The Role of Nitrogen Doping in Enhancing the Thermal Stability and Wear Resistance of AlSi Coatings at Elevated Temperatures
Abstract
1. Introduction
2. Experimental Details
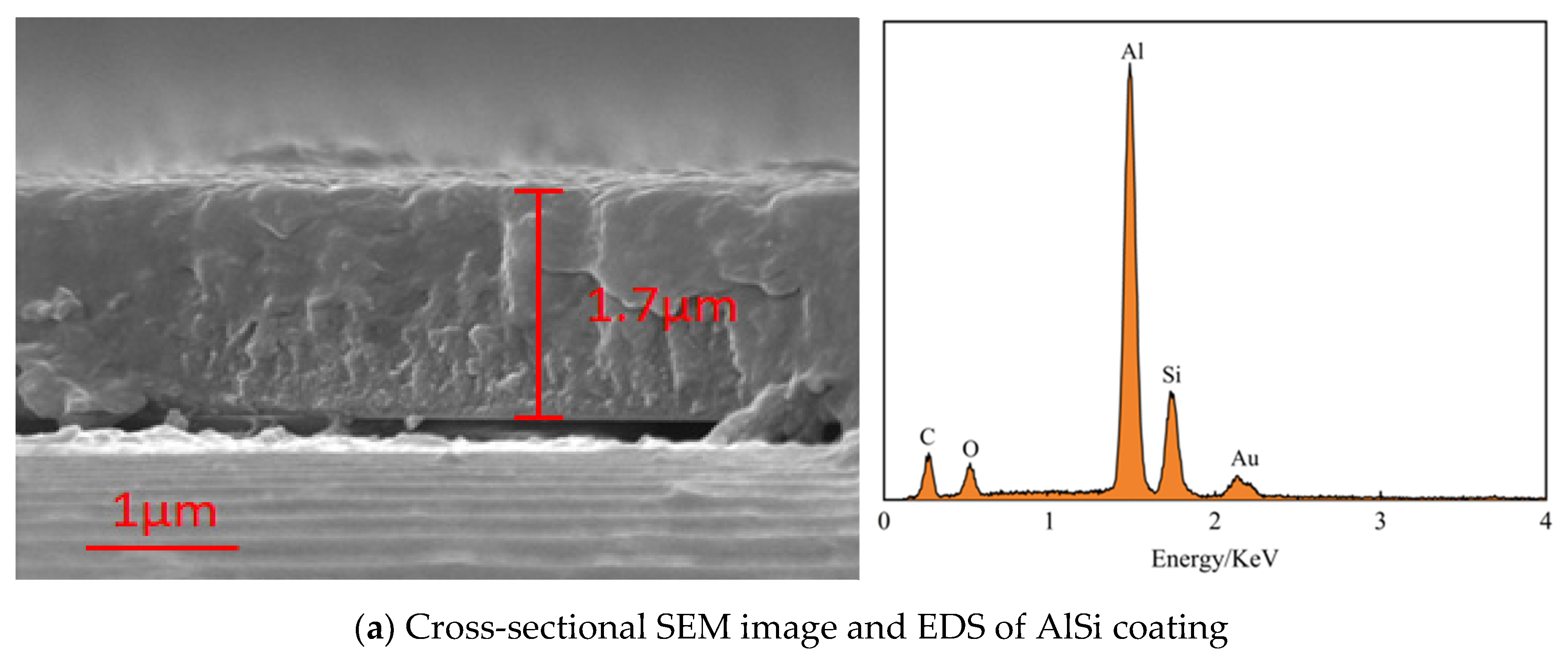
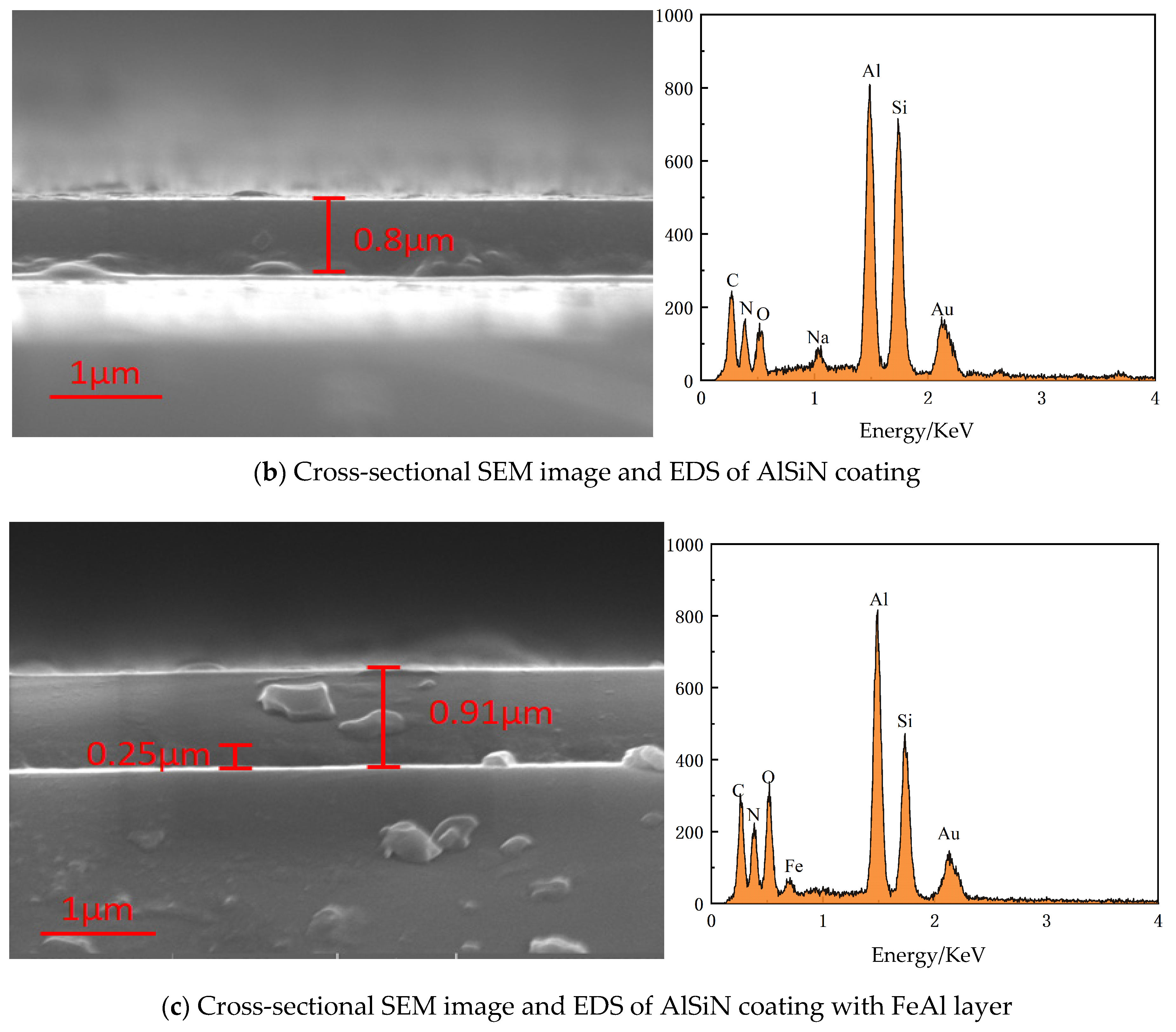
2.1. Deposition of AlSi Coatings and N Doping
2.2. Microstructure
2.3. Friction and Wear Behaviors
3. Results and Discussion
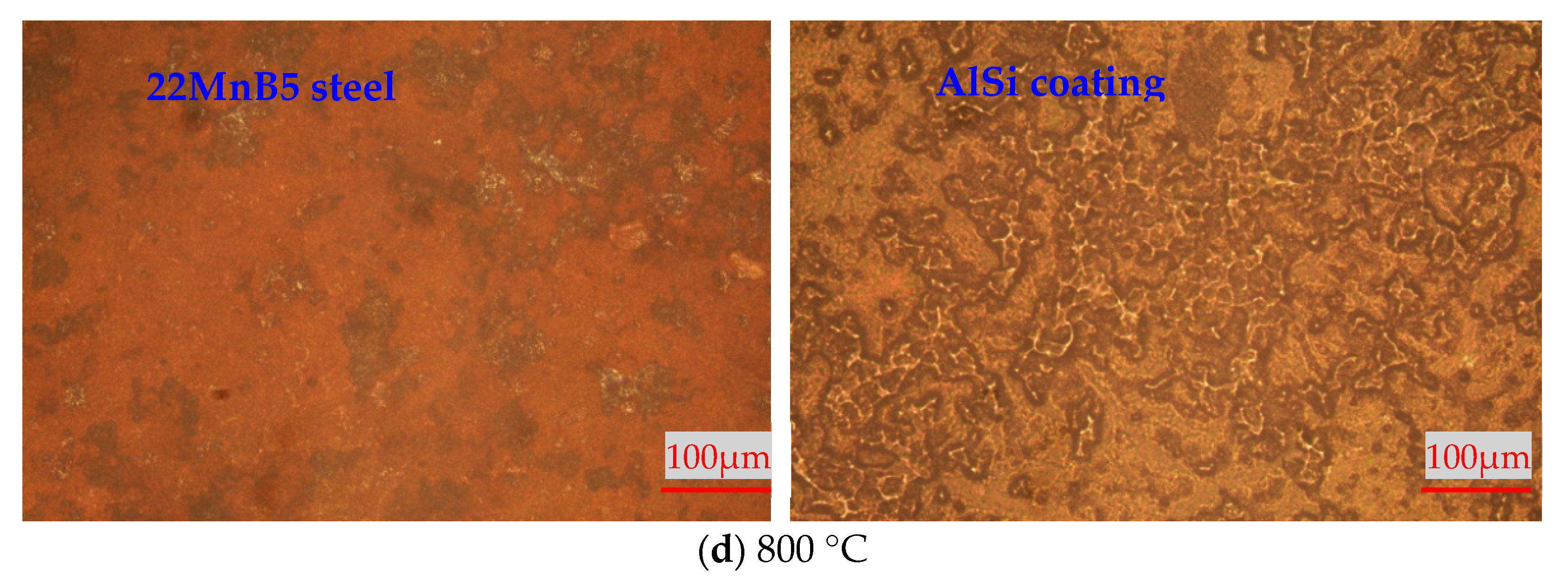
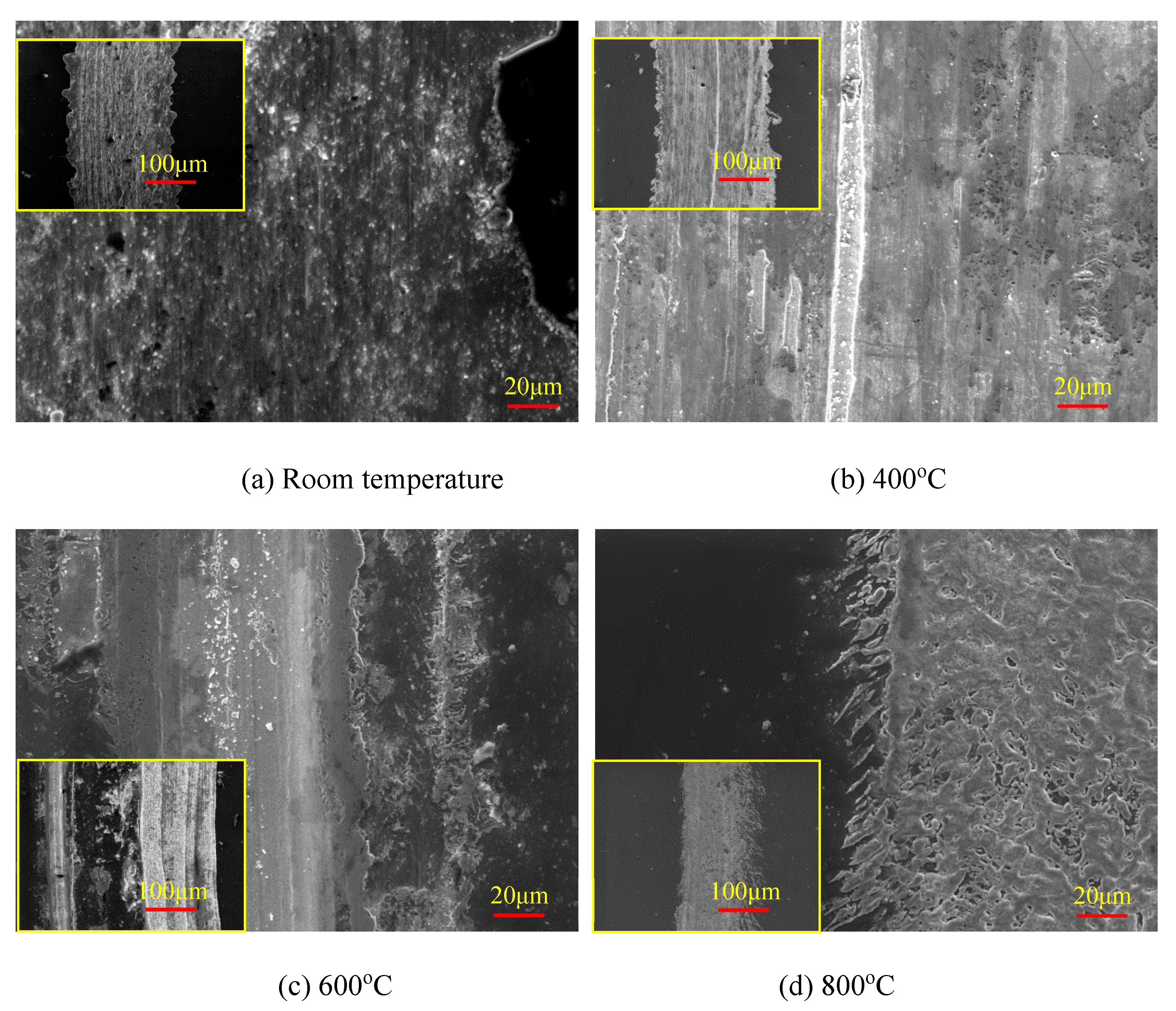
3.1. High-Temperature Thermal Stability of 22MnB5 Steel and AlSi Coatings
- (a)
- High-temperature thermal stability of AlSi coatings
- (b)
- High-temperature thermal stability of AlSiN coatings
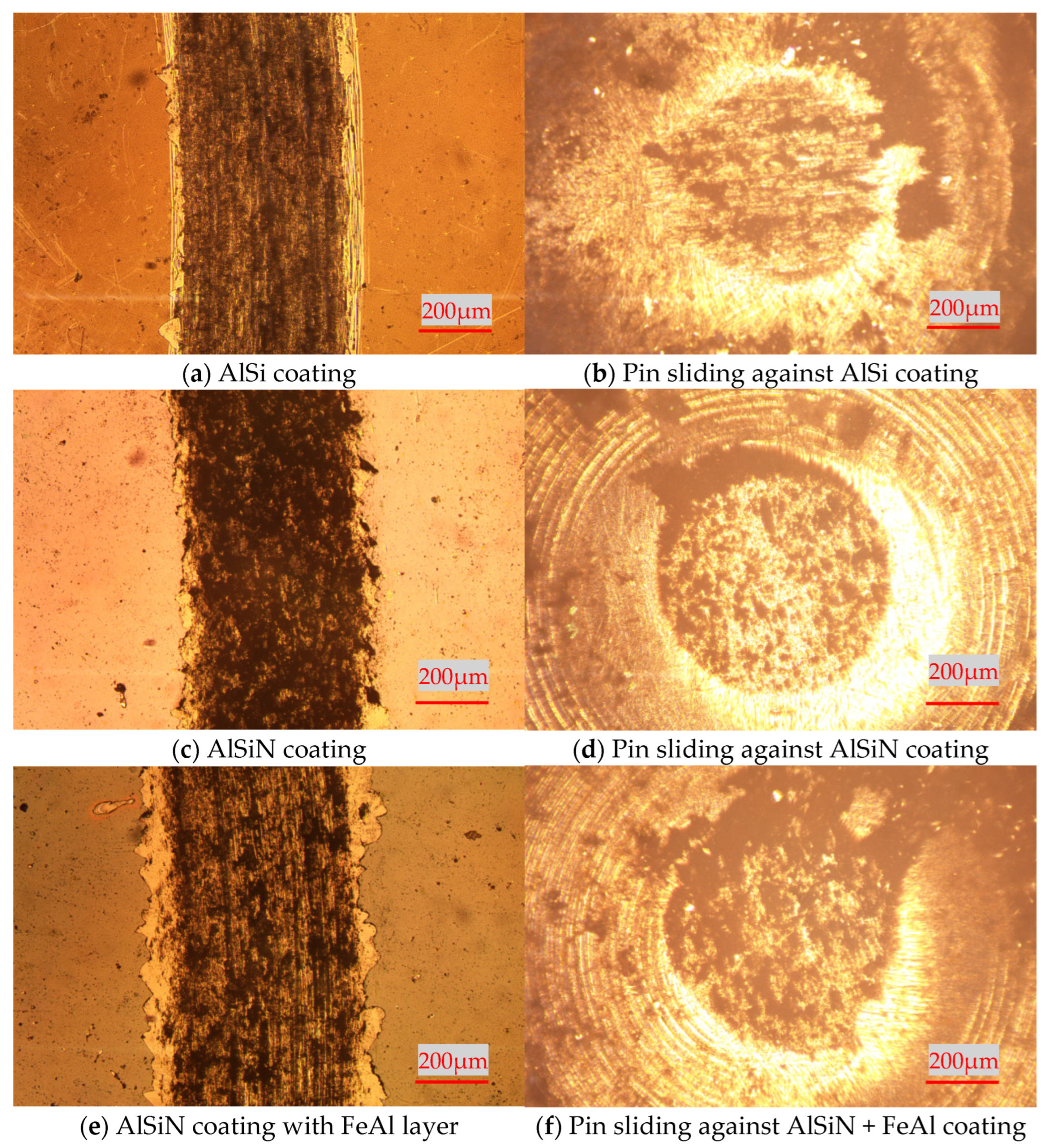
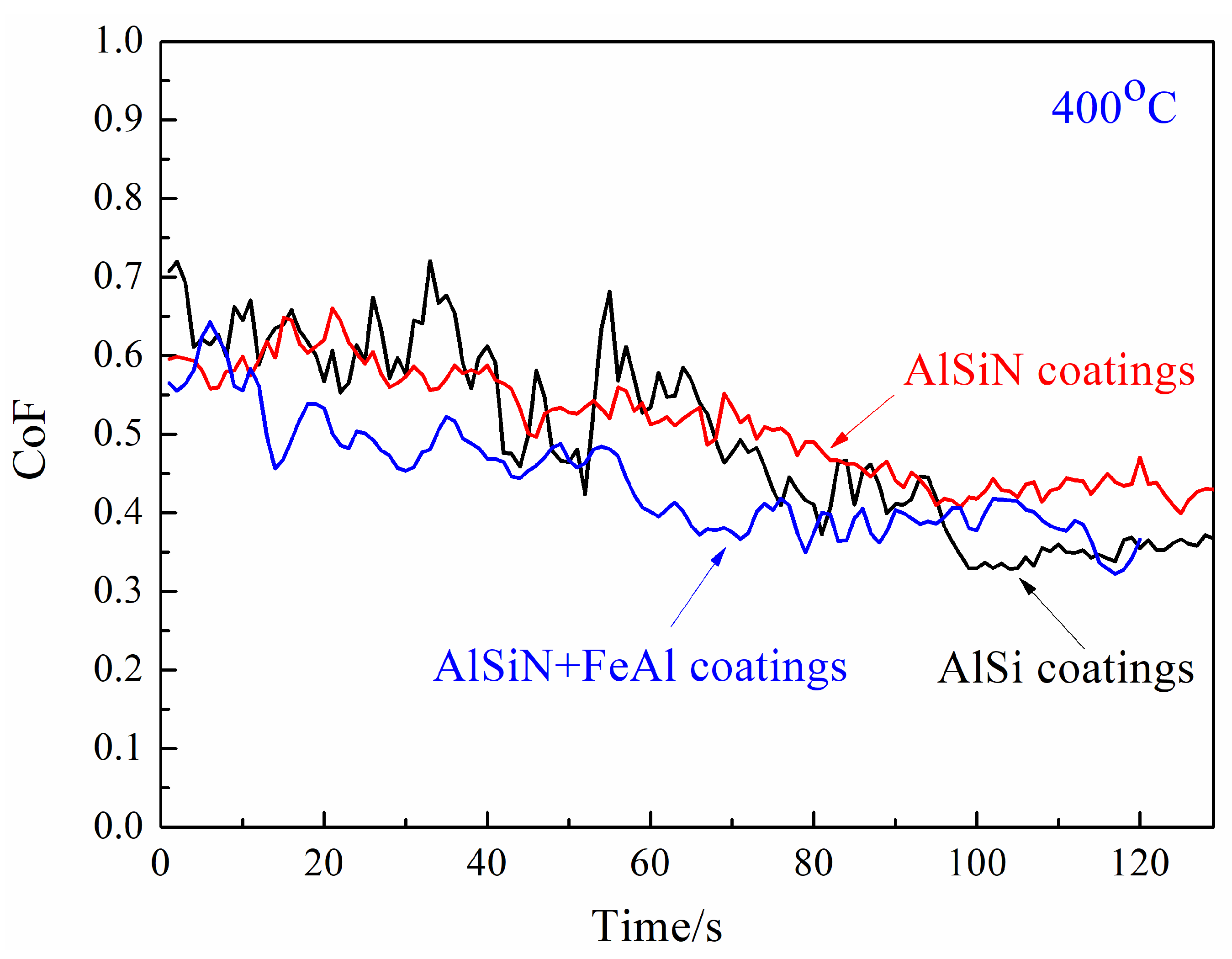
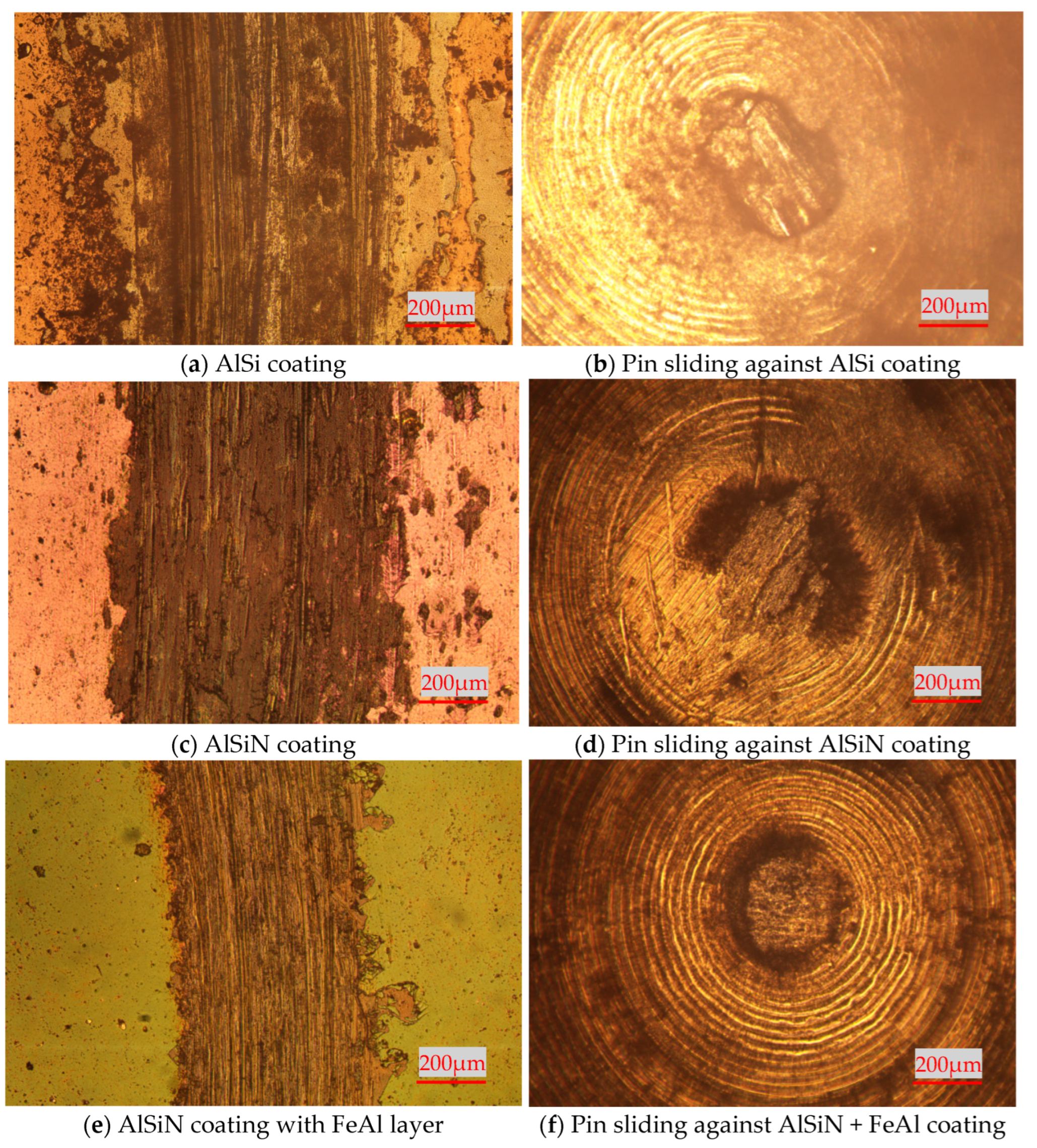
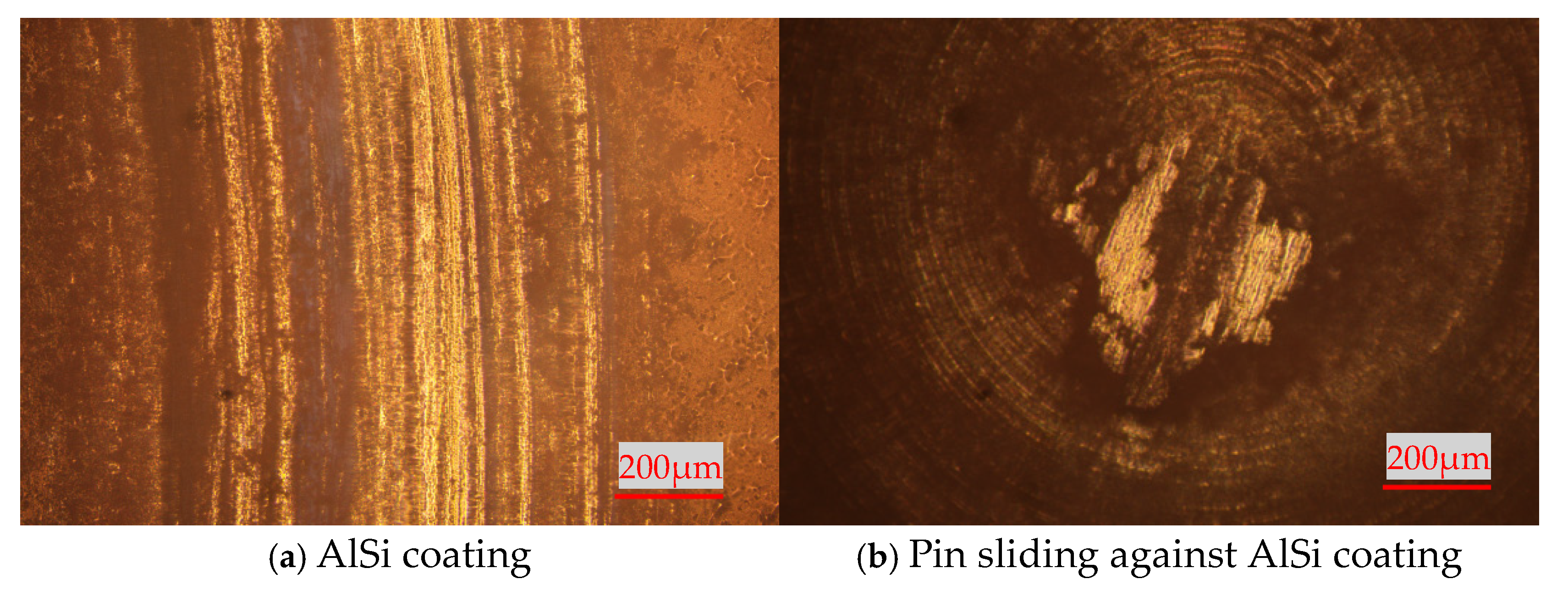
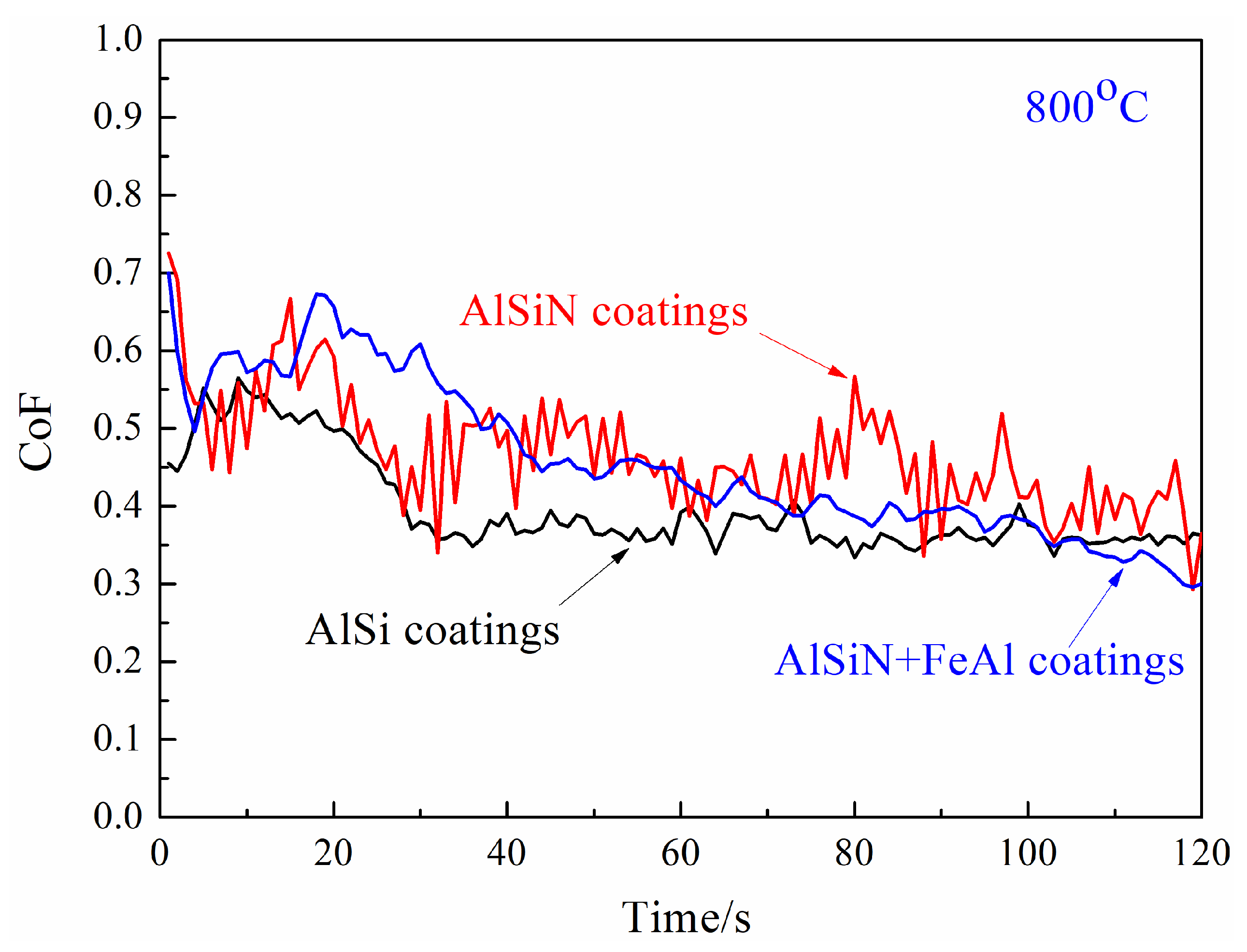
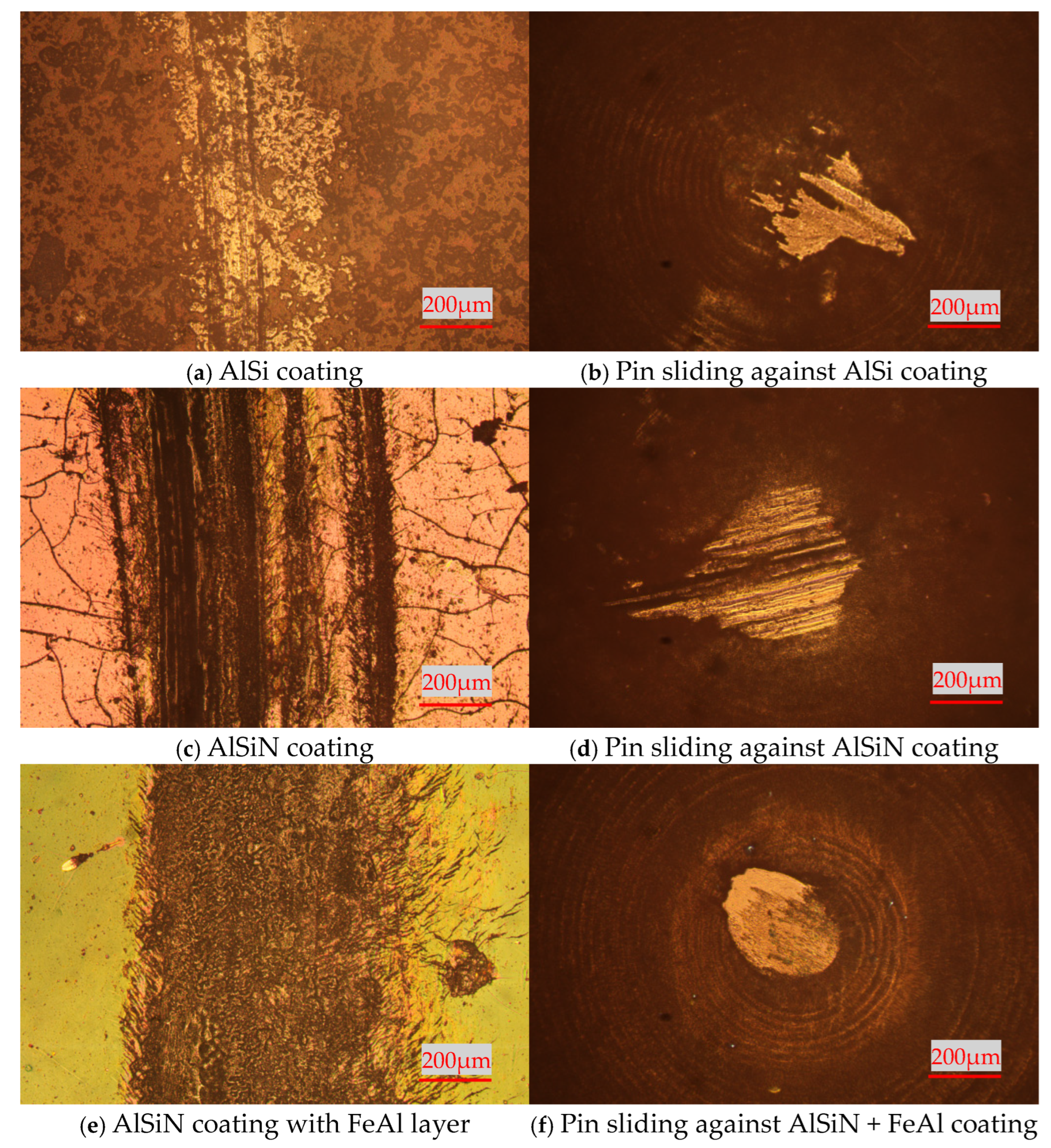
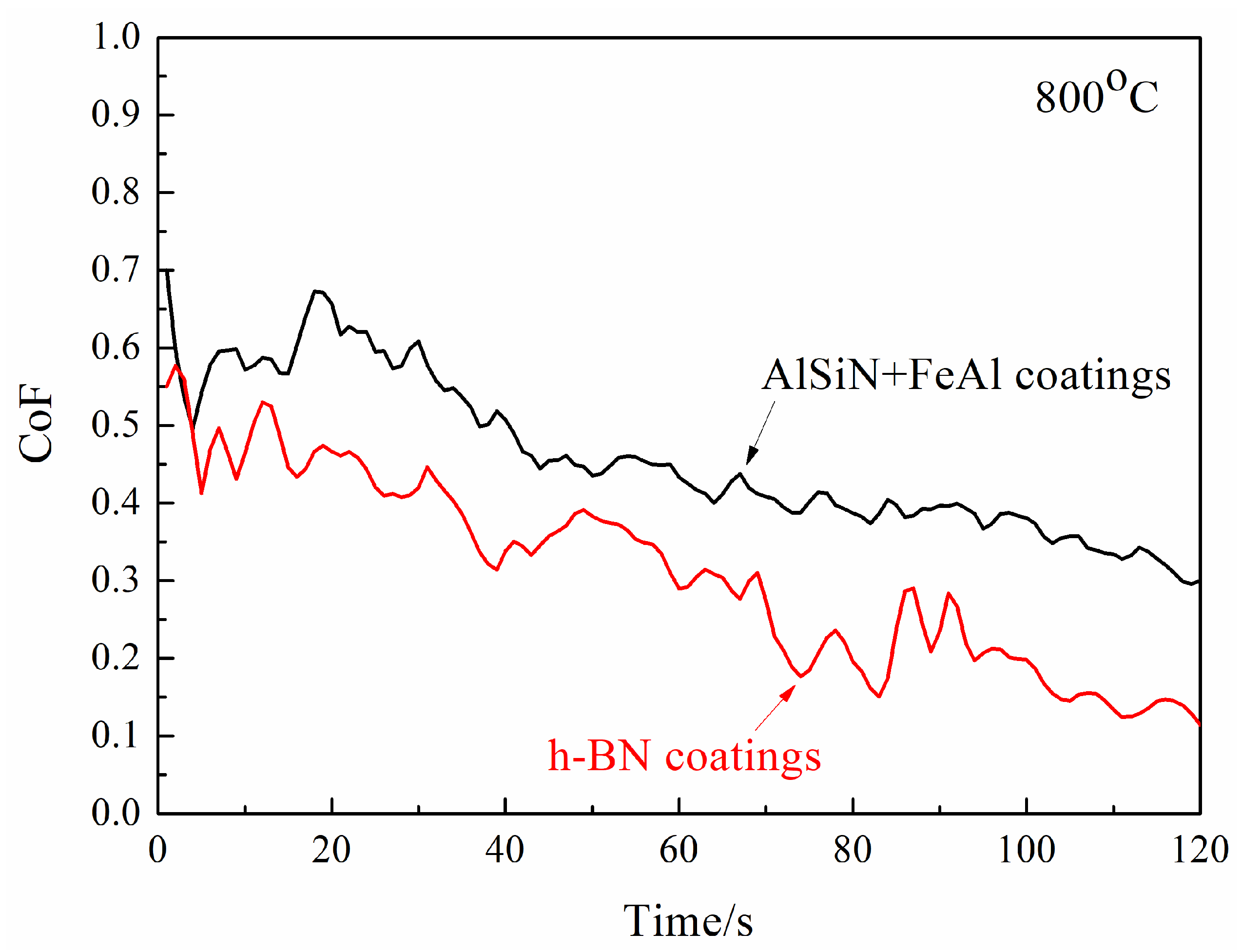
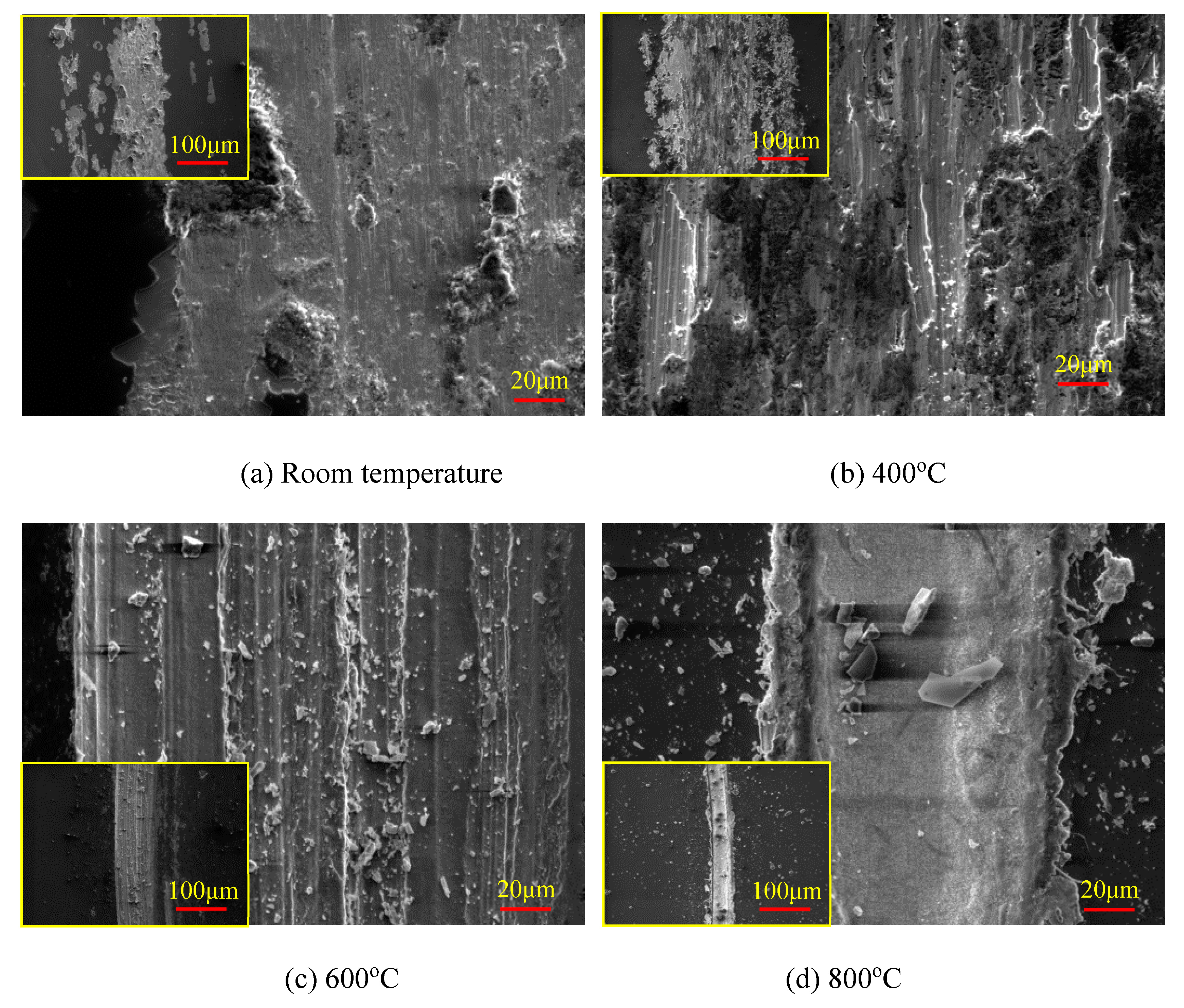
3.2. High-Temperature Tribological Properties of Coatings
- (a)
- High-temperature tribological properties of AlSi coatings and AlSiN coatings
- (1)
- Room temperature
- (2)
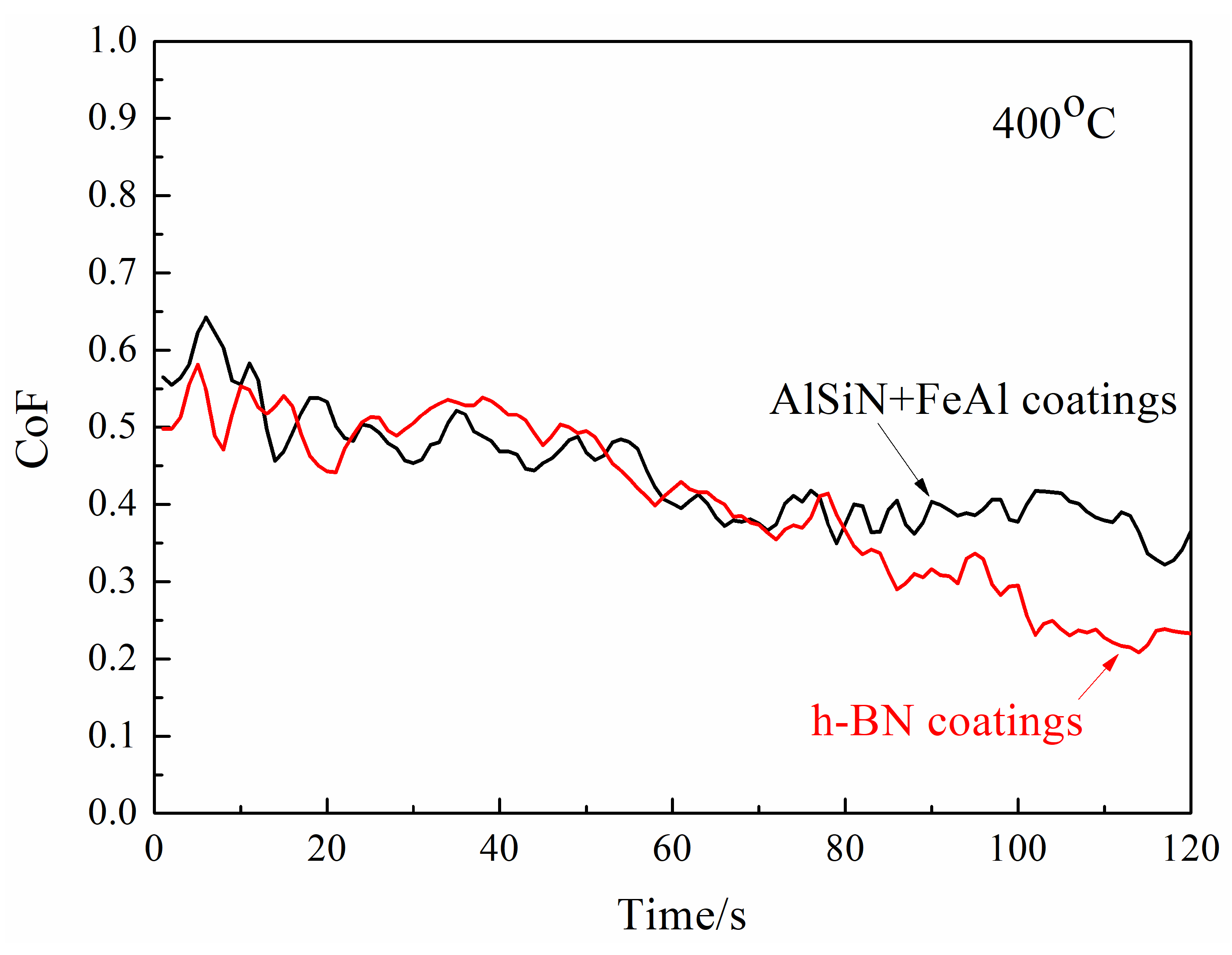
- Temperature of 400 °C
- (3)
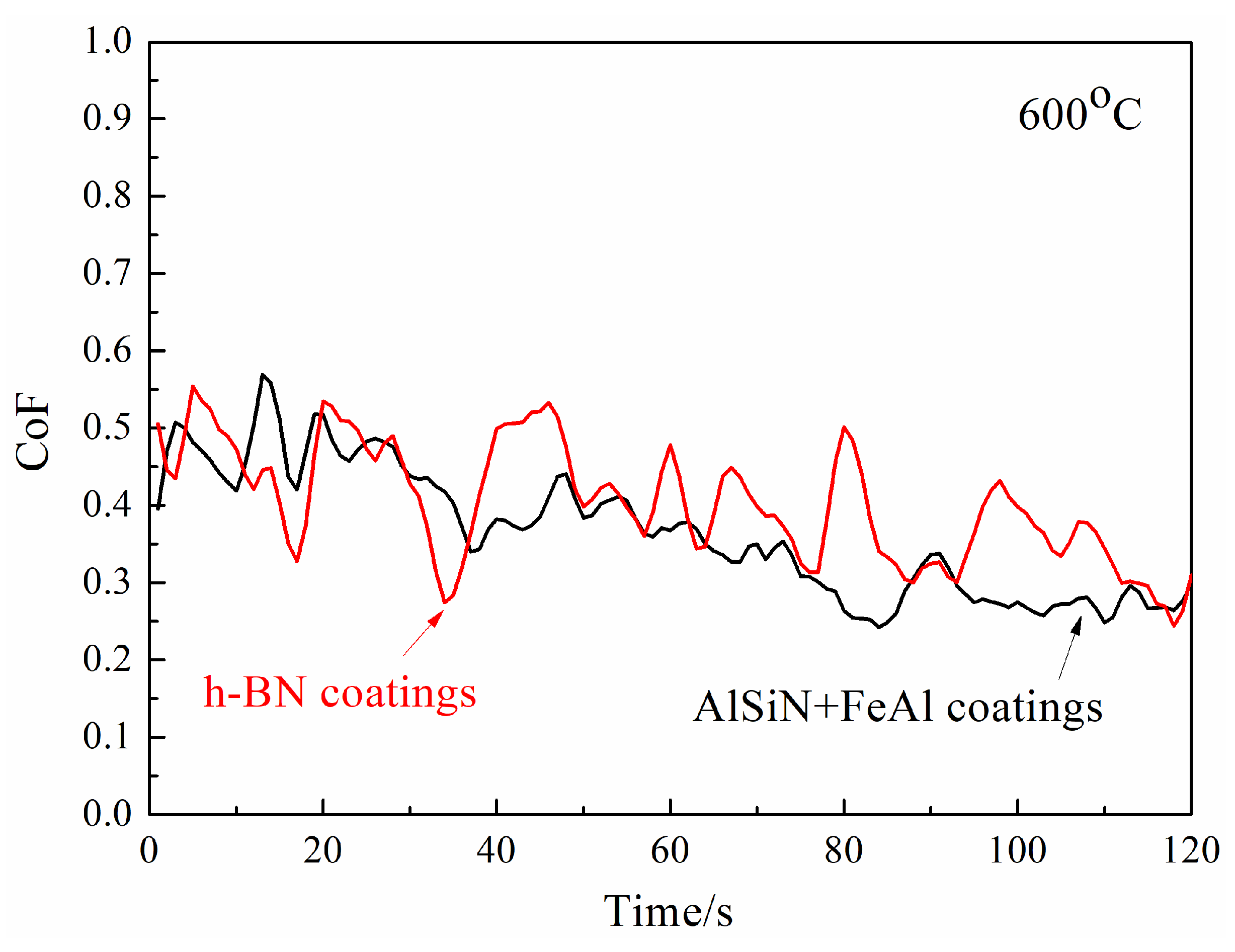
- Temperature of 600 °C
- (4)
- Temperature of 800 °C
- (b)
- High-temperature tribological properties of h-BN coatings
- (1)
- Room temperature
- (2)
- Temperature of 400 °C
- (3)
- Temperature of 600 °C
- (4)
- Temperature of 800 °C
4. Conclusions
- (1)
- The CoF of the AlSi coatings was relatively high compared with that of uncoated 22MnB5 steel. The wear mechanisms of the coatings varied with temperature, exhibiting abrasive wear at RT, adhesive wear at 400 °C, and adhesive wear at 600 °C and 800 °C.
- (2)
- The role of nitrogen doping in AlSi coatings is helpful, possibly, to enhance the hardness and wear resistance of the AlSi coatings and optimize the thermal conductivity performance of the coatings. The AlSiN coatings with an FeAl transition layer exhibited the best thermal stability behaviors. The FeAl layer significantly enhanced the performance of AlSiN-coated steel.
- (3)
- The friction pair of N-doped AlSi coatings and h-BN coatings demonstrated a lower CoF compared with the friction pair of AlSiN coatings and steel, showing a pronounced anti-friction effect.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.S.; Razmpoosh, M.H.; Biro, E.; Zhou, Y. A review on the laser welding of coated 22MnB5 press-hardened steel and its impact on the production of tailor-welded blanks. Sci. Technol. Weld. Join. 2020, 25, 447–467. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, C. Effect of silicon on the formation of intermetallic phases in aluminide coating on mild steel. Intermetallics 2011, 19, 1455–1460. [Google Scholar] [CrossRef]
- Basak, S.; Das, H.; Pal, T.; Shome, M. Characterization of intermetallics in aluminum to zinc coated interstitial free steel joining by pulsed MIG brazing for automotive application. Mater. Charact. 2016, 112, 229–237. [Google Scholar] [CrossRef]
- Kab, M.; Mendil, S.; Taibi, K. Evolution of the microstructure of intermetallic compounds formed on mild steel during hot dipping in molten aluminum alloy baths. Metallogr. Microstruct. Anal. 2020, 9, 476–483. [Google Scholar] [CrossRef]
- Zeng, Q.; Sun, S.; Jia, Q. Influence of Ag Doping on wide-emperature tribological properties of γ-Fe2O3@SiO2 nanocomposite coatings on steel. Metals 2024, 14, 996. [Google Scholar] [CrossRef]
- Hardell, J.; Prakash, B. High-temperature friction and wear behaviour of different tool steels during sliding against Al–Si-coated high-strength steel. Tribol. Int. 2008, 41, 663–671. [Google Scholar] [CrossRef]
- Pelcastre, L.; Hardell, J.; Rolland, A.; Prakash, B. Influence of microstructural evolution of Al-Si coated UHSS on its tribological behaviour against tool steel at elevated temperatures. J. Mater. Process. Technol. 2016, 228, 117–124. [Google Scholar] [CrossRef]
- Ding, J.C.; Wang, Q.M.; Liu, Z.R.; Jeong, S.; Zhang, T.F.; Kim, K.H. Influence of bias voltage on the microstructure, mechanical and corrosion properties of AlSiN films deposited by HiPIMS technique. J. Alloys Compd. 2019, 772, 112–121. [Google Scholar] [CrossRef]
- Petkov, N.; Bakalova, T.; Cholakova, T.; Bahchedzhiev, H.; Louda, P.; Ryšánek, P.; Kormunda, M.; Čapková, P.; Kejzlar, P. Study of surface morphology, structure, mechanical and tribological properties of an AlSiN coating obtained by the cathodic arc deposition method. Superlattices Microstruct. 2017, 109, 402–413. [Google Scholar] [CrossRef]
- Zeng, Q.; Qi, W. High temperature superlubricity behaviors achieved by AlSiN coatings against WS2 coatings at 600 °C. Ceram. Int. 2024, 50, 3787–3796. [Google Scholar] [CrossRef]
- Anwar, S.; Anwar, S.; Priyadarshini, B. Microscopic evidences of nanocomposite structure in hard and optically transparent ternary AlSiN coating. Thin Solid Film. 2023, 777, 139891. [Google Scholar] [CrossRef]
- Anwar, S.; Sonali, S.; Anwar, S. Microscopic studies on transparent AlSiN ternary hard coating. Ceram. Int. 2023, 49, 16868–16878. [Google Scholar] [CrossRef]
- Zeng, Q. High-temperature superlubricity performance of h-BN coating on the textured inconel X750 alloy. Lubricants 2023, 11, 258. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, Q.; Zhang, B.; Yan, C.; He, W.; Lin, N. Improving the lubrication of WSNb nanocomposite coatings by the in-situ oxygen shielding effect at elevated temperatures: A combined study with density functional theory simulations. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2023, 237, 1181–1196. [Google Scholar] [CrossRef]
- Zeng, Q. High temperature low friction behavior of h-BN coatings against ZrO2. Coatings 2022, 12, 1772. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, Q.; Zhang, B.; Yan, C.; He, W. Elevated-temperature super-lubrication performance analysis of dispersion-strengthened WSN coatings: Experimental research and first-principles calculation. Surf. Coat. Technol. 2021, 406, 126651. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, Q.; Wang, Y.; Yan, C.; He, W. Nano-crystallization driven high temperature self-lubricating properties of magnetron sputtered WS2 coatings. Tribol. Lett. 2020, 68, 50. [Google Scholar] [CrossRef]
- Zhu, J.; Zeng, Q.; Yan, C.; He, W. WS2 nanopowders as high-temperature lubricants: An experimental and theoretical study. ACS Appl. Nano Mater. 2019, 2, 5604–5613. [Google Scholar] [CrossRef]
- Zeng, Q. Thermally induced superlow friction of DLC films in ambient air. High Temp. Mater. Process. 2018, 37, 725–731. [Google Scholar] [CrossRef]
- Zeng, Q.; Erdemir, A.; Erylimaz, O. Ultralow friction of ZrO2 ball sliding against DLC films under various environments. Appl. Sci. 2017, 7, 938. [Google Scholar] [CrossRef]
- Zeng, Q.; Eryilmaz, O.; Erdemir, A. Superlubricity of the DLC films-related friction system at elevated temperature. RSC Adv. 2015, 5, 93147–93154. [Google Scholar] [CrossRef]
- Karbasian, H.; Tekkaya, A. A review on hot stamping. J. Mater. Process. Technol. 2010, 210, 2103–2118. [Google Scholar] [CrossRef]
- Merklein, M.; Wieland, M.; Lechner, M. Hot stamping of boron steel sheets with tailored properties: A review. J. Mater. Process. Technol. 2016, 228, 11–24. [Google Scholar] [CrossRef]
- Ghiotti, A.; Bruschi, S.; Borsetto, F. Tribological characteristics of high strength steel sheets under hot stamping conditions. J. Mater. Process. Technol. 2011, 211, 1694–1700. [Google Scholar] [CrossRef]
- Luo, Q.; Lu, C.; Liu, L.; Zhu, M. A review on the synthesis of transition metal nitride nanostructures and their energy related applications. Green Energy Environ. 2023, 8, 406–437. [Google Scholar] [CrossRef]
- Yang, H.-Q.; Yao, Z.-J.; Luo, X.-X.; Zhang, Z.-L.; Chen, Y. Effect of Nb addition on structure and mechanical properties of FeAl coating. Surf. Coat. Technol. 2015, 270, 221–226. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Pan, H.; Hou, J.; Mao, H.; Liu, B.; Xu, H.; Bai, P.; Misra, R.D.K. Phase formation and mechanical properties of iron-based intermetallic/steel laminate composites. Adv. Compos. Hybrid Mater. 2022, 5, 2171–2183. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Liu, M.; Zhou, Y.; Zha, Z.; Li, C.; Yang, Y.; Wang, J.; Jiang, F. Microstructure characterization and mechanical properties of the 304SS-(NiTif/FeAl) metal-intermetallic laminate composites. J. Alloys Compd. 2023, 943, 169117. [Google Scholar] [CrossRef]
- Eiler, J.; Ehtiati, K.; Sørensen, I.E.; Thormann, E. Measuring the salt content of sweat inside a sweat-absorbing skin adhesive. ACS Appl. Bio. Mater. 2023, 7, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Gong, D. Improved the elevated temperature mechanical properties of Al-Si alloy deposited with Al-Si coating by magnetron sputtering. Vacuum 2018, 150, 1–7. [Google Scholar] [CrossRef]
- Liu, X.; Lan, S.; Ni, J. Analysis of process parameters effects on friction stir welding of dissimilar aluminum alloy to advanced high strength steel. Mater. Des. 2014, 59, 50–62. [Google Scholar] [CrossRef]
- Mittal, D.; Hostaša, J.; Silvestroni, L.; Esposito, L.; Mohan, A.; Kumar, R.; Sharma, S.K. Tribological behaviour of transparent ceramics: A review. J. Eur. Ceram. Soc. 2022, 42, 6303–6334. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Mitterer, C.; Hultman, L.; Clemens, H. Microstructural design of hard coatings. Prog. Mater. Sci. 2006, 51, 1032–1114. [Google Scholar] [CrossRef]
- Veprek, S.; Veprek-Heijman, M. Industrial applications of superhard nanocomposite coatings. Surf. Coat. Technol. 2008, 202, 5063–5073. [Google Scholar] [CrossRef]
- Musil, J. Hard nanocomposite coatings: Thermal stability, oxidation resistance and toughness. Surf. Coat. Technol. 2012, 207, 50–65. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Mitterer, C.; Hultman, L.; Clemens, H. Wear performance of (nc-AlTiN)/(a-Si3N4) coating and (nc-AlCrN)/(a-Si3N4) coating in high-speed machining of titanium alloys under dry and minimum quantity lubrication (MQL) conditions. Wear 2013, 305, 249–259. [Google Scholar]
- Petkov, N.; Bakalova, T.; Bahchedzhiev, H.; Kejzlar, P.; Rysanek, P.; Kormunda, M.; Capkova, P. Influence of the addition of the elements Ti and Cr on the structure, and mechanical and tribological properties of AlSiN coatings. J. Nano Res. 2022, 73, 175–196. [Google Scholar] [CrossRef]
- Sharma, S.; Suman, M. The effect of Si content on structural, mechanical and optical behaviour of magnetron sputtered Al–Si–N nanocomposite thin films. J. Alloys Compd. 2020, 831, 154686. [Google Scholar]
- Kolaklieva, L.; Kakanakov, R.; Kovacheva, D.; Chitanov, V.; Cholakova, T.; Bahchedjiev, C.; Kolchev, S. Effect of the post-deposition thermal treatment on the mechanical properties of a compositionally modulated CrAlSiN-AlSiN coating. Coatings 2021, 11, 1311. [Google Scholar] [CrossRef]
- Kolchev, S.; Kolaklieva, L.; Chitanov, V.; Cholakova, T.; Zlatareva, E.; Kovacheva, D.; Atanasova, G.; Kakanakov, R. The role of period modulation on the structure, composition and mechanical properties of nanocomposite multilayer TiAlSiN/AlSiN coatings. Coatings 2023, 13, 1546. [Google Scholar] [CrossRef]
- Zeng, Q. Influence of CePO4 on high temperature anti-friction and anti-wear behaviors of nickel-based h-BN composite coatings. Diam. Relat. Mater. 2024, 148, 111421. [Google Scholar] [CrossRef]
- Polcar, T.; Parreira, N.M.G.; Novák, R. Friction and wear behaviour of CrN coating at temperatures up to 500 C. Surf. Coat. Technol. 2007, 201, 5228–5235. [Google Scholar] [CrossRef]
- Williams, J. Wear and wear particles—Some fundamentals. Tribol. Int. 2005, 38, 863–870. [Google Scholar] [CrossRef]
- Kolchev, S.; Kolaklieva, L.; Chitanov, V.; Cholakova, T.; Zlatareva, E.; Kovacheva, D.; Atanasova, G.; Kakanakov, R. Al2O3–cBN composites sintered by SPS and HPHT methods. J. Eur. Ceram. Soc. 2016, 36, 1783–1789. [Google Scholar]
- Zhao, Y.; Wang, Y.; Yu, Z.; Planche, M.-P.; Peyraut, F.; Liao, H.; Lasalle, A.; Allimant, A.; Montavon, G. Microstructural, mechanical and tribological properties of suspension plasma sprayed YSZ/h-BN composite coating. J. Eur. Ceram. Soc. 2018, 38, 4512–4522. [Google Scholar] [CrossRef]
- Taheri, M.; Shirvani, K. Design and processing of hard and self-lubricating NiCr/hBN-cBN composite coatings by laser cladding: Investigation of microstructure, hardness, and wear. Photonics 2025, 12, 265. [Google Scholar] [CrossRef]
- Tyagi, R.; Pandey, K.; Das, A.K.; Mandal, A. Deposition of hBN+ Cu layer through electrical discharge process using green compact electrode. Mater. Manuf. Process. 2019, 34, 1035–1048. [Google Scholar] [CrossRef]
- Eßler, J.; Woelk, D.; Utu, I.-D.; Marginean, G. Impact of hBN content on the tribological behavior and thermal diffusivity of HVOF-sprayed Cr3C2-NiCr coatings. Materials 2024, 17, 5470. [Google Scholar] [CrossRef]
- Sousa, V.F.; Da Silva, F.J.G.; Pinto, G.F.; Baptista, A.; Alexandre, R. Characteristics and wear mechanisms of TiAlN-based coatings for machining applications: A comprehensive review. Metals 2021, 11, 260. [Google Scholar] [CrossRef]
- Schulz, W.; Joukov, V.; Köhn, F.; Engelhart, W.; Schier, V.; Schubert, T.; Albrecht, J. The behavior of TiAlN and TiAlCrSiN films in abrasive and adhesive Tribological contacts. Coatings 2023, 13, 1603. [Google Scholar] [CrossRef]
- Zhang, X.; Tian, X.-B.; Zhao, Z.-W.; Gao, J.-B.; Zhou, Y.-W.; Gao, P.; Guo, Y.-Y.; Lv, Z. Evaluation of the adhesion and failure mechanism of the hard CrN coatings on different substrates. Surf. Coat. Technol. 2019, 364, 135–143. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Huang, T.; Guo, F.; Dai, L.; Liu, Y.; Zhang, B. Tribological properties of CrN/DLC and CrN coatings under different testing conditions. Coatings 2024, 14, 1002. [Google Scholar] [CrossRef]
- Wang, D.; Lin, S.; Lu, J.; Huang, S.-Q.; Yin, Z.-F.; Yang, H.-Z.; Bian, P.-Y.; Zhang, Y.-L.; Dai, M.-J.; Dai, M.-J.; et al. Research on high temperature wear resistance mechanism of CrN/CrAlN multilayer coatings. Tribol. Int. 2023, 180, 108184. [Google Scholar] [CrossRef]
- Krella, A. Resistance of PVD coatings to erosive and wear processes: A review. Coatings 2020, 10, 921. [Google Scholar] [CrossRef]
- Cai, J. Modelling of Phase Transformation in Hot Stamping of Boron Steel; Imperial College London: London, UK, 2011. [Google Scholar]
- Chkhartishvili, L.; Tabatadze, G.; Nackebia, D.; Bzhalava, T.; Kalandadze, I. Hexagonal boron nitride as a solid lubricant additive (an overview). Nano Stud. 2016, 14, 2016. [Google Scholar]
- Torres, H.; Podgornik, B.; Jovičević-Klug, M.; Ripoll, M.R. Compatibility of graphite, hBN and graphene with self-lubricating coatings and tool steel for high temperature aluminium forming. Wear 2022, 490, 204187. [Google Scholar] [CrossRef]
- Fallah, P.; Hensley, C.; Beall, C.J.; Islasencalada, A.; Chromik, R.R.; Wuthrich, R.; Stoyanov, P. Environmentally friendly MoS2-hBN solid lubricants: A comprehensive tribological evaluation. J. Tribol. 2025, 147, 041403. [Google Scholar] [CrossRef]
- Podgornik, B.; Kosec, T.; Kocijan, A.; Donik, Č. Tribological behaviour and lubrication performance of hexagonal boron nitride (h-BN) as a replacement for graphite in aluminium forming. Tribol. Int. 2015, 81, 267–275. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, H.; Zhang, P.; Yu, Z.; Lu, Q.; Guo, J. Microstructural evolution and wear behaviors of laser-clad Stellite 6/NbC/h-BN self-lubricating coatings. Surf. Coat. Technol. 2019, 372, 218–228. [Google Scholar] [CrossRef]
- Windmann, M.; Röttger, A.; Theisen, W. Formation of intermetallic phases in Al-coated hot-stamped 22MnB5 sheets in terms of coating thickness and Si content. Surf. Coat. Technol. 2014, 246, 17–25. [Google Scholar] [CrossRef]
- Kucera, V.; Cabibbo, M.; Prusa, F.; Fojt, J.; Petr-Soini, J.; Pilvousek, T.; Kolarikova, M.; Vojtech, D. Phase Composition of Al-Si Coating from the Initial State to the Hot-Stamped Condition. Materials 2021, 14, 1125. [Google Scholar] [CrossRef] [PubMed]
- Gui, Z.X.; Liang, W.K.; Zhang, Y.S. Enhancing ductility of the Al-Si coating on hot stamping steel by controlling the Fe-Al phase transformation during austenitization. Sci. China Technol. Sci. 2014, 57, 1785–1793. [Google Scholar] [CrossRef]
- Soni; Kumari, S.; Sharma, S.K.; Mishra, S.K. Effect of deposition pressure, nitrogen content and substrate temperature on optical and mechanical behavior of nanocomposite Al-Si-N hard coatings for solar thermal applications. J. Mater. Eng. Perform. 2018, 27, 6729–6736. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Mahdizadeh, S.J. Thermal conductivity and heat transport properties of nitrogen-doped graphene. J. Mol. Graph. Model. 2015, 62, 74–80. [Google Scholar] [CrossRef] [PubMed]








| Temperature (°C) | Atomic Percentage (at.%) | ||||
|---|---|---|---|---|---|
| C | O | Al | Si | Fe | |
| RT | 22.78 | 6.49 | 34.11 | 12.16 | 0.85 |
| 400 | 13.25 | 15.51 | 56.79 | 13.48 | 0.98 |
| 600 | 20.37 | 42.72 | 31.28 | 3.67 | 1.96 |
| 800 | 18.56 | 50.65 | 25.14 | 5.65 | 2.81 |
| Temperature (°C) | Atomic Percentage (at.%) | ||||
|---|---|---|---|---|---|
| N | O | Al | Si | Fe | |
| RT | 42.38 | 6.49 | 28.11 | 12.16 | 10.86 |
| 400 | 40.43 | 8.31 | 27.20 | 12.54 | 11.52 |
| 600 | 37.56 | 7.49 | 28.02 | 13.11 | 13.82 |
| 800 | 37.44 | 11.21 | 24.33 | 11.30 | 15.72 |
| Temperature (°C) | Atomic Percentage (at.%) | ||||
|---|---|---|---|---|---|
| N | O | Al | Si | Fe | |
| RT | 45.09 | 13.26 | 23.93 | 9.94 | 7.77 |
| 400 | 43.26 | 15.48 | 23.20 | 9.54 | 8.52 |
| 600 | 41.13 | 14.87 | 24.15 | 9.16 | 10.15 |
| 800 | 39.79 | 15.35 | 23.54 | 10.41 | 10.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Q. The Role of Nitrogen Doping in Enhancing the Thermal Stability and Wear Resistance of AlSi Coatings at Elevated Temperatures. Coatings 2025, 15, 1296. https://doi.org/10.3390/coatings15111296
Zeng Q. The Role of Nitrogen Doping in Enhancing the Thermal Stability and Wear Resistance of AlSi Coatings at Elevated Temperatures. Coatings. 2025; 15(11):1296. https://doi.org/10.3390/coatings15111296
Chicago/Turabian StyleZeng, Qunfeng. 2025. "The Role of Nitrogen Doping in Enhancing the Thermal Stability and Wear Resistance of AlSi Coatings at Elevated Temperatures" Coatings 15, no. 11: 1296. https://doi.org/10.3390/coatings15111296
APA StyleZeng, Q. (2025). The Role of Nitrogen Doping in Enhancing the Thermal Stability and Wear Resistance of AlSi Coatings at Elevated Temperatures. Coatings, 15(11), 1296. https://doi.org/10.3390/coatings15111296






