A Study on the Structure and Properties of NiCr-DLC Films Prepared by Filtered Cathodic Vacuum Arc Deposition
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Characteristics of NiCr–DLC Films
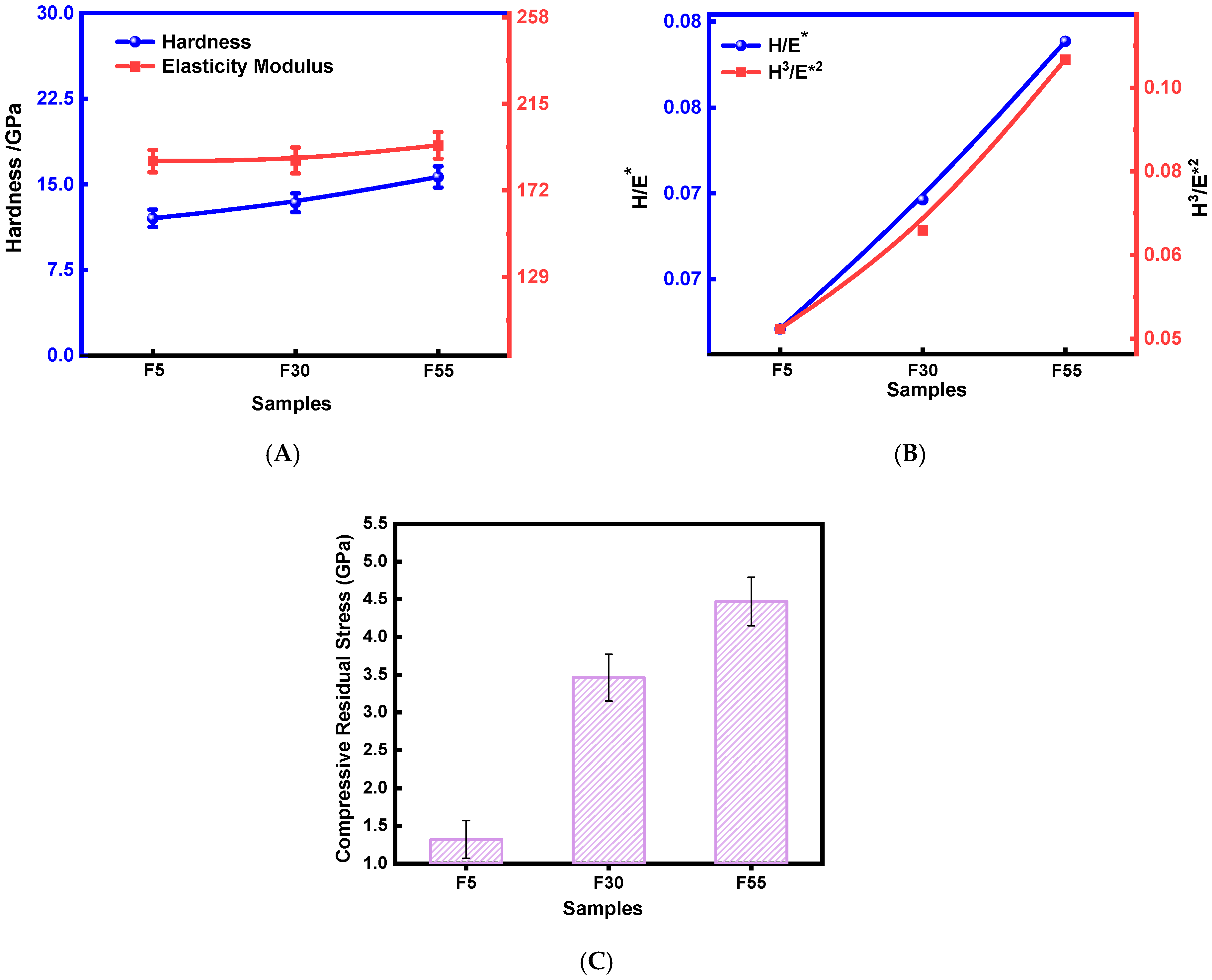
3.2. Mechanical Properties of NiCr–DLC Films
| Samples | Friction Coefficient | Wear Rate mm3/(N·m) |
|---|---|---|
| F5 | 0.415 ± 0.003 | 1.18 × 10−4 |
| F30 | 0.182 ± 0.002 | 2.30 × 10−6 |
| F55 | 0.115 ± 0.001 | 4.45 × 10−7 |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rajak, D.K.; Kumar, A.; Behera, A.; Menezes, P.L. Diamond-Like Carbon (DLC) Coatings: Classification, Properties, and Applications. Appl. Sci. 2021, 11, 4445. [Google Scholar] [CrossRef]
- Sahay, S.; Pandey, M.K.; Kar, A.K. Nickel concentration dependent mechanical and tribological properties of subsurface layer of electrodeposited Ni-C nanocomposite thin films. Thin Solid Film. 2023, 773, 139818. [Google Scholar] [CrossRef]
- Liu, X.; Jin, P.; Shu, Z.; Yang, Y.; Gui, B.; Cui, Q.; Wang, J.; Ding, J.; Zhang, S.; Zheng, J. Regulating the structure and performance of amorphous carbon-based films by introducing different interlayers for applications in PEMFC bipolar plates. Surf. Coat. Technol. 2024, 487, 131000. [Google Scholar] [CrossRef]
- Dai, W.; Wang, A. Synthesis, characterization and properties of the DLC films with low Cr concentration doping by a hybrid linear ion beam system. Surf. Coat. Technol. 2011, 205, 2882–2886. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Yang, S.-J.; Wang, D.-Y. Characterization of TiCr(C,N)/amorphous carbon coatings synthesized by a cathodic arc deposition process. Thin Solid Film. 2007, 515, 4722–4726. [Google Scholar] [CrossRef]
- Zhang, S.; Bui, X.L.; Jiang, J.; Li, X. Microstructure and tribological properties of magnetron sputtered nc-TiC/a-C nanocomposite. Surf. Coat. Technol. 2005, 198, 206–211. [Google Scholar] [CrossRef]
- Dub, S.; Pauleau, Y.; Thièry, F. Mechanical properties of nanostructured copper-hydrogenated amorphous carbon composite films studied by nanoindentation. Surf. Coat. Technol. 2004, 180–181, 551–555. [Google Scholar] [CrossRef]
- Dai, W.; Ke, P.; Wang, A. Microstructure and property evolution of Cr-DLC films with different Cr content deposited by a hybrid beam technique. Vacuum 2011, 85, 792–797. [Google Scholar] [CrossRef]
- Dovydaitis, V.; Milieška, M.; Chimborazo, J.; Gnecco, E.; Marcinauskas, L. The Effect of Chromium on the Microstructure and Transparency of Diamond-like Carbon Films. Processes 2025, 13, 1098. [Google Scholar] [CrossRef]
- Łukaszczyk, A.; Zimowski, S.; Pawlak, W.; Dubiel, B.; Moskalewicz, T. Microstructure, Micro-Mechanical and Tribocorrosion Behavior of Oxygen Hardened Ti–13Nb–13Zr Alloy. Materials 2021, 14, 2088. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Zhang, Y.; Cai, W.; Wang, L.; Tang, L. Effect of substrate bias on the microstructure and mechanical and tribological properties of ZrNbTiMo refractory high entropy alloy film. Surf. Coat. Technol. 2023, 455, 129214. [Google Scholar] [CrossRef]
- Liu, J.; Xiang, J.; Zuo, Z.; Xie, G.; Luo, J.; Sheng, Y. Tribological Properties of Ti-DLC Coatings on Piston-pin Surfaces. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2023, 38, 1136–1146. [Google Scholar] [CrossRef]
- Bootkul, D.; Saenphinit, N.; Supsermpol, B.; Aramwit, C.; Intarasiri, S. Synthesis of Ti-doped DLC film on SS304 steels by Filtered Cathodic Vacuum Arc (FCVA) technique for tribological improvement. Appl. Surf. Sci. 2014, 310, 293–299. [Google Scholar] [CrossRef]
- Zemek, J.; Houdkova, J.; Jiricek, P.; Jelinek, M. Amorphous carbon nanocomposite films doped by titanium: Surface and sub-surface composition and bonding. Diam. Relat. Mater. 2018, 81, 61–69. [Google Scholar] [CrossRef]
- Li, X.; Ke, P.; Wang, A. Ab initio molecular dynamics simulation on stress reduction mechanism of Ti-doped diamond-like carbon films. Thin Solid Film. 2015, 584, 204–207. [Google Scholar] [CrossRef]
- Jelinek, M.; Kocourek, T.; Zemek, J.; Mikšovský, J.; Kubinová, Š.; Remsa, J.; Kopeček, J.; Jurek, K. Chromium-doped DLC for implants prepared by laser-magnetron deposition. Mater. Sci. Eng. C 2015, 46, 381–386. [Google Scholar] [CrossRef]
- Zhu, L.-n.; Li, J.-c.; Kang, J.-j.; Tang, L.; Ma, G.-z.; Han, C.-h.; Shi, J.-d.; Wang, H.-d. Different Cr Contents on the Microstructure and Tribomechanical Properties of Multi-Layered Diamond-Like Carbon Films Prepared by Unbalanced Magnetron Sputtering. J. Mater. Eng. Perform. 2020, 29, 7131–7140. [Google Scholar] [CrossRef]
- Zheng, J.; Shang, J.; Zhuang, W.; Ding, J.C.; Mei, H.; Yang, Y.; Ran, S. Structural and tribomechanical properties of Cr-DLC films deposited by reactive high power impulse magnetron sputtering. Vacuum 2024, 230, 113611. [Google Scholar] [CrossRef]
- Constantinou, M.; Pervolaraki, M.; Koutsokeras, L.; Prouskas, C.; Patsalas, P.; Kelires, P.; Giapintzakis, J.; Constantinides, G. Enhancing the nanoscratch resistance of pulsed laser deposited DLC films through molybdenum-doping. Surf. Coat. Technol. 2017, 330, 185–195. [Google Scholar] [CrossRef]
- Shen, Y.; Luo, J.; Liao, B.; Zhang, X.; Zhao, Y.; Zeng, X.; Chen, L.; Pang, P.; Bao, F. Tribocorrosion and tribological behavior of Ti-DLC coatings deposited by filtered cathodic vacuum arc. Diam. Relat. Mater. 2022, 125, 108985. [Google Scholar] [CrossRef]
- Zou, C.W.; Wang, H.J.; Feng, L.; Xue, S.W. Effects of Cr concentrations on the microstructure, hardness, and temperature-dependent tribological properties of Cr-DLC coatings. Appl. Surf. Sci. 2013, 286, 137–141. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Alam, M.S.; Mukherjee, N. Cu incorporated amorphous diamond like carbon (DLC) composites: An efficient electron field emitter over a wide range of temperature. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 97, 120–125. [Google Scholar] [CrossRef]
- Zhang, S.; Ali, N. Nanocomposite Thin Films and Coatings: Processing, Properties and Performance; Imperial College Press: London, UK, 2007; pp. 1–615. [Google Scholar]
- Mohammadinia, E.; Elahi, S.M.; Shahidi, S. Structural and optical properties of Ni-embedded hydrogenated diamond-like carbon (Ni-DLC) prepared by co-deposition of RF-Sputtering and RF-PECVD method. Mater. Sci. Semicond. Process. 2018, 74, 7–12. [Google Scholar] [CrossRef]
- Balestra, R.M.; Castro, A.M.G.; Evaristo, M.; Escudeiro, A.; Mutafov, P.; Polcar, T.; Cavaleiro, A. Carbon-based coatings doped by copper: Tribological and mechanical behavior in olive oil lubrication. Surf. Coat. Technol. 2011, 205, S79–S83. [Google Scholar] [CrossRef]
- Sun, H.; Yang, L.; Wu, H.; Zhao, L. Effects of Element Doping on the Structure and Properties of Diamond-like Carbon Films: A Review. Lubricants 2023, 11, 186. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, P.; He, X.; Li, L.; Wang, A.; Li, H. Developing transparent copper-doped diamond-like carbon films for marine antifouling applications. Diam. Relat. Mater. 2016, 69, 144–151. [Google Scholar] [CrossRef]
- Zhou, H.; Hou, Q.; Xiao, T.; Wang, Y.; Liao, B.; Zhang, X. The composition, microstructure and mechanical properties of Ni/DLC nanocomposite films by filtered cathodic vacuum arc deposition. Diam. Relat. Mater. 2017, 75, 96–104. [Google Scholar] [CrossRef]
- Chen, S.N.; Yan, W.Q.; Zhao, Y.M.; Li, Q.; Chen, L.; Ouyang, X.; Hua, Q.S.; Wu, X.Y.; Zhang, Y.F.; Liao, B.; et al. Strong amorphization of AlCrNiTiV high-entropy alloy films deposited by cofilter cathode vacuum arc deposition. Appl. Surf. Sci. 2022, 592, 153318. [Google Scholar] [CrossRef]
- Shen, Y.; Zhou, H.; Wang, H.; Liao, B.; Wu, X.; Zhang, X. Tribological behavior of diamond-like carbon coatings with patterned structure deposited by the filtered cathodic vacuum arc. Thin Solid Film. 2019, 685, 123–130. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Liu, W.; Wu, B.; Liu, H.; Liu, R.; Mo, Y.; Tian, Z.; Hou, Z.; Xi, T.; Wan, Z.; Huang, C.; et al. Simulation on microstructure evolution and mechanical properties of Mg−Y alloys: Effect of trace Y. Trans. Nonferr. Met. Soc. China 2022, 32, 812–823. [Google Scholar] [CrossRef]
- Zhou, F.; Fu, K.; Liao, B.; Yu, J.; Yang, C.; Zhang, X. Effect of carbon content on nanostructural, mechanical and electrochemical characteristics of self-organized nc-ZrCN/a-CNx nanocomposite films. Appl. Surf. Sci. 2015, 327, 350–357. [Google Scholar] [CrossRef]
- Nedfors, N.; Tengstrand, O.; Lewin, E.; Furlan, A.; Eklund, P.; Hultman, L.; Jansson, U. Structural, mechanical and electrical-contact properties of nanocrystalline-NbC/amorphous-C coatings deposited by magnetron sputtering. Surf. Coat. Technol. 2011, 206, 354–359. [Google Scholar] [CrossRef]
- Pelleg, J.; Zevin, L.Z.; Lungo, S.; Croitoru, N. Reactive-sputter-deposited TiN films on glass substrates. Thin Solid Film. 1991, 197, 117–128. [Google Scholar] [CrossRef]
- Arab Pour Yazdi, M.; Lomello, F.; Wang, J.; Sanchette, F.; Dong, Z.; White, T.; Wouters, Y.; Schuster, F.; Billard, A. Properties of TiSiN coatings deposited by hybrid HiPIMS and pulsed-DC magnetron co-sputtering. Vacuum 2014, 109, 43–51. [Google Scholar] [CrossRef]
- Jo, Y.J.; Zhang, T.F.; Son, M.J.; Kim, K.H. Synthesis and electrochemical properties of Ti-doped DLC films by a hybrid PVD/PECVD process. Appl. Surf. Sci. 2018, 433, 1184–1191. [Google Scholar] [CrossRef]
- Di, Z.; Huang, A.; Fu, R.K.Y.; Chu, P.K.; Shao, L.; Höchbauer, T.; Nastasi, M.; Zhang, M.; Liu, W.; Shen, Q.; et al. Thermal stability of diamondlike carbon buried layer fabricated by plasma immersion ion implantation and deposition in silicon on insulator. J. Appl. Phys. 2005, 98, 053502. [Google Scholar] [CrossRef]
- Ong, C.W.; Zhao, X.A.; Cheung, J.T.; Lam, S.K.; Liu, Y.; Choy, C.L.; Chan, P.W. Thermal stability of pulsed laser deposited diamond-like carbon films. Thin Solid Film. 1995, 258, 34–39. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Berndt, M.; Abrasonis, G.; Kovács, G.J.; Krause, M.; Munnik, F.; Heller, R.; Kolitsch, A.; Möller, W. Bulk diffusion induced structural modifications of carbon-transition metal nanocomposite films. J. Appl. Phys. 2011, 109, 063503. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. The same chemical state of carbon gives rise to two peaks in X-ray photoelectron spectroscopy. Sci. Rep. 2021, 11, 11195. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. Reliable determination of chemical state in x-ray photoelectron spectroscopy based on sample-work-function referencing to adventitious carbon: Resolving the myth of apparent constant binding energy of the C 1s peak. Appl. Surf. Sci. 2018, 451, 99–103. [Google Scholar] [CrossRef]
- Singh, V.; Jiang, J.C.; Meletis, E.I. Cr-diamondlike carbon nanocomposite films: Synthesis, characterization and properties. Thin Solid Film. 2005, 489, 150–158. [Google Scholar] [CrossRef]
- Dai, W.; Zheng, H.; Wu, G.; Wang, A. Effect of bias voltage on growth property of Cr-DLC film prepared by linear ion beam deposition technique. Vacuum 2010, 85, 231–235. [Google Scholar] [CrossRef]
- Bayer, B.C.; Bosworth, D.A.; Michaelis, F.B.; Blume, R.; Habler, G.; Abart, R.; Weatherup, R.S.; Kidambi, P.R.; Baumberg, J.J.; Knop-Gericke, A.; et al. In Situ Observations of Phase Transitions in Metastable Nickel (Carbide)/Carbon Nanocomposites. J. Phys. Chem. C 2016, 120, 22571–22584. [Google Scholar] [CrossRef]
- Zhao, Y.; Ju, P.; Liu, H.; Pei, L.; Ji, L.; Li, H.; Xue, D.; Zhou, H.; Chen, J. A strategy to construct long-range fullerene-like nanostructure in amorphous carbon film with improved toughness and carrying capacity. J. Phys. D Appl. Phys. 2020, 53, 335205. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the significance of the H/E ratio in wear control: A nanocomposite coating approach to optimised tribological behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Chen, S.N.; Zhao, Y.M.; Zhang, Y.F.; Chen, L.; Liao, B.; Zhang, X.; Ouyang, X.P. Influence of carbon content on the structure and tribocorrosion properties of TiAlCN/TiAlN/TiAl multilayer composite coatings. Surf. Coat. Technol. 2021, 411, 126886. [Google Scholar] [CrossRef]
- Aoki, Y.; Ohtake, N. Tribological properties of segment-structured diamond-like carbon films. Tribol. Int. 2004, 37, 941–947. [Google Scholar] [CrossRef]
- Xu, P.; Cao, X.; Zhang, M.; Yue, W.; Zhang, G. Friction and wear behaviors of different DLC films sliding against SiC and Si3N4 balls under high relative humidity. Diam. Relat. Mater. 2020, 108, 107977. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, Y.; Chen, Z. Fretting tribological performance of DLC, TiAlN and DLC/TiAlN coatings deposited on carburized 18CrNi4A steel. Surf. Topogr. Metrol. Prop. 2022, 10, 015009. [Google Scholar] [CrossRef]
- Mussa, A.; Krakhmalev, P.; Bergström, J. Sliding wear and fatigue cracking damage mechanisms in reciprocal and unidirectional sliding of high-strength steels in dry contact. Wear 2020, 444–445, 203119. [Google Scholar] [CrossRef]
- Rani, R.; Panda, K.; Kumar, N.; Titovich, K.A.; Ivanovich, K.V.; Vyacheslavovich, S.A.; Lin, I.N. Tribological Properties of Ultrananocrystalline Diamond Films: Mechanochemical Transformation of Sliding Interfaces. Sci. Rep. 2018, 8, 283. [Google Scholar] [CrossRef]
- Erdemir, A.; Martin, J.M. Superior wear resistance of diamond and DLC coatings. Curr. Opin. Solid State Mater. Sci. 2018, 22, 243–254. [Google Scholar] [CrossRef]
- Li, L.; Liu, L.-L.; Li, X.; Guo, P.; Ke, P.; Wang, A. Enhanced Tribocorrosion Performance of Cr/GLC Multilayered Films for Marine Protective Application. ACS Appl. Mater. Interfaces 2018, 10, 13187–13198. [Google Scholar] [CrossRef]
- Aliyu, A.; Srivastava, C. Microstructure-corrosion property correlation in electrodeposited AlCrFeCoNiCu high entropy alloys-graphene oxide composite coatings. Thin Solid Film. 2019, 686, 137434. [Google Scholar] [CrossRef]
- Zhou, H.; Chhin, D.; Morel, A.; Gallant, D.; Mauzeroll, J. Potentiodynamic polarization curves of AA7075 at high scan rates interpreted using the high field model. npj Mater. Degrad. 2022, 6, 20. [Google Scholar] [CrossRef]
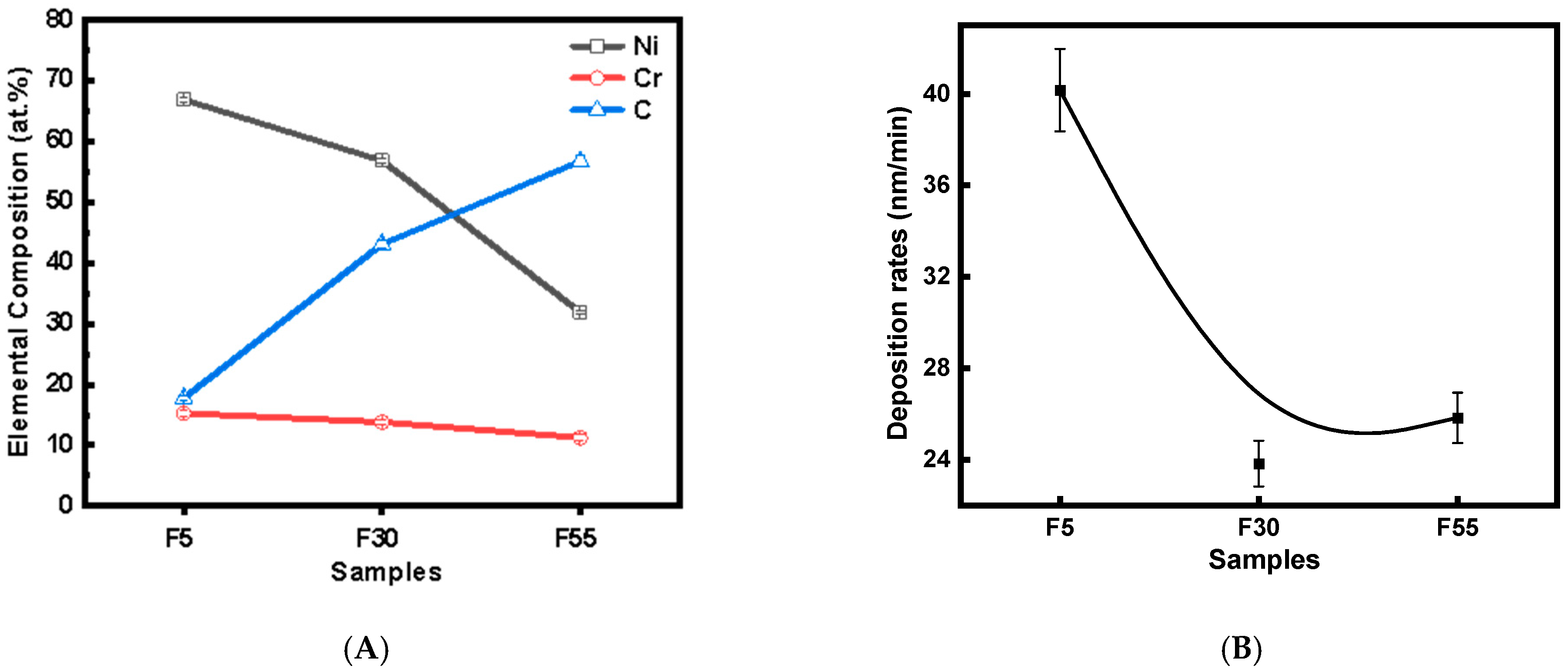

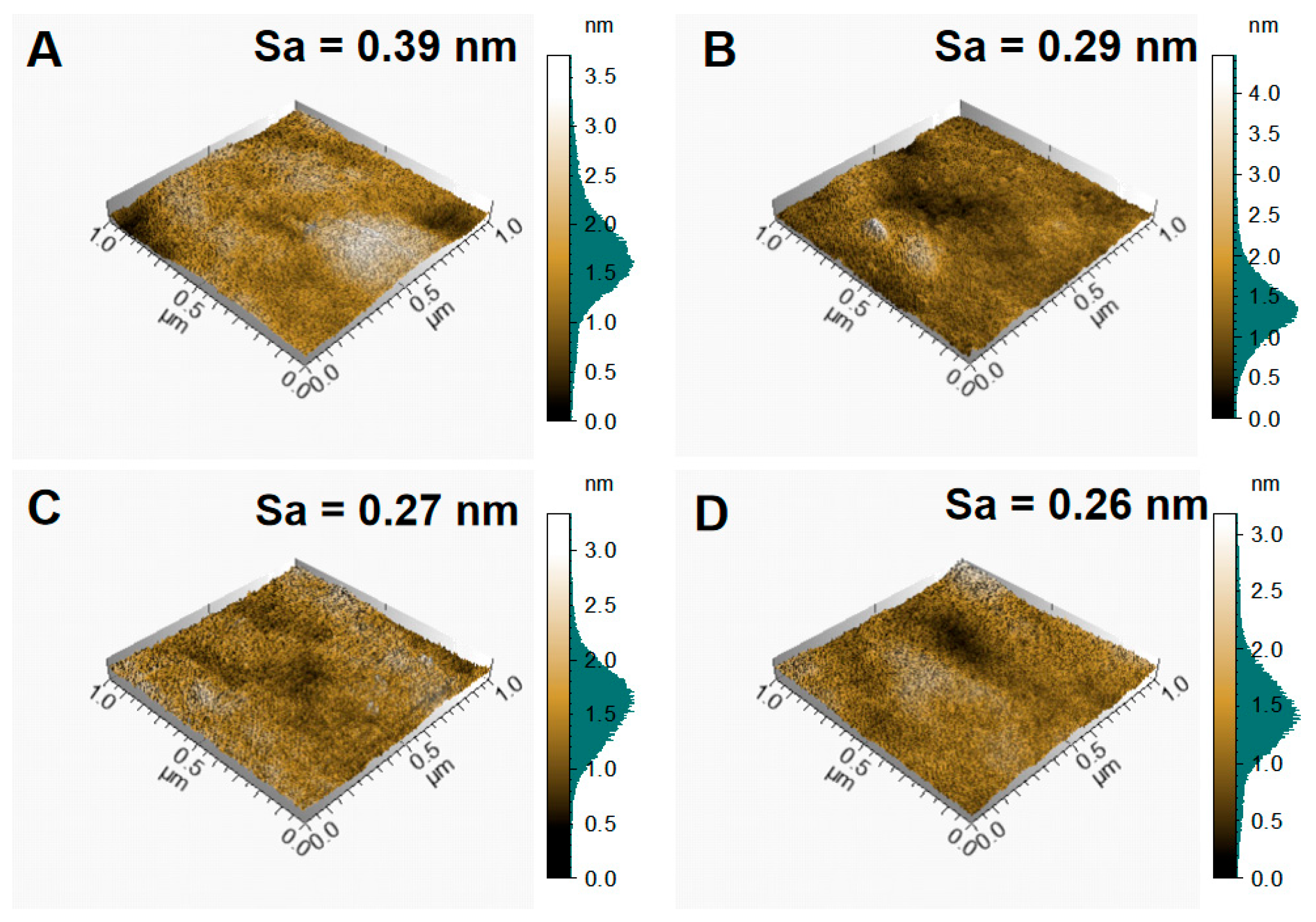

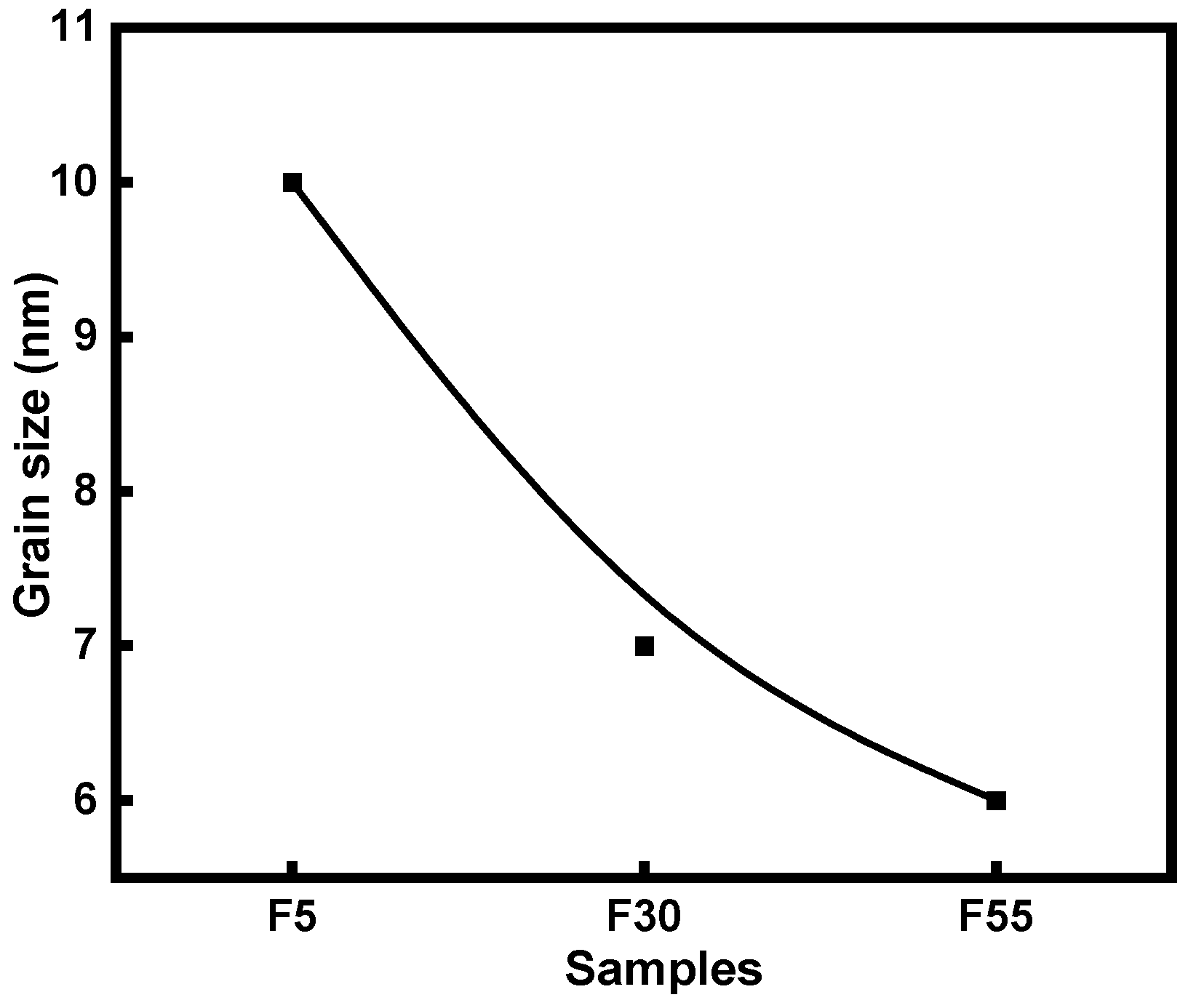

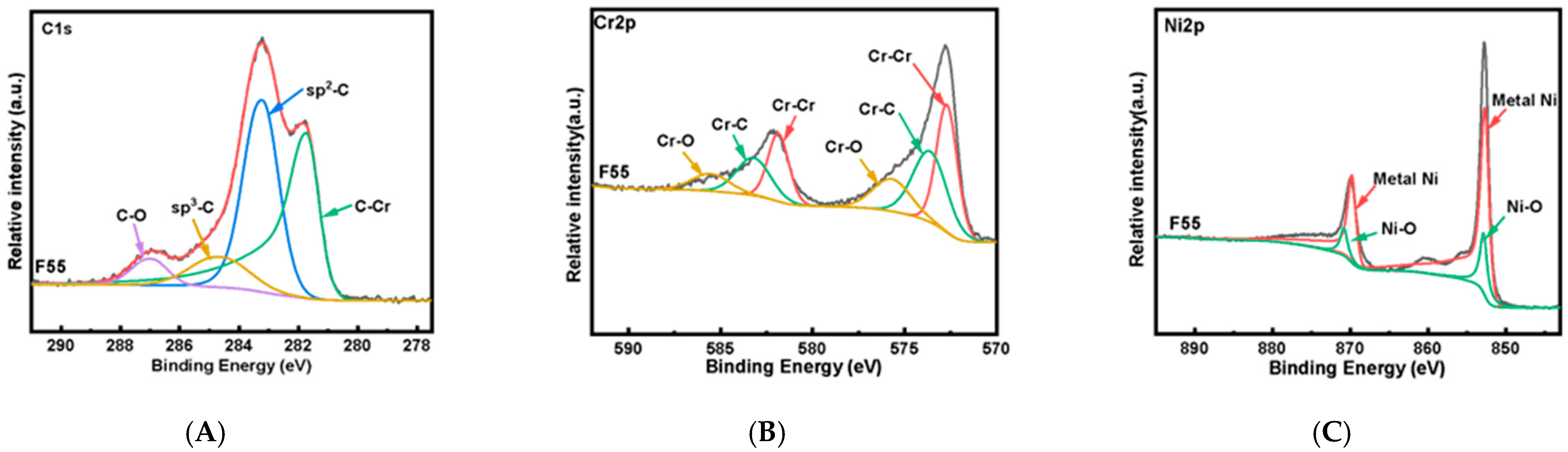



| Sample | D Peak Position/cm−1 | G Peak Position/cm−1 | FWHM of D Peak | FWHM of G Peak | ID/IG |
|---|---|---|---|---|---|
| F5 | 1417.31 | 1572.84 | 258.03 | 112.15 | 0.63 |
| F30 | 1376.03 | 1572.25 | 82.38 | 67.58 | 0.66 |
| F55 | 1347.16 | 1565.92 | 347.12 | 116.48 | 1.17 |
| Samples | (V) | (A/cm2) | (mV) | (mV) | (Ω/cm2) | η |
|---|---|---|---|---|---|---|
| AISI304L | −0.22 ± 0.005 | 9.94 × 10−7 ± 0.22 | 436.95 | 142.91 | 4.79 × 104 | — |
| F5 | −0.08 ± 0.004 | 6.82 × 10−7 ± 0.12 | 176.70 | 236.60 | 7.36 × 104 | 31.4% |
| F30 | 0.003 ± 0.001 | 2.27 × 10−7 ± 0.15 | 244.53 | 181.84 | 1.87 × 105 | 77.2% |
| F55 | 0.083 ± 0.001 | 1.22 × 10−7 ± 0.17 | 183.21 | 280.78 | 2.76 × 105 | 87.7% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhang, L.; Wu, S.; Peng, X.; Ouyang, X.; Liao, B.; Zhang, X. A Study on the Structure and Properties of NiCr-DLC Films Prepared by Filtered Cathodic Vacuum Arc Deposition. Coatings 2025, 15, 1136. https://doi.org/10.3390/coatings15101136
Zhang B, Zhang L, Wu S, Peng X, Ouyang X, Liao B, Zhang X. A Study on the Structure and Properties of NiCr-DLC Films Prepared by Filtered Cathodic Vacuum Arc Deposition. Coatings. 2025; 15(10):1136. https://doi.org/10.3390/coatings15101136
Chicago/Turabian StyleZhang, Bo, Lan Zhang, Shuai Wu, Xue Peng, Xiaoping Ouyang, Bin Liao, and Xu Zhang. 2025. "A Study on the Structure and Properties of NiCr-DLC Films Prepared by Filtered Cathodic Vacuum Arc Deposition" Coatings 15, no. 10: 1136. https://doi.org/10.3390/coatings15101136
APA StyleZhang, B., Zhang, L., Wu, S., Peng, X., Ouyang, X., Liao, B., & Zhang, X. (2025). A Study on the Structure and Properties of NiCr-DLC Films Prepared by Filtered Cathodic Vacuum Arc Deposition. Coatings, 15(10), 1136. https://doi.org/10.3390/coatings15101136






