TBC Development on Ti-6Al-4V for Aerospace Application
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
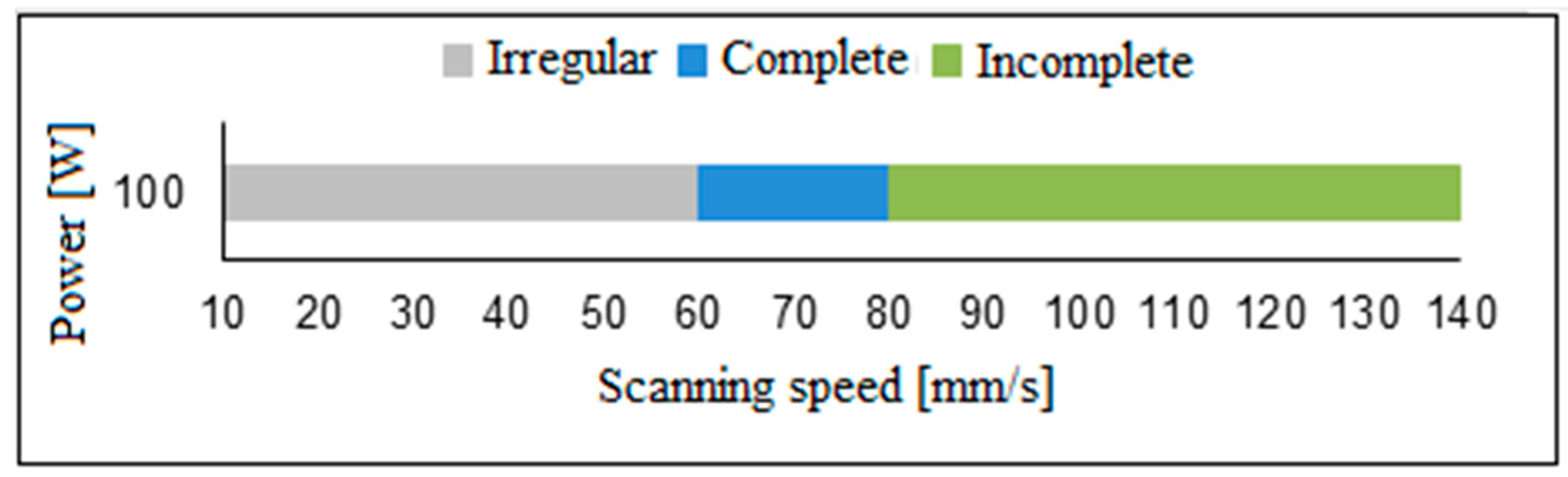
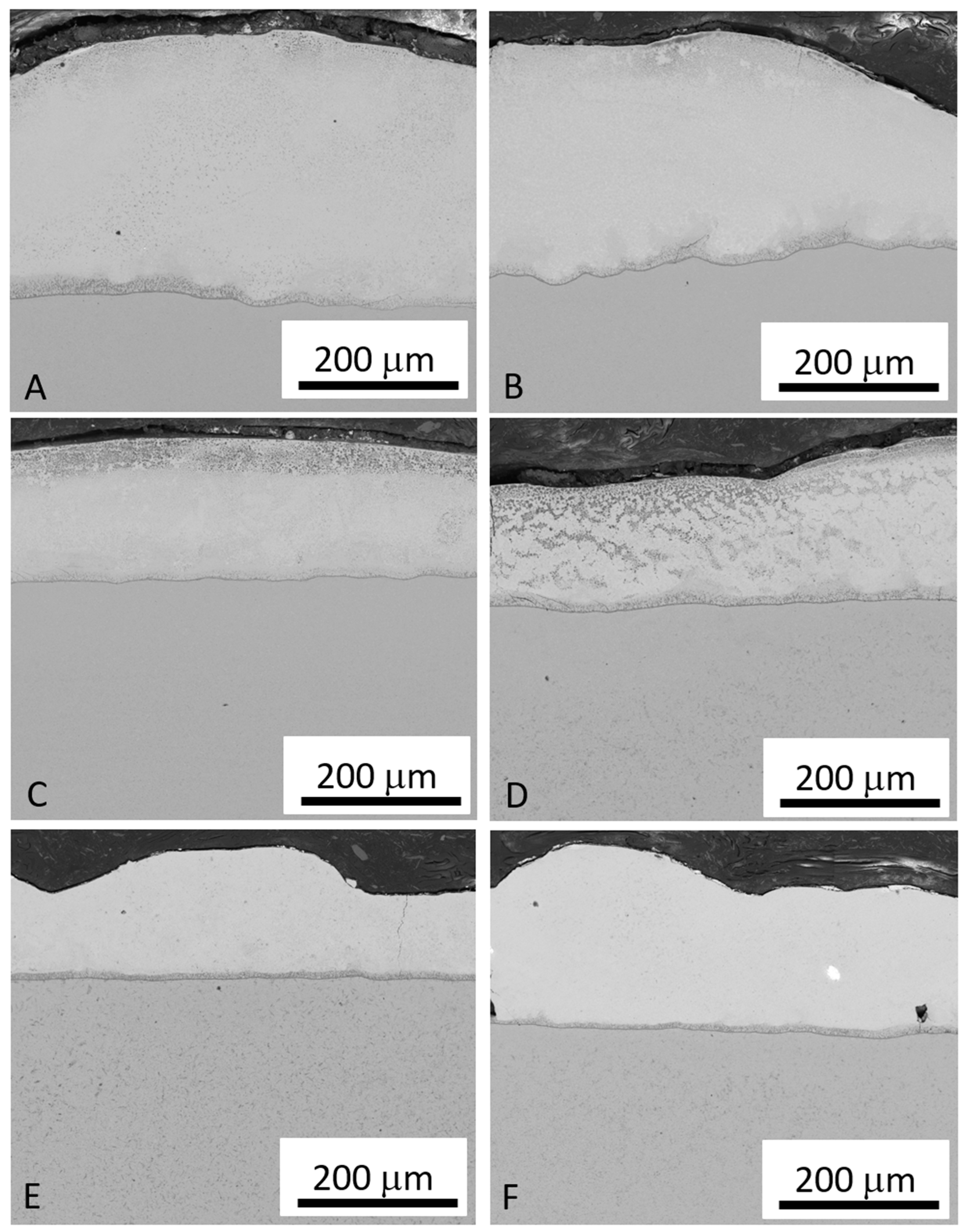
3.1. Bond Coating—NiCrAlY
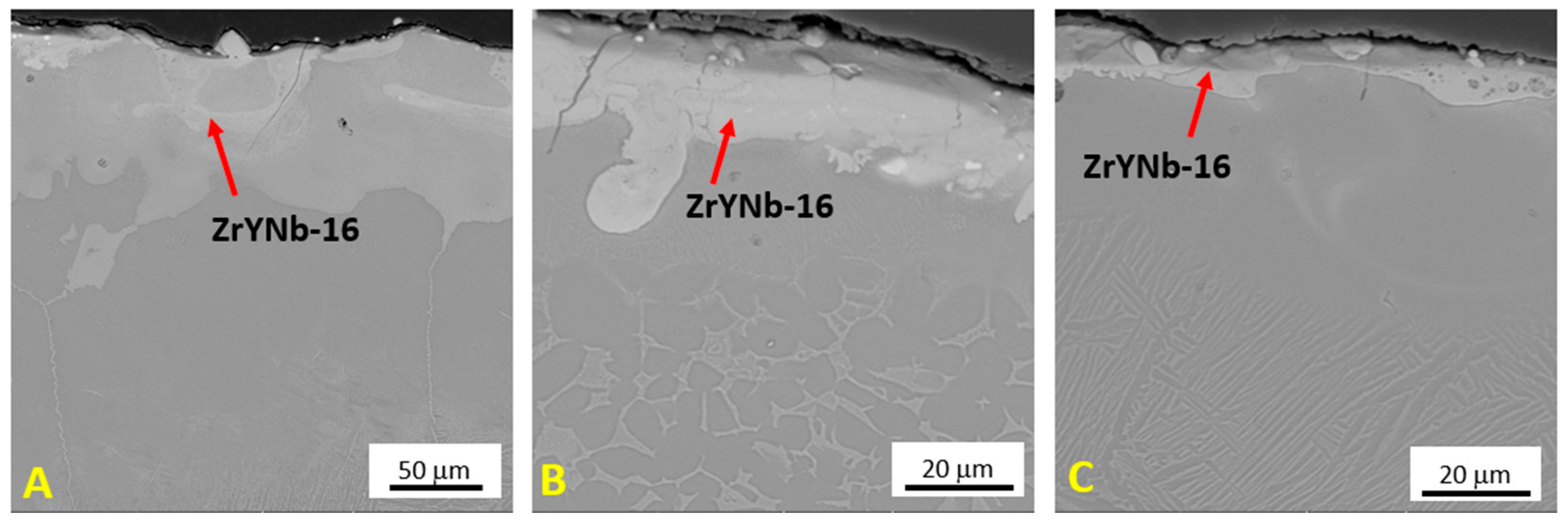
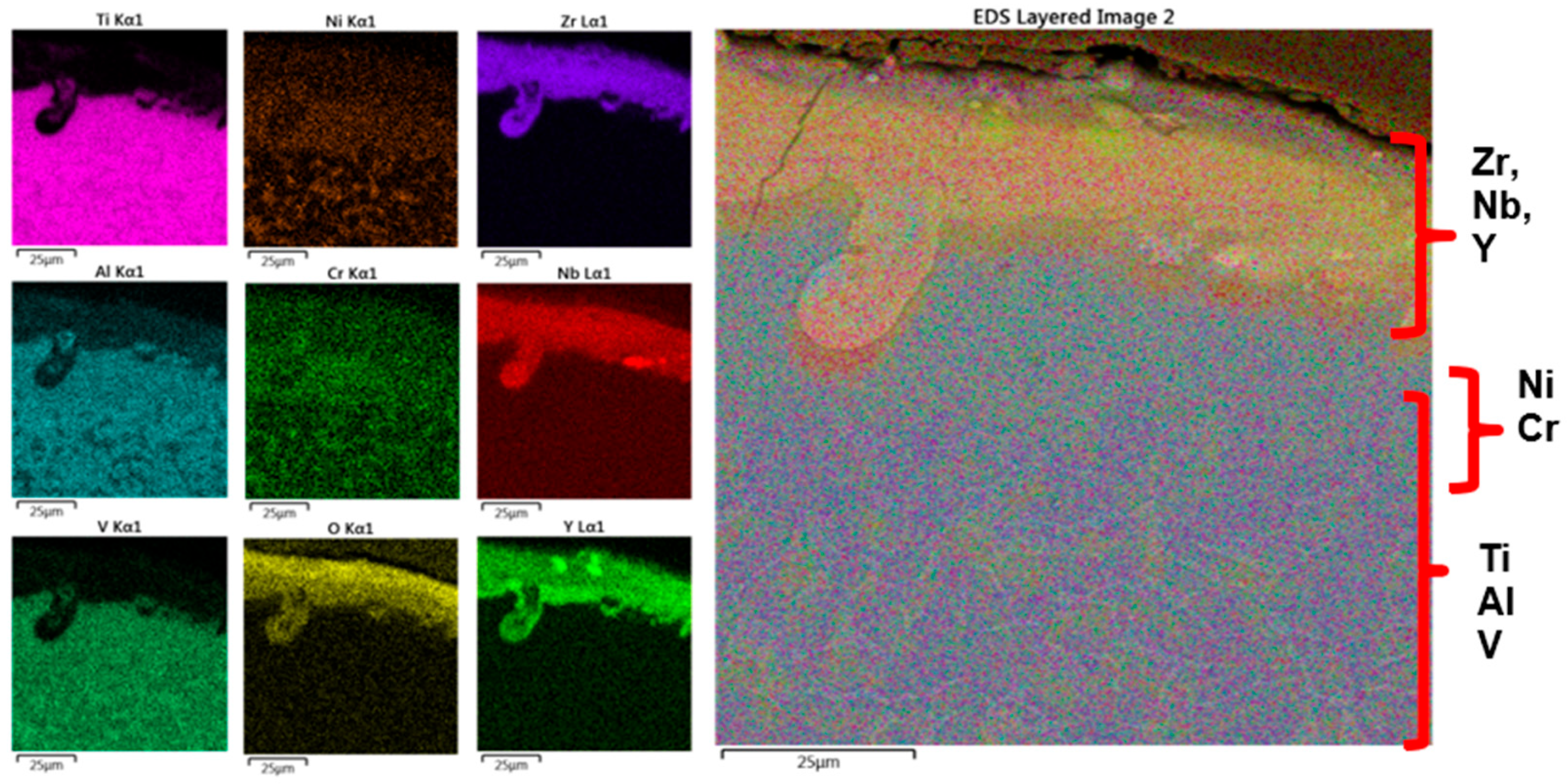
3.2. Top Coating—ZrYNb-16
4. Conclusions
- The thickness of the NiCrAlY layer decreased as the scanning speed of the CO2 laser beam increased despite maintaining a constant power of 100 W. This reduction is attributed to the shorter interaction time between the laser and the material, which affects the energy transfer necessary for layer densification.
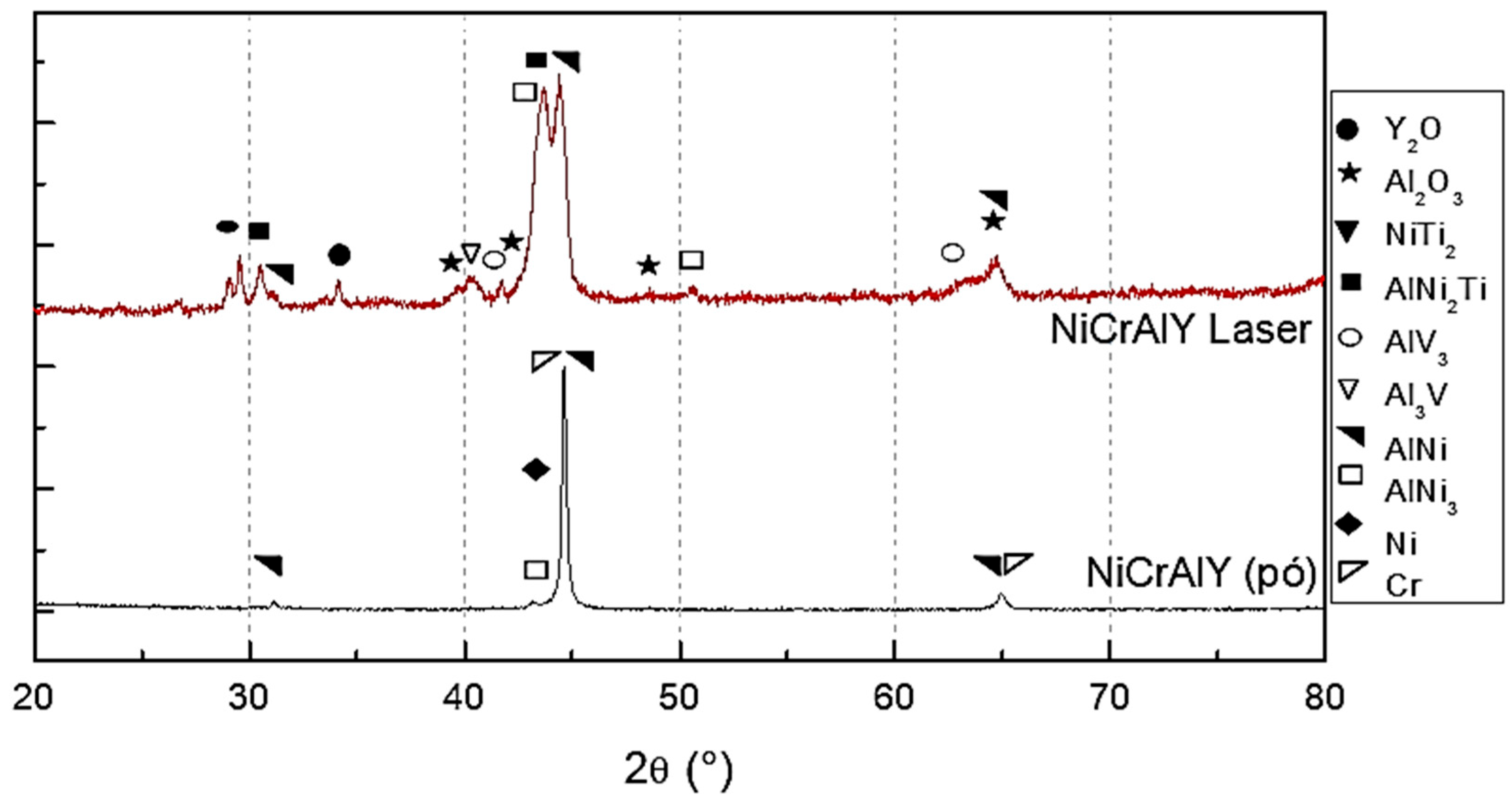
- In the consolidation of the NiCrAlY layer on Ti-6Al-4V, the most suitable deposition parameters were identified as a scanning speed of 70 mm/s, a laser power of 100 W, and a beam overlap of 50% per track. These conditions resulted in an average layer thickness of 170.1 μm, characterized by a uniformly homogeneous surface, minimal large cracks, and no significant porosity. The X-ray diffraction (XRD) analysis revealed that the predominant phases in the layer were β-NiAl and γ’-Ni3Al.
- After laser irradiation, a TGO layer was formed on the substrate. This layer, composed of aluminum and yttrium oxides, was uniformly distributed across the entire surface of the top layer, contributing to the thermal protection and bonding of the TBC system.
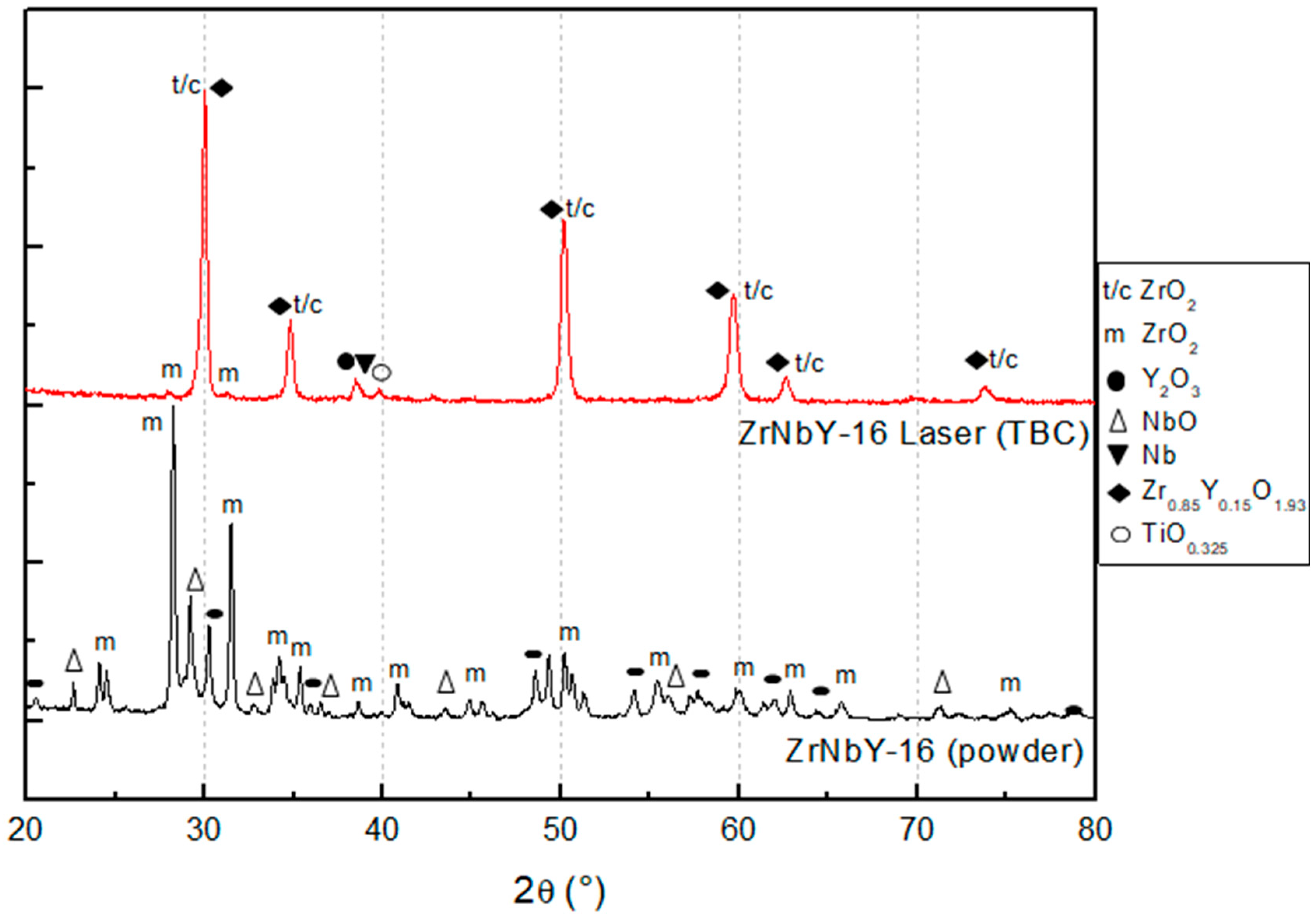
- The ZrYNb-16 ceramic layer was optimally consolidated with a laser process speed of 550 mm/s, a laser power of 70 W, and a beam overlap of 50% per track. The resulting layer had the best average thickness of 20.72 μm compared to other parameters. XRD analysis identified the presence of monoclinic (m-ZrO2), tetragonal (t-ZrO2, pseudocubic), and cubic (c-ZrO2) zirconia phases in the ceramic layer.
- Laser processing can effectively consolidate layers to thicknesses suitable for TBC applications in a precise, efficient, and rapid manner while minimizing the heat-affected zone.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Evans, H.E. Oxidation failure of TBC systems: An assessment of mechanisms. Surf. Coat. Technol. 2011, 206, 1512–1521. [Google Scholar] [CrossRef]
- Funatani, K. Emerging technology in surface modification of light metals. Surf. Coat. Technol. 2000, 133–134, 264–272. [Google Scholar] [CrossRef]
- Grant, P. Thermal barrier coatings. In Aerospace Materials; Chapter 22 Series in Materials Science and Engineering; Cantor, B., Grand, P., Assender, H., Eds.; Taylor & Francis: Bristol, UK, 2001. [Google Scholar]
- Levi, C.G. Emerging materials and processes for thermal barrier systems. Curr. Opin. Solid State Mater. Sci. 2004, 8, 77–91. [Google Scholar] [CrossRef]
- Schulz, U.; Leyens, C.; Fritscher, K.; Peters, M.; Saruhan-Brings, B.; Lavigne, O.; Dorvaux, J.M.; Poulain, M.; Mévrel, R.; Caliez, M. Some recent trends in research and technology of advanced thermal barrier coatings. Aerosp. Sci. Technol. 2003, 7, 73–80. [Google Scholar] [CrossRef]
- Seo, D.; Ogawa, K.; Nakao, Y.; Miura, H.; Shoji, T. Influence of high-temperature creep stress on growth of thermally grown oxide in thermal barrier coatings. Surf. Coat. Technol. 2009, 203, 1979–1983. [Google Scholar] [CrossRef]
- Almeida, D.S.; Silva, C.R.M.; Nono, M.C.A.; Cairo, C.A. Thermal conductivity investigation of zirconia co-doped with yttria and niobia EB-PVD TBCs. Mater. Sci. Eng. A 2007, 443, 60–65. [Google Scholar] [CrossRef]
- Takahashi, R.J.; Assis, J.M.K.; Piorino Neto, F.; Reis, D.A.P. Thermal conductivity study of ZrO2-YO1.5-NbO2.5 TBC. J. Mater. Res. Technol. 2022, 19, 4932–4938. [Google Scholar] [CrossRef]
- Almeida, D.S.D.; Neto, F.P.; Henriques, V.A.R.; Assis, J.M.K.d.; Gonςalves, P.A.R.; Takahashi, R.J.; Reis, D.A.P. Study of Non-Transformable t’-YSZ by Addition of Niobia for TBC Application. Coatings 2024, 14, 249. [Google Scholar] [CrossRef]
- Assis, J.M.K. Estudo da Estabilização de Fases Cristalinas de Cerâmicas do Sistema Nióbia–Ítria–Zircônia. Ph.D. Thesis, INPE (Instituto Nacional de Pesquisas Espaciais), São José dos Campos, Brazil, 2014. [Google Scholar]
- ANM: Agência Nacional de Mineração. Nióbio. 2017. Available online: http://www.anm.gov.br/dnpm/publicacoes/serie-estatisticas-e-economia-mineral/sumario-mineral/sumario-brasileiro-mineral-2017/niobio_sm_2017/ (accessed on 7 May 2019).
- Bellippady, M.; Björklund, S.; Li, X.-H.; Frykholm, R.; Kjellman, B.; Joshi, S.; Markocsan, N. Performance of Atmospheric Plasma-Sprayed Thermal Barrier Coatings on Additively Manufactured Super Alloy Substrates. Coatings 2024, 14, 626. [Google Scholar] [CrossRef]
- Heimann, R.B. Plasma-Spray Coating:Principles and Applications, 2nd ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Fauchais, P.; Montavon, G.; Bertrand, G. From Powders to Thermally Sprayed Coatings. J. Therm. Spray Technol. 2010, 19, 56–80. [Google Scholar] [CrossRef]
- Lima, C.C.; Trevisan, R. Aspersão Térmica Fundamentos e Aplicações, 2nd ed.; Artliber: São Paulo, Brazil, 2007; 152p. [Google Scholar]
- Movchan, M.; Rudoy, Y. Composition, structure and properties of gradient thermal barrier coatings (TBCs) produced by electron beam physical vapor deposition EB-PVD. Mater. Des. 1998, 19, 253–258. [Google Scholar] [CrossRef]
- Warren, J.; Hsiung, L.M.; Wadley, H.N.G. High temperature deformation behavior of physical vapor deposited Ti-6Al-4V. Acta Metall. Mater. 1995, 43, 2773–2787. [Google Scholar] [CrossRef]
- Takahashi, R.J.; Assis, J.M.K.; Reis, D.A.P. Microstructural Characterization of Zirconia Co-Doped with Yttria and Niobia by Laser Deposition on Ti-6Al-4V as a Thermal Barrier for Application in Turbines; SAE Technical Paper. 2018-36-0332; SAE International: Warrendale, PA, USA, 2018. [Google Scholar]
- Takahashi, R.J.; de Assis, J.M.K.; Riva, R.; de Oliveira, A.C.; Reis, D.A.P. Caracterização microestrutural da camada de NiCrAlY sobre Ti-6Al-4V processado por laser de Yb: Fibra. Tecnol. Metal. Mater. Mineração 2022, 19, 1–8. [Google Scholar]
- De Freitas, F.E.; Briguente, F.P.; dos Reis, A.G.; de Vasconcelos, G.; Reis, D.A.P. Investigation on the microstructure and creep behavior of laser remelted thermal barrier coating. Surf. Coat. Technol. 2019, 369, 257–264. [Google Scholar] [CrossRef]
- Xu, Q.L.; Liu, K.C.; Wang, K.Y.; Lou, L.Y.; Zhang, Y.; Li, C.J.; Li, C.X. TGO and Al diffusion behavior of CuAlxNiCrFe high-entropy alloys fabricated by high-speed laser cladding for TBC bond coats. Corros. Sci. 2021, 192, 109781. [Google Scholar] [CrossRef]
- Suárez, A.; Tobar, M.J.; Yáñez, A.; Pérez, I.; Sampedro, J.; Amigó, V.; Candel, J.J. Modeling of phase transformations of Ti6Al4V during laser metal deposition. Phys. Procedia 2011, 12, 666–673. [Google Scholar] [CrossRef][Green Version]
- Meng, Q.; Geng, L.; Ni, D. Laser cladding NiCoCrAlY coating on Ti-6Al-4V. Mater. Lett. 2005, 59, 2774–2777. [Google Scholar] [CrossRef]
- Jiang, C.; Zhu, Z.; Chen, J. Laser texturing at interface for improved strain tolerance and thermal insulation performance of thermal barrier coatings. Surf. Coat. Technol. 2023, 459, 129385. [Google Scholar] [CrossRef]
- Ryan Cottam, R.; Brandt, M. Laser Cladding of Ti-6Al-4V Powder on Ti-6Al-4V Substrate: Effect of laser cladding parameters on microstructure. Phys. Procedia 2011, 12, 323–329. [Google Scholar] [CrossRef]
- Takahashi, R.J.; Assis, J.M.K.; Neto, F.P.; Reis, D.A.P. Heat treatment for TGO growth on NiCrAlY for TBC application. Mater. Res. Express 2019, 6, 126442. [Google Scholar] [CrossRef]
- Li, J.F.; Li, L.; Stott, F.H. Multi-layered surface coatings of refractory ceramics prepared by combined laser and flame spraying. Surf. Coat. Technol. 2004, 180, 500–505. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Hu, X.B.; Zheng, S.J.; Zhu, Y.L.; Wei, H.; Ma, X.L. Microstructural evolution of the interface between NiCrAlY coating and superalloy during isothermal oxidation. Mater. Des. 2015, 80, 63–69. [Google Scholar] [CrossRef]
- Wu, Y.N.; Zhang, G.; Feng, Z.C.; Zhang, B.C.; Liang, Y.; Liu, F.J. Oxidation behavior of laser remelted plasma sprayed NiCrAlY and NiCrAlY-Al2O3 coatings. Surf. Coat. Technol. 2001, 138, 56–60. [Google Scholar] [CrossRef]
- Partes, K.; Giolli, C.; Borgioli, F.; Bardi, U.; Seefeld, T.; Vollertsen, F. High temperature behaviour of NiCrAlY coatings made by laser cladding. Surf. Coat. Technol. 2008, 202, 2208–2213. [Google Scholar] [CrossRef]
- Tobar, M.J.; Amado, J.M.; Yáñez, A.; Pereira, J.C.; Amigó, V. Laser cladding of MCrAlY coatings on stainless steel. Phys. Procedia 2014, 56, 276–283. [Google Scholar] [CrossRef]
- Tawancy, H.M. Communication Oxidation of an Ni-Cr-AI-Fe-Y Alloy. Metall. Trans. A 1991, 22, 1463–1465. [Google Scholar] [CrossRef]
- Gil, A.; Shemet, V.; Vassen, R.; Subanovic, M.; Toscano, J.; Naumenko, D.; Singheiser, L.; Quadakkers, W.J. Effect of surface condition on the oxidation behaviour of MCrAlY coatings. Surf. Coat. Technol. 2009, 201, 3824–3828. [Google Scholar] [CrossRef]

| Sample—CO2 Laser Irradiation Speed (mm/s) | Thicknesses (μm) |
|---|---|
| 10 | 335.60 ± 6.65 |
| 20 | 223.11 ± 2.77 |
| 30 | 306.81 ± 4.82 |
| 40 | 298.54 ± 8.52 |
| 50 | 230.45 ± 10.66 |
| 60 | 175.80 ± 7.78 |
| 70 | 170.12 ± 4.31 |
| 80 | 168.22 ± 6.26 |
| 90 | 159.74 ± 11.12 |
| 100 | 155.28 ± 7.23 |
| 110 | 152.30 ± 6.95 |
| 120 | 146.90 ± 10.75 |
| 130 | 145.62 ± 5.68 |
| 140 | 150.35 ± 13.23 |
| Sample—CO2 Laser Irradiation Speed (mm/s) | Thicknesses (μm) |
|---|---|
| 60 | 2.15 ± 2.78 |
| 70 | 20.72 ± 6.48 |
| 80 | 18.52 ± 13.26 |
| 90 | 21.36 ± 13.98 |
| 100 | 20.89 ± 15.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, R.J.; Assis, J.M.K.d.; Fazan, L.H.; Rodríguez, L.A.A.; Capella, A.G.; Reis, D.A.P. TBC Development on Ti-6Al-4V for Aerospace Application. Coatings 2025, 15, 47. https://doi.org/10.3390/coatings15010047
Takahashi RJ, Assis JMKd, Fazan LH, Rodríguez LAA, Capella AG, Reis DAP. TBC Development on Ti-6Al-4V for Aerospace Application. Coatings. 2025; 15(1):47. https://doi.org/10.3390/coatings15010047
Chicago/Turabian StyleTakahashi, Renata Jesuina, João Marcos Kruszynski de Assis, Leonardo Henrique Fazan, Laura Angélica Ardila Rodríguez, Aline Gonçalves Capella, and Danieli Aparecida Pereira Reis. 2025. "TBC Development on Ti-6Al-4V for Aerospace Application" Coatings 15, no. 1: 47. https://doi.org/10.3390/coatings15010047
APA StyleTakahashi, R. J., Assis, J. M. K. d., Fazan, L. H., Rodríguez, L. A. A., Capella, A. G., & Reis, D. A. P. (2025). TBC Development on Ti-6Al-4V for Aerospace Application. Coatings, 15(1), 47. https://doi.org/10.3390/coatings15010047






