Effect of Laser Surface Melting on the Microstructure and Corrosion Resistance of Laser Powder Bed Fusion and Wrought Ti-6Al-4V Alloys
Abstract
1. Introduction
2. Materials and Methods
- Slow scan rate (10 mV min−1) to minimise capacitive artefacts at the electrode/solution interface.
- Wide Tafel span for the linear region to cover at least one decade of current density; only the cathodic branch was used, because it showed the clearest linearity free of concentration polarisation or roughness effects.
- Starting point ≥ 50 mV from the open circuit potential to ensure pure Tafel behaviour without overlap of anodic and cathodic reactions.
- Linear fit (R2 > 0.98)—fits not meeting this criterion were rejected.
3. Results
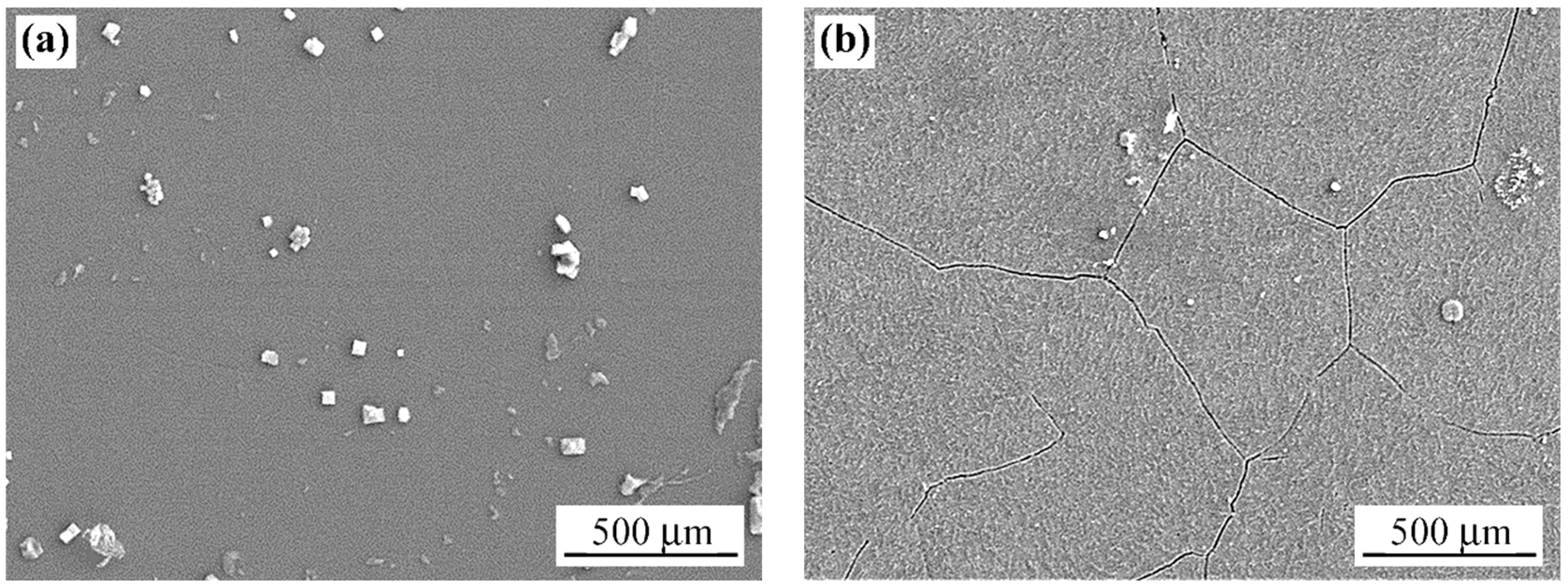
3.1. Microstructure Characterisation
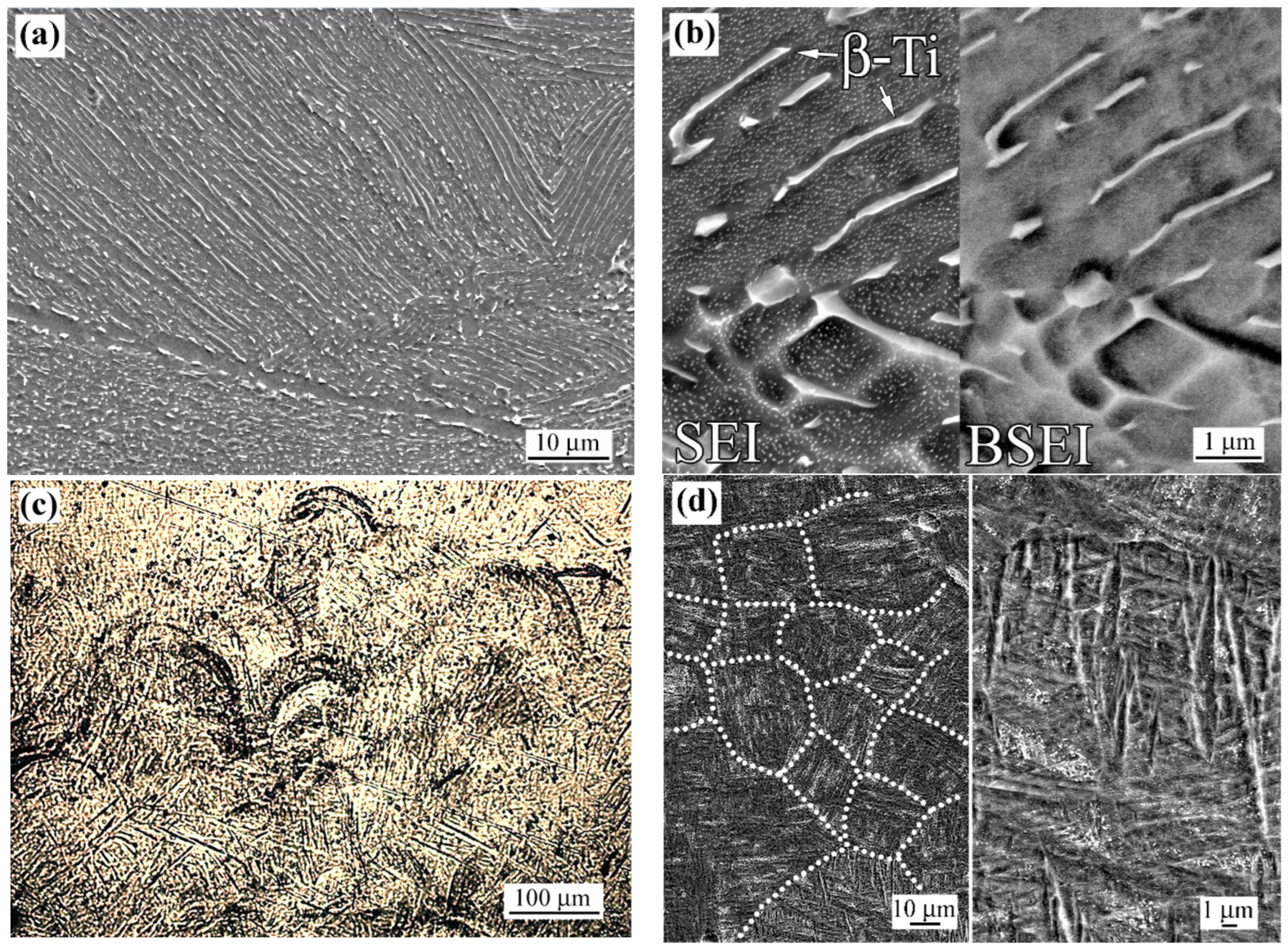
3.1.1. As-Fabricated Microstructure
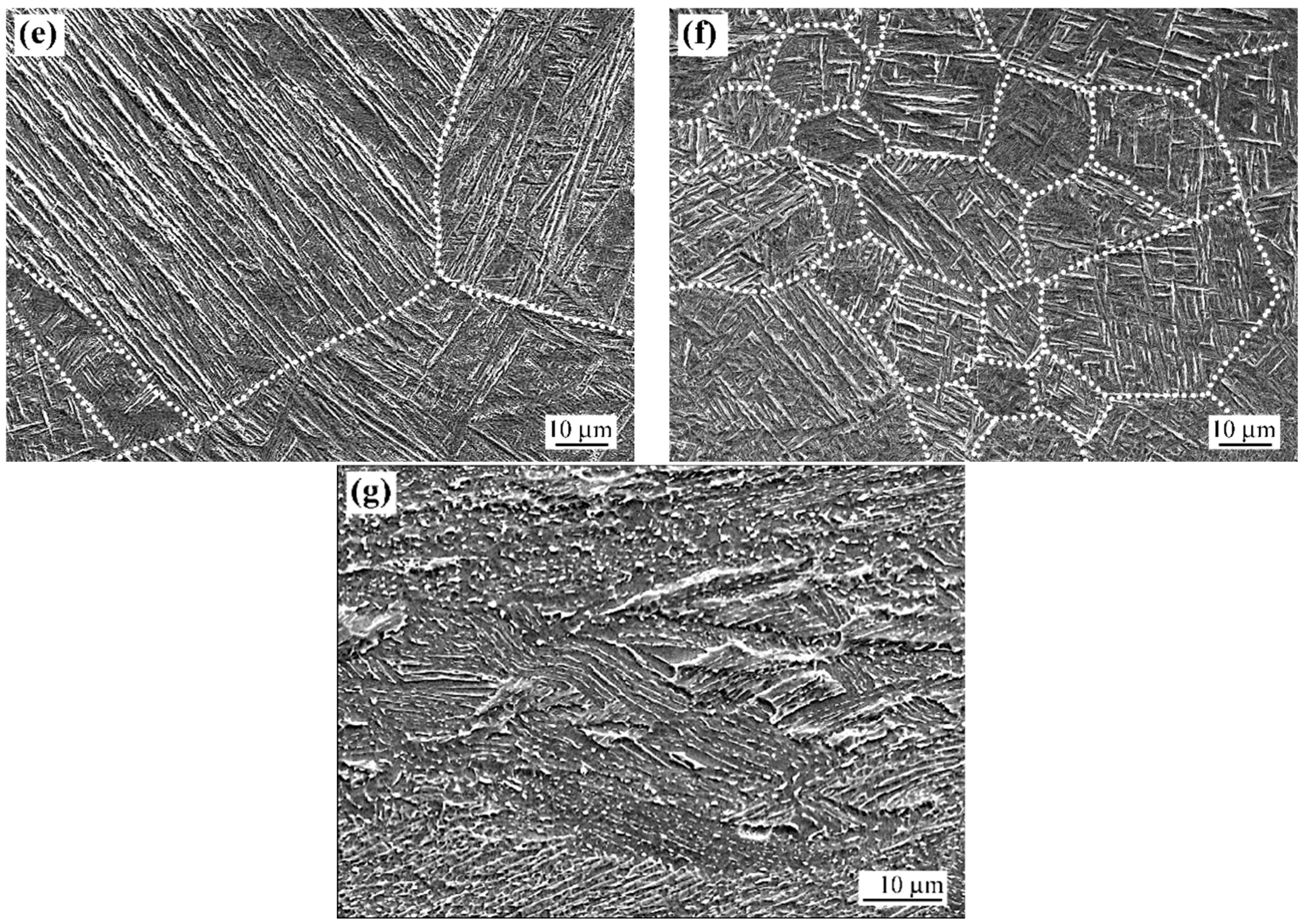
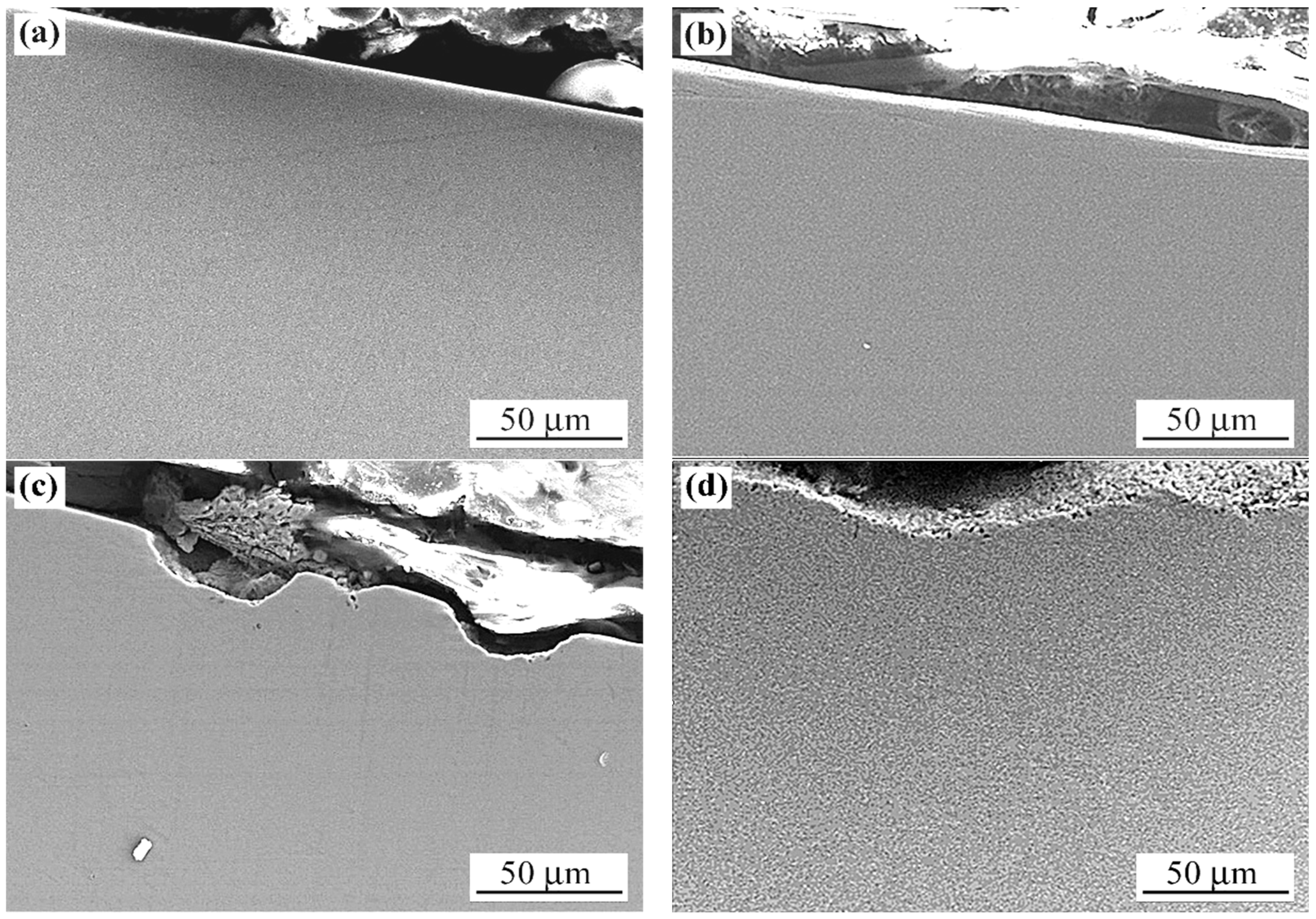
3.1.2. Laser Beam Surface-Modified Microstructure
3.1.3. Electron Backscattered Diffraction Analysis
3.1.4. X-Ray Diffraction
3.2. Microhardness Distribution
3.3. Electrochemical Testing
3.3.1. Effect of Laser Surface Melting on the Cyclic Polarisation Behaviours of LPBF and Wrought Ti-6-4
3.3.2. Comparison of Cyclic Polarisation Behaviour of LSM-LPBF with LSM-WR Specimens
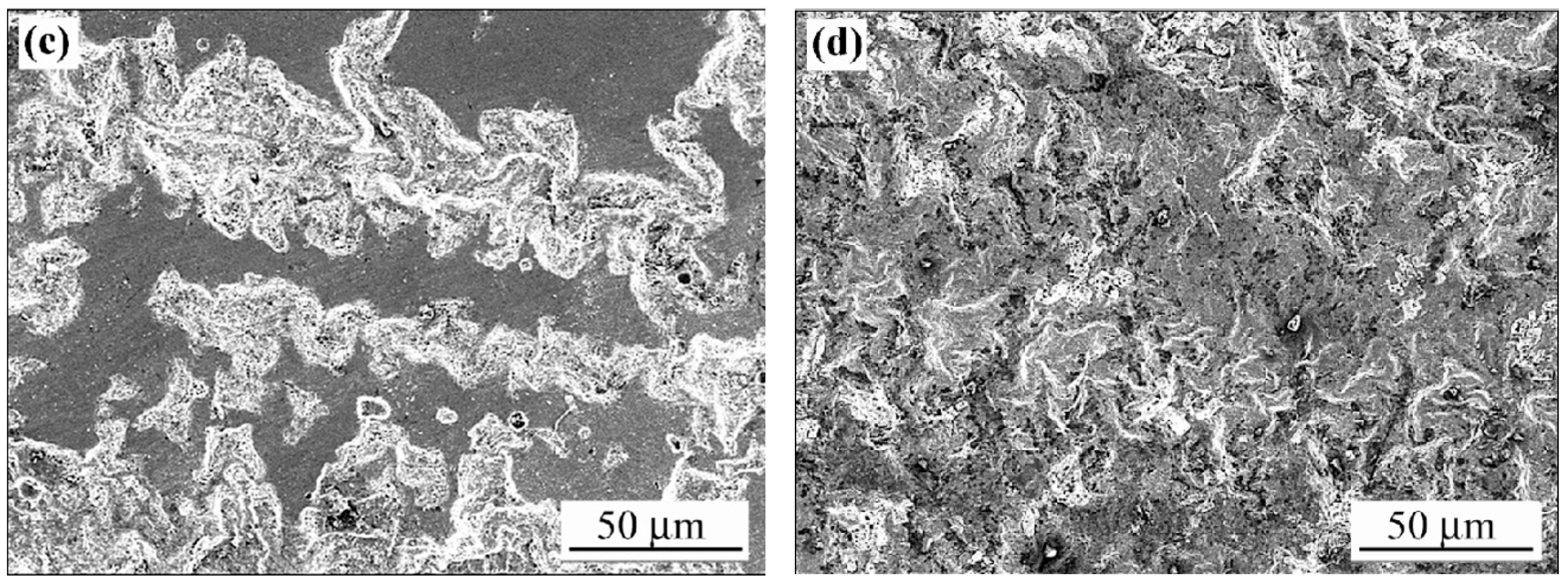
3.4. Microstructure of Corrosion
3.4.1. Effect of Laser Treatment (LSM) on the Cyclic Polarisation Behaviours of LPBF and Wrought Ti-6-4
3.4.2. Comparison of LSM-WR and LSM-LBPF Ti-6-4 After Corrosion
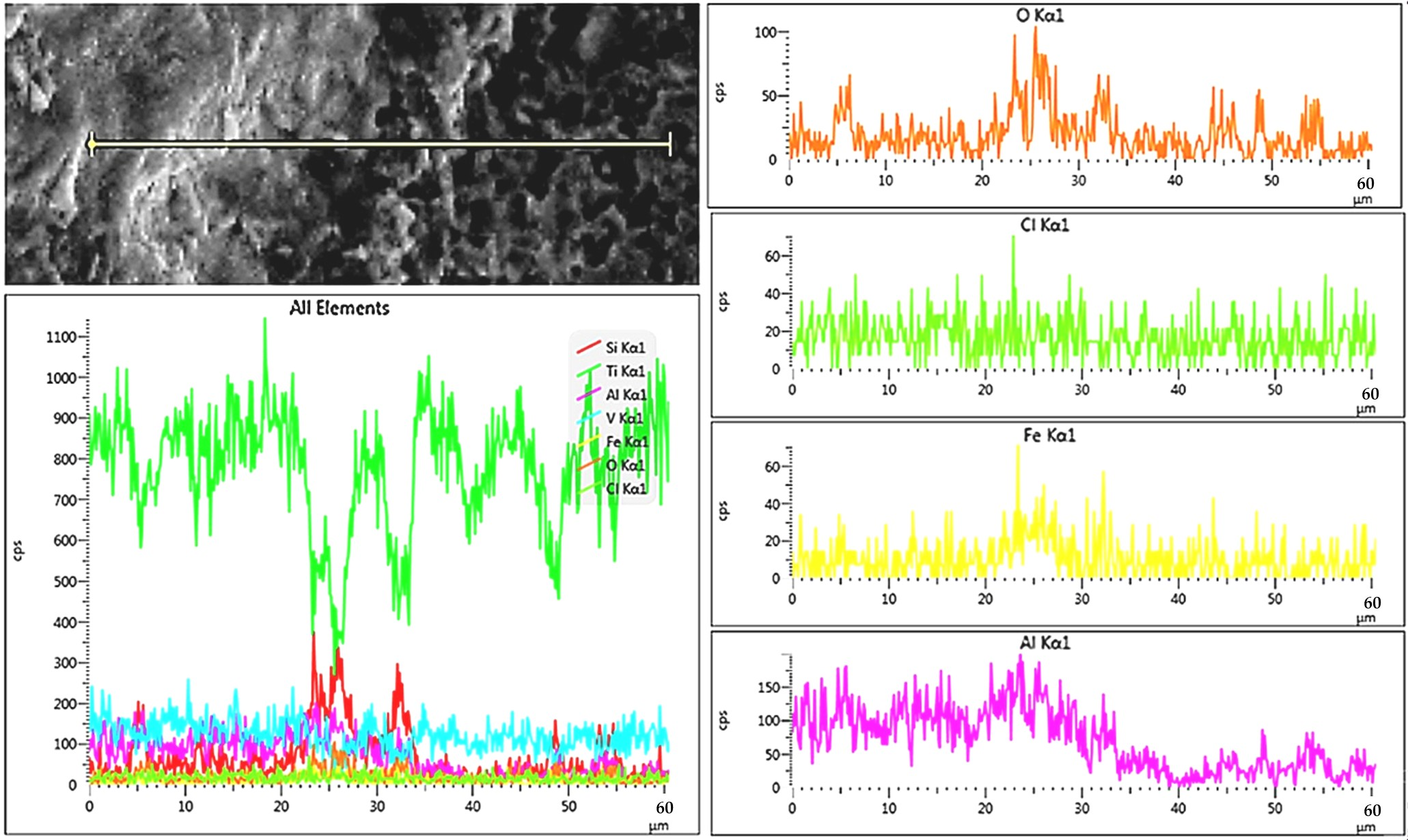
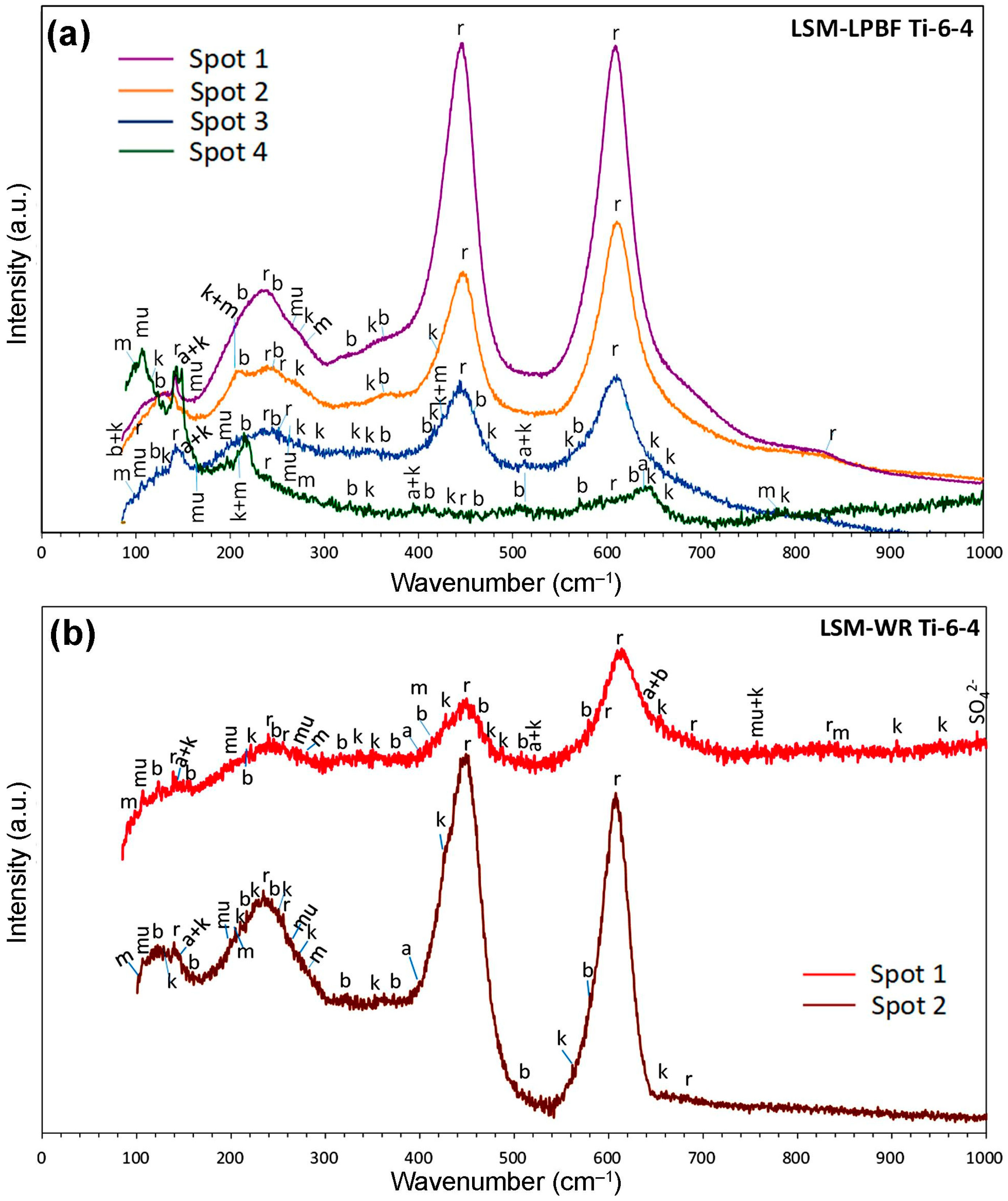
3.4.3. Nature of Corrosion Products-Raman Spectroscopy
4. Discussion
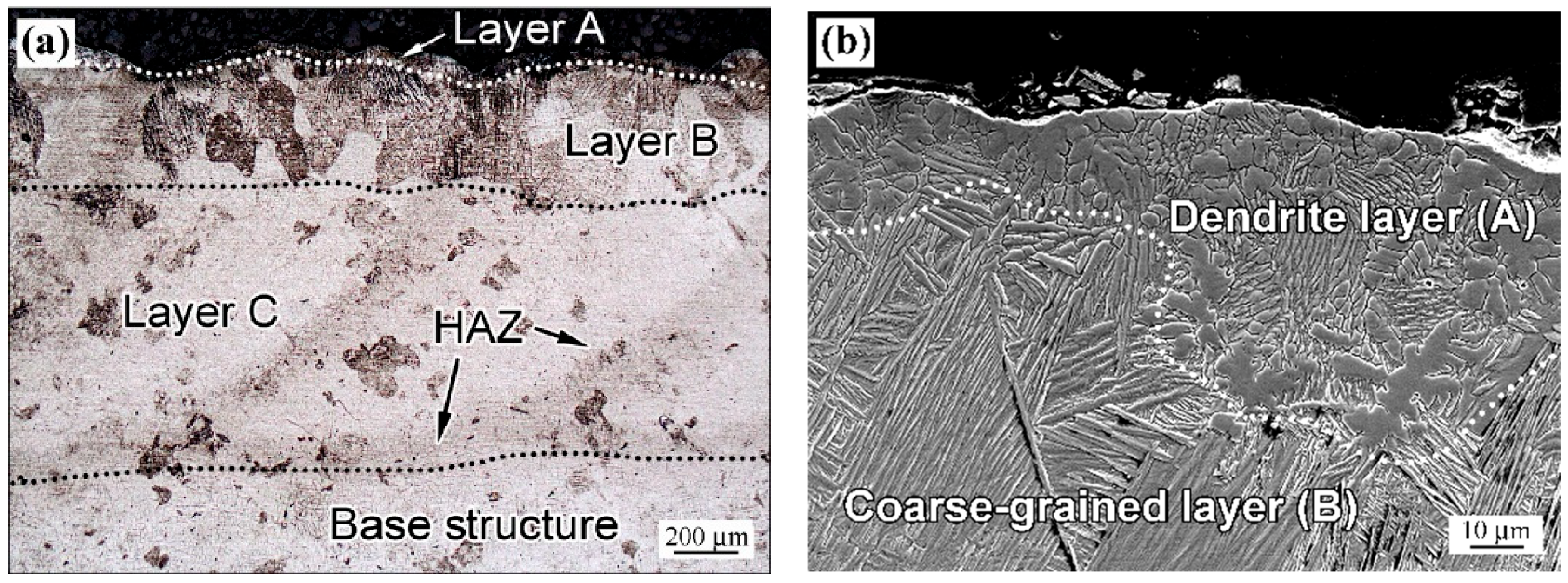
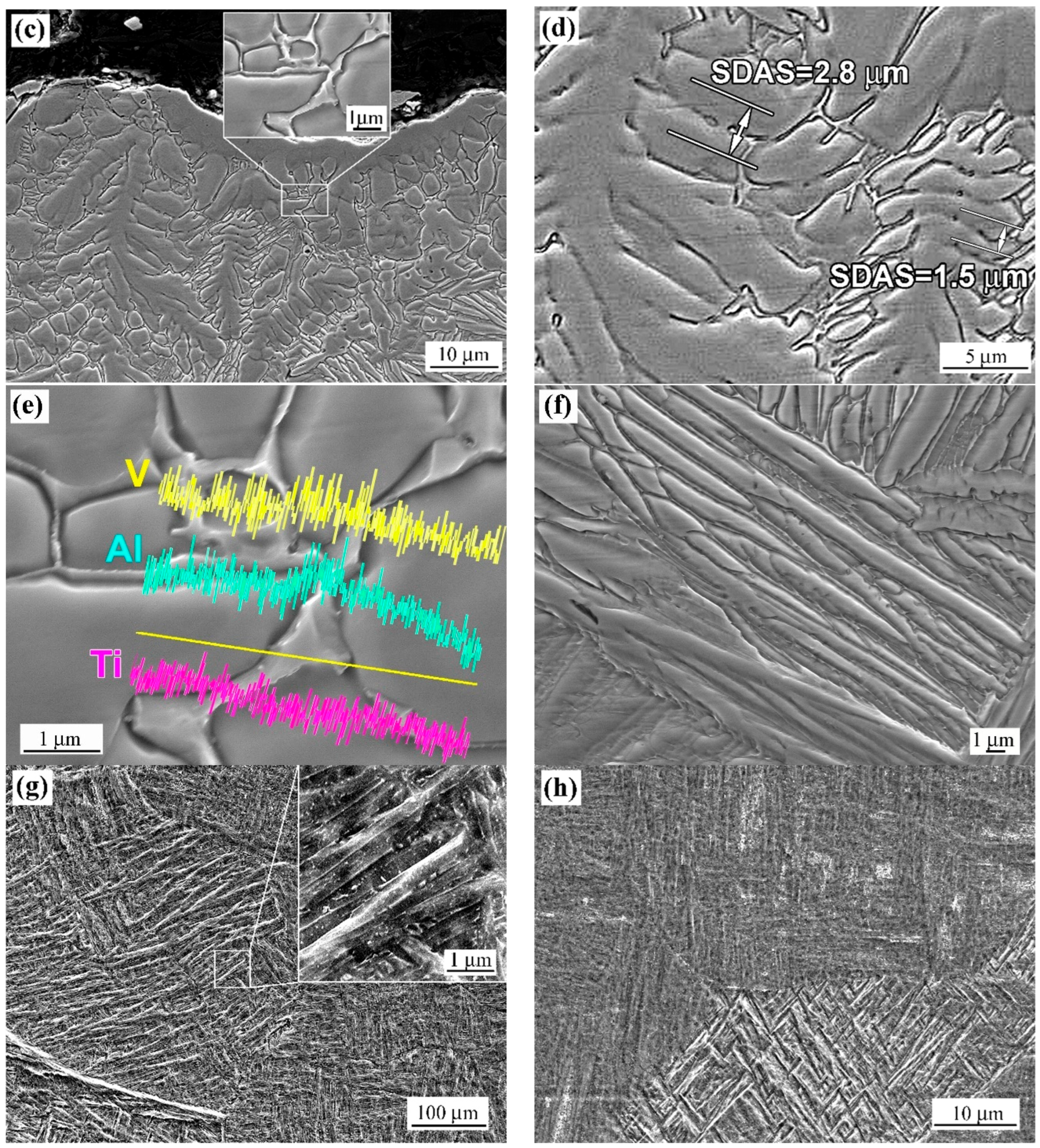
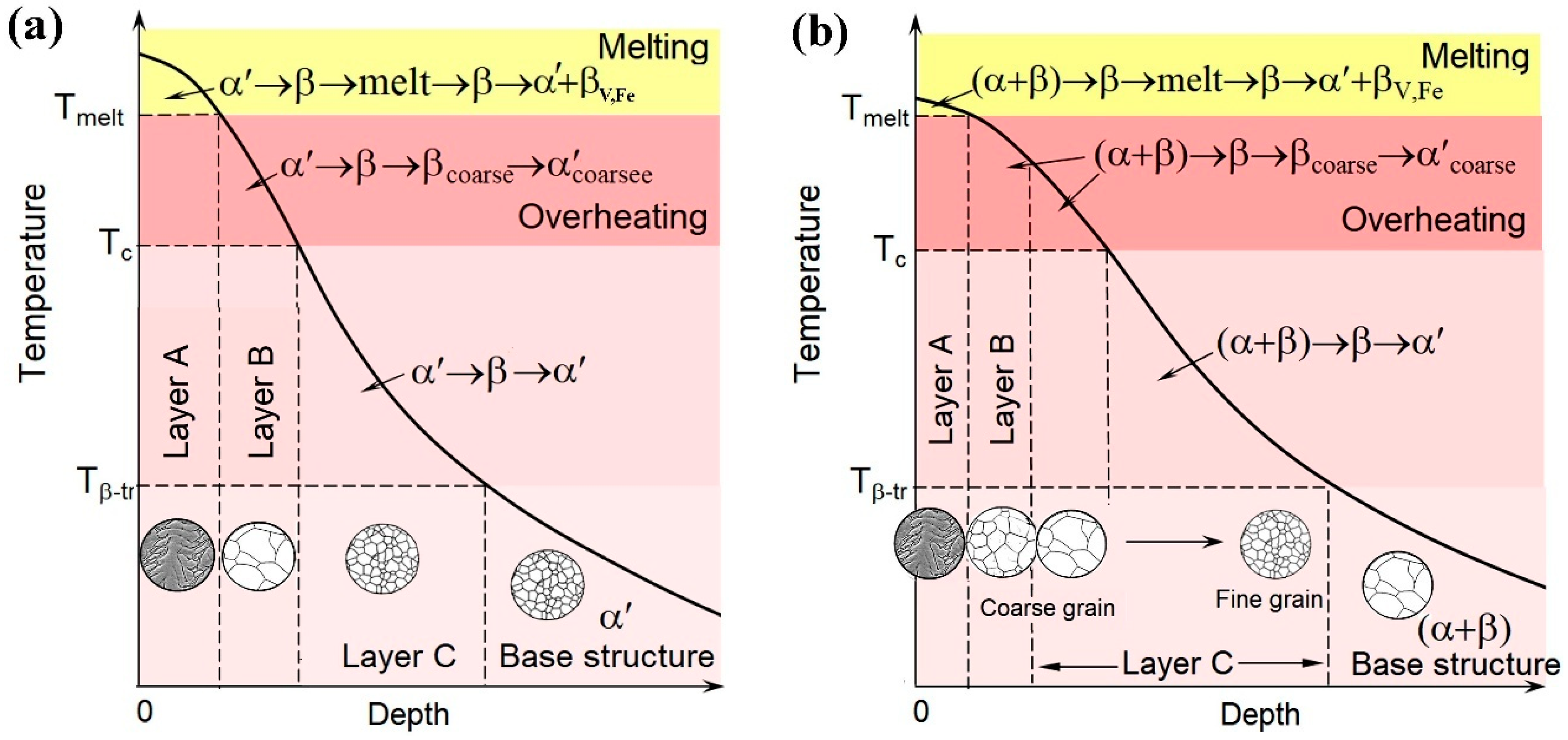
4.1. Microstructure Modification Mechanisms
- (a)
- LPBF sample: dendritic zone A → coarse-grained zone B (grain size of 140–175 µm) → fine-grained zone C → base (unmodified) structure.
- (b)
- WR sample: dendritic zone A → medium-grained zone B (grain size of 75.1 µm) → zone C with variable grain size (ranging from 123 µm to 16.5 µm) → base structure.
- (a)
- β-annealing → lamellar α-phase and retained β-phase; and/or
- (b)
- quenching → metastable β-solid solution enriched in α-stabilising Al (around the dendrites) [90].
4.2. Effect of LSM on the Hardness of LPBF and WR Ti-6-4
4.3. Effect of LSM on the Corrosion Performance of LPBF and WR Ti-6-4
- (a)
- These oxides originate from Fe and Si impurities in the Ti-6-4 alloy. The low density of Si (almost half that of Ti) facilitates its diffusion to the surface, where it readily binds with oxygen. Although SiO2 has slightly less negative free energy of formation than TiO2 [101], its higher electronegativity compared to Ti [102] enables stronger covalent bonding with O. Similarly, Al, being lighter and more reactive with oxygen [101], forms oxides more readily than Ti. However, Al-silicates are less resistant to aqueous Cl− than TiO2, especially when bearing iron [103], consistent with Cl detection in them (Figure 12 and Figure 13). LSM has also been reported to cause the formation of Al-silicate layers on Inconel-718 [104] and 316L stainless steel [105].
- (b)
- These oxides may also derive from residual sandblasting media (aluminium silicate with Fe2O3, Cao and K2O) used to remove any surface contamination during the laser treatment. Indeed, the XRD of the LSM specimens revealed a minor presence of Al2SiO5 (Figure 7a), while SEM/EDX showed angular Si-, Al-, Fe- and O-rich particles (Figure 4). RS on the corroded surfaces identified aluminium silicate hydrates, such as kaolinite, likely formed through the transformation of Al2SiO5 (Figure 7a) to kaolinite (Figure 14) [106]. Kaolin minerals may also form through weathering of muscovite and smectites [107], whereas smectites and micas often coexist with kaolinite [108].
4.4. Comparison of LSM-WR and LSM-LBPF Ti-6-4 After Corrosion
5. Conclusions
- Laser processing produced modified layers of 1250–1350 µm (LPBF) and 1530–1600 µm (WR) thickness. The modified layers presented a structural gradient—from a thin (tens of micrometres), remelted dendritic zone to a coarse acicular structure, and further to fine-acicular α′ martensite. Within the melted layer, microhardness increased to 655–680 HV, attributed to lattice parameter expansion, likely due to oxygen interstitial dissolution, increased lattice distortion owing to crystallite refinement and higher dislocation density, and aluminium silicate surface oxides originating from oxidation of Al and impurities during LSM or sandblasting residues.
- The laser surface-melted (LSM) specimens (both LPBF and wrought) exhibited higher corrosion rates and less stable passive films compared to the untreated ones. This deterioration is primarily attributed to several factors induced by LSM: increased surface roughness, enhanced martensite formation, elevated residual stresses, lattice strain and dislocation density, and microstructural heterogeneity (dendrite (martensite)/interdendritic (oversaturated βTi)) in the melted top layer. Additionally, the presence of aluminium silicate surface films containing iron further weakened passivity.
- Raman spectroscopy supported by EDX indicated that post-polarisation surface films were mainly composed of TiO2 polymorphs, dominated by rutile. Aluminium silicate hydrates—primarily kaolin-type, with minor mica (muscovite), and smectite (montmorillonite)—were also present, especially in the rough zones of the LSM-LPBF specimens.
- Nevertheless, the LSM specimens (LPBF and wrought) presented very low corrosion current densities (order of 10−4 mA/cm2) and true passivity ((order of 10−3–10−4 mA/cm2) without breakdown in the studied range of potentials (up to 1000 mV vs. Ag/AgCl). Furthermore, both LSM Ti-6-4 alloys appeared resistant to localised corrosion, primarily owing to the extensive presence of TiO2 polymorphs.
- The LSM-LPBF Ti-6-4 specimens presented slightly inferior resistance to general corrosion compared with the wrought counterparts in SBF at 37 °C, mainly due to (a) intensive surface relief, with alternating smooth and rough zones; (b) differences in phase and chemical composition between the alternating zones; and (c) higher microstrains and dislocation density.
- Sandblasting with aluminium silicates as the final surface treatment should be followed by meticulous surface cleaning as it leaves oxide residues that are susceptible to Cl− penetration and hydrolysis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AM | Additive manufacturing |
| CSAM | Cold spraying additive manufacturing |
| DED | Directed energy deposition |
| EBSD | Electron backscattered diffraction |
| EDX | Energy-dispersive X-ray spectroscopy |
| HAZ | Heat-affected zone |
| LMD | Laser metal wire/powder deposition |
| LPBF | Laser powder bed fusion |
| LSM | Laser surface melting |
| LSR | Laser surface remelting |
| RS | Raman spectroscopy |
| SBF | Simulated body fluid |
| Ti-6-4 | Ti-6Al-4V |
| TZ | Treated zone |
| WAAM | Wire arc additive manufacturing |
| WR | Wrought |
Appendix A. Some Extra Details in the XRD Patterns and Raman Spectra Illustrated in Figure 7 and Figure 14, Respectively
References
- ASTM F136; Standard Specification for Wrought Titanium-6Aluminum-4Vanadium ELI (Extra Low Interstitial) Alloy for Surgical Implant Applications (UNS R56401). ASTM International: West Conshohocken, PA, USA, 2021.
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti Based Biomaterials, the Ultimate Choice for Orthopaedic Implants—A Review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Abd-Elaziem, W.; Darwish, M.A.; Hamada, A.; Daoush, W.M. Titanium-Based Alloys and Composites for Orthopedic Implants Applications: A Comprehensive Review. Mater. Des. 2024, 241, 112850. [Google Scholar] [CrossRef]
- Adesina, O.; Popoola, P.; Fatoba, O. Laser Surface Modification—A Focus on the Wear Degradation of Titanium Alloy. In Fiber Laser; Paul, M.C., Ed.; IntechOpen: London, UK, 2016; pp. 367–381. [Google Scholar] [CrossRef]
- Chen, L.; Tong, Z.; Luo, H.; Qu, Y.; Gu, X.; Si, M. Titanium Particles in Peri-Implantitis: Distribution, Pathogenesis and Prospects. Int. J. Oral Sci. 2023, 15, 49. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General Review of Titanium Toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Chen, L.-Y.; Wang, L. Surface Modification of Titanium and Titanium Alloys: Technologies, Developments, and Future Interests. Adv. Eng. Mater. 2020, 22, 1901258. [Google Scholar] [CrossRef]
- Zhang, L.-C.; Chen, L.-Y.; Zhou, S.; Luo, Z. Powder Bed Fusion Manufacturing of Beta-Type Titanium Alloys for Biomedical Implant Applications: A Review. J. Alloys Compd. 2023, 936, 168099. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Pramanik, A.; Basak, A.K.; Dong, Y.; Prakash, C.; Debnath, S.; Shankar, S.; Jawahir, I.S.; Dixit, S.; Buddhi, D. A Critical Review on Additive Manufacturing of Ti-6Al-4V Alloy: Microstructure and Mechanical Properties. J. Mater. Res. Technol. 2022, 18, 4641–4661. [Google Scholar] [CrossRef]
- Tshephe, T.S.; Akinwamide, S.O.; Olevsky, E.; Olubambi, P.A. Additive Manufacturing of Titanium-Based Alloys—A Review of Methods, Properties, Challenges, and Prospects. Heliyon 2022, 8, e09041. [Google Scholar] [CrossRef]
- Jin, B.; Wang, Q.; Zhao, L.; Pan, A.; Ding, X.; Gao, W.; Song, Y.; Zhang, X. A Review of Additive Manufacturing Techniques and Post-Processing for High-Temperature Titanium Alloys. Metals 2023, 13, 1327. [Google Scholar] [CrossRef]
- Liu, S.; Shin, Y. Additive Manufacturing of Ti6Al4V Alloy: A Review. Mater. Des. 2019, 164, 107552. [Google Scholar] [CrossRef]
- Neikter, M.; Åkerfeldt, P.; Pederson, R.; Antti, M.L.; Sandell, V. Microstructural Characterization and Comparison of Ti-6Al-4V Manufactured with Different Additive Manufacturing Processes. Mater. Charact. 2018, 143, 68–75. [Google Scholar] [CrossRef]
- Balbaa, M.; Mekhiel, S.; Elbestawi, M.; McIsaac, J. On Selective Laser Melting of Inconel 718: Densification, Surface Roughness, and Residual Stresses. Mater. Des. 2020, 193, 108818. [Google Scholar] [CrossRef]
- Menci, G.; Gökhan, A.; Waugh, D.G.; Lawrence, J.; Previtali, B. Laser Surface Texturing of β-Ti Alloy for Orthopaedics: Effect of Different Wavelengths and Pulse Durations. Appl. Surf. Sci. 2019, 489, 175–186. [Google Scholar] [CrossRef]
- Laketić, S.; Rakin, M.; Momčilović, M.; Ciganović, J.; Veljović, Đ.; Trifković, B. Surface Modifications of Biometallic Commercially Pure Ti and Ti-13Nb-13Zr Alloy by Picosecond Nd:YAG Laser. Int. J. Miner. Metall. Mater. 2021, 28, 285–295. [Google Scholar] [CrossRef]
- Sypniewska, J.; Szkodo, M. Influence of Laser Modification on the Surface Character of Biomaterials: Titanium and Its Alloys—A Review. Coatings 2022, 12, 1371. [Google Scholar] [CrossRef]
- Yan, X.; Cao, W.; Li, H. Biomedical Alloys and Physical Surface Modifications: A Mini-Review. Materials 2022, 15, 66. [Google Scholar] [CrossRef]
- Liu, W.; Liu, S.; Wang, L. Surface Modification of Biomedical Titanium Alloy: Micromorphology, Microstructure Evolution and Biomedical Applications. Coatings 2019, 9, 249. [Google Scholar] [CrossRef]
- Wei, G.; Tan, M.; Attarilar, S.; Li, J.; Uglov, V.V.; Wang, B.; Liu, J.; Lu, L.; Wang, L. An Overview of Surface Modification, A Way toward Fabrication of Nascent Biomedical Ti–6Al–4V Alloys. J. Mater. Res. Technol. 2023, 24, 5896–5921. [Google Scholar] [CrossRef]
- Ge, J.; Pillay, S.; Ning, H. Post-Process Treatments for Additive-Manufactured Metallic Structures: A Comprehensive Review. J. Mater. Eng. Perform. 2023, 32, 7073–7122. [Google Scholar] [CrossRef]
- Das, B.; Srivastava, S.K.; Manna, I.; Majumdar, J.D. Mechanically Tailored Surface of Titanium Based Alloy (Ti6Al4V) by Laser Surface Treatment. Surf. Coat. Technol. 2024, 479, 130560. [Google Scholar] [CrossRef]
- Jażdżewska, M.; Kwidzińska, D.B.; Seyda, W.; Fydrych, D.; Zieliński, A. Mechanical Properties and Residual Stress Measurements of Grade IV Titanium and Ti-6Al-4V and Ti-13Nb-13Zr Titanium Alloys after Laser Treatment. Materials 2021, 14, 6316. [Google Scholar] [CrossRef]
- Lu, Y.J.; Zhang, Z.L.; Liu, Y.J.; Yu, C.; Zhang, X.; Liu, X.C. Improving Mechanical Properties and Corrosion Behavior of Biomedical Ti-3Zr-2Sn-3Mo-25Nb Alloy through Laser Surface Remelting. Surf. Coat. Technol. 2024, 490, 131135. [Google Scholar] [CrossRef]
- Huang, S.; Zeng, J.; Wang, W.; Zhao, Z. Study on Laser Polishing of Ti6Al4V Fabricated by Selective Laser Melting. Micromachines 2024, 15, 336. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, S.R.; Abdelfatah, A. Corrosion Behavior of a Laser Surface-Treated Alpha-Beta 6/4 Titanium Alloy. Metallogr. Microstruct. Anal. 2020, 9, 553–560. [Google Scholar] [CrossRef]
- Majumdar, J.D.; Manna, I. 21-Laser Surface Engineering of Titanium and Its Alloys for Improved Wear, Corrosion and High-Temperature Oxidation Resistance. In Laser Surface Engineering; Lawrence, J., Waugh, D.G., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 483–521. [Google Scholar] [CrossRef]
- Amaya-Vazquez, M.R.; Sánchez-Amaya, J.M.; Boukha, Z.; Botana, F.J. Microstructure, Microhardness and Corrosion Resistance of Remelted TiG2 and Ti6Al4V by a High Power Diode Laser. Corros. Sci. 2012, 56, 36–48. [Google Scholar] [CrossRef]
- Lekatou, A.G.; Efremenko, B.V.; Haoui, V.; Efremenko, V.G.; Emmanouilidou, S.; Zurnadzhy, V.I.; Petryshynets, I.; Chabak, Y.G.; Sili, I.I. Microstructure, Electrochemical, Wear and Corrosive Wear Performance of Laser-Based Powder Bed Fusion and Wrought Biomedical Ti-6Al-4V Alloys. Trans. Nonferrous Met. Soc. China 2025, 35, 2612–2631. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, L.-C.; Zhang, J.; Chen, Q.; Wu, M. Corrosion Behavior of Selective Laser Melted Ti-6Al-4V Alloy in NaCl Solution. Corros. Sci. 2016, 102, 484–489. [Google Scholar] [CrossRef]
- Metikos-Hukovic, M.; Kwokal, A.; Piljac, J. The Influence of Niobium and Vanadium on Passivity of Titanium-Based Implants in Physiological Solution. Biomaterials 2003, 24, 3765–3775. [Google Scholar] [CrossRef]
- Mohazzab, B.F.; Jaleh, B.; Fattah-alhosseini, A.; Mahmoudi, F.; Momeni, A. Laser Surface Treatment of Pure Titanium: Microstructural Analysis, Wear Properties, and Corrosion Behavior of Titanium Carbide Coatings in Hank’s Physiological Solution. Surf. Interfaces 2020, 20, 100597. [Google Scholar] [CrossRef]
- Ataiwi, A.H.; Majed, R.A.; Muhsin, A.A. Effect of Laser Surface Modification on the Corrosion Resistance of Dental Alloys in Artificial Saliva Containing Alcoholic Beverages. Iraqi J. Laser 2019, 12, 43–52. [Google Scholar] [CrossRef]
- Jażdżewska, M.; Zieliński, A.; Skalski, I.; Majkowska-Marzec, B. Effects of the Preheating Laser Treatment on Microstructure and Corrosion Resistance of Ti6Al4V Bioalloy. Eng. Biomater. 2013, 16, 122–123. Available online: https://bibliotekanauki.pl/articles/284890.pdf (accessed on 26 February 2025).
- Gil, F.J.; Delgado, L.; Espinar, E.; Llamas, J.M. Corrosion and Corrosion-Fatigue Behavior of cp-Ti and Ti-6Al-4V Laser-Marked Biomaterials. J. Mater. Sci. Mater. Med. 2012, 23, 885–890. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, A.; Serbiński, W.; Majkowska, B.; Jażdżewska, M.; Skalski, I. Conditions on Corrosion Resistance of Non-Ferrous Alloys. Adv. Mater. Sci. 2009, 9, 22–28. [Google Scholar] [CrossRef]
- Singh, R.; Chowdhury, S.G.; Tiwari, S.K.; Dahotre, N.B. Laser Surface Processing of Ti6Al4V in Gaseous Nitrogen: Corrosion Performance in Physiological Solution. J. Mater. Sci. Mater. Med. 2008, 19, 1363–1369. [Google Scholar] [CrossRef]
- Khosroshahi, M.E.; Mahmoodi, M.; Saeedinasab, H.; Tahriri, M. Evaluation of Mechanical and Electrochemical Properties of Laser Surface Modified Ti–6Al–4V for Biomedical Applications: In Vitro Study. Surf. Eng. 2008, 24, 209–218. [Google Scholar] [CrossRef]
- Porwal, A.; Dhara, S.; Kumar, C.S. Surface Modification of LPBF Ti6Al4V through in Situ Nitrogen-Assisted Laser Surface Remelting. J. Manuf. Sci. Eng. 2025, 147, 031012. [Google Scholar] [CrossRef]
- Feng, W.; Wang, N.; Weng, F.; Su, J.; Lim, P.G.H.; Yan, L.; O’Neill, G.K. The Influence of Laser Surface Remelting on the In Vitro Cell Viability of Additively Manufactured Ti–6Al–4V Plates. Prog. Addit. Manuf. 2023, 8, 997–1005. [Google Scholar] [CrossRef]
- Lu, W.Q.; Liu, Y.J.; Wu, X.; Liu, X.C.; Wang, J.C. Corrosion and Passivation Behavior of Ti-6Al-4V Surfaces Treated with High-Energy Pulsed Laser: A Comparative Study of Cast and 3D-Printed Specimens in a NaCl Solution. Surf. Coat. Technol. 2023, 470, 129849. [Google Scholar] [CrossRef]
- Pradeep, G.V.K.; Duraiselvam, M.; Sivaprasad, K. Tribological Behavior of Laser Surface Melted γ-TiAl Fabricated by Electron Beam Additive Manufacturing. J. Mater. Eng. Perform. 2022, 31, 1009–1020. [Google Scholar] [CrossRef]
- Molatlhegi, G.; Hoosain, S.; Popoola, A.P.I.; Malatjia, N.; Pityana, S. The Effect of Laser Surface Re-melting on the Surface Roughness and Micro-Hardness of Selective Laser Melting (SLM) Fabricated Ti-6Al-4V Samples. S. Afr. Tydskr. Natuurwet. Tegnol. 2021, 40, 186–189. [Google Scholar] [CrossRef]
- Kahlin, M.; Ansell, H.; Basu, D.; Kerwin, A.; Newton, L.; Smith, B.; Moverare, J.J. Improved Fatigue Strength of Additively Manufactured Ti6Al4V by Surface Post Processing. Int. J. Fatigue 2020, 134, 105497. [Google Scholar] [CrossRef]
- Vaithilingam, J.; Goodridge, R.D.; Hague, R.J.M.; Christie, S.D.R.; Edmondson, S. The Effect of Laser Remelting on the Surface Chemistry of Ti6Al4V Components Fabricated by Selective Laser Melting. J. Mater. Process. Technol. 2016, 232, 1–8. [Google Scholar] [CrossRef]
- Efremenko, B.V.; Petryshynets, I.; Chabak, Y.G.; Zurnadzhy, V.I.; Wu, K.; Efremenko, V.G.; Fedun, V.I.; Kromka, F.; Kulyk, V.V. Structure and Wet-Sliding Characterization of a Laser Powder Bed Fusion Ti-6Al-4V Biomedical Alloy: Effect of Laser Surface Modification. Rom. J. Phys. 2024, 69, 613. [Google Scholar] [CrossRef]
- Sun, Z.; Annergren, I.; Pan, D.; Mai, T.A. Effect of Laser Surface Remelting on the Corrosion Behavior of Commercially Pure Titanium Sheet. Mater. Sci. Eng. A 2003, 345, 293–300. [Google Scholar] [CrossRef]
- ISO 11126; Preparation of Steel Substrates Before Application of Paints and Related Products—Specifications for Non-Metallic Blast-Cleaning Abrasives—Part 1: General Introduction and Classification. ISO: Geneva, Switzerland, 2025.
- Bellini, D.; Cencetti, C.; Meraner, J.; Stoppoloni, D.; D’Abusco, A.S.; Matricardi, P. An In-Situ Gelling System for Bone Regeneration of Osteochondral Defects. Eur. Polym. J. 2015, 72, 642–650. [Google Scholar] [CrossRef]
- Emmanouilidou, S.N.; Lekatou, A.G.; Papagiannopoulos, A.D.; Siaraka, Z.Z.; Tzala, I.E.; Lekatou, A.G. Effect of Nb Low-Alloying on the Microstructure, Corrosion and Wear Performance of a Co-28Cr-6Mo Alloy Fabricated by Vacuum Arc Melting. Int. J. Refract. Met. Hard Mater. 2025, 133, 107330. [Google Scholar] [CrossRef]
- Lekatou, A.G.; Sfikas, A.K.; Karantzalis, A.E. The Influence of the Fabrication Route on the Microstructure and Surface Degradation Properties of Al Reinforced by Al9Co2. Mater. Chem. Phys. 2017, 200, 33–39. [Google Scholar] [CrossRef]
- Lekatou, A.G.; Sioulas, D.; Grimanelis, D. Corrosion and Wear of Coatings Fabricated by HVOF-Spraying of Nanostructured and Conventional WC–10Co-4Cr Powders on Al7075-T6. Int. J. Refract. Met. Hard Mater. 2023, 112, 106164. [Google Scholar] [CrossRef]
- ASTM G102; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2015.
- Dietrich, K.; Diller, J.; Dubiez-Le Goff, S.; Bauer, D.; Forêt, P.; Witt, G. The Influence of Oxygen on the Chemical Composition and Mechanical Properties of Ti-6Al-4V during Laser Powder Bed Fusion (L-PBF). Addit. Manuf. 2020, 32, 100980. [Google Scholar] [CrossRef]
- Chabak, Y.; Efremenko, B.; Petryshynets, I.; Efremenko, V.; Lekatou, A.G.; Zurnadzhy, V.; Bogomol, I.; Fedun, V.; Koval’, K.; Pastukhova, T. Structural and Tribological Assessment of Biomedical 316 Stainless Steel Subjected to Pulsed-Plasma Surface Modification: Comparison of LPBF 3D Printing and Conventional Fabrication. Materials 2021, 14, 7671. [Google Scholar] [CrossRef] [PubMed]
- Ettefagh, A.H.; Guo, S.; Rauch, J. Corrosion Behavior of Additively Manufactured Ti-6Al-4V Parts and the Effect of Post Annealing. Addit. Manuf. 2019, 28, 252–258. [Google Scholar] [CrossRef]
- Efremenko, V.G.; Lekatou, A.G.; Chabak, Y.G.; Efremenko, B.V.; Petryshynets, I.; Zurnadzhy, V.I.; Emmanouilidou, S.; Vojtko, M. Micromechanical, Corrosion and Wet Sliding Wear Behaviours of Co-28Cr-6Mo Alloy: Wrought vs. LPBF. Mater. Today Commun. 2023, 35, 105936. [Google Scholar] [CrossRef]
- Efremenko, B.V.; Zurnadzhy, V.I.; Chabak, Y.G.; Efremenko, V.G.; Kudinova, K.V.; Mazur, V.A. A Comparison Study on the Effect of Counter Ball Material on Sliding Wear Response of SLM-Printed Biomedical 316L Steel. Mater. Today Proc. 2022, 66, 2587–2593. [Google Scholar] [CrossRef]
- Derimow, N.; Hrabe, N. Oxidation in Reused Powder Bed Fusion Additive Manufacturing Ti-6Al-4V Feedstock: A Brief Review. JOM 2021, 73, 3618–3638. [Google Scholar] [CrossRef]
- Li, Y.; Song, L.; Xie, P.; Cheng, M.; Xiao, H. Enhancing Hardness and Wear Performance of Laser Additive Manufactured Ti6Al4V Alloy through Achieving Ultrafine Microstructure. Materials 2020, 13, 1210. [Google Scholar] [CrossRef]
- Gubicza, J. Reliability and Interpretation of the Microstructural Parameters Determined by X-ray Line Profile Analysis for Nanostructured Materials. Eur. Phys. J. Spec. Top. 2022, 231, 4153–4165. [Google Scholar] [CrossRef]
- Aubry, S.; Rhee, M.; Hommes, G.; Bulatov, V.V.; Arsenlis, A. Dislocation dynamics in hexagonal close-packed crystals. J. Mech. Phys. Solids 2016, 94, 105–126. [Google Scholar] [CrossRef]
- Muiruri, A.; Maringa, M.; du Preez, W. Evaluation of Dislocation Densities in Various Microstructures of Additively Manufactured Ti6Al4V (ELI) by the Method of X-ray Diffraction. Materials 2020, 13, 5355. [Google Scholar] [CrossRef]
- Lekatou, A.G.; Tsouli, S. Cyclic Polarization of Corrugated Austenitic Stainless Steel Rebars in Acid Rain: Effect of Fly Ash, pH and Steel Type. Corros. Mater. Degrad. 2022, 3, 75–100. [Google Scholar] [CrossRef]
- Fontana, M.G. Corrosion Engineering, 3rd ed.; McGraw-Hill: New York, NY, USA, 1986; p. 172. ISBN 978-0070214637. [Google Scholar]
- Beard, M.A.; Ghita, O.R.; Evans, K.E. Using Raman spectroscopy tomonitor surface finish and roughness of components manufactured by selective laser sintering. J. Raman Spectrosc. 2011, 42, 744–748. [Google Scholar] [CrossRef]
- Medel-Ruiz, C.I.; Chiu, R.; Sevilla-Escoboza, J.R.; Casillas-Rodríguez, F.J. Nanoscale Surface Roughness Effects on Photoluminescence and Resonant Raman Scattering of Cadmium Telluride. Appl. Sci. 2024, 14, 7680. [Google Scholar] [CrossRef]
- Frank, O.; Zukalova, M.; Laskova, B.; Kürti, J.; Koltai, J.; Kavan, L. Raman Spectra of Titanium Dioxide (Anatase, Rutile) with Identified Oxygen Isotopes (16, 17, 18). Phys. Chem. Chem. Phys. 2012, 14, 14567–14572. [Google Scholar] [CrossRef]
- Tompsett, G.A.; Bowmaker, G.A.; Cooney, R.P.; Metson, J.B.; Rodgers, K.A.; Seakins, J.M. The Raman Spectrum of Brookite, TiO2 (Pbca, Z = 8). J. Raman Spectrosc. 1995, 26, 57–62. [Google Scholar] [CrossRef]
- Iliev, M.N.; Hadjiev, V.G.; Litvinchuk, A.P. Raman and Infrared Spectra of Brookite (TiO2): Experiment and Theory. Vib. Spectrosc. 2013, 64, 148–152. [Google Scholar] [CrossRef]
- Tian, F.; Zhang, Y.; Zhang, J.; Pan, C. Raman Spectroscopy: A New Approach to Measure the Percentage of Anatase TiO2 Exposed (001) Facets. J. Phys. Chem. C 2012, 116, 7515–7519. [Google Scholar] [CrossRef]
- Kloprogge, J.T. Raman and Infrared Spectroscopies of Intercalated Kaolinite Groups Minerals. In Infrared and Raman Spectroscopies of Clay Minerals; Madejova, J., Bergaya, F., Gates, W., Kloprogge, J.T., Eds.; Developments in Clay Science Series; Elsevier: Amsterdam, The Netherlands, 2017; Volume 8, pp. 343–410. ISBN 978-0081003558. [Google Scholar]
- Frost, R.L.; Tran, T.H.; Rintoul, L.; Kristof, J. Raman Microscopy of Dickite, Kaolinite and Their Intercalates. Analyst 1998, 123, 611–616. [Google Scholar] [CrossRef]
- Ritz, M.; Vaculíková, L.; Kupková, J.; Plevová, E.; Bartoňová, L. Different Level of Fluorescence in Raman Spectra of Montmorillonites. Vib. Spectrosc. 2016, 84, 7–15. [Google Scholar] [CrossRef]
- McKeown, D.A.; Bell, M.I.; Etz, E.S. Vibrational Analysis of the Dioctahedral Mica: 2M1 Muscovite. Am. Mineral. 1999, 84, 1041–1048. [Google Scholar] [CrossRef]
- Lekatou, A.; Zois, D.; Karantzalis, A.E.; Grimanelis, D. Electrochemical Behaviour of Cermet Coatings with a Bond Coat on Al7075: Pseudopassivity, Localized Corrosion and Galvanic Effect Considerations in a Saline Environment. Corros. Sci. 2010, 52, 2616–2635. [Google Scholar] [CrossRef]
- Sfikas, A.K.; Lekatou, A.G. Electrochemical Behavior of Al–Al9Co2 Alloys in Sulfuric Acid. Corros. Mater. Degrad. 2020, 1, 249–272. [Google Scholar] [CrossRef]
- Addison, O.; Davenport, A.J.; Newport, R.J.; Kalra, S.; Monir, M.; Mosselmans, J.F.W.; Proops, D.; Martin, R.A. Do “Passive” Medical Titanium Surfaces Deteriorate in Service in the Absence of Wear? J. R. Soc. Interface 2012, 9, 3161–3164. [Google Scholar] [CrossRef] [PubMed]
- Kuphasuk, C.; Oshida, Y.; Andres, C.J.; Hovijitra, S.T.; Barco, M.T.; Brown, D.T. Electrochemical Corrosion of Titanium and Titanium-Based Alloys. J. Prosthet. Dent. 2001, 85, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Madapana, D.; Bathe, R.; Manna, I.; Majumdar, J.D. Effect of Process Parameters on the Corrosion Kinetics and Mechanism of Nanosecond Laser Surface Structured Titanium Alloy (Ti6Al4V). Appl. Surf. Sci. Adv. 2024, 20, 100580. [Google Scholar] [CrossRef]
- Ghasemi, A.; Fereiduni, E.; Balbaa, M.; Elbestawi, M.; Habibi, S. Unraveling the Low Thermal Conductivity of the LPBF Fabricated Pure Al, AlSi12, and AlSi10Mg Alloys through Substrate Preheating. Addit. Manuf. 2022, 59, 103148. [Google Scholar] [CrossRef]
- Bartsch, K.; Bossen, B.; Chaudhary, W.; Landry, M.; Herzog, D. Thermal Conductivity of Ti-6Al-4V in Laser Powder Bed Fusion. Front. Mech. Eng. 2022, 8, 830104. [Google Scholar] [CrossRef]
- Strumza, E.; Landau, P.; Kimmel, G.; Ganor, Y.I.; Yeheskel, O.; Hayun, S. Thermophysical Properties of Ti-6Al-4V Fabricated by Powder Bed Fusion Methods. Addit. Manuf. 2022, 58, 103045. [Google Scholar] [CrossRef]
- Bolzoni, L.; Ruiz-Navas, E.M.; Gordo, E. Flexural Properties, Thermal Conductivity and Electrical Resistivity of Prealloyed and Master Alloy Addition Powder Metallurgy Ti–6Al–4V. Mater. Des. 2013, 52, 888–895. [Google Scholar] [CrossRef]
- Saini, A.; Pabla, B.S.; Dhami, S.S. Developments in Cutting Tool Technology in Improving Machinability of Ti6Al4V Alloy: A Review. Proc. Inst. Mech. Eng. Part B 2016, 230, 1977–1989. [Google Scholar] [CrossRef]
- Yang, J.; Yu, H.; Yin, J.; Gao, M.; Wang, Z.; Zeng, X. Formation and Control of Martensite in Ti-6Al-4V Alloy Produced by Selective Laser Melting. Mater. Des. 2016, 108, 308–318. [Google Scholar] [CrossRef]
- Kenel, C.; Grolimund, D.; Li, X.; Panepucci, E.; Samson, V.A.; Sanchez, D.F.; Marone, F.; Leinenbach, C. In Situ Investigation of Phase Transformations in Ti-6Al-4V under Additive Manufacturing Conditions Combining Laser Melting and High-Speed Micro-X-ray Diffraction. Sci. Rep. 2017, 7, 16358. [Google Scholar] [CrossRef]
- Doridot, E.; Hans, S.; Jardy, A.; Bellot, J.-P. Industrial Applications of Modelling Tools to Simulate the PAMCHR Casting and VAR Process for Ti64. MATEC Web Conf. 2020, 321, 10011. [Google Scholar] [CrossRef]
- Kurz, W.; Fisher, D.J. Fundamentals of Solidification, 4th ed.; Chapters 4–5; Trans Tech Publications: Aedermannsdorf, Switzerland, 1984; ISBN 978-0878495221. [Google Scholar]
- Lekatou, A. Engineering Alloys; Papasotiriou Publications: Athens, Greece, 2005. (In Greek) [Google Scholar]
- Mizukami, H.; Shirai, Y.; Kawakami, A.; Mitchell, A. Solidification Behavior of Ti-6Al-4V Alloy. ISIJ Int. 2020, 60, 2455–2461. [Google Scholar] [CrossRef]
- Ding, L.; Hu, R.; Gu, Y.; Zhou, D.; Chen, F.; Zhou, L.; Chang, H. Effect of Fe Content on the As-Cast Microstructures of Ti–6Al–4V–xFe Alloys. Metals 2020, 10, 989. [Google Scholar] [CrossRef]
- Mitchell, A.; Kawakami, A.; Cockcroft, S.L. Segregation in Titanium Alloy Ingots. High Temp. Mater. Process. 2007, 26, 59–77. [Google Scholar] [CrossRef]
- Montanari, R.; Costanza, G.; Tata, M.E.; Testani, C. Lattice Expansion of Ti-6Al-4V by Nitrogen and Oxygen Absorption. Mater. Charact. 2008, 59, 334–337. [Google Scholar] [CrossRef]
- Fang, Z.Z.; Pamore, J.D.; Sun, P.; Chadran, K.S.R.; Zhang, Y.; Xia, Y.; Cao, F.; Koopman, M. Powder Metallurgy of Titanium—Past, Present, and Future. Int. Mater. Rev. 2018, 63, 407–459. [Google Scholar] [CrossRef]
- Basak, S.; Lee, S.-H.; Lee, J.-R.; Kim, D.-H.; Lee, J.H.; Byun, M.; Kim, D.-H. Modification of Mechanical Properties of Ti–6Al–4V Using L-PBF for Anatomical Plates. Metals 2025, 15, 32. [Google Scholar] [CrossRef]
- Schumann, W. Gemstones of the World, Revised & Expanded ed.; Sterling Publishing Co., Inc.: New York, NY, USA, 1997; p. 21. ISBN 978-0806994611. [Google Scholar]
- Dahotre, N.B.; Harimkar, S. Laser Fabrication and Machining of Materials; Springer: New York, NY, USA, 2008; p. 558. ISBN 978-0-387-72344-0. [Google Scholar] [CrossRef]
- Pham, D.-P.; Tran, H.-C. Multi-Physics Simulation for Predicting Surface Roughness of Laser Powder Bed Fused Parts after Laser Polishing. Addit. Manuf. 2024, 94, 104486. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, N.; Yang, X.; Zhang, R.; Feng, Q. Microstructure and Inclusion of Ti–6Al–4V Fabricated by Selective Laser Melting. Front. Mater. Sci. 2016, 10, 428–431. [Google Scholar] [CrossRef]
- Chase, M.W. (Ed.) NIST-JANAF Thermochemical Tables, 4th ed.; Journal of Physical and Chemical Reference Data, Monograph 9; American Chemical Society and American Institute of Physics: Washington, DC, USA, 1998; ISBN 978-1563968310. [Google Scholar]
- National Center for Biotechnology Information. Electronegativity in the Periodic Table of Elements. Available online: https://pubchem.ncbi.nlm.nih.gov/periodic-table/electronegativity (accessed on 26 February 2025).
- Frankel, G.S.; Vienna, J.D.; Lian, J.; Scully, J.R.; Gin, S.; Ryan, J.V.; Wang, J.; Kim, S.H.; Windl, W.; Du, J. A Comparative Review of the Aqueous Corrosion of Glasses, Crystalline Ceramics, and Metals. npj Mater. Degrad. 2018, 2, 15. [Google Scholar] [CrossRef]
- Efremenko, V.G.; Lekatou, A.G.; Efremenko, B.V.; Chabak, Y.G.; Petryshynets, I.; Margariti, D.; Emmanouilidou, S.N.; Puchy, V. Laser Beam Surface Modification of Additively Manufactured Ni-Based Superalloy: Correlations of Microstructure and Tribological/Corrosion Properties. J. Mater. Res. Technol. 2025, 35, 4390–4411. [Google Scholar] [CrossRef]
- Carboni, C.; Peyre, P.; Béranger, G.; Lemaitre, C. Influence of High Power Diode Laser Surface Melting on the Pitting Corrosion Resistance of Type 316L Stainless Steel. J. Mater. Sci. 2002, 37, 3715–3723. [Google Scholar] [CrossRef]
- Jiménez-Millán, J.; Velilla, N.; Vázquez, M. Two-Stage Formation of Kaolinite in Shear-Zone Slates, Southern Iberian Massif, SE Spain. Clay Miner. 2007, 42, 273–286. [Google Scholar] [CrossRef]
- Dill, H.G. Kaolin: Soil, Rock and Ore from the Mineral to the Magmatic, Sedimentary and Metamorphic Environments. Earth-Sci. Rev. 2016, 161, 16–129. [Google Scholar] [CrossRef]
- Rengasamy, P. Substitution of Iron and Titanium in Kaolinites. Clays Clay Miner. 1976, 24, 265–266. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Chen, L.-Y.; Qin, P.; Li, R.; Zang, Q.; Peng, J.; Zhang, L.; Lu, S.; Wang, L.; Zhang, L.-C. Metastable Pitting Corrosion Behavior of Laser Powder Bed Fusion Produced Ti-6Al-4V in Hank’s Solution. Corros. Sci. 2022, 203, 110333. [Google Scholar] [CrossRef]
- Vignal, V.; Delrue, O.; Heintz, O.; Peultier, J. Influence of the Passive Film Properties and Residual Stresses on the Micro-Electrochemical Behavior of Duplex Stainless Steels. Electrochim. Acta 2010, 55, 7118–7125. [Google Scholar] [CrossRef]
- Popov, K.I.; Nikolić, N.D.; Živković, P.M.; Branković, G. The Effect of the Electrode Surface Roughness at Low Level of Coarseness on the Polarization Characteristics of Electrochemical Processes. Electrochim. Acta 2010, 55, 1919–1925. [Google Scholar] [CrossRef]
- Gnilitskyi, I.; Bellucci, S.; Marrani, A.G.; Shepida, M.; Mazur, A.; Zozulya, G.; Kordan, V.; Babizhetskyy, V.; Sahraoui, B.; Kuntyi, O. Femtosecond Laser-Induced Nano- and Microstructuring of Cu Electrodes for CO2 Electroreduction in Acetonitrile Medium. Sci. Rep. 2023, 13, 8837. [Google Scholar] [CrossRef]
- Manjaiah, M.; Laubscher, R.F. Review of the Surface Modifications of Titanium Alloys for Biomedical Applications. Mater. Technol. 2017, 51, 181–193. [Google Scholar] [CrossRef]
- Karacs, A.; Joob Fancsaly, A.; Divinyi, T.; Pet, G.; Kovách, G. Morphological and Animal Study of Titanium Dental Implant Surface Induced by Blasting and High Intensity Pulsed Nd-Glass Laser. Mater. Sci. Eng. C 2003, 23, 431–435. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, C. In-Situ Development of a Sandwich Microstructure with Enhanced Ductility by Laser Reheating of a Laser Melted Titanium Alloy. Sci. Rep. 2020, 10, 15870. [Google Scholar] [CrossRef]

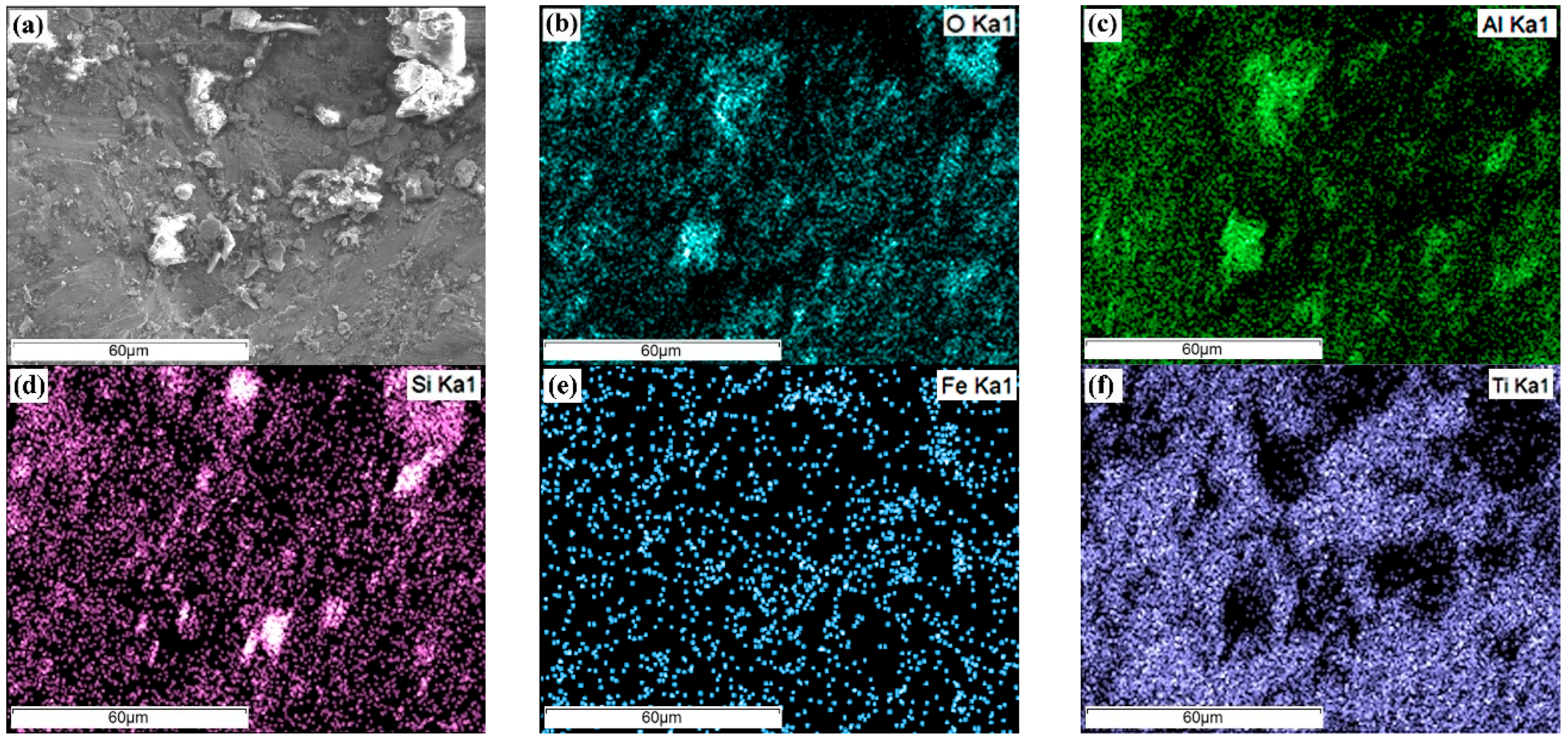
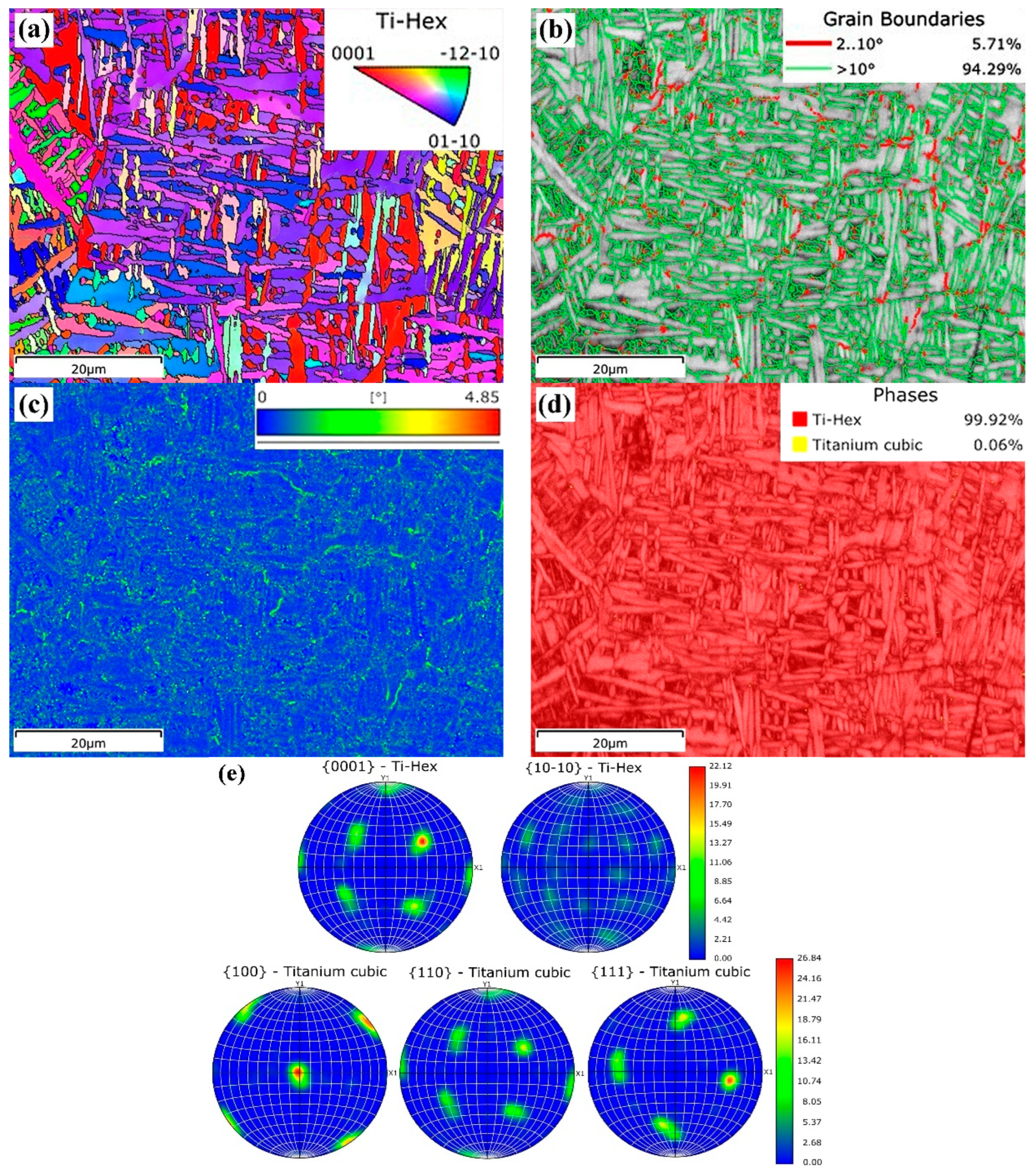
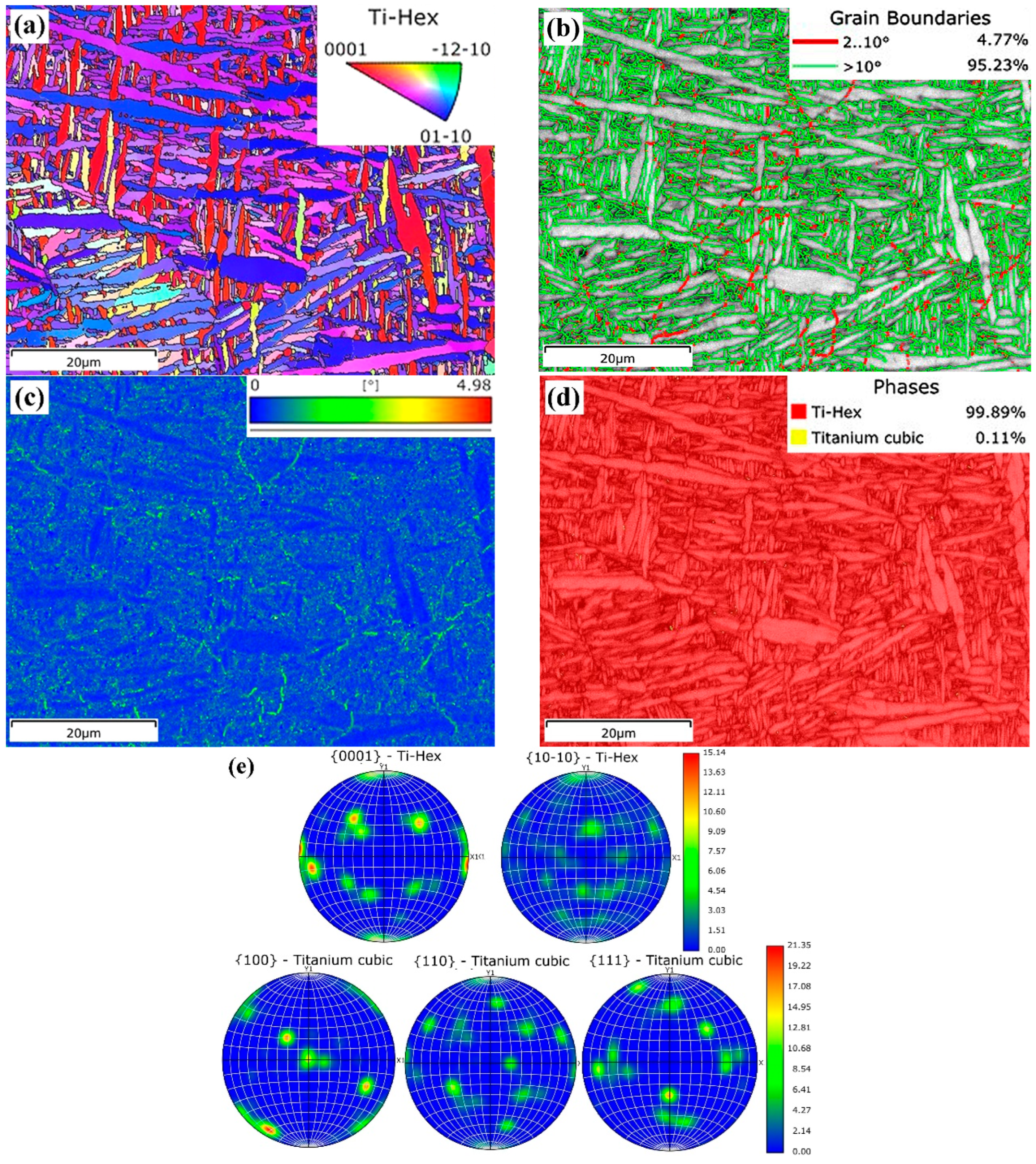
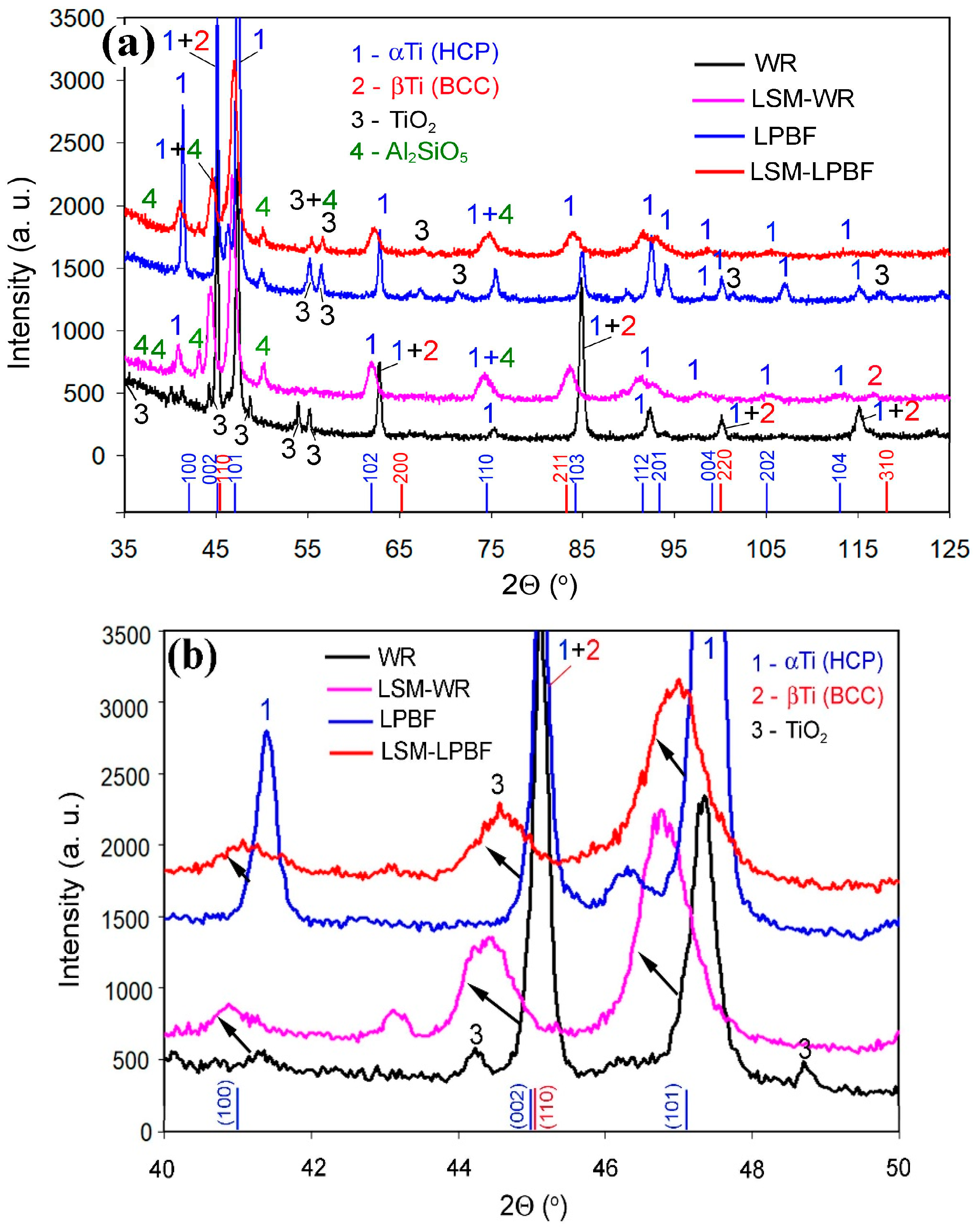



| Specimen | Phase | Al | V | Si | Fe | Ti |
|---|---|---|---|---|---|---|
| LSM-LPBF | Dendrite | 3.8 ± 0.4 | 1.7 ± 0.6 | 0.6 ± 0.1 | – | 93.9 ± 1.1 |
| Interdendritic network | 8.3 ± 0.2 | 6.9 ± 0.3 | 0.7 ± 0.3 | 1.0 ± 0.3 | 83.1 ± 0.1 | |
| LSM-WR | Dendrite | 6.0 ± 0.3 | 2.7 ± 0.1 | 1.1 ± 0.2 | – | 90.2 ± 0.2 |
| Interdendritic network | 6.4 ± 0.2 | 10.3 ± 0.3 | 0.9 ± 0.2 | 0.3 ± 0.1 | 82.1 ± 0.1 |
| α-Ti Line | WR | LSM-WR | (LSM-WR)/WR | LPBF | LSM-LPBF | (LSM-LPBF)/LPBF |
|---|---|---|---|---|---|---|
| (100) | 0.47 | 0.50 | 1.06 | 0.34 | 0.77 | 2.26 |
| (101) | 0.42 | 0.68 | 1.62 | 0.29 | 0.87 | 3.00 |
| (102) | 0.43 | 1.00 | 2.33 | 0.37 | 1.07 | 2.89 |
| (110) | 0.63 | 1.40 | 2.22 | 0.56 | 1.41 | 2.52 |
| (112) | 0.73 | 2.47 | 3.38 | 0.67 | 2.50 | 3.73 |
| Specimen | a (nm) | c (nm) | c/a | D (nm) | Microstrains | ρXRD (m−2) |
|---|---|---|---|---|---|---|
| WR | 0.2935 ± 0.193 × 10−4 | 0.4676 ± 0.195 × 10−4 | 1.593 | 57.2 ± 2.1 | 0.0014 ± 6.34 × 10−5 | 1.57 × 1014 |
| LSM-WR | 0.2964 ± 0.287 × 10−4 | 0.4744 ± 0.577 × 10−4 | 1.611 | 21.5 ± 0.6 | 0.0046 ± 7.70 × 10−5 | 1.37 × 1015 |
| LPBF | 0.2931 ± 0.114 × 10−4 | 0.4675 ± 0.269 × 10−4 | 1.595 | 88.3 ± 5.4 | 0.0018 ± 5.34 × 10−5 | 1.31 × 1014 |
| LSM-LPBF | 0.2956 ± 0.344 × 10−4 | 0.4720 ± 0.878 × 10−4 | 1.597 | 16.7 ± 0.3 | 0.0053 ± 2.76 × 10−5 | 2.03 × 1015 |
| Values | LPBF | LSM-LPBF | WR | LSM-WR |
|---|---|---|---|---|
| Ecorr (mV, Ag/AgCl) | −468 ± 41 | −110 ± 8 | −489 ± 23 | −157 ± 2 |
| Ea/c tr (mV, Ag/AgCl) | 130 ± 46 | 432 ± 47 | 109 ± 24 | 503 ± 47 |
| Ecp (mV, Ag/AgCl) | −184 ± 18 | 175 ± 5 | −92 ± 14 | −112 ± 6 |
| icorr (mA/cm2) | (5.3 ± 2.0) × 10−5 | (32.0 ± 4.9) × 10−5 | (2.0 ± 0.8) × 10−5 | (11.0 ± 1.3) × 10−5 |
| ip (mA/cm2) | (4.5 ± 0.4) × 10−4 | (16 ± 1.9) × 10−4 | (3.0 ± 0.3) × 10−4 | (8.5 ± 2.1) × 10−4 |
| rcorr (mm/y) | (4.5 ± 1.7) × 10−4 | (27 ± 4.2) × 10−4 | (1.7 ± 0.7) × 10−4 | (9.4 ± 1.1) × 10−4 |
| R2 | 0.989 ± 0.003 | 0.992 ± 0.001 | 0.991 ± 0.004 | 0.988 ± 0.006 |
| Pitting (x out of four replicates) | No (4/4) | No (4/4) | No (4/4) | No (4/4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lekatou, A.G.; Sarika, V.; Efremenko, B.; Chabak, Y.; Efremenko, V.; Petrišinec, I.; Emmanouilidou, S.; Tsirka, K. Effect of Laser Surface Melting on the Microstructure and Corrosion Resistance of Laser Powder Bed Fusion and Wrought Ti-6Al-4V Alloys. Coatings 2025, 15, 1285. https://doi.org/10.3390/coatings15111285
Lekatou AG, Sarika V, Efremenko B, Chabak Y, Efremenko V, Petrišinec I, Emmanouilidou S, Tsirka K. Effect of Laser Surface Melting on the Microstructure and Corrosion Resistance of Laser Powder Bed Fusion and Wrought Ti-6Al-4V Alloys. Coatings. 2025; 15(11):1285. https://doi.org/10.3390/coatings15111285
Chicago/Turabian StyleLekatou, Angeliki G., Vaia Sarika, Bohdan Efremenko, Yuliia Chabak, Vasily Efremenko, Ivan Petrišinec, Sevasti Emmanouilidou, and Kyriaki Tsirka. 2025. "Effect of Laser Surface Melting on the Microstructure and Corrosion Resistance of Laser Powder Bed Fusion and Wrought Ti-6Al-4V Alloys" Coatings 15, no. 11: 1285. https://doi.org/10.3390/coatings15111285
APA StyleLekatou, A. G., Sarika, V., Efremenko, B., Chabak, Y., Efremenko, V., Petrišinec, I., Emmanouilidou, S., & Tsirka, K. (2025). Effect of Laser Surface Melting on the Microstructure and Corrosion Resistance of Laser Powder Bed Fusion and Wrought Ti-6Al-4V Alloys. Coatings, 15(11), 1285. https://doi.org/10.3390/coatings15111285







