Laser Fabricated MgO-TiO2 Based Photocatalytic Antifogging and Self-Cleaning Surface in Air
Abstract
1. Introduction
2. Experimental Methods
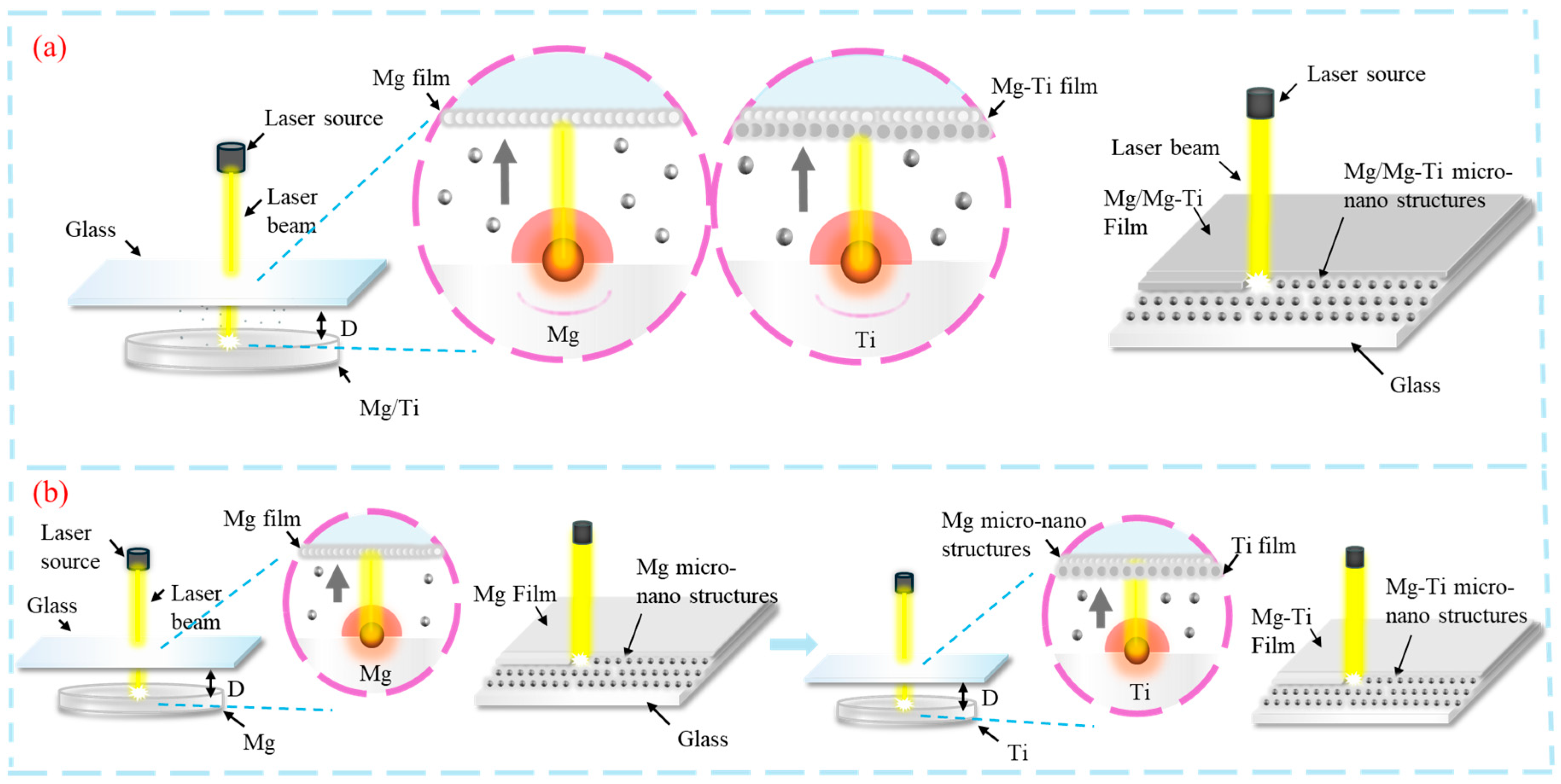
2.1. Materials and Methods
2.2. Characterization
2.2.1. Self-Cleaning Test
2.2.2. Antifog Durability Test
2.2.3. Aging Test
2.2.4. Photocatalytic Decomposition of MO
2.2.5. Abrasion Resistance Test
3. Results and Analysis
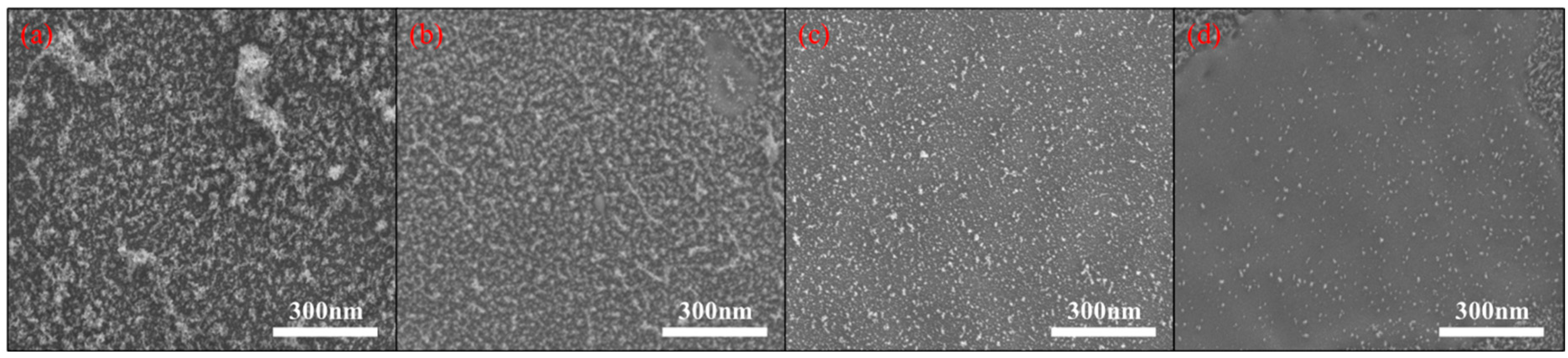
3.1. Surface Morphology
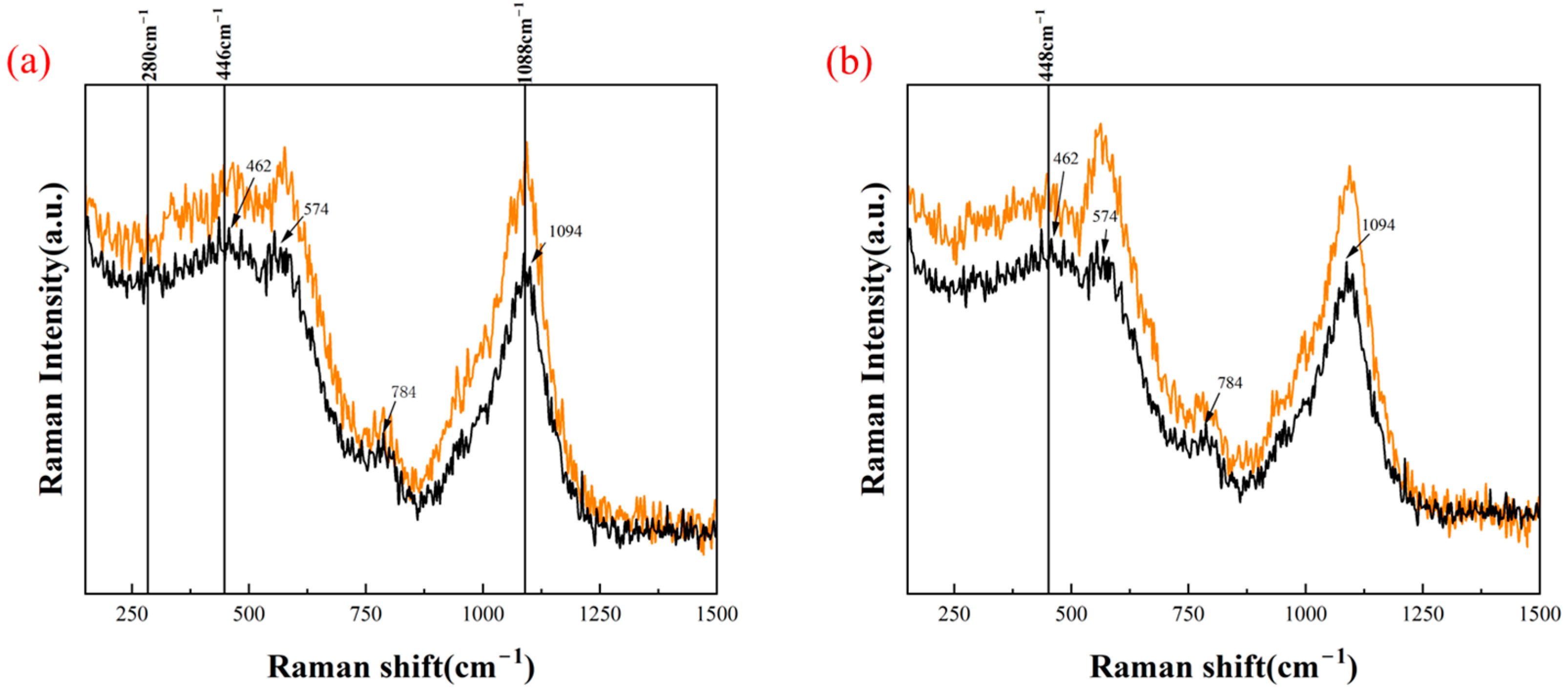
3.2. Surface Structure Analysis
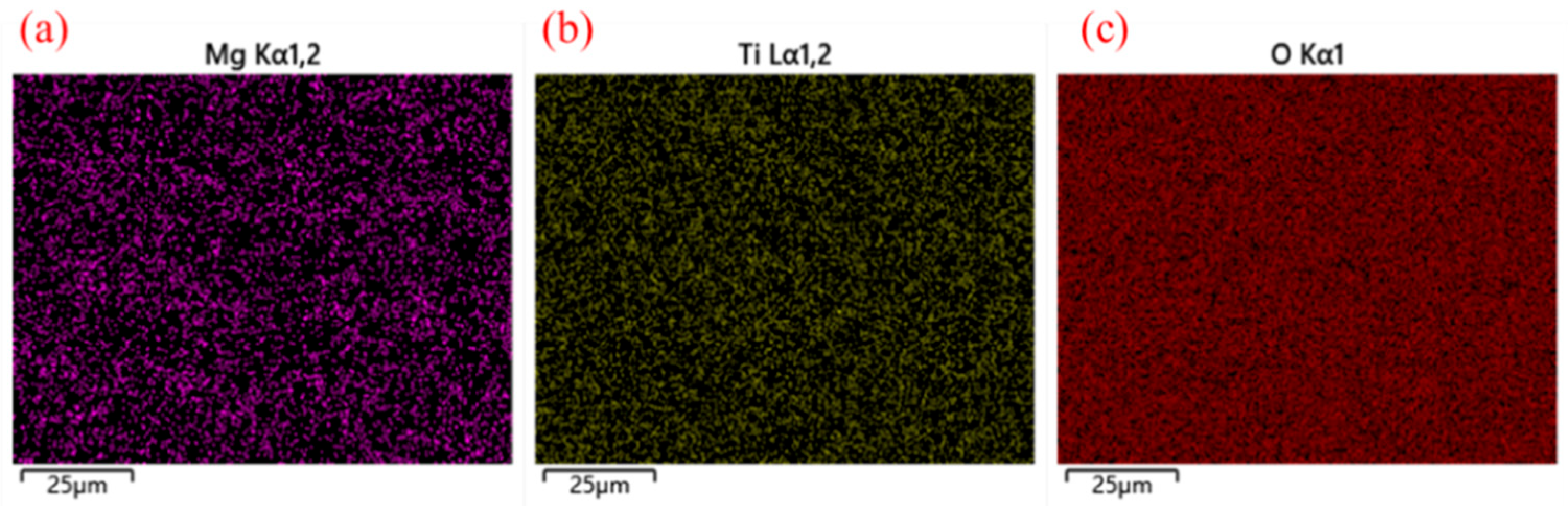
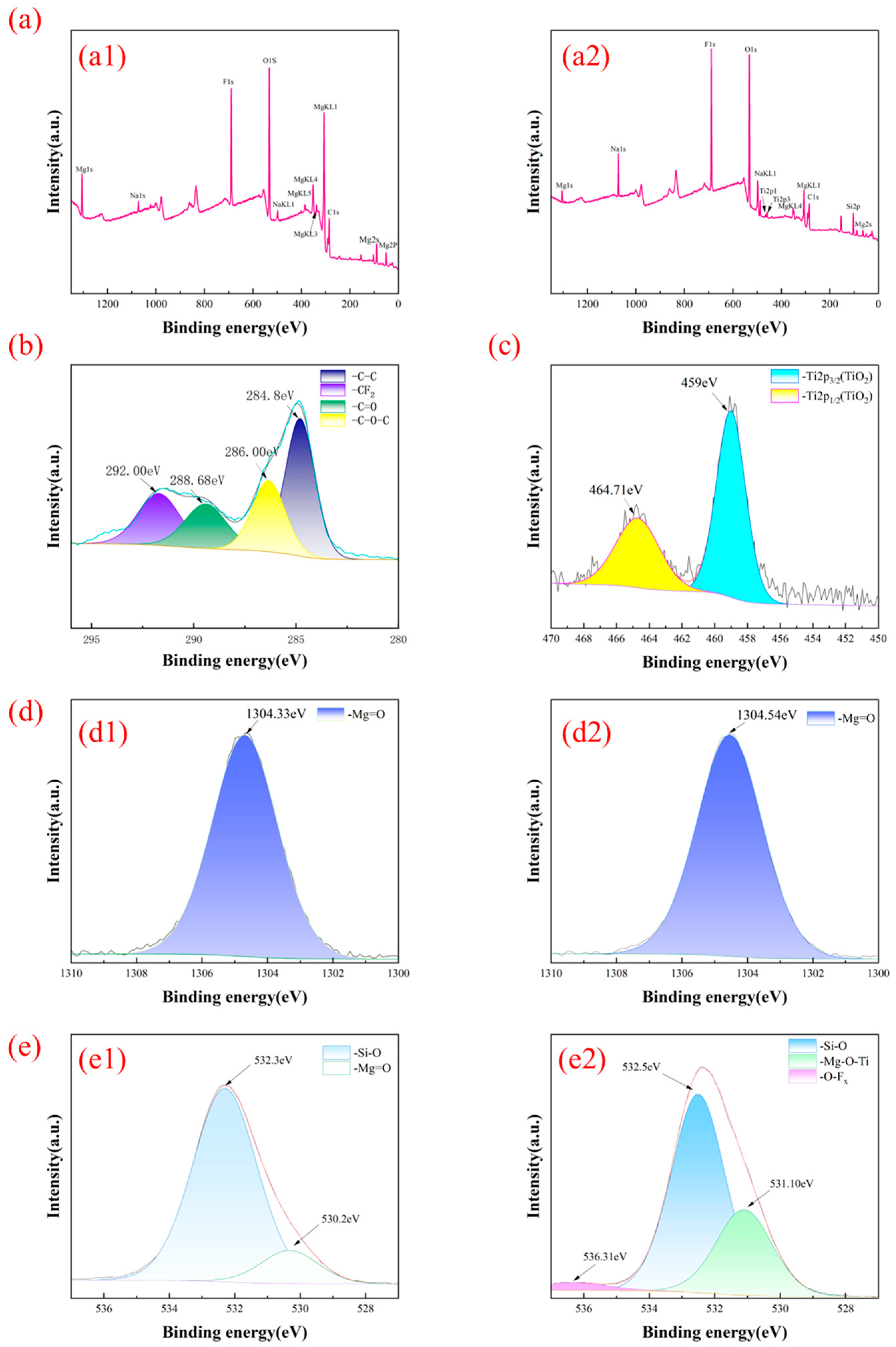
3.3. Chemical Composition Analysis
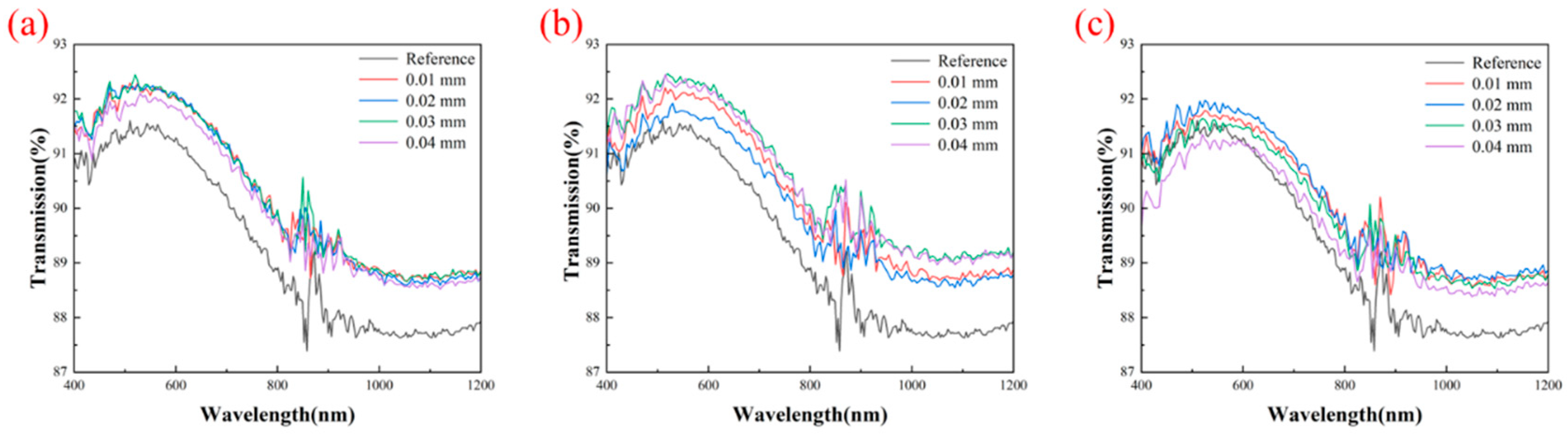
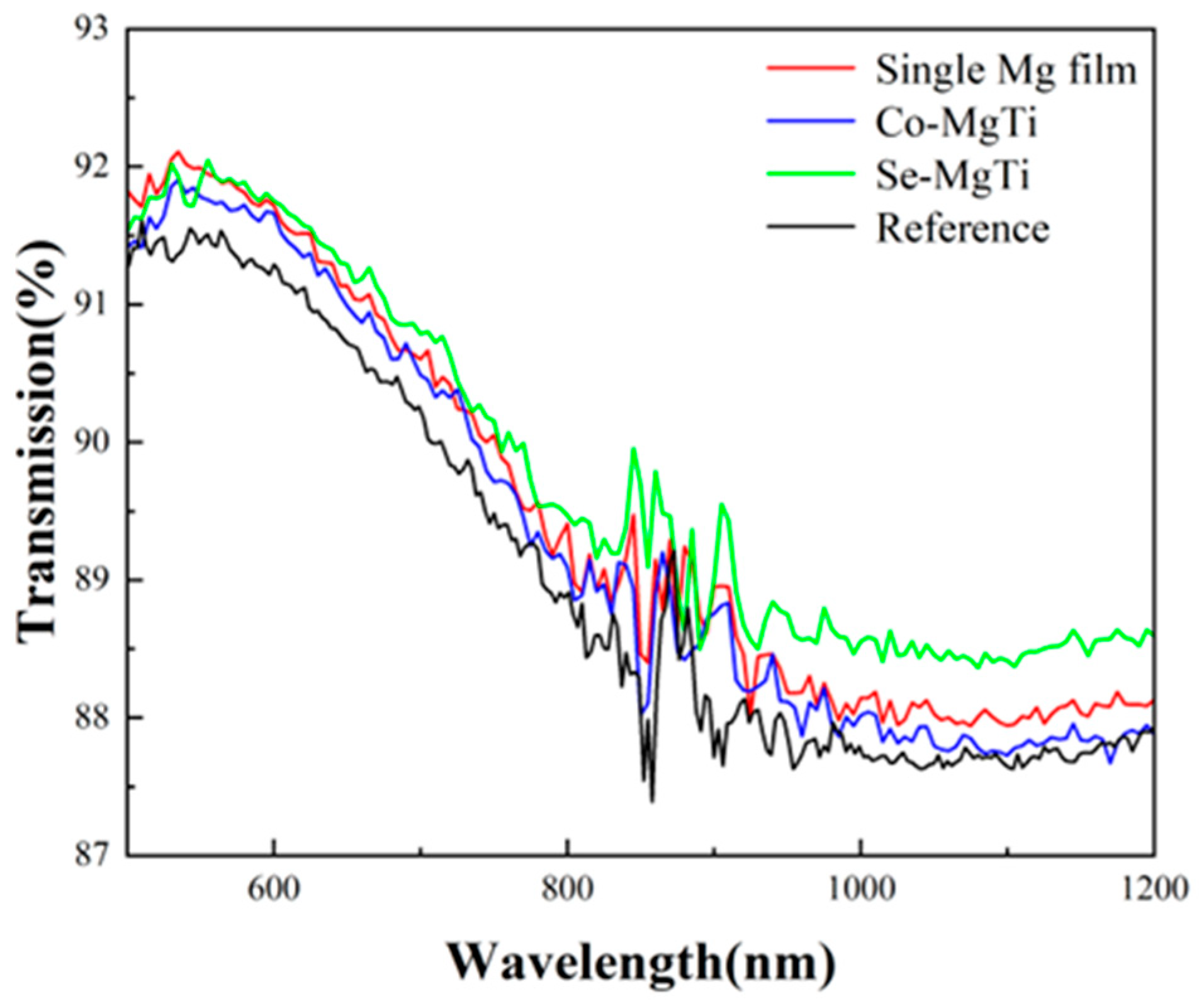
3.4. Optical Properties
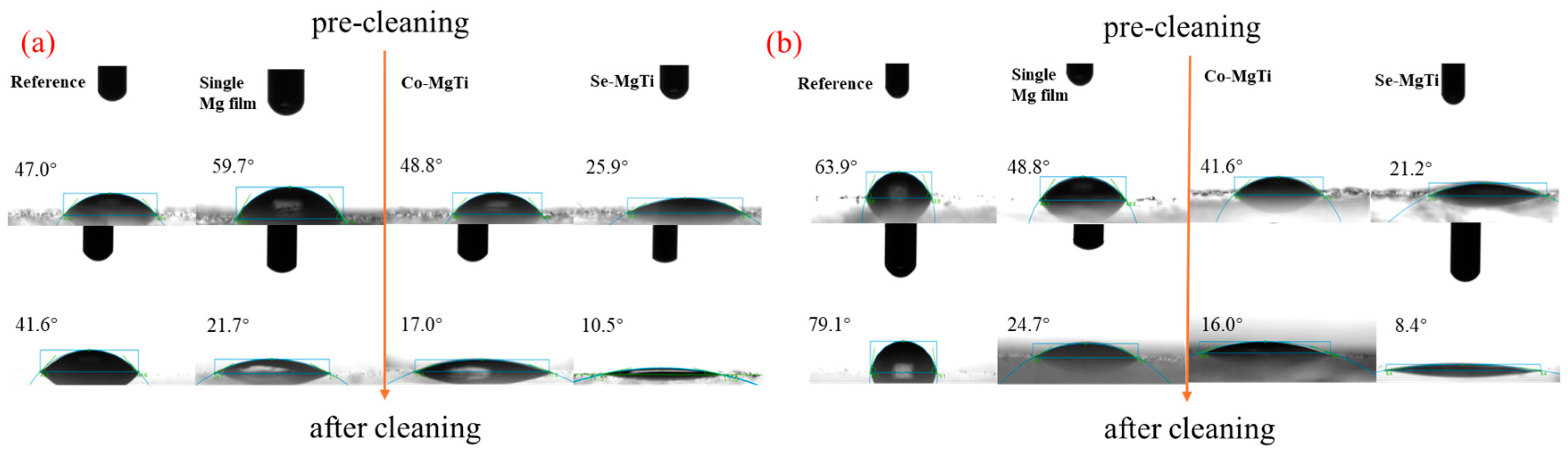
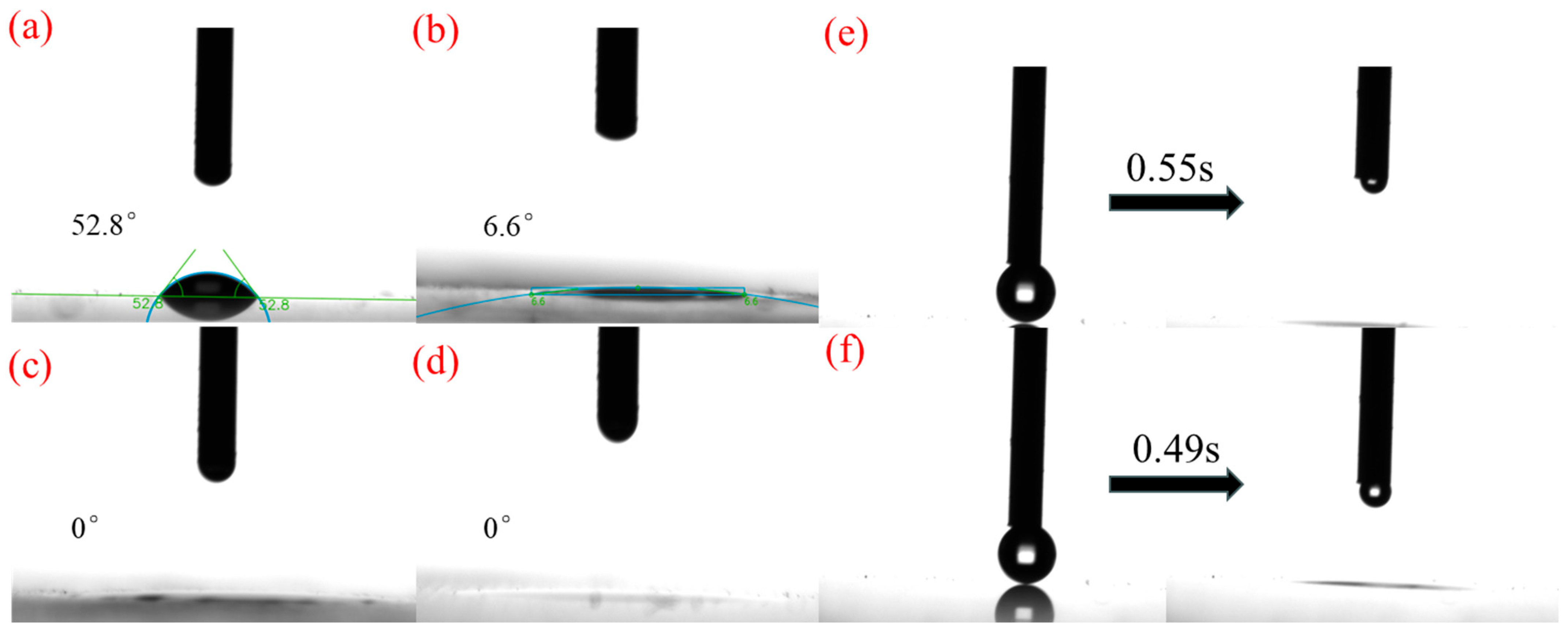
3.5. Wettability Test
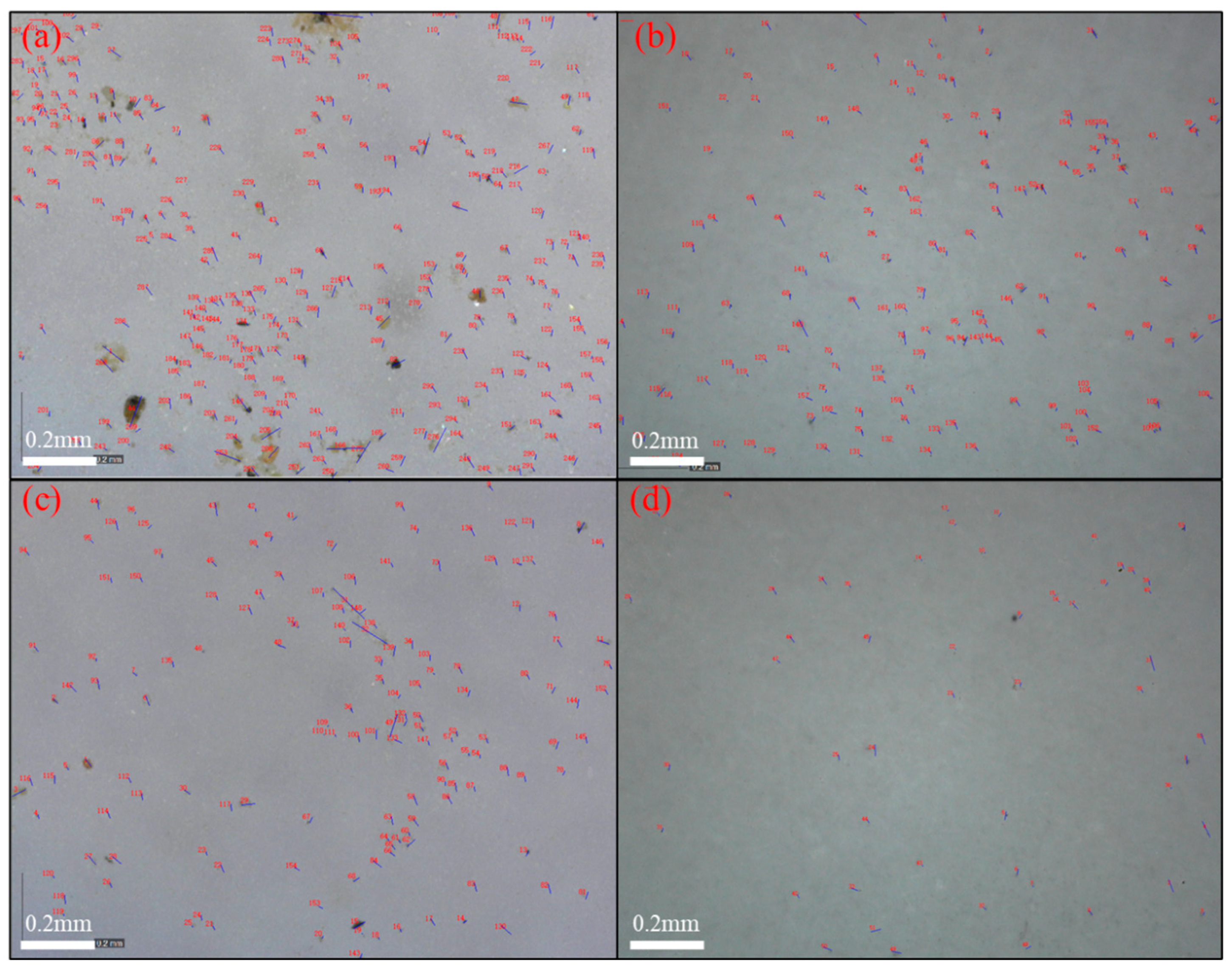
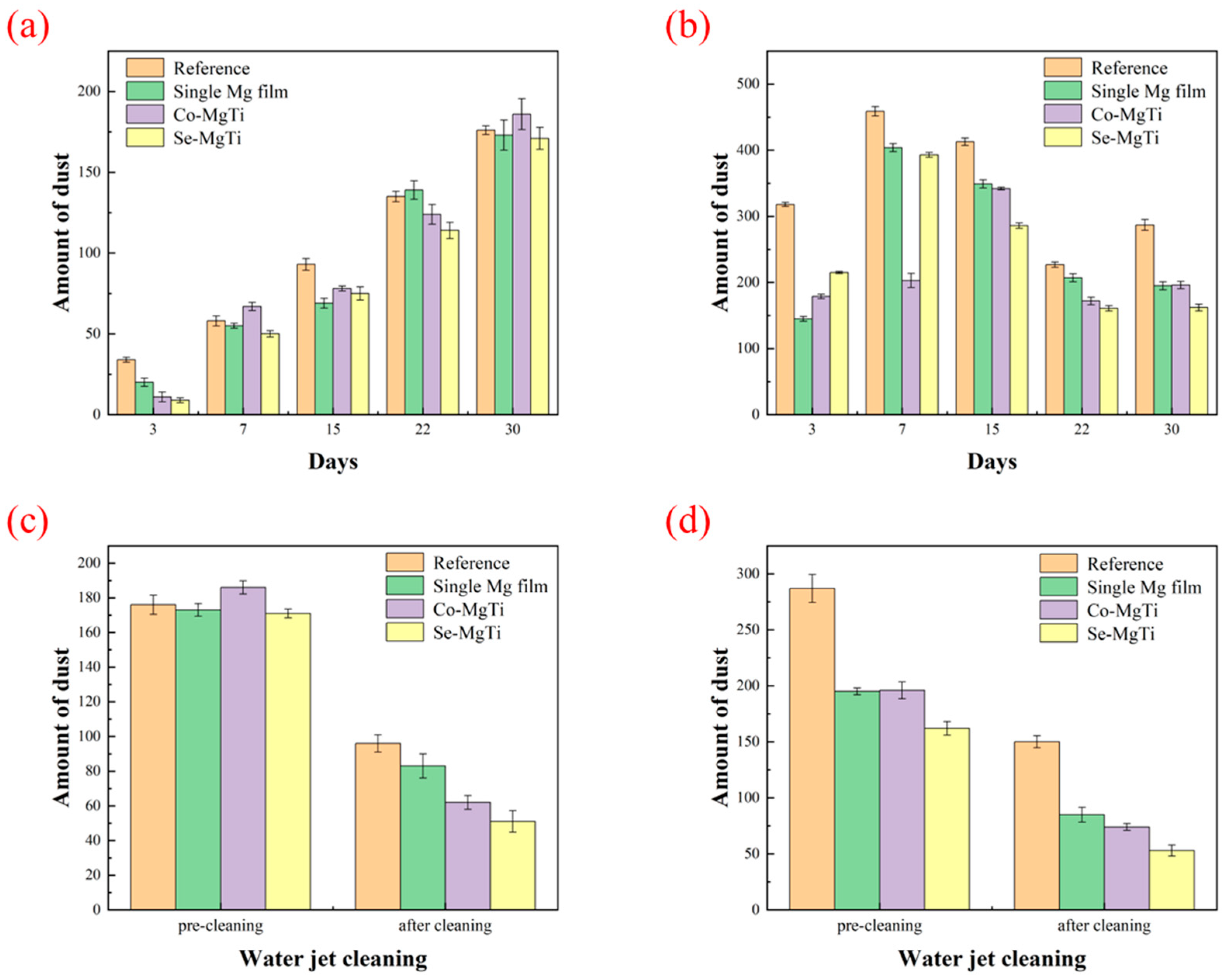
3.6. Self-Cleaning Performance Test
3.7. Antifog Performance Test
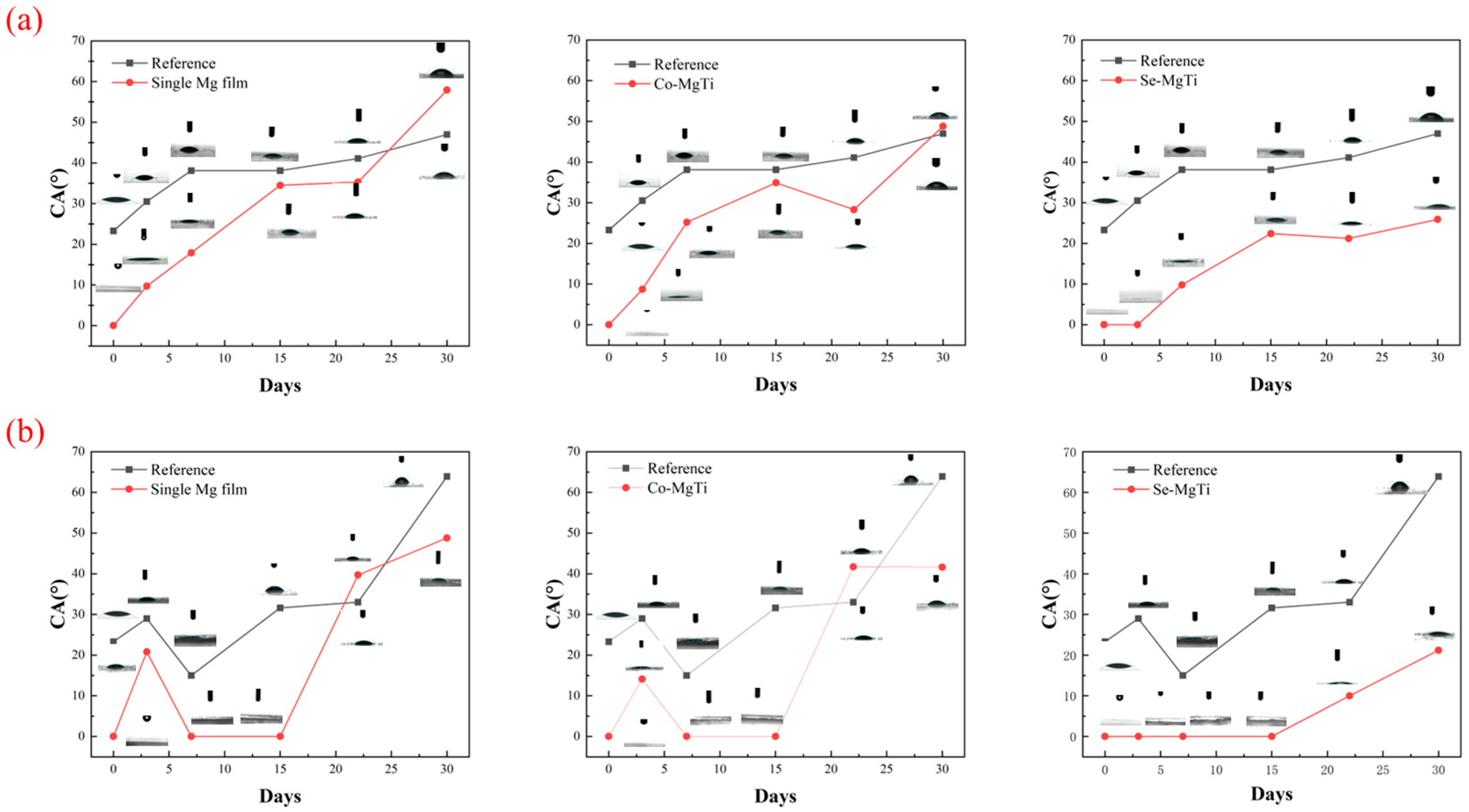
3.8. Aging Test
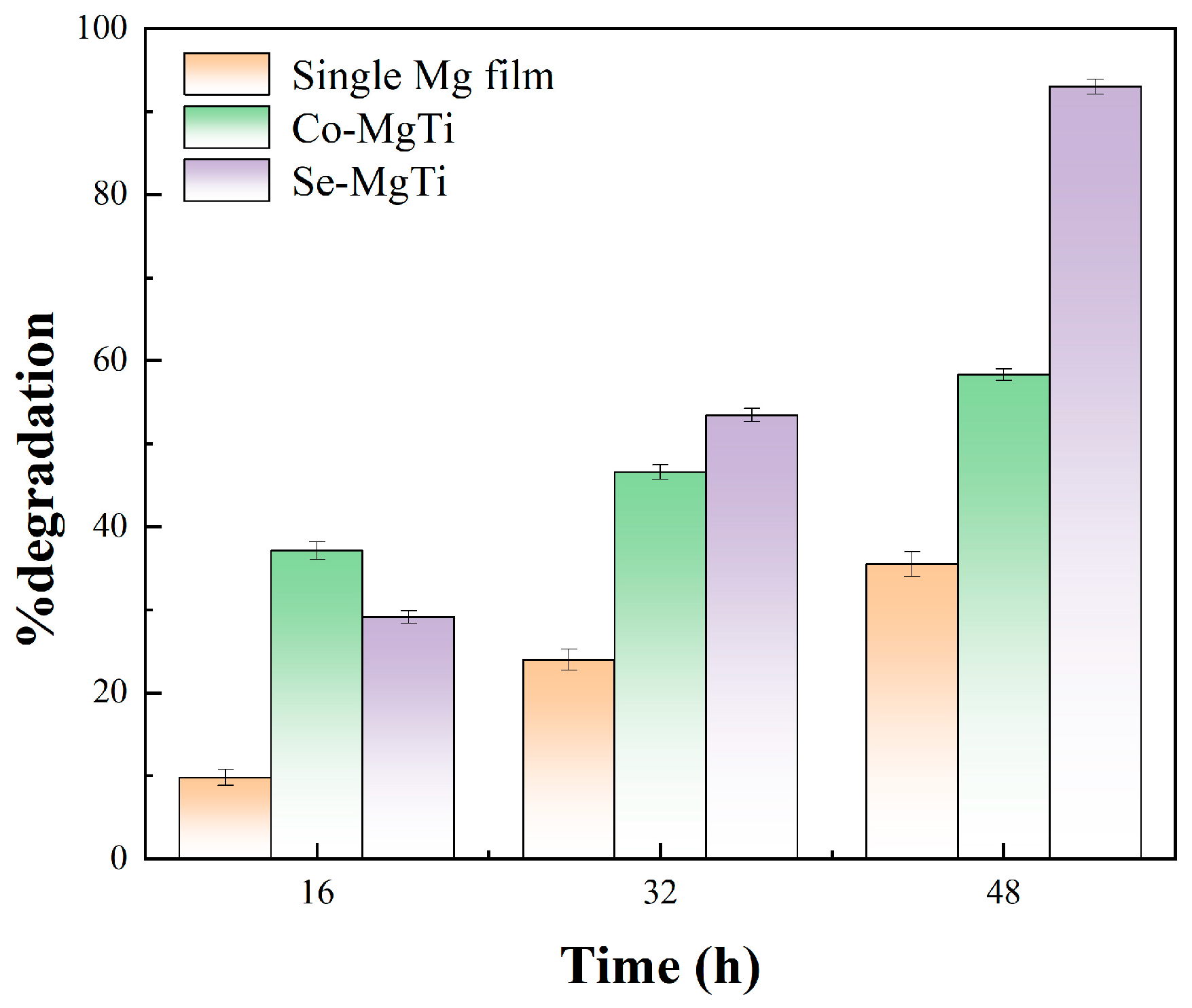
3.9. Photocatalytic Degradation Test of MO
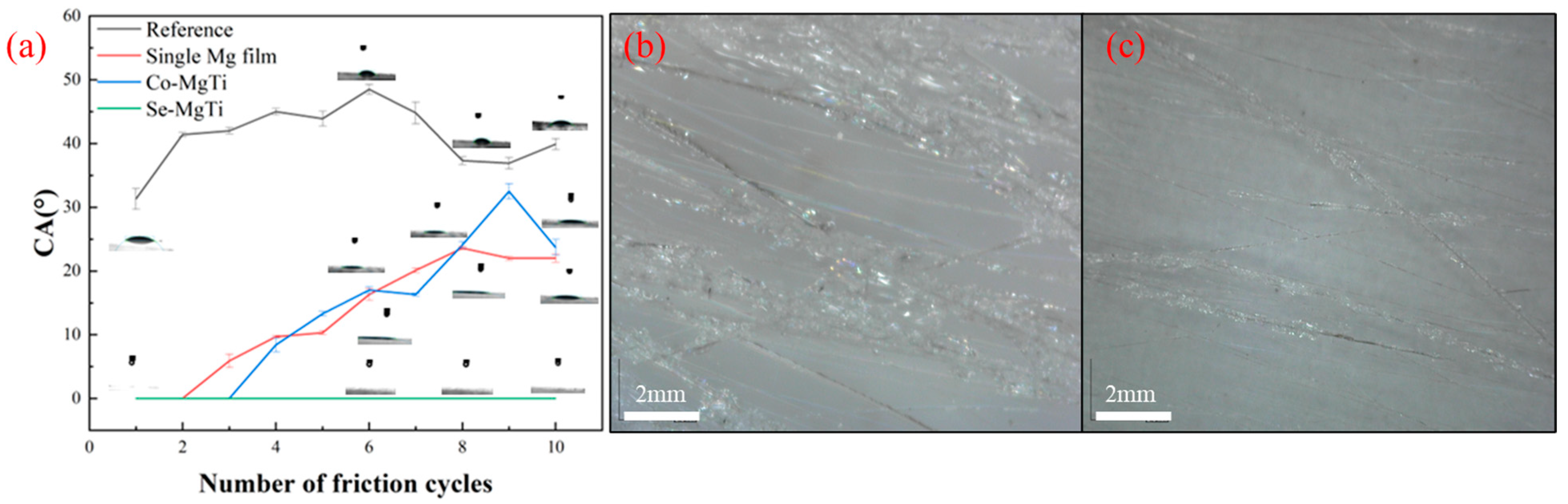
3.10. Abrasion Resistance Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- de Araujo, C.B.; Kassab, L.R.P.; da Silva, D.M. Optical properties of glasses and glass-ceramics for optical amplifiers, photovoltaic devices, color displays, optical limiters, and Random Lasers. Opt. Mater. 2022, 131, 112648. [Google Scholar] [CrossRef]
- Talu, S.; Yadav, R.P.; Sik, O.; Sobola, D.; Dallaev, R.; Solaymani, S.; Man, O. How topographical surface parameters are correlated with CdTe monocrystal surface oxidation. Mater. Sci. Semicond. Process. 2018, 85, 15–23. [Google Scholar] [CrossRef]
- He, X.; Punpongjareorn, N.; Wu, C.Y.; Davydov, I.A.; Yang, D.S. Ultrafast Carrier Dynamics of CdTe: Surface Effects. J. Phys. Chem. C 2016, 120, 9350–9356. [Google Scholar] [CrossRef]
- Zaihidee, F.M.; Mekhilef, S.; Seyedmahmoudian, M.; Horan, B. Dust as an unalterable deteriorative factor affecting PV panel’s efficiency: Why and how. Renew. Sustain. Energy Rev. 2016, 65, 1267–1278. [Google Scholar] [CrossRef]
- Nayshevsky, I.; Xu, Q.F.; Barahman, G.; Lyons, A.M. Fluoropolymer coatings for solar cover glass: Anti-soiling mechanisms in the presence of dew. Sol. Energy Mater. Sol. Cells 2020, 206, 110281. [Google Scholar] [CrossRef]
- Ilse, K.; Werner, M.; Naumann, V.; Figgis, B.W.; Hagendorf, C.; Bagdahn, J. Microstructural analysis of the cementation process during soiling on glass surfaces in arid and semi-arid climates. Phys. Status Solidi-Rapid Res. Lett. 2016, 10, 525–529. [Google Scholar] [CrossRef]
- Kazmerski, L.L.; Diniz, A.S.A.C.; Maia, C.B.; Viana, M.M.; Costa, S.C.; Brito, P.P.; Campos, C.D.; Macheto Neto, L.V.; Hanriot, S.d.M.; de Oliveira Cruz, L.R. Fundamental Studies of Adhesion of Dust to PV Module Surfaces: Chemical and Physical Relationships at the Microscale. IEEE J. Photovolt. 2016, 6, 719–729. [Google Scholar] [CrossRef]
- Sarver, T.; Al-Qaraghuli, A.; Kazmerski, L.L. A comprehensive review of the impact of dust on the use of solar energy: History, investigations, results, literature, and mitigation approaches. Renew. Sustain. Energy Rev. 2013, 22, 698–733. [Google Scholar] [CrossRef]
- Dalawai, S.P.; Aly, M.A.S.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.-S.; Sadasivuni, K.K.; Liu, S. Recent Advances in durability of superhydrophobic self-cleaning technology: A critical review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Wang, X.-S.; Cui, S.-W.; Zhou, L.; Xu, S.-H.; Sun, Z.-W.; Zhu, R.-Z. A generalized Young’s equation for contact angles of droplets on homogeneous and rough substrates. J. Adhes. Sci. Technol. 2014, 28, 161–170. [Google Scholar] [CrossRef]
- Kim, D.; Pugno, N.M.; Ryu, S. Wetting theory for small droplets on textured solid surfaces. Sci. Rep. 2016, 6, 37813. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Z.; Li, C.; Du, H.; Fang, N.X.; Wu, L.; Song, Y. Continuous 3D printing from one single droplet. Nat. Commun. 2020, 11, 4685. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Luo, X.; Chang, W.; Tian, Y.; Wang, Z.; Gao, J.; Cai, Y.; Qin, Y.; Duxbury, M. Manufacturing of anti-fogging super-hydrophilic microstructures on glass by nanosecond laser. J. Manuf. Process. 2020, 59, 557–565. [Google Scholar] [CrossRef]
- Zhi, J.; Zhang, L.-Z. Durable superhydrophobic surface with highly antireflective and self-cleaning properties for the glass covers of solar cells. Appl. Surf. Sci. 2018, 454, 239–248. [Google Scholar] [CrossRef]
- Duran, I.R.; Laroche, G. Current trends, challenges, and perspectives of anti-fogging technology: Surface and material design, fabrication strategies, and beyond. Prog. Mater. Sci. 2019, 99, 106–186. [Google Scholar] [CrossRef]
- Blossey, R. Self-cleaning surfaces—Virtual realities. Nat. Mater. 2003, 2, 301–306. [Google Scholar] [CrossRef]
- Xu, F.; Wang, T.; Bohling, J.; Maurice, A.M.; Chen, H.; Wu, L.; Zhou, S. Insight into the dependence of dirt adsorption/desorption on the surface wetting behavior of TiO2-based nanocomposite coatings. Prog. Org. Coat. 2019, 131, 137–144. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Z.; Yang, X.; Xu, X.; Zhang, L.; Zhao, N.; Xu, J. Antifogging and antireflective silica film and its application on solar modules. Surf. Coat. Technol. 2011, 206, 1490–1494. [Google Scholar] [CrossRef]
- Huang, Y.; Hu, Y.; Zhu, C.; Zhang, F.; Li, H.; Lu, X.; Meng, S. Long-Lived Multifunctional Superhydrophobic Heterostructure Via Molecular Self-Supply. Adv. Mater. Interfaces 2016, 3, 1500727. [Google Scholar] [CrossRef]
- Hwang, G.B.; Patir, A.; Allan, E.; Nair, S.P.; Parkin, I.P. Superhydrophobic and White Light-Activated Bactericidal Surface through a Simple Coating. Acs Appl. Mater. Interfaces 2017, 9, 29002–29009. [Google Scholar] [CrossRef]
- Jeung, Y.; Yong, K. Underwater superoleophobicity of a superhydrophilic surface with unexpected drag reduction driven by electrochemical water splitting. Chem. Eng. J. 2020, 381, 122734. [Google Scholar] [CrossRef]
- Huang, S.; Ras, R.H.A.; Tian, X. Antifouling membranes for oily wastewater treatment: Interplay between wetting and membrane fouling. Curr. Opin. Colloid Interface Sci. 2018, 36, 90–109. [Google Scholar] [CrossRef]
- Mi, L.; Wang, Y.; Fang, X.; Qi, X.; Chen, L.; Cui, H. Laser-fabricated core-shell Cu/Zn-based photocatalytic films with superhydrophilic self-cleaning properties. J. Alloys Compd. 2025, 1026, 180422. [Google Scholar] [CrossRef]
- Armakovic, S.J.; Savanovic, M.M.; Armakovic, S. Titanium Dioxide as the Most Used Photocatalyst for Water Purification: An Overview. Catalysts 2023, 13, 26. [Google Scholar] [CrossRef]
- Shayegan, Z.; Lee, C.-S.; Haghighat, F. TiO2 photocatalyst for removal of volatile organic compounds in gas phase—A review. Chem. Eng. J. 2018, 334, 2408–2439. [Google Scholar] [CrossRef]
- Yao, L.; He, J. Facile dip-coating approach to fabrication of mechanically robust hybrid thin films with high transmittance and durable superhydrophilicity. J. Mater. Chem. A 2014, 2, 6994–7003. [Google Scholar] [CrossRef]
- Piispanen, M.; Hupa, L. Comparison of self-cleaning properties of three titania coatings on float glass. Appl. Surf. Sci. 2011, 258, 1126–1131. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Wu, S. Room-temperature fabrication of TiO2-PHEA nanocomposite coating with high transmittance and durable superhydrophilicity. Chem. Eng. J. 2019, 371, 609–617. [Google Scholar] [CrossRef]
- Cui, H.; Fang, X.; Qi, X.; Wang, Y.; Wang, H.; Zhai, Z.; Zhang, F.; Hu, Q.; Liu, J. Laser-fabricated core-shell Ti/W based photocatalytic films with superhydrophilic self-cleaning properties. Surf. Interfaces 2025, 61, 106147. [Google Scholar] [CrossRef]
- Zhong, H.; Liu, X.; Yu, B.; Zhou, S. Fast UV-Curable Zwitter-Wettable Coatings with Reliable Antifogging/Frost-Resisting Performances. Biomimetics 2022, 7, 162. [Google Scholar] [CrossRef]
- Wang, P.; Qi, X.; Fang, X.; Teng, C.; Guo, Y.; Liu, C.; Chen, X.; Cui, H. Durable self-cleaning anti-fog and antireflective micro-nano structures fabricated by laser marker ablation of Si coated glass. Ceram. Int. 2024, 50, 31402–31417. [Google Scholar] [CrossRef]
- Cui, H.; Teng, C.; Zhao, Y.; Wang, P.; Guo, Y.; Qi, X. Durable self-cleaning antireflective and antifog Al micro-nano structure on glass by laser marker ablation. Opt. Mater. 2024, 147, 114675. [Google Scholar] [CrossRef]
- Akimov, Y.A. Mie scattering theory: A review of physical features and limitations. arXiv 2024, arXiv:2401.04146. [Google Scholar] [CrossRef]
- Guo, L.; Huang, J.; Xue, L.; Zhang, R. Research on the arc stability of dry hyperbaric GMAW from the perspective of arc energy and electrical conductivity. J. Manuf. Process. 2025, 138, 14–23. [Google Scholar] [CrossRef]
- Abildina, A.A.; Kurbatov, A.P.; Bakhytzhan, Y.G.; Jumanova, R.Z.; Argimbayeva, A.M.; Avchukir, K.; Rakhymbay, G.S. Corrosion behavior of magnesium in aqueous sulfate-containing electrolytes. J. Magnes. Alloys 2023, 11, 2125–2141. [Google Scholar] [CrossRef]
- Aghion, E. Biodegradable Metals. Metals 2018, 8, 804. [Google Scholar] [CrossRef]
- She, J.; Chen, J.; Xiong, X.; Yang, Y.; Peng, X.; Chen, D.; Pan, F. Research advances of magnesium and magnesium alloys globally in 2023. J. Magnes. Alloys 2024, 12, 3441–3475. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.M.; Chen, J.; Peng, X.D.; Chen, D.L.; Pan, F.S. Research advances of magnesium and magnesium alloys worldwide in 2022. J. Magnes. Alloys 2023, 11, 2611–2654. [Google Scholar] [CrossRef]
- Feng, L.; Zhu, Y.; Wang, J.; Shi, X. One-step hydrothermal process to fabricate superhydrophobic surface on magnesium alloy with enhanced corrosion resistance and self-cleaning performance. Appl. Surf. Sci. 2017, 422, 566–573. [Google Scholar] [CrossRef]
- Ishizaki, T.; Hieda, J.; Saito, N.; Saito, N.; Takai, O. Corrosion resistance and chemical stability of super-hydrophobic film deposited on magnesium alloy AZ31 by microwave plasma-enhanced chemical vapor deposition. Electrochim. Acta 2010, 55, 7094–7101. [Google Scholar] [CrossRef]
- Kiahosseini, S.R.; Afshar, A.; Larijani, M.M.; Yousefpour, M. Structural and corrosion characterization of hydroxyapatite/zirconium nitride-coated AZ91 magnesium alloy by ion beam sputtering. Appl. Surf. Sci. 2017, 401, 172–180. [Google Scholar] [CrossRef]
- Peng, M.K.; Zhang, X.D.; Xiao, X.; Dong, M.J.; Zhao, G.W.; Liu, P.; Chen, Y.S.; Wang, C.H. Polyelectrolytes fabrication on magnesium alloy surface by layer-by-layer assembly technique with antiplatelet adhesion and antibacterial activities. J. Coat. Technol. Res. 2019, 16, 857–868. [Google Scholar] [CrossRef]
- Yeganeh, M.; Mohammadi, N. Superhydrophobic surface of Mg alloys: A review. J. Magnes. Alloys 2018, 6, 59–70. [Google Scholar] [CrossRef]
- Syafiq, A.; Balakrishnan, V.; Abd Rahim, N.A. Durable self-cleaning nano-titanium dioxide superhydrophilic coating with anti-fog property. Pigment Resin Technol. 2024, 53, 261–270. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Lv, G.C.; Zhou, R.; Ma, R.X.; Hou, X.F.; Zhang, J.M.; Li, W. Fabrication of superhydrophilic self-cleaning SiO2-TiO2 coating and its photocatalytic performance. Ceram. Int. 2022, 48, 20033–20040. [Google Scholar] [CrossRef]
- Chinthala, M.; Balakrishnan, A.; Venkataraman, P.; Gowtham, V.M.; Polagani, R.K. Synthesis and applications of nano-MgO and composites for medicine, energy, and environmental remediation: A review. Environ. Chem. Lett. 2021, 19, 4415–4454. [Google Scholar] [CrossRef]
- Liu, C.L.; Fang, X.L.; Chen, X.J.; Qi, X.W.; Teng, C.; Xie, X.Y.; Wang, Y.F.; Lu, G.X.; Chen, L.Z.; Mi, L.F.; et al. Flash regeneration of superhydrophilicity and antifog performance of laser induced molybdenum based micro-nano structures by flame treatment. J. Manuf. Process. 2025, 141, 169–180. [Google Scholar] [CrossRef]
- Cui, H.; Fang, X.; Qi, X.; Wang, Y.; Wang, H.; Li, C. Nature-inspired wettability-mixed titanium surface for efficient fog collection. Appl. Mater. Today 2025, 44, 102792. [Google Scholar] [CrossRef]
- Pescheux, A.C.; Raccurt, O.; Bourdon, D.; Le Baron, E. Accelerated aging tests and characterizations of innovated anti-soiling coatings for solar receiver glasses. Mater. Chem. Phys. 2020, 256, 123646. [Google Scholar] [CrossRef]
- Xie, X.; Qi, X.; Chen, X.; Cui, H. Durable self-cleaning anti-fog and antireflective titanium-based micro-nano structures on glass by laser marker ablation. Surf. Interfaces 2024, 51, 104644. [Google Scholar] [CrossRef]
- Vorobyev, A.Y.; Guo, C. Direct femtosecond laser surface nano/microstructuring and its applications. Laser Photonics Rev. 2013, 7, 385–407. [Google Scholar] [CrossRef]
- Hauschwitz, P.; Jagdheesh, R.; Rostohar, D.; Brajer, J.; Kopecek, J.; Jiricek, P.; Houdkova, J.; Mocek, T. Hydrophilic to ultrahydrophobic transition of Al 7075 by affordable ns fiber laser and vacuum processing. Appl. Surf. Sci. 2020, 505, 144523. [Google Scholar] [CrossRef]
- He, W.; Yao, P.; Chu, D.; Sun, H.; Lai, Q.; Wang, Q.; Wang, P.; Qu, S.; Huang, C. Controllable hydrophilic titanium surface with micro-protrusion or micro-groove processed by femtosecond laser direct writing. Opt. Laser Technol. 2022, 152, 108082. [Google Scholar] [CrossRef]
- Guo, S.; Liu, M.D.; You, L.M.; Cheng, G.; Li, J.; Zhou, K. Oxygen vacancy induced peroxymonosulfate activation by Mg-doped Fe2O3 composites for advanced oxidation of organic pollutants. Chemosphere 2021, 279, 130482. [Google Scholar] [CrossRef] [PubMed]
- Nithya, N.; Gopi, S.; Bhoopathi, G. An Amalgam of Mg-Doped TiO2 Nanoparticles Prepared by Sol-Gel Method for Effective Antimicrobial and Photocatalytic Activity. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4594–4607. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.P.; Tian, Y.L. Insights into the wettability transition of nanosecond laser ablated surface under ambient air exposure. J. Colloid Interface Sci. 2019, 533, 268–277. [Google Scholar] [CrossRef]
- Dekermenjian, M.; Ruediger, A.P.; Merlen, A. Raman spectroscopy investigation of magnesium oxide nanoparticles. Rsc Adv. 2023, 13, 26683–26689. [Google Scholar] [CrossRef]
- Kernazhitsky, L.; Shymanovska, V.; Gavrilko, T.; Naumov, V.; Fedorenko, L.; Kshnyakin, V.; Baran, J. Room temperature photoluminescence of anatase and rutile TiO2 powders. J. Lumin. 2014, 146, 199–204. [Google Scholar] [CrossRef]
- Cao, Y.Q.; Long, H.J.; Chen, Y.M.; Cao, Y.A. Photocatalytic Activity of TiO2 Films with Rutile/Anatase Mixed Crystal Structures. Acta Phys. -Chim. Sin. 2009, 25, 1088–1092. [Google Scholar]
- Liu, B.-T.; Yeh, W.-D. Reflective properties of nanoparticle-arrayed surfaces. Thin Solid Film. 2010, 518, 6015–6021. [Google Scholar] [CrossRef]
- Kumar, M.; Gupta, A.K.; Kumar, D. Mg-doped TiO2 thin films deposited by low cost technique for CO gas monitoring. Ceram. Int. 2016, 42, 405–410. [Google Scholar] [CrossRef]
- He, X.; Li, G.; Zhang, Y.; Lai, X.; Zhou, M.; Xiao, L.; Tang, X.; Hu, Y.; Liu, H.; Yang, Y.; et al. Bioinspired functional glass integrated with multiplex repellency ability from laser-patterned hexagonal texturing. Chem. Eng. J. 2021, 416, 129113. [Google Scholar] [CrossRef]





| Sample | Frequency (kHz) | Power Output (%) | Scanning Speed (m/s) | D (mm) | Deposition Line Spacing (mm) | Etching Line Spacing (mm) |
|---|---|---|---|---|---|---|
| Single Mg film | 20, 50, 100, 200 | 50 | 2 | 2 | 0.5 | 0.01–0.04 |
| Co-MgTi | 20, 50, 100, 200 | 50 | 2 | 2 | 0.5 | 0.01–0.04 |
| Se-MgTi | 20, 50, 100, 200 | 50 | 2 | 2 | 0.5 | 0.01–0.04 |
| Sample | Band Gap (eV) |
|---|---|
| Single Ti film | 3.5859 |
| Co-MgTi | 3.441 |
| Se-MgTi | 3.3712 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Z.; Zhang, F.; Gao, Y.; Chen, L.; Liu, J.; Wang, Y.; Sun, C.; Cui, H. Laser Fabricated MgO-TiO2 Based Photocatalytic Antifogging and Self-Cleaning Surface in Air. Coatings 2025, 15, 1214. https://doi.org/10.3390/coatings15101214
Zhai Z, Zhang F, Gao Y, Chen L, Liu J, Wang Y, Sun C, Cui H. Laser Fabricated MgO-TiO2 Based Photocatalytic Antifogging and Self-Cleaning Surface in Air. Coatings. 2025; 15(10):1214. https://doi.org/10.3390/coatings15101214
Chicago/Turabian StyleZhai, Zhenze, Feiyue Zhang, Yongjian Gao, Longze Chen, Jia Liu, Yu Wang, Chaoran Sun, and Hongtao Cui. 2025. "Laser Fabricated MgO-TiO2 Based Photocatalytic Antifogging and Self-Cleaning Surface in Air" Coatings 15, no. 10: 1214. https://doi.org/10.3390/coatings15101214
APA StyleZhai, Z., Zhang, F., Gao, Y., Chen, L., Liu, J., Wang, Y., Sun, C., & Cui, H. (2025). Laser Fabricated MgO-TiO2 Based Photocatalytic Antifogging and Self-Cleaning Surface in Air. Coatings, 15(10), 1214. https://doi.org/10.3390/coatings15101214







