1. Introduction
In order to achieve glassy solidification, BMGs are designed as multicomponent systems with three or more elements [
1,
2,
3]. The inherent atomic disorder in multicomponent systems promotes dense stochastic packing configurations, kinetically stabilizing supercooled liquids through viscosity elevation and crystallization retardation. This multicomponent effect on BMG formation constitutes the fundamental “confusion principle”—a topological frustration mechanism impeding long-range ordering. Minor addition and elemental substitution are effective ways for improving the GFA and T-A of metallic glasses [
4,
5,
6,
7,
8]. The elements employed in these approaches are typically selected from those with large or small atomic radii. While intermediate-sized atoms like Fe, Ni, Co, Cu, Ti, and Ta have been incorporated as minor alloying additions in different systems, they generally exhibit an adverse effect on GFA [
9]. A beneficial role in bulk glass formation is only observed when their concentration surpasses approximately 3 at.%, transforming them into primary components of the alloy system.
Rapid quenching from the liquid temperature is generally essential for forming BMGs, a process in which the initial melt condition and applied cooling rate are critical. The resulting solid, derived from the rapidly cooled undercooled melts, possesses a stochastic atomic arrangement. This amorphous structure imparts exceptional characteristics, including a high elastic modulus, superior strength, and excellent corrosion resistance [
10,
11,
12,
13,
14,
15,
16,
17,
18,
19]. However, the amorphous structure of BMGs usually has brittle characteristics, namely, poor deformation behavior. Severe brittle fracture would occur once the applied stress exceeds the yielding strength of BMGs [
20,
21,
22]. Therefore, the engineering application of BMGs is seriously restricted because they lack plasticity. Several treatment methods, such as surface treatment, synthesis of heterogeneous metallic glass composites, selective laser melting, minor element addition, fabrication of metallic glass-based composites, melting, and cryogenic thermal cycling, have been proven to improve the plasticity of BMGs [
22,
23,
24,
25,
26,
27,
28,
29].
Melting is an efficient method to adjust the plasticity of BMGs. During the quenching of BMGs, their thermal history (which includes the cooling rate and melt overheating temperature) plays an important role in their microstructure and mechanical property [
10]. A high cooling rate could result in a relatively loose microstructure with a large free volume, thus enhancing plasticity [
30]. Meanwhile, the microstructure of BMGs is usually inherited from their melt structure, which is strongly affected by the overheating temperature. Therefore, T-A, GFA, and mechanical behavior strongly depend on the melt state [
31,
32,
33].
Similar to the effect of the cooling rate, the melt state impacts the amount of free volume and thus the plasticity of BMGs [
32,
33]. The dynamic crossover temperature of Au-based BMG melt was detected to be approximately 2.3 times the glass transition temperature using nuclear magnetic resonance; based on the experimental results and theoretical analysis, Cu and Si atoms tend to form strong covalent-like bonds below the crossover temperature [
34]. The dynamic heterogeneity of a Pd-based BMG increases gradually with decreasing temperature until a dynamic crossover arises [
35]. Another variation of the melt state of BMGs is liquid–liquid phase transition: for Cu
46Zr
46Al
8 BMG prepared below the transition temperature, CuZr
2 nanocrystals with good plasticity were formed and the elastic modulus was increased [
36]. Jonas et al. demonstrated that the LLPT kinetics in BMGs under cooling conditions occur within a 10–100 s timeframe, with the transition onset temperature exhibiting negligible dependence on applied cooling rates [
37]. In the present work, the correlation between the liquid state and structural as well as mechanical behavior of a Zr
50Cu
40Al
10 BMG was systematically explored. The effect of melt temperature on glass-forming ability, thermal stability, and mechanical behavior was meticulously analyzed. Mechanical and structural tests revealed a significant increase in plasticity under the optimal melt conditions. The findings underscore the critical role of melt state in tailoring BMG properties for specific applications.
2. Experimental Procedure
An ingot of Zr
50Cu
40Al
10 alloy was produced via arc melting in a Ti-gettered, high-purity argon environment. Prior to melting, constituent metal particles of purity exceeding 99.9 at% were ultrasonically cleaned. The CuZrAl ternary system is one of the rare ternary alloy systems capable of forming centimeter-scale bulk metallic glasses. The specifically selected Zr
50Cu
40Al
10 alloy composition lies near the ternary eutectic point, exhibiting a relatively low melting point of 1273 K that facilitates the preparation of samples in a superheated molten state [
38]. To achieve chemical homogeneity, the melting procedure was repeated four times with concurrent electromagnetic stirring. Cylindrical rods measuring φ 5 mm × 50 mm were fabricated by ejection casting into a copper mold. The overheating temperature was monitored with an infrared pyrometer, which was set at three different levels relative to the liquidus temperature (
Tl): 1.2
Tl, 1.3
Tl, and 1.4
Tl, with the corresponding samples labeled accordingly. A separate rod sample (φ 5 mm × 100 mm) was also produced by suction casting in an arc melting furnace, denoted as
SCTl.
To ensure the amorphous structural characteristics of the prepared as-cast φ 5 mm rods, X-ray diffraction (XRD) testing was performed. The sample for XRD analysis was a small, flat fragment taken from the central region of the as-cast rod to ensure representative sampling. In order to maintain the intrinsic as-cast structure and prevent any potential surface crystallization, the sample surface was gently cleaned with ethanol in an ultrasonic cleaner to remove any contaminants or oxide layers. No mechanical polishing or etching was conducted, which could otherwise induce stress or alter the surface structure. The prepared fragment was then mounted on a zero-background silicon crystal sample holder using a thin layer of vacuum grease to ensure a flat and stable surface for analysis. The XRD measurement was performed using a Rigaku D/MAX 2500 V X-ray diffractometer was used with a Cu-Kα source in a reflection (Bragg–Brentano) mode with θ-θ scan. The scanning range (2θ) was from 20° to 80°, with a scanning speed of 2°/min adopted to enhance the signal-to-noise ratio and ensure a high-quality pattern for detailed lineshape analysis.
Fine structural analysis was performed using high resolution transmission electron microscopy (HRTEM). The initial bulk sample was first mechanically ground and polished to a thickness of approximately 40 μm. To avoid introducing mechanical artifacts or crystallization, minimal applied pressure and sufficient coolant flow were maintained during this process. The final thinning to electron transparency was performed using a Gatan 691 precision ion polishing system at low temperatures. A small disk (3 mm in diameter) was subjected to argon ion milling at a low incident angle (typically 3°–5°) and a low accelerating voltage (initially 4 keV, followed by a final polish at 2 keV). This low-energy ion milling is crucial for minimizing ion-beam-induced damage, amorphization, or preferential thinning, which are particular concerns for BMGs. The sample was periodically rotated to ensure uniform thinning. The HRTEM study was conducted using a FEI Tecnai G2 F20 operated at an accelerating voltage of 200 kV to provide sufficient penetration power and high spatial resolution while minimizing electron beam-induced effects. Prior to high-resolution imaging, the sample was screened in selected-area electron diffraction (SAED) mode to locate regions of unambiguous amorphous structure, identified by the presence of broad, diffuse halos and the absence of sharp diffraction spots or rings. Once a suitable area was identified, HRTEM imaging was performed.
Thermal characterization of the metallic glass specimens was performed using a TA Q2000 differential scanning calorimeter (DSC) equipped with an alumina crucible under flowing ultrahigh-purity nitrogen. The sample was in the form of a thin, plate-like fragment, characteristic of the high brittleness exhibited by many as-cast monolithic BMGs. To ensure representative thermal analysis, a small, flat piece weighing approximately 15.0 ± 0.5 mg was carefully selected and weighed. The pristine as-cast surface of the sample was utilized without mechanical polishing to avoid introducing artificial nucleation sites or stress-induced crystallization that could alter thermal behavior. The instrument was calibrated for temperature and heat flow using high-purity indium and zinc standards under identical gas conditions. All measurements were conducted under a high-purity nitrogen atmosphere at a constant flow rate of 50 mL/min to prevent oxidative degradation at elevated temperatures. A fixed heating rate of 10 K/min was applied from room temperature to 873 K.
The plastic behavior under uniaxial compression was evaluated with the MTS 809 testing system at room temperature, using a fixed strain rate of 1 × 10−4 s−1. The cylindrical compressive samples were machined to a precise geometry with an aspect ratio (height-to-diameter ratio, h/d) of 2:1, typically with dimensions of d2 mm × h4 mm. This aspect ratio is critical to ensure stable deformation and minimize the confounding effects of end constraints and buckling. The machining process was conducted with great care under copious coolant supply to prevent excessive heating and the introduction of machining-induced stresses or crystallization. The parallelism of the two end faces was precision-ground to within 5 μm to ensure uniform stress distribution during loading. Prior to formal testing, a small pre-load of approximately ~30 N was applied to ensure stable and full contact between the sample and the platens. The test was then conducted under displacement control mode at a constant crosshead speed, corresponding to an initial nominal strain rate of 1 × 10−4 s−1. The deformation process was continued until a significant load drop, indicative of final fracture, was observed. The load and crosshead displacement data were acquired synchronously at a high sampling rate throughout the test. By plotting the data from the compression test, the stress-strain curve was obtained. Plastic strain (ε), defined as the deformation from the elastic limit to the fracture point, was derived from this curve.
3. Results and Discussion
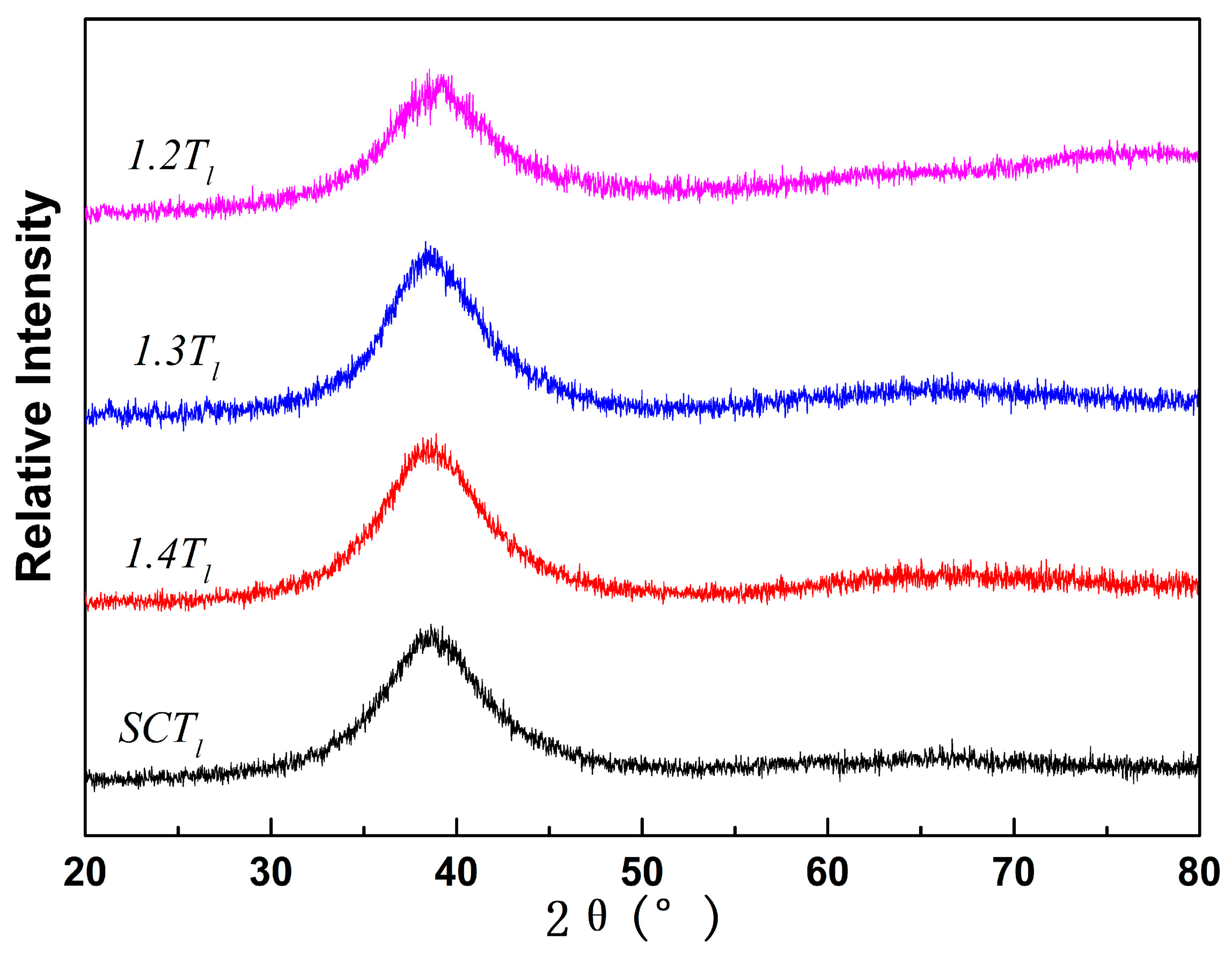
The XRD patterns of Zr
50Cu
40Al
10 BMGs fabricated under different melt conditions in
Figure 1 indicate a broad diffraction halo typical of amorphous structure, and the absence of sharp peaks verifies the amorphous nature of the samples. The DSC curves in
Figure 2 reveal distinct exothermic peaks corresponding to different melt states. With elevated melt temperature, the injection-cast samples exhibited a clear increasing trend in both glass transition temperature (
Tg) and crystallization temperature (
Tx). The
Tg and
Tx are determined by extrapolated onset temperature, which is the temperature corresponding to the intersection point of the tangent of the previous baseline and the extended line of the midpoint line.
Table 1 demonstrates an increase in
Tg from 708.6 K to 713.7 K, accompanied by a rise in
Tx from 769.2 K to 776.9 K. This correlation underscores the critical role of melt temperature in optimizing BMGs’ GFA and thermal properties and highlights the necessity for its precise control during manufacturing to achieve the desired material performance. The data further support the hypothesis that high melt temperatures promote uniform atomic arrangements, thereby enhancing the overall structural integrity and reliability of BMGs.
Similar studies on other BMG compositions have shown that the melt state significantly influences structural characteristics, and low melt temperatures usually induce the emergence of nano-grained phases [
39,
40]. The heterogeneity of alloy melts decreases with the increase in the melt temperature; under low melt temperatures, the heterogeneous structure facilitates nucleation during cooling [
41]. This phenomenon can guide future research and production strategies to maximize the potential of these advanced materials. Such refined control over melt temperatures ensures consistent quality and opens avenues for tailoring BMGs to specific applications where durability and T-A are paramount. By fine-tuning these parameters, manufacturers can unlock the full spectrum of BMGs’ capabilities, paving the way for innovations in various high-performance industries.
The
Tg and
Tx of Zr
50Cu
40Al
10 BMG prepared using suction casting were 712.3 K and 774.6 K, respectively. These values indicate that the melt maintains an intermediate temperature during arc melting. The upper part of the melt has direct contact with the electric arc during arc melting before suction casting, while its bottom has direct contact with the water-cooled copper mold. The lower middle part of the melt could be sucked into the water-cooled copper mold, resulting in an appropriate melt temperature range for the
SCTl sample. The
Tg and
Tx shown in
Figure 2 and
Table 1 prove this deduction. During the overheating process, the oxygen content in the alloy melt and the resulting BMGs rises with increasing temperature. Although this correlation exists, Inoue et al. reported that the improvement in glass-forming ability associated with higher casting temperatures exhibits only a weak dependence on the oxygen content [
42]. Notably, when the melt temperature exceeds 770 K (surpassing the liquidus temperature of Zr-based BMGs), the glass transition is suppressed, indicating a critical threshold for the effect of oxygen on glass formation. Hence, the oxygen content should not be regarded as a major influencing factor of the impact of melt temperature on the GFA and T-A of Zr
50Cu
40Al
10 BMG.
The above results indicate that the optimal casting temperature affects the melt state, thermal behavior, and GFA of Zr
50Cu
40Al
10 BMG. The structure of BMGs is closely related to their melt state because the atomic arrangement and bonding strength vary significantly with the melt temperature, a phenomenon known as LLPT [
36,
37,
43]. For the specific case of Cu
46Zr
46Al
8 BMG, Hu et al. [
36] found that quenching below the LLPT temperature resulted in a 12% higher elastic modulus and a 40% greater number of activation of the shear localization pathways during deformation compared to samples quenched above this threshold. This observation aligns with their broader findings from experiments and simulations on metallic glasses, which demonstrated identical trends. This notable enhancement in mechanical properties underscores the pivotal role of LLPT in tuning atomic configurations and bonding strengths, thereby optimizing the structural integrity and deformation resistance of BMGs.
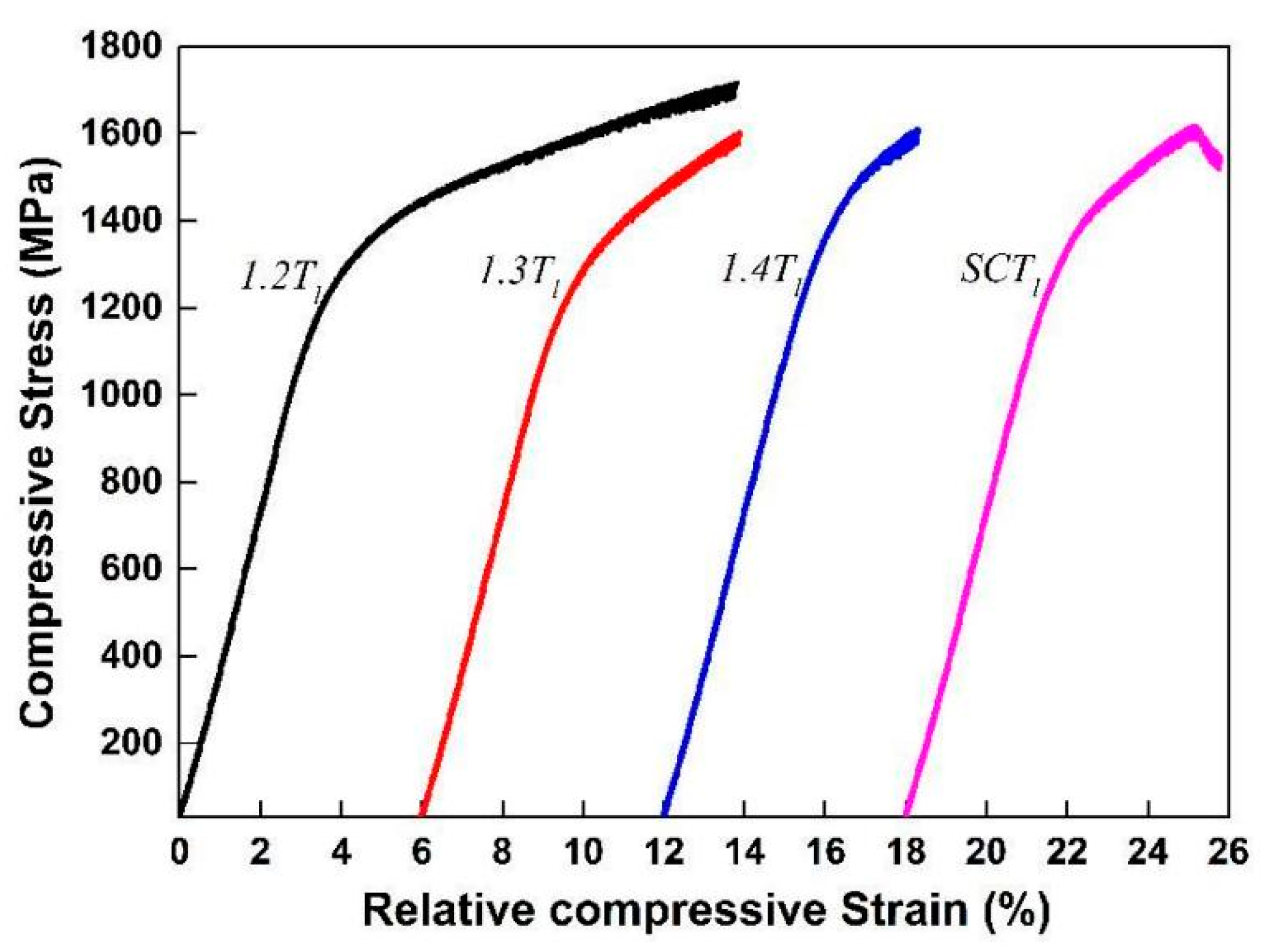
Compression testing was conducted on the CuZrAl BMGs with different melt states to further study the relationship between melt state and plasticity, especially plastic deformation capacity. The BMG rods with an initial diameter of 5 mm underwent cutting and polishing processes to reduce their diameter to 2 mm, each with a height-to-diameter ratio of 2:1. Compression testing was performed on these specimens at a fixed strain rate of 1 × 10
−4 s
−1 to obtain accurate stress–strain behavior measurements. As depicted in
Figure 3, ZrCuAl BMGs produced with different melt states display distinct engineering stress-strain curves, corresponding plastic deformation behavior indicators are tabulated in
Table 1. The highest plasticity (9.7% plastic strain) occurred in rods quenched from the lowest melt temperature. The specimens with intermediate melt temperatures exhibited moderate plasticity, averaging approximately 4.4% plastic strain, and those with the highest melt temperature showed the least plastic deformation of 2.4%. This trend underscores the critical role of melt temperature in determining the microstructural evolution and plasticity of ZrCuAl BMGs.
The results are in agreement with those for other Zr-based MGs that prepared with distinct melt states [
39,
44]. However, the contrary was observed in a La-based BMG [
44]. A in-homogeneous structure is necessary to realize the plastic behavior of metallic glasses. Structural heterogeneity within bulk metallic glasses (BMGs) is often conceptualized as structural defects, emerging as manifestations such as liquid-like sites, soft areas, flow units, and free volume [
28]. Hence, free volume in metallic glasses serves as nucleation centers for shear band multiplication during deformation. This self-organized branching mechanism effectively suppresses catastrophic propagation of major shear bands [
28].
The difference in melt temperatures likely alters the free volume distribution and influences plasticity; low melt temperature induces a high free volume, thus enhancing plasticity in BMGs [
39,
44]. Conversely, high melt temperatures seem to stabilize the structure of La-based BMGs, reducing their free volume and thus limiting their plastic deformation [
45]. Therefore, the relationship between melt state and plasticity varies for different alloy systems. This disparity underscores the nuanced interplay between melt states and microstructure in Zr-based BMGs, highlighting the need for tailored approaches in optimizing their mechanical behaviors. The intricate balance of free volume and melt temperature necessitates the precise control of casting parameters to achieve desired mechanical properties. Further studies on the solidification process at varying temperatures are crucial to elucidate the underlying mechanisms and refine casting methodologies [
36,
42].
Given that the amorphous structure for metal alloy usually needs rapid cooling, the microstructure of BMGs could be regarded as melt quenched. Advanced characterization techniques such as HRTEM were employed to further investigate the relationship of melt state with microstructural evolution and mechanical properties.
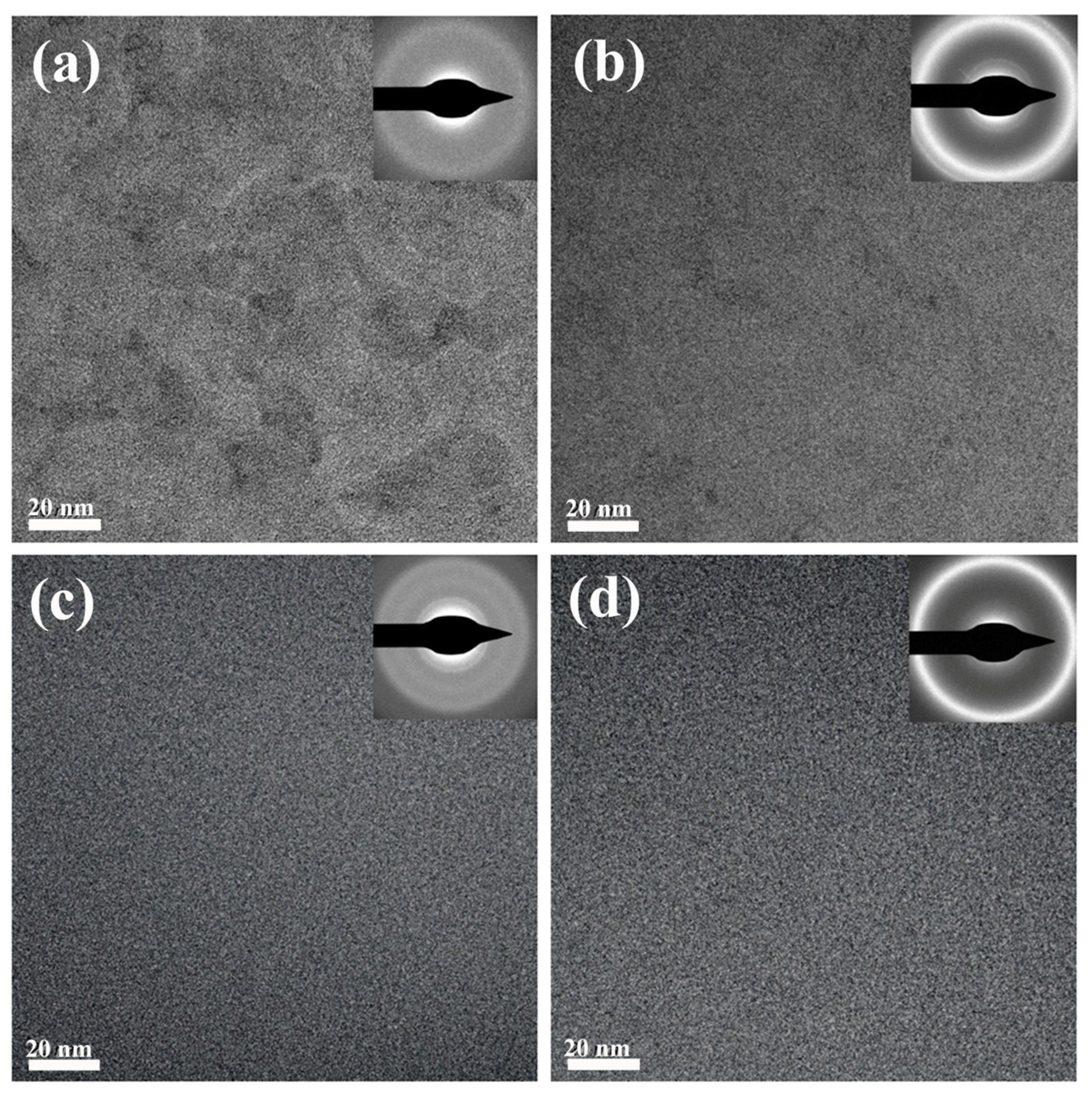
Figure 4a–d demonstrates the intricate details of BMG microstructures, with the selected area electron diffraction patterns revealing the amorphous nature for all ZrCuAl BMGs prepared with different melt states. However, the structure variations shown in
Figure 4a for the 1.2
Tl sample indicate distinct structural/chemical heterogeneity in the HRTEM images, suggesting that melt state significantly influences the local atomic arrangement. For the 1.3
Tl sample shown in
Figure 4b, the heterogeneous structure is weak. However, the heterogeneous structure cannot be observed for the 1.3
Tl and
SCTl samples. This heterogeneity likely affects the mechanical properties of the samples, as observed in their varied plasticity behaviors. Further analysis using dark-field HRTEM images and EDS mapping acquired in scanning transmission electron microscopy (STEM) mode for the 1.2
Tl sample confirmed the segregation of alloying elements as shown in
Figure 5. The heterogeneous Cu distribution is responsible for the observed differences in local atomic configurations, which in turn influences the overall mechanical behavior. The experimental results are consistent with those obtained from molecular dynamics simulations for CuZrAl BMGs, indicating that Cu segregation is crucial in determining the microstructural heterogeneity [
46].
During the rapid quenching of the alloy melt, the heterogeneous distribution of Cu in the BMG matrix quenched at a low melt temperature is inherited from the corresponding alloy melt, in which the local clusters exist. The increase in temperature induces LLPT, which is closely related to the arrangement of medium-range order [
36,
37,
43]. LLPT represents a kinetically sluggish process necessitating extensive atomic diffusion over macroscopic distances. As the melting temperature exceeds the LLPT threshold temperature, the local segregation of alloy clusters disappears and a uniformly distributed alloy structure is obtained by quenching the melt as shown in
Figure 4c,d.
The LLPT observed in metallic glass melts upon heating fundamentally involves structural reorganization from high-coordination polyhedral clusters to low-coordination atomic configurations [
47]. This transition mechanism was experimentally validated through thermal cycling tests on a Zr-based metallic glass melt by Inoue et al., with their results confirming excellent repeatability of the LLPT phenomenon [
42]. Thus, controlling the melt temperature prior to quenching is crucial to achieve a heterogeneous microstructure in BMGs. Low temperatures below the LLPT threshold effectively promote local segregations, ensuring the inhomogeneous distribution of alloy elements. This methodology substantially improves both structural heterogeneity and mechanical performance in fabricated metallic glasses, with the uniform morphological features observed in
Figure 3 and
Figure 4 providing definitive experimental verification. While the arc-melting process generates ultra-high temperatures at the molten alloy’s upper surface, the central region of the melt may become entrained into the water-cooled copper crucible during suction casting operations. The thermal parameters shown in
Figure 2 and HRTEM images in
Figure 4 confirm that the melt temperature of the
SCTl sample lies between those of the 1.3
Tl and 1.4
Tl samples, and the element distribution is homogeneous. In addition, the plastic deformation capacity is lower than that of the sample prepared before LLPT, which is controlled by adjusting the melting temperature.
At the atomic scale, even a stable superheated melt is not entirely disordered. It dynamically exhibits fluctuations in local atomic clusters with distinct energies and structures, which can be primarily categorized into two types: one is the “solid-like” clusters (e.g., characterized by icosahedral ordering) with lower energy, dense packing, and sluggish dynamics; the other is the “liquid-like” clusters with higher energy, loose packing, and rapid dynamics. At elevated temperatures, these two types of clusters interconvert rapidly, resulting in macroscopic homogeneity. When the melt is cooled from high temperatures and enters the critical temperature range, the total free energy tends to minimize, the melt would abandons its macroscopic homogeneity and initiates phase separation. Kinetically, this phase separation process manifests as the amplification and stabilization of nanoscale structural fluctuations. The originally transient “solid-like” clusters become stabilized by the thermodynamic driving force and act as “embryos”, growing persistently by incorporating surrounding similar atoms and components. This process could proceed via a nucleation and growth mechanism, leading to the precipitation of a high-density, high-viscosity, low-entropy liquid phase within another low-density, low-viscosity, high-entropy liquid phase. Alternatively, within the unstable region, it may occur via a spinodal decomposition mechanism, spontaneously interconnecting and coarsening to form a bicontinuous two-phase structure.
Ultimately, the melt separates into two distinct phases: one is a “high-order” liquid enriched with solid-like ordered structures, whose configuration more closely resembles the final amorphous solid; the other is a relatively “disordered” liquid. This mechanism is robustly supported by experimental evidence [
35,
36,
37,
42,
43,
44]. In summary, the LLPT mechanism profoundly reveals the structural polymorphism of the liquid state. It establishes a critical link between the microscopic local structure of the melt and the macroscopic glass-forming ability, providing a fundamental theoretical basis for understanding and designing high-performance metallic glasses.
In this work, the inherent microstructural heterogeneity due to Cu segregation significantly impacts the mechanical properties of Zr50Cu40Al10 BMG. These experimental results underscore the critical role of melt state in determining the microstructural evolution and resultant properties, highlighting the need for its precise control during synthesis. Moreover, the findings align with previous research, reinforcing the idea that tailored melt treatments can systematically manipulate alloy microstructures. By understanding these interdependencies and fine-tuning the melting and quenching process, researchers can develop effective strategies for manipulating BMG structures and ultimately synthesize materials with superior performance and broad applications.