The Effect of Current Density and Cathode Position on the Corrosion Resistance and Thermal Emission Properties of Nickel Electroplated Layers on Brass Surfaces
Abstract
1. Introduction
2. Experimental
2.1. Experiment Materials and Preparation
2.2. Coating Preparation
2.3. Morphology Characterization and Performance Assessment
3. Results and Discussion
3.1. Surface Morphology Analysis
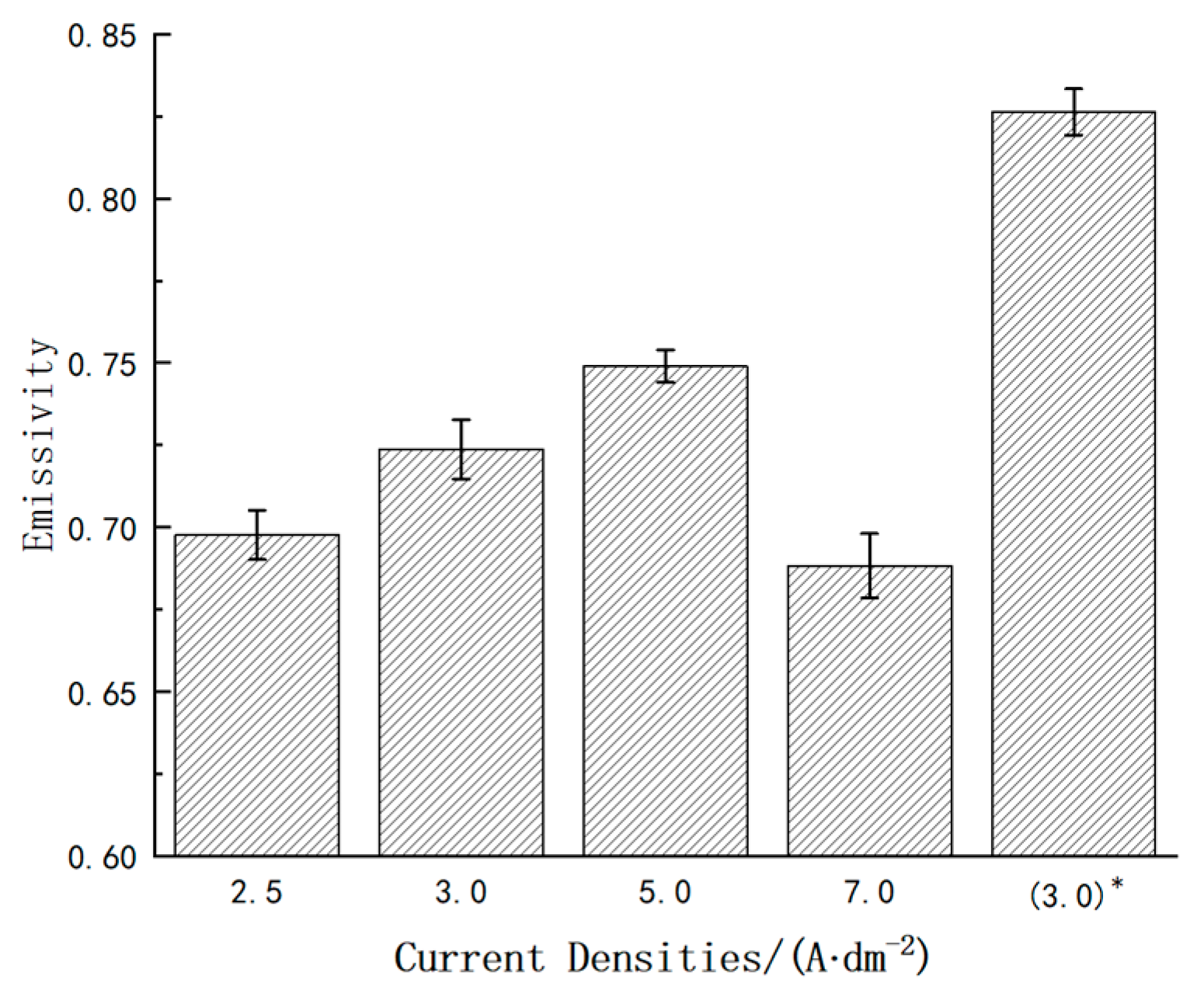
3.2. Emissivity of the Coatings
3.3. Corrosion Resistance
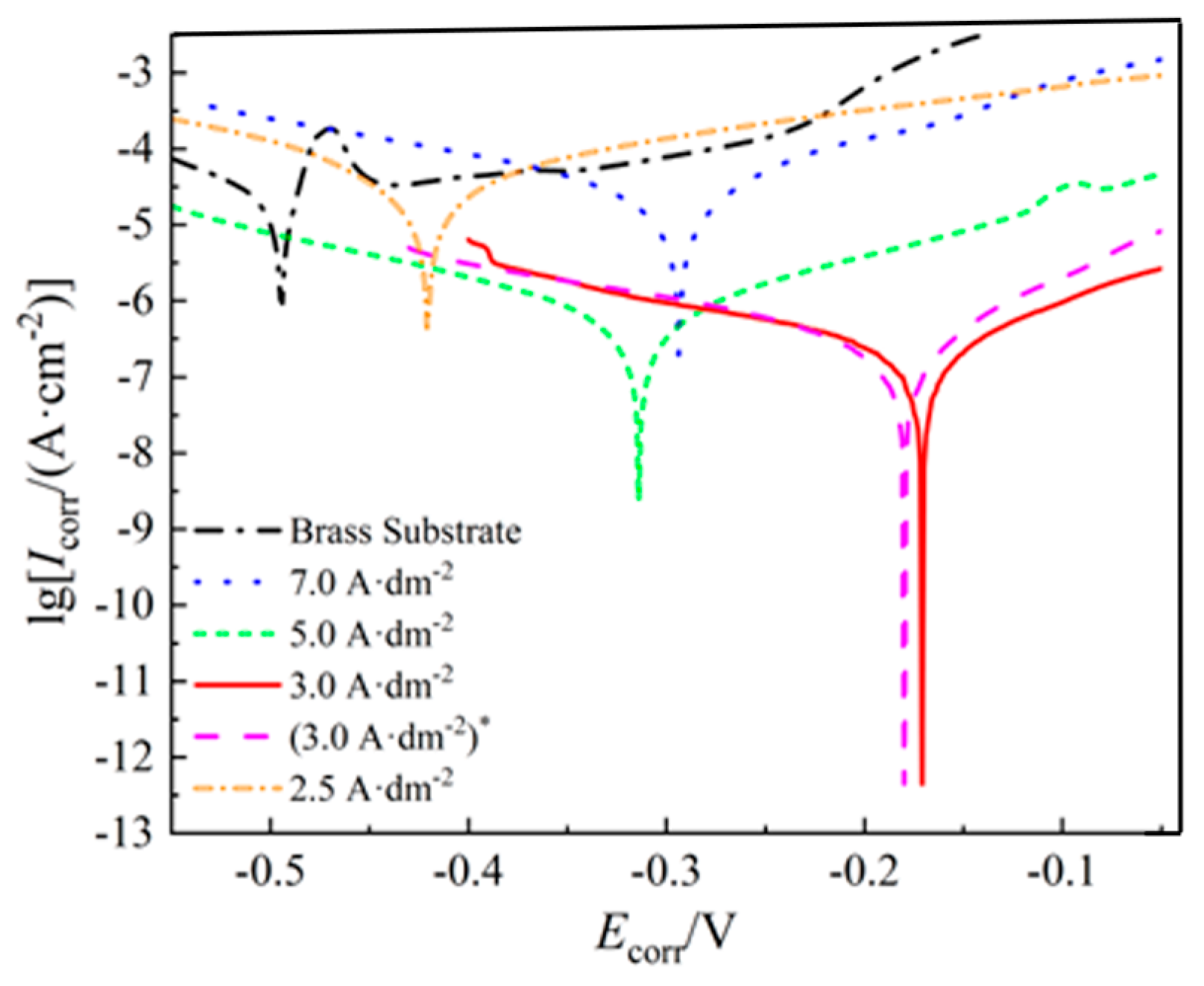
3.3.1. Polarization Curve Analysis
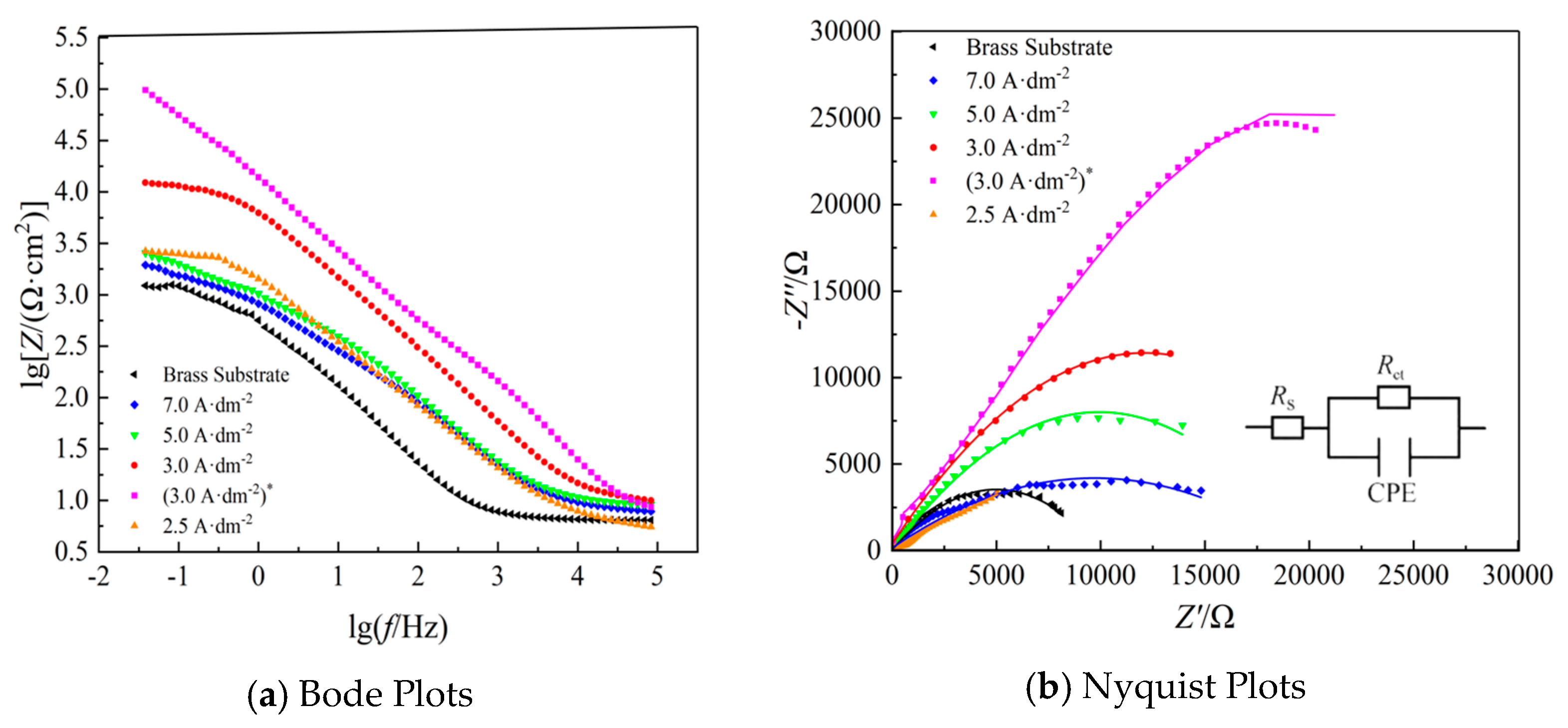
3.3.2. EIS Curve Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Zhang, M.; Jiang, H.; Chen, L.; Han, D.; Hu, X.; Qiu, L. Tribological behavior and wear mechanism of TiO2/MoS2 nanocomposite coatings fabricated on TC6 alloys by micro-arc oxidation and duplex surface technologies. Surf. Coat. Technol. 2025, 510, 132227. [Google Scholar] [CrossRef]
- Ma, X.; Qi, N.; Zhang, M. First-principles investigation of Zn-doped β-Ga2O3: Electronic, optoelectronic, and thermodynamic properties. Phys. B: Condens. Matter 2025, 715, 417557. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, M.; Ma, X.; Lv, M.; Zhou, X.; Kim, K.H. Structural, optical property and solar-blind photoelectric detection performance of high-quality Mg-doped β-Ga2O3 thin films prepared using RF magnetron sputtering. Surf. Coat. Technol. 2025, 506, 132144. [Google Scholar] [CrossRef]
- Chai, D.; Jia, Y.; Zhao, M.; Li, J.; Feng, W. Preparation of gradient nickel coating on copper by electrodeposition and study on its hydrogen evolution property. Electroplat. Finish. 2019, 38, 641–646. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, M.; Pan, Y.; Huang, Y.; Cheng, S.; Ding, Y.; Pei, W. Effect of Bath Temperature on Microstructure of Ni-SiC Nanocomposite Coating by Electrochemical Deposition. China Surf. Eng. 2013, 26, 70–74. [Google Scholar]
- Gao, M.; Xie, H.; Fang, Y.; Wang, H.T.; Liu, J.B. Progress in surface treatment techniques of copper and copper alloys. Chin. J. Nonferr. Met. 2021, 31, 1121–1133. [Google Scholar]
- Kan, H.; Meng, Y.; Cui, S.; Kong, L.; Wang, X.; Long, H. Research Progress on Electrodeposited Nickel-Based Composite Coatings. Mater. Mech. Eng. 2021, 45, 1–7. [Google Scholar]
- Wang, H.; Zhai, X.; Sun, Y. Microstructure and corrosion resistance of in-suit coating on copper alloy using laser shock melt injection of fine-CeO2 particles. Mater. Res. Express 2020, 7, 116–302. [Google Scholar] [CrossRef]
- Li, Q.; Xu, G.; Chen, Y.; Xu, F.; Jiang, Y. Study of Thermal Endurance and Thermal Shock Resistance of High-emissivity Coating on Metal Substrate. Infrared Technol. 2011, 33, 284–288. [Google Scholar]
- Wang, Q.; Guo, X.; Wu, W.; Zhang, J.; Liu, L. Preparation and properties of metal oxide coatings with high emissivity on stainless steel. Trans. Mater. Heat Treat. 2012, 33, 137–141. [Google Scholar]
- Lee, J.; Bae, K.; Jung, K.; Jeong, J.H.; Ko, J.S. Creation of microstructured surfaces using Cu–Ni composite electrodeposition and their application to superhydrophobic surfaces. Appl. Surf. Sci. 2014, 289, 14–20. [Google Scholar] [CrossRef]
- Xiao, Q.; Chen, W.; Chen, Y.; Zou, J.; Cheng, B.; Zhang, X. Corrosion Resistance of Electrodeposited Ni-W-Ti3C2Tx Composite Coatings with Different Current Densities. Rear Met. 2022, 46, 1298–1305. [Google Scholar]
- Cheng, H.; Guo, H.; Wang, Q.; Wang, C.; Tang, Y. Electrodeposition Technology and Corrosion Resistance of Ni-Fe Alloy Coating. Plat. Finish. 2011, 6, 1–4. [Google Scholar]
- Li, T.; Liu, X.; Liu, W.; Qu, M. Nickel electroplating on pure copper tube heat exchanger. Electroplat. Finish. 2022, 41, 1049–1052. [Google Scholar]
- Somasundaram, S.; Pillai, A.M.; Rajendra, A.; Sharma, A.K. High emittance black nickel coating on copper substrate for space applications. J. Alloys Compd. 2015, 643, 263–269. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A. Electrochemical polarization: I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, Y.; Shi, X.; Meng, F.; Li, C.; Liu, S. Effect of Deposition Current on Electrocatalytic Hydrogen Evolution Performance of Electroplated Nickel. Plat. Finish. 2022, 10, 49–53. [Google Scholar]
- Li, P.; Ren, C.; Zhang, J.; Chen, T. Effect of current density on nickel electroplating of silicon carbide. Electroplat. Finish. 2022, 41, 770–773. [Google Scholar]
- Li, H.; Lu, S.; Qin, W.; Wu, X. Current Density on Microstructure and Thermal Control Performances of MgO-ZnO Ceramic Coatings. J. Inorg. Mater. 2017, 32, 1292–1298. [Google Scholar]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-inhibiting coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef]
- Yang, C.; Yao, Z.; Zhang, F.; Oleksandr, M. Repairing the specified track on the copper coating surface via area-selective electrodeposition. J. Mater. Res. 2021, 36, 812–821. [Google Scholar] [CrossRef]
- Li, C.; Du, S.; Zeng, Z.; Liu, E.; Wang, F.; Ma, F. Effect of Current Density on Microstructure, Wear and Corrosion Resistance of Electrodeposited Ni-Co-B Coating. J. Chin. Soc. Corros. Prot. 2020, 40, 439–447. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, X.; Zhang, S.; Hou, L.; Kim, K.H. One-step fabrication of wear resistant and friction-reducing Al2O3/MoS2 nanocomposite coatings on 2A50 aluminum alloy by plasma electrolytic oxidation with MoS2 nanoparticle additive. Surf. Coat. Technol. 2025, 497, 131796. [Google Scholar] [CrossRef]
- Hu, J.; Chen, J.; Chen, L.; Li, S.; Huang, D.; Chen, Z.; Chen, H.; Xie, W. Effect of Electroplating Current Density on Corrosion Resistance of Al2O3-Ni Composite Coating. Guangdong Chem. Ind. 2023, 50, 24–26. [Google Scholar]
- Li, Y.; Lin, L.; Dai, W.; Chen, Z.; Liu, F.; Chen, L. Preparation of copper–nickel–molybdate alloy electrode by electrodeposition at gradient current densities and study on its properties. Electroplat. Finish. 2020, 39, 521–526. [Google Scholar]
- Liang, H.; Hou, J.; Jiang, L.; Qi, Z.; Zhang, M.; Cao, Z. The Effect of Heat Treatment on the Microstructure and Mechanical Properties of Al0.6CoFeNi2V0.5 High Entropy Alloy. Coatings 2024, 14, 658. [Google Scholar] [CrossRef]
- Ye, C.; You, D.; Qiu, C.; Li, Q. The Preparation and Corrosion Resistance of Electroless Ni-Cu-P Coating on Nd-Fe-B Permanent Magnet. Guangdong Chem. Ind. 2019, 46, 44–45. [Google Scholar]



| Samples | Time (min) | Temperature (°C) | Current Density (A·dm−2) | Starting Voltage (V) | End Voltage (V) | Coating Thickness (μm) |
|---|---|---|---|---|---|---|
| 1 | 30 | RT | 2.5 | 1.8 | 1.7 | 7.1 ± 1.5 |
| 2 | 3.0 | 2.1 | 2.0 | 10.6 ± 0.8 | ||
| 3 | 5.0 | 2.4 | 2.2 | 16.4 ± 1.1 | ||
| 4 | 7.0 | 3.2 | 2.9 | 18.2 ± 1.2 |
| Sample No. | Time (min) | Temperature (°C) | Current Density (A·dm−2) | Placement Method | Starting Voltage (V) | End Voltage (V) | Coating Thickness (μm) |
|---|---|---|---|---|---|---|---|
| 2 | 30 | RT | 3.0 | (a) Horizontal | 2.1 | 2.0 | 10.6 ± 0.8 |
| 5 | (b) Vertical | 2.6 | 2.5 | 13.8 ± 0.6 |
| Samples | Icorr/(A·cm−2) | Ecorr/V | me/g | Vc/(mm/a) |
|---|---|---|---|---|
| Brass substrate | 3.46 × 10−4.9 | −0.497 | 13.61 | 0.564 |
| 7.0 A·dm−2 | 2.77 × 10−5 | −0.294 | 10.27 | 0.252 |
| 5.0 A·dm−2 | 7.63 × 10−7 | −0.314 | 10.70 | 0.072 |
| 2.5 A·dm−2 | 4.47 × 10−5 | −0.421 | 11.37 | 0.423 |
| 3.0 A·dm−2 | 3.10 × 10−7 | −0.171 | 10.10 | 0.028 |
| (3.0 A·dm−2)* | 2.59 × 10−7 | −0.180 | 10.32 | 0.023 |
| Samples | Rp/(Ω·cm−2) | Rs/(Ω·cm−2) | Rct/(Ω·cm−2) | Q (CPE-T) (F·sn−1·cm−2) | n |
|---|---|---|---|---|---|
| Brass substrate | 1638.06 | 5.012 | 1063 | 2.49 × 10−4 | 0.37 |
| 7.0 A·dm−2 | 3157.14 | 6.854 | 2058 | 1.72 × 10−4 | 0.64 |
| 5.0 A·dm−2 | 3875.86 | 5.053 | 4033 | 1.70 × 10−4 | 0.67 |
| 2.5 A·dm−2 | 2596.35 | 6.003 | 1148 | 1.38 × 10−4 | 0.74 |
| 3.0 A·dm−2 | 4233.02 | 8.822 | 4133 | 7.67 × 10−4 | 0.79 |
| (3.0 A·dm−2)* | 6381.55 | 5.990 | 5488 | 1.00 × 10−3 | 0.95 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Lv, M.; Zhang, H.; Zhang, X.; Zhao, M.; Zhang, M. The Effect of Current Density and Cathode Position on the Corrosion Resistance and Thermal Emission Properties of Nickel Electroplated Layers on Brass Surfaces. Coatings 2025, 15, 1276. https://doi.org/10.3390/coatings15111276
Zhang L, Lv M, Zhang H, Zhang X, Zhao M, Zhang M. The Effect of Current Density and Cathode Position on the Corrosion Resistance and Thermal Emission Properties of Nickel Electroplated Layers on Brass Surfaces. Coatings. 2025; 15(11):1276. https://doi.org/10.3390/coatings15111276
Chicago/Turabian StyleZhang, Lin, Mingyue Lv, Haoqian Zhang, Xuan Zhang, Mingyue Zhao, and Min Zhang. 2025. "The Effect of Current Density and Cathode Position on the Corrosion Resistance and Thermal Emission Properties of Nickel Electroplated Layers on Brass Surfaces" Coatings 15, no. 11: 1276. https://doi.org/10.3390/coatings15111276
APA StyleZhang, L., Lv, M., Zhang, H., Zhang, X., Zhao, M., & Zhang, M. (2025). The Effect of Current Density and Cathode Position on the Corrosion Resistance and Thermal Emission Properties of Nickel Electroplated Layers on Brass Surfaces. Coatings, 15(11), 1276. https://doi.org/10.3390/coatings15111276







