Development and Experimental Validation of a Filament-Assisted Chemical Vapor Deposition (FACVD) Reactor Using a Plastic Chamber
Abstract
1. Introduction
2. FACVD and Experimental Setup
2.1. FACVD Process
- (a)
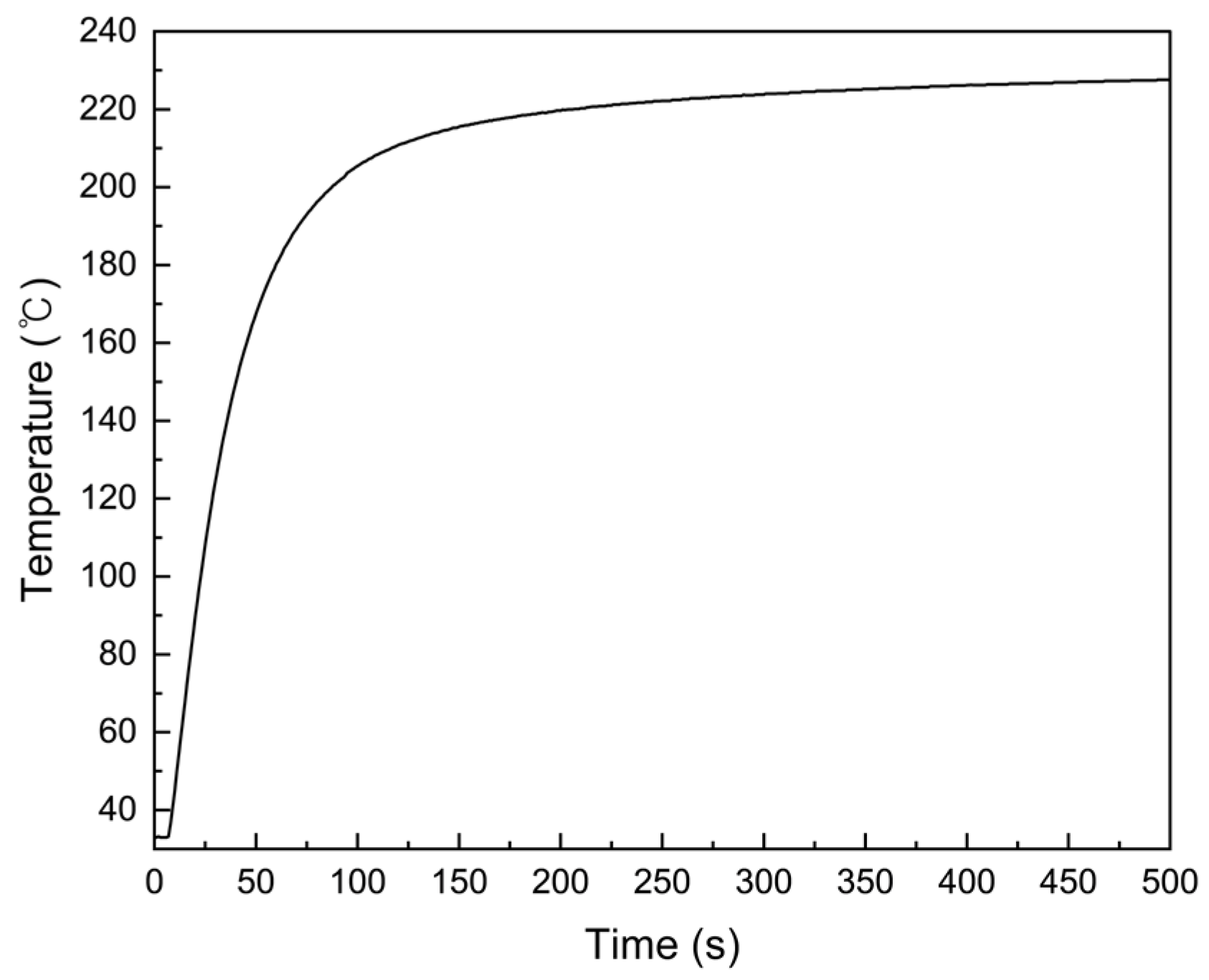
- Radical Generation: Vapor-phase initiators (e.g., TBPO) are thermally decomposed on a filament heated to approximately 220 °C, generating reactive radicals. These radicals remain stable in the low-pressure vapor phase and diffuse toward the substrate surface.
- (b)
- Monomer Adsorption: The substrate is maintained at approximately 25–30 °C, allowing monomer vapors to physically adsorb onto the surface and increase the local monomer concentration. The adsorbed monomers enhance the surface polymerization efficiency and establish the surface reaction-dominant conditions.
- (c)
- Surface Polymerization: Vapor-phase radicals react with the vinyl groups of the monomers condensed on the substrate surface, initiating free-radical polymerization. Radicals continuously add to the monomers on the surface, promoting chain growth and forming a thin-film network structure as bonds are formed.
- (d)
- Thin-Film Growth: As polymerization proceeds, a uniform polymer thin film grows on the surface. The film thickness and composition can be controlled by adjusting the monomer/initiator feed ratio, radical generation rate, substrate temperature, and reaction time, thereby enabling high-purity film deposition without solvent residues [5,19,20].
2.2. FACVD System and Chamber Configuration
2.3. Deposition Procedure
2.4. Analysis Methods
3. Results
4. Discussion
5. Conclusions
- A plastic acrylic chamber was successfully applied to a FACVD system as a vacuum reactor, demonstrating feasibility for vapor-phase polymerization under moderate vacuum conditions.
- The devised reactor showed a successful demonstration of growing pGMA thin films by controlling the growth rate within 85% of thickness uniformity for a 4-inch wafer’s area.
- Polymerization of pGMA thin films was validated by using FTIR and XPS analysis.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, N.; Kim, D.H.; Kovacik, P.; Sojoudi, H.; Wang, M.; Gleason, K.K. Polymer Thin Films and Surface Modification by Chemical Vapor Deposition: Recent Progress. Annu. Rev. Chem. Biomol. Eng. 2016, 7, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Barra, G.; Guadagno, L.; Raimondo, M.; Santonicola, M.G.; Toto, E.; Ciprioti, S.V. A Comprehensive Review on the Thermal Stability Assessment of Polymers and Composites for Aeronautics and Space Applications. Polymers 2023, 15, 3786. [Google Scholar] [CrossRef] [PubMed]
- Ohring, M. Materials Science of Thin Films: Deposition and Structure, 2nd ed.; Academic Press: Cambridge, MA, USA, 2001; ISBN 978-0125249751. [Google Scholar]
- Gleason, K.K. Controlled Release Utilizing Initiated Chemical Vapor Deposited (iCVD) of Polymeric Nanolayers. Front. Bioeng. Biotechnol. 2021, 9, 632753. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.K.S.; Gleason, K.K. Initiated Chemical Vapor Deposition (iCVD) of Poly(alkyl acrylates): A Kinetic Model. Macromolecules 2006, 39, 3695–3703. [Google Scholar] [CrossRef]
- Chan, K.; Gleason, K.K. Initiated Chemical Vapor Deposition of Linear and Cross-linked Poly(2-hydroxyethyl methacrylate) for Use as Thin-Film Hydrogels. Langmuir 2005, 21, 8930–8939. [Google Scholar] [CrossRef]
- Song, Q.; Zhu, M.; Chen, X.; Liu, T.; Xie, M.; Mao, Y. Flexible Membranes Fabricated by Initiated Chemical Vapor Deposition for Water Treatment, Battery, and Drug Delivery. Chem. Eng. J. 2023, 477, 146911. [Google Scholar] [CrossRef]
- Heikkinen, I.T.; Marin, G.; Bihari, N.; Ekstrum, C.; Mayville, P.J.; Fei, Y.; Hu, Y.H.; Karppinen, M.; Savin, H.; Pearce, J.M. Atomic layer deposited aluminum oxide mitigates outgassing from fused filament fabrication–based 3-D printed components. Surf. Coat. Technol. 2020, 386, 125459. [Google Scholar] [CrossRef]
- Gkinis, T.; Tsoukalas, D.; Chroneos, A. Design and implementation of a rotating disk chemical vapor deposition reactor. J. Vac. Sci. Technol. A 2017, 35, 21503. [Google Scholar]
- Zhao, Z.; Chen, Y.; Liu, X. Optimization of gas inlet design for uniform thin film deposition in CVD reactors. J. Vac. Sci. Technol. A 2023, 41, 33409. [Google Scholar]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Kim, H.R.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5(8), 574–578. [Google Scholar]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.A.; Lichtenberg, A.J. Principles of Plasma Discharges and Materials Processing, 2nd ed.; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar]
- Martin, T.P.; Gleason, K.K. Initiated chemical vapor deposition (iCVD) of polymer thin films. Langmuir 2007, 23, 3238–3244. [Google Scholar]
- Xu, J.; Gleason, K.K. Mechanistic study of initiated chemical vapor deposition (iCVD) of polymer thin films. Macromolecules 2011, 44, 6610–6618. [Google Scholar]
- Asatekin, A.; Gleason, K.K. Polymeric nanostructured membranes by initiated chemical vapor deposition (iCVD). J. Am. Chem. Soc. 2008, 130, 4554–4555. [Google Scholar]
- Mao, Y.; Gleason, K.K. Vapor-deposited fluorinated glycidyl copolymer thin films with low surface energy and improved mechanical properties. Macromolecules 2006, 39, 3895–3900. [Google Scholar] [CrossRef]
- Mao, Y.; Gleason, K.K. Hot filament chemical vapor deposition of poly (glycidyl methacrylate) thin films using tert-butyl peroxide as an initiator. Langmuir 2004, 20, 2484–2488. [Google Scholar] [CrossRef]
- Lau, K.K.S.; Gleason, K.K. Initiated chemical vapor deposition (iCVD) of poly(alkyl acrylates): An experimental study. Macromolecules 2006, 39, 3688–3694. [Google Scholar] [CrossRef]
- Baxamusa, S.H.; Gleason, K.K. Thin polymer films with high step coverage in microtrenches by initiated CVD. Chem. Vap. Depos. 2008, 14, 313–318. [Google Scholar] [CrossRef]
- Yagüe, J.L.; Perry, J.W.; Flynn, E.R.; Gleason, K.K. Chemical vapor deposition for solvent-free polymerization at surfaces. Macromol. Chem. Phys. 2013, 214, 302–312. [Google Scholar]
- Lewis, H.G.P.; Lau, K.K.S.; Mao, Y.; Gleason, K.K. Initiated Chemical Vapor Depositioin (iCVD) of polymer thin films. In Proceedings of the Annual Technical Conference-Society of Vacuum Coaters, Denver, CO, USA, 23–28 April 2005; pp. 90–94. [Google Scholar]
- Zhou, J.; Ohno, T.R.; Wolden, C.A. High-temperature stability of nichrome in reactive environments. J. Vac. Sci. Technol. A Vac. Surf. Film. 2003, 21, 756–761. [Google Scholar]
- Burkey, D.; Anastasio, D.; Suresh, A. A New Take on Kinetics: Initiated Chemical Vapor Deposition as a Chemical Engineering Capstone Laboratory. Chem. Eng. Educ. 2014, 48, 98–106. [Google Scholar]
- Graur, V.; Mukherjee, A.; Sebakhy, K.O.; Bose, R.K. Initiated chemical vapor deposition (iCVD) of bio-based poly (tulipalin A) coatings: Structure and material properties. Polymers 2022, 14, 3993. [Google Scholar] [CrossRef]
- Asatekin, A.; Barr, M.C.; Baxamusa, S.H.; Lau, K.K.; Tenhaeff, W.; Xu, J.; Gleason, K.K. Designing polymer surfaces via vapor deposition. Mater. Today 2010, 13, 26–33. [Google Scholar] [CrossRef]
- Tao, R.; Anthamatten, M. Condensation and polymerization of supersaturated monomer vapor. Langmuir 2012, 28, 16580–16587. [Google Scholar] [CrossRef]
- ISO 21360-1:2020; Vacuum Technology—Standard Methods for Measuring Vacuum-Pump Performance—Part 1: General Description. ISO: Geneva, Switzerland, 2020.
- Liu, W.; Yin, P.; Liu, X.; Dong, X.; Zhang, J.; Xu, Q. Thermodynamics, kinetics, and isotherms studies for gold(III) adsorption using silica functionalized by diethylenetriaminemethylenephosphonic acid. Chem. Eng. Res. Des. 2013, 91, 2748–2758. [Google Scholar] [CrossRef]
- Darvishi, A.; Mehr, M.J.Z.; Marandi, G.B.; Kabiri, K.; Bouhendi, H.; Bakhshi, H. Copolymers of glycidyl methacrylate and octadecyl acrylate: Synthesis, characterization, swelling properties, and reactivity ratios. Des. Monomers Polym. 2013, 16, 79–88. [Google Scholar] [CrossRef]
- Abdollahi, H.; Salimi, A.; Barikani, M.; Samadi, A.; Rad, S.H.; Zanjanijam, A.R. Systematic investigation of mechanical properties and fracture toughness of epoxy networks: Role of the polyetheramine structural parameters. J. Appl. Polym. Sci. 2019, 136, 47121. [Google Scholar] [CrossRef]
- Seah, M.P. Ultrathin SiO2 on Si. VI. Evaluation of uncertainties in thickness measurement using XPS. Surf. Interface Anal. Int. J. Devoted Dev. Appl. Tech. Anal. Surf. Terfaces Thin Film. 2005, 37, 300–309. [Google Scholar] [CrossRef]
- Beamson, G.; Briggs, D. High resolution xps of organic polymers. Anal. Chim. Acta 1993, 276, 469–470. [Google Scholar] [CrossRef]
- Barbey, R.; Klok, H.-A. Room temperature, aqueous post-polymerization modification of glycidyl methacrylate-containing polymer brushes prepared via surface-initiated atom transfer radical polymerization. Langmuir 2010, 26, 18219–18230. [Google Scholar]
- Hong, S.-G. The thermal-oxidative degradation of an epoxy adhesive on metal substrates: XPS and RAIR analyses. Polym. Degrad. Stab. 1995, 48, 211–218. [Google Scholar] [CrossRef]








| Structure | Name (Abbreviation) | Molecular Weight (g/mol) | Chemical Formula |
|---|---|---|---|
 | Tert-Butyl peroxide (TBPO) | 146.23 | C8H18O2 |
 | Glycidyl methacrylate (GMA) | 142.15 | C7H10O3 |
| Specimen 1 | Specimen 2 | Specimen 3 | |
|---|---|---|---|
| FI (sccm) | 1.513 | 1.797 | 1.671 |
| FM (sccm) | 0.931 | 0.716 | 0.430 |
| PCh (mTorr) | 950 | 800 | 800 |
| Sub (°C) | 25 | 25 | 25 |
| PM/PSAT | 1.167 | 0.735 | 0.528 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.C.; Lee, J.H.; Kwak, J.B. Development and Experimental Validation of a Filament-Assisted Chemical Vapor Deposition (FACVD) Reactor Using a Plastic Chamber. Coatings 2025, 15, 1213. https://doi.org/10.3390/coatings15101213
Kang HC, Lee JH, Kwak JB. Development and Experimental Validation of a Filament-Assisted Chemical Vapor Deposition (FACVD) Reactor Using a Plastic Chamber. Coatings. 2025; 15(10):1213. https://doi.org/10.3390/coatings15101213
Chicago/Turabian StyleKang, Him Chan, Jeong Heon Lee, and Jae B. Kwak. 2025. "Development and Experimental Validation of a Filament-Assisted Chemical Vapor Deposition (FACVD) Reactor Using a Plastic Chamber" Coatings 15, no. 10: 1213. https://doi.org/10.3390/coatings15101213
APA StyleKang, H. C., Lee, J. H., & Kwak, J. B. (2025). Development and Experimental Validation of a Filament-Assisted Chemical Vapor Deposition (FACVD) Reactor Using a Plastic Chamber. Coatings, 15(10), 1213. https://doi.org/10.3390/coatings15101213






