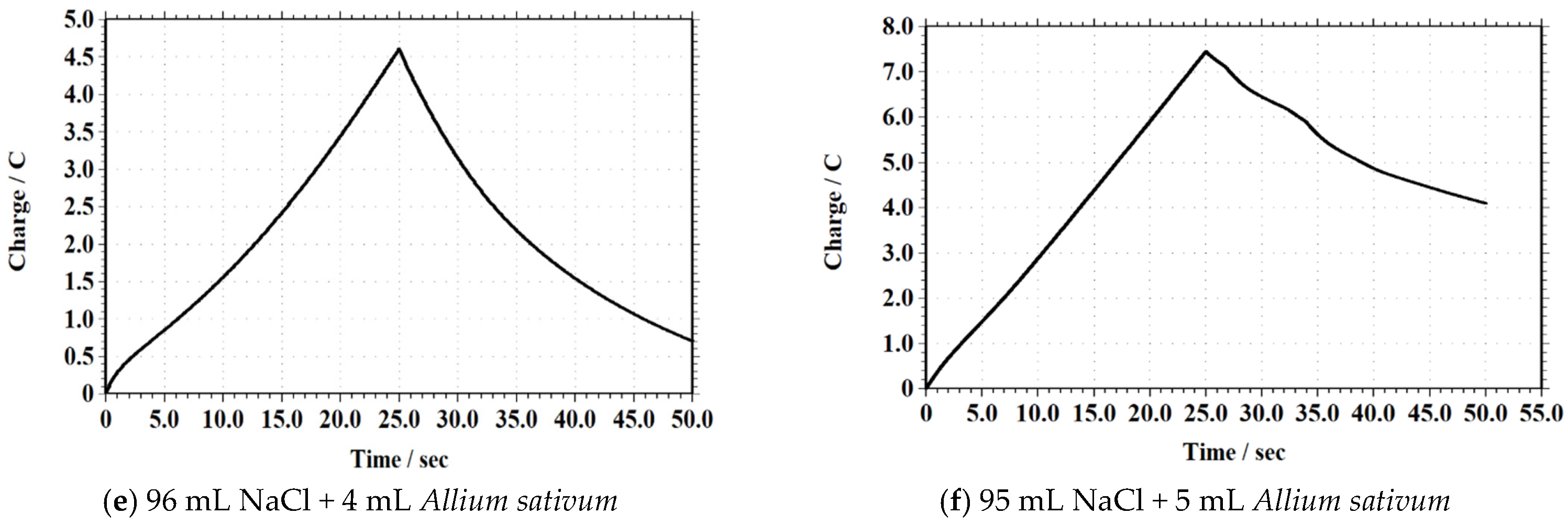
| Sample Composition | Maximum Charge (C) |
|---|---|
| 100 mL NaCl (Blank) | 1.1 |
| 99 mL NaCl + 1 mL Allium sativum | 0.48 |
| 98 mL NaCl + 2 mL Allium sativum | 0.88 |
| 97 mL NaCl + 3 mL Allium sativum | 0.22 |
| 96 mL NaCl + 4 mL Allium sativum | 4.7 |
| 95 mL NaCl + 5 mL Allium sativum | 7.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pitchiah, I.; Navasingh, R.J.H.; Devaraj, M.G.; Nikolova, M.P. Improved Mechanical Performance and Green Corrosion Inhibition of Copper Matrix Composites Reinforced with Crassostrea Madrasensis via Powder Metallurgy and Allium sativum Extract. Coatings 2025, 15, 1303. https://doi.org/10.3390/coatings15111303
Pitchiah I, Navasingh RJH, Devaraj MG, Nikolova MP. Improved Mechanical Performance and Green Corrosion Inhibition of Copper Matrix Composites Reinforced with Crassostrea Madrasensis via Powder Metallurgy and Allium sativum Extract. Coatings. 2025; 15(11):1303. https://doi.org/10.3390/coatings15111303
Chicago/Turabian StylePitchiah, Issac, Rajesh Jesudoss Hynes Navasingh, Merlin Gethsy Devaraj, and Maria P. Nikolova. 2025. "Improved Mechanical Performance and Green Corrosion Inhibition of Copper Matrix Composites Reinforced with Crassostrea Madrasensis via Powder Metallurgy and Allium sativum Extract" Coatings 15, no. 11: 1303. https://doi.org/10.3390/coatings15111303
APA StylePitchiah, I., Navasingh, R. J. H., Devaraj, M. G., & Nikolova, M. P. (2025). Improved Mechanical Performance and Green Corrosion Inhibition of Copper Matrix Composites Reinforced with Crassostrea Madrasensis via Powder Metallurgy and Allium sativum Extract. Coatings, 15(11), 1303. https://doi.org/10.3390/coatings15111303







