Efficient and Controllable Preparation of Super-Hydrophobic Alumina-Based Ceramics Coating on Aviation Al-Li Alloy Surface for Corrosion Resistance and Anti-Icing Behavior
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation
2.3. Technical Method Characterization
3. Results and Discussion
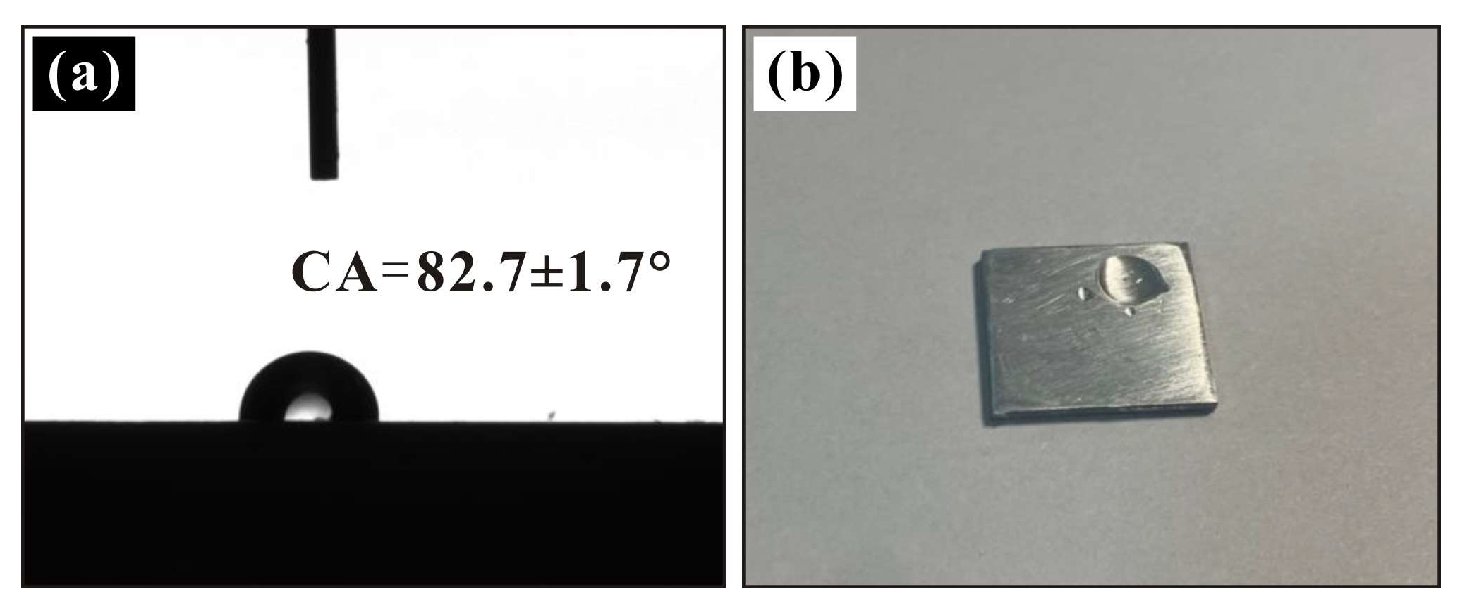
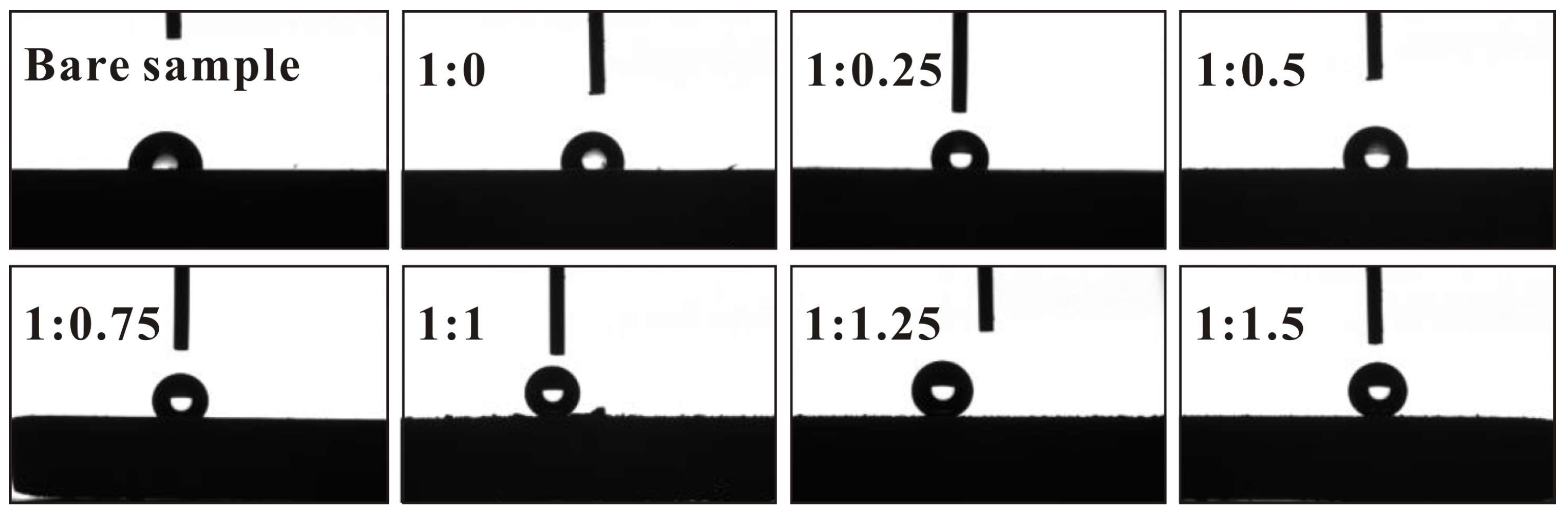
3.1. Wettability of Sprayed Coatings
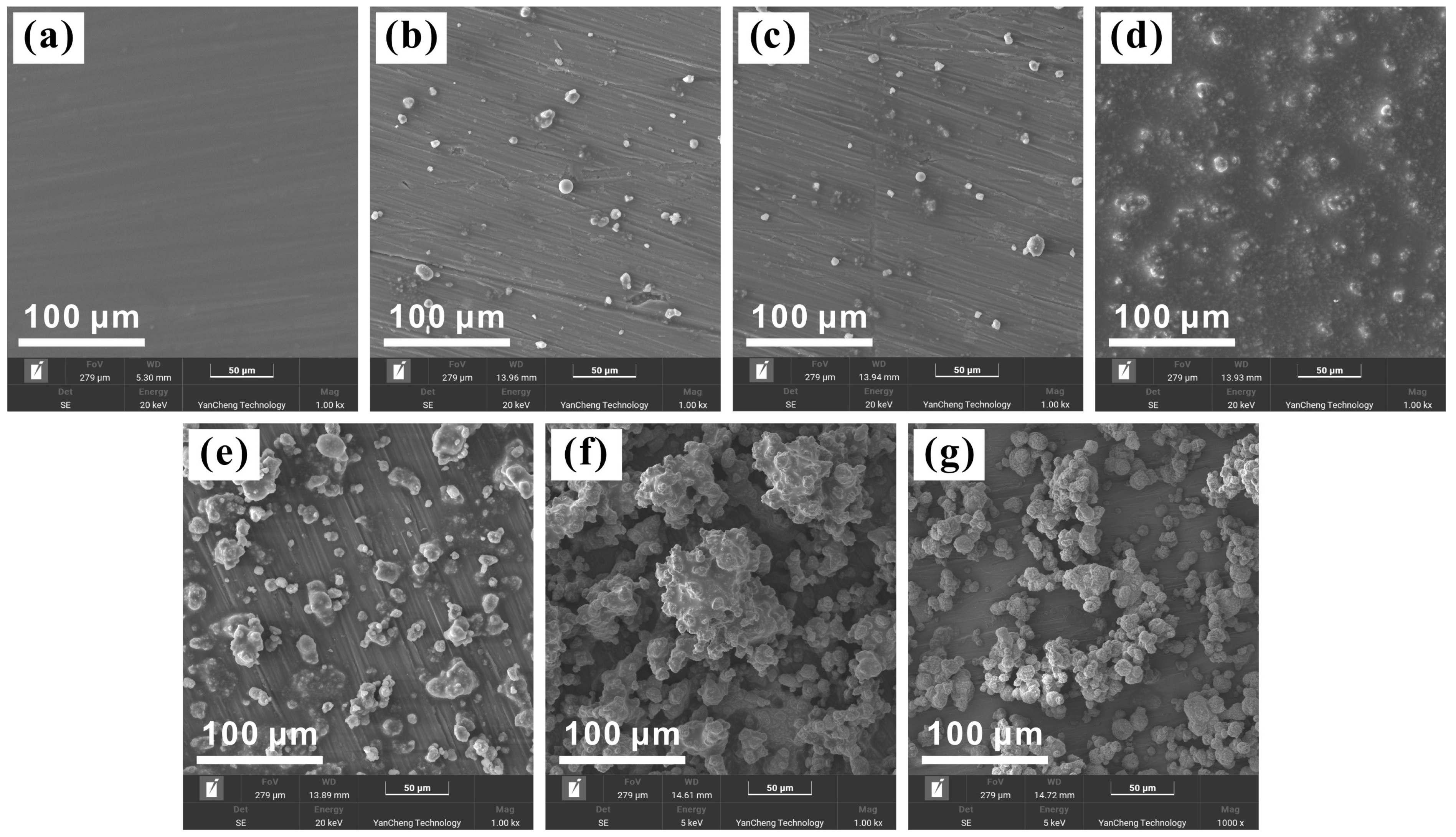
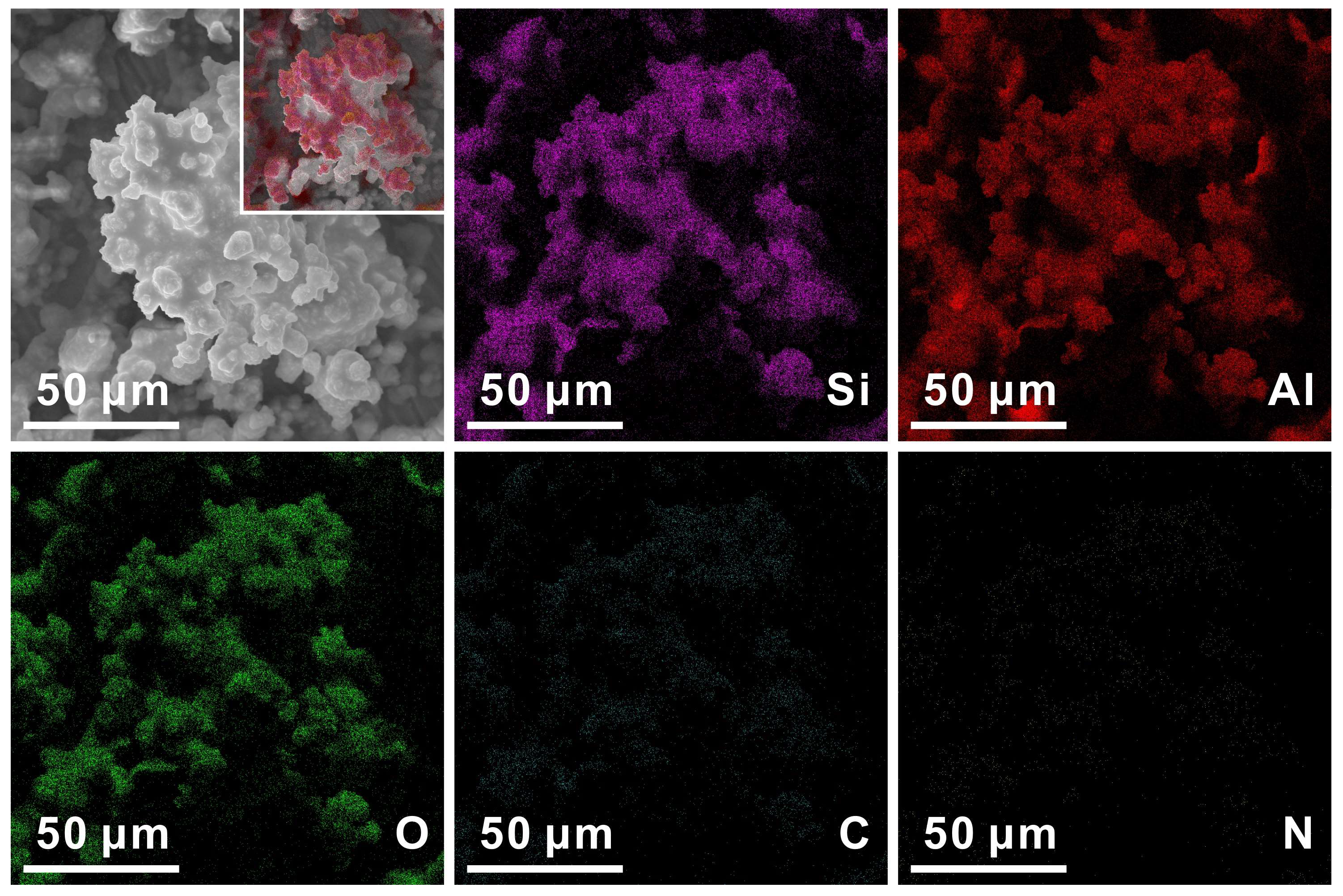
3.2. Morphologies of Sprayed Coatings
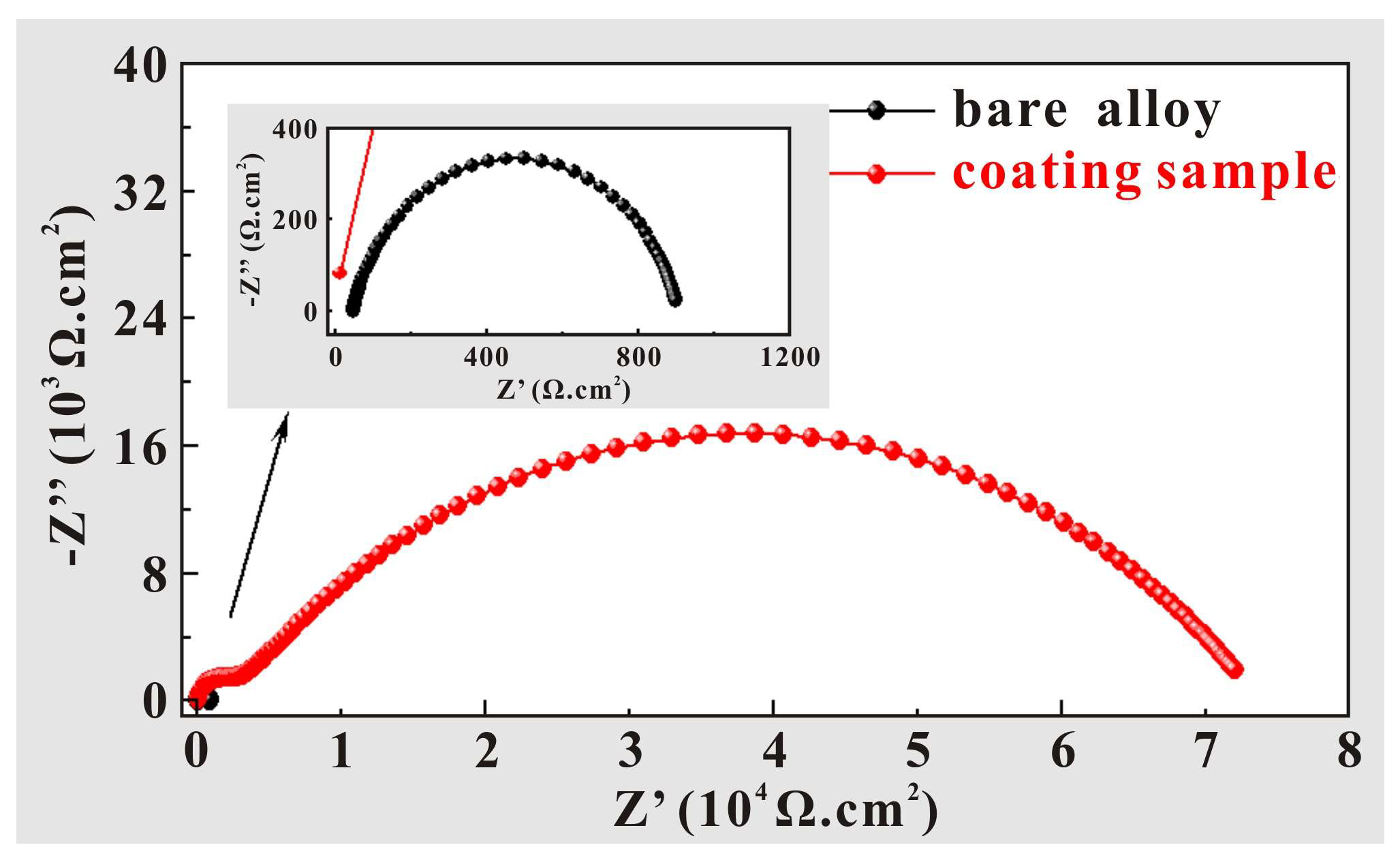
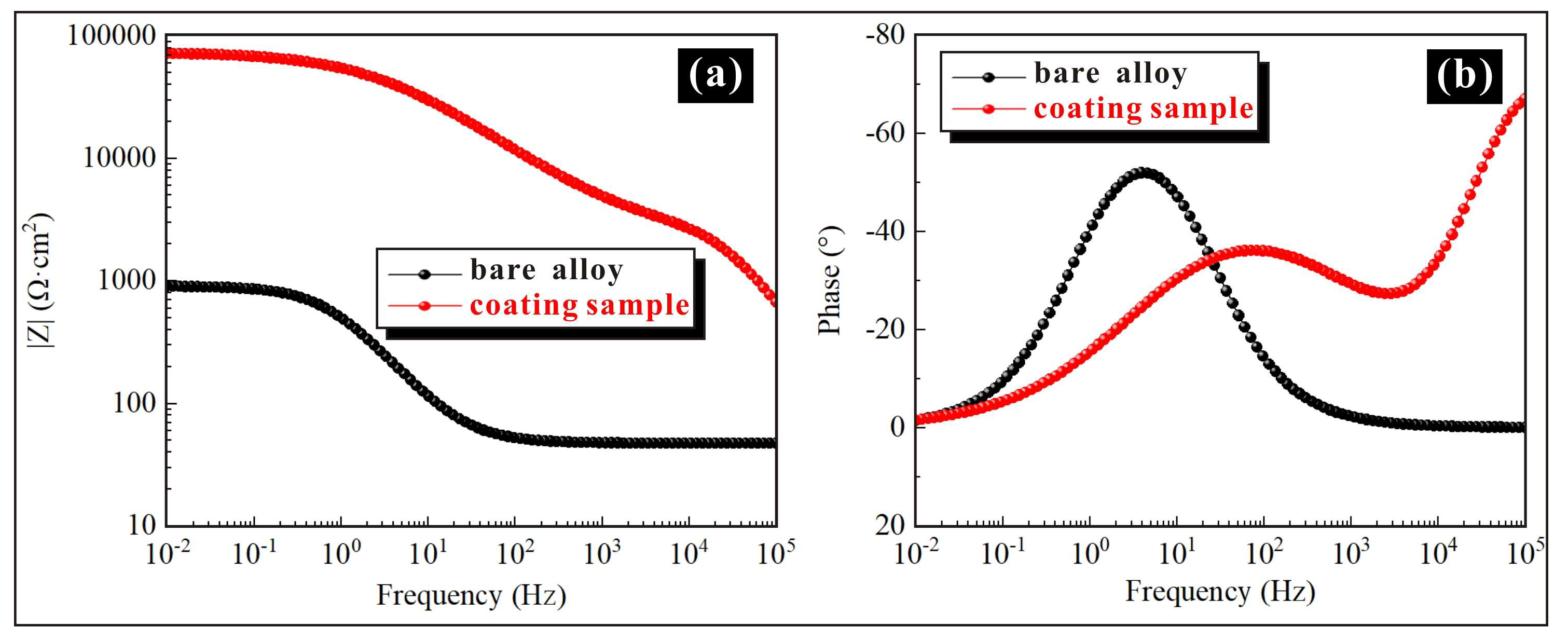
3.3. Multiple Functionalities of Sprayed Coatings
4. Conclusions
- The coating prepared by air spraying can capture a large amount of air phase, thus generating a cushion effect to lift the water droplet. Therefore, the high-viscosity solid–liquid contact interface disappears, and a solid–gas–liquid composite contact interface is formed, which reduces the direct contact proportion between the coating and the liquid. This greatly decreases the viscous resistance of the sample to liquid, thereby exhibiting excellent super-hydrophobic performance and anti-pollution characteristics.
- The corrosion potential of the super-hydrophobic coating is higher than that of the substrate, while the corrosion current density is lower than that of the substrate, which indicates that the corrosion resistance of the sample with super-hydrophobic coating has been significantly improved. On the one hand, the super-hydrophobic coating provides excellent physical isolation against corrosive media. On the other hand, the prepared microstructures can capture air and then enhance the removal effect of corrosive media, thereby further enhancing the corrosion resistance.
- Compared with the alloy substrate, the droplet freezing time on super-hydrophobic coating is greatly delayed, indicating that the anti-icing behavior is enhanced. This is due to the different heat transfer coefficients between the two samples. The super-hydrophobic coating can capture a large amount of air, so its heat transfer coefficient is much lower than that of the alloy substrate. This will prolong the non-uniform nucleation time at the solid–liquid interface under a low-temperature condition, thus achieving a delayed freezing process.
- The corrosion resistance of the super-hydrophobic coating prepared in this work was significantly improved compared to the previous structural strategy. For future work, it is necessary to have a deeper simulative understanding of the de-icing mechanism for such super-hydrophobic coating. Meanwhile, it may also be useful to consider the multifunctional coating for thermal and fluid transport applications.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, X.Y.; Cui, Z.F.; Pan, X.C.; Chen, Z.; Dong, T.J.; Chen, Y.J. Post-impact Fatigue Performance of 2198-T8 Aluminum-Lithium Alloy Sheet with Pre-crack. J. Test. Eval. 2023, 51, 4328–4339. [Google Scholar] [CrossRef]
- Su, W.; Zhu, H.M. In situ corrosion fatigue life of 2198-T8 Al-Li alloy based on tests and DIC technique. Mater. Test. 2022, 64, 1383–1396. [Google Scholar] [CrossRef]
- Chen, Y.; Pan, X.; Peng, J.; Wang, F. Effect of coupling damage on fatigue behavior of 2198-T8 aluminum-lithium alloy thin sheet for fuselage. Fatigue Fract. Eng. Mater. Struct. 2022, 45, 754–769. [Google Scholar] [CrossRef]
- Pang, Z.; Yang, J.; Cai, Y. Effects of Rotational Speed on the Microstructure and Mechanical Properties of 2198-T8 Al-Li Alloy Processed by Friction Spot Welding. Materials 2023, 16, 1807. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Cui, Z.; Wang, F.; Dong, T.; Meng, X. Effect of impact-corrosion coupling damage on fatigue properties of 2198-T8 aluminum-lithium alloy. Eng. Fail. Anal. 2024, 163, 108471. [Google Scholar] [CrossRef]
- de Souza Carvalho Machado Machado, C.; Klumpp, R.E.; Ayusso, V.H.; Donatus, U.; Milagre, M.X.; de SAraujo, J.V.; Machado, G.A.F.; Costa, I.S. Effect of surface treatments on the localized corrosion resistance of the AA2198-T8 aluminum lithium alloy welded by FSW process. Surf. Interface Anal. 2019, 51, 1231–1239. [Google Scholar] [CrossRef]
- de Sousa Araujo, J.V.; Milagre, M.X.; Klumpp, R.E.; Hugo Ayusso, V.; Donatus, U.; Costa, I. TSA anodising voltage effects on the near-surface coarse intermetallic particles in the AA2024-T3 and AA2198-T8 alloys. Corros. Eng. Sci. Technol. 2022, 57, 380–396. [Google Scholar] [CrossRef]
- Sergienko, V.P.; Kozhushko, V.V.; Bukharov, S.N.; Merinov, V.K. Effect of Corrosion Inhibitors in Compositions of Friction Composites on Corrosion Resistance of the Metal Counterbody and Noise Generation during Friction. J. Frict. Wear 2024, 44, 259–265. [Google Scholar] [CrossRef]
- Sahoo, B.; Das, T.; Paul, J. Thermal spraying and related technologies for the surface modification of al alloys: Review. Surf. Rev. Lett. 2022, 29, 2230009. [Google Scholar] [CrossRef]
- Wondwosen, D.; Jiajun, X.; Pawan, L.T. Review of internal and external surface finishing technologies for additively manufactured metallic alloys components and new frontiers. Prog. Addit. Manuf. 2023, 8, 1433–1453. [Google Scholar]
- Gong, S.H.; Lim, H.J.; Lee, J.H.; Yoo, Y.; Yu, S.; Lim, H.D.; Jung, H.W.; Ko, J.S.; Kim, I.S.; Kim, H.S. Electrochemical assessment of highly reversible SnO2-coated Zn metal anodes prepared via atomic layer deposition for aqueous Zn-ion batteries. Appl. Surf. Sci. 2023, 611, 155633. [Google Scholar] [CrossRef]
- Xia, D.H.; Deng, C.M.; Macdonald, D.; Jamali, S.; Mills, D.; Luo, J.L.; Strebl, M.G.; Amiri, M.; Jin, W.; Song, S.; et al. Electrochemical measurements used for assessment of corrosion and protection of metallic materials in the field: A critical review. J. Mater. Sci. Technol. 2022, 112, 151–183. [Google Scholar] [CrossRef]
- Hino, M.; Hori, T. Phosphate Anodization for Magnesium Alloys and Its Anticorrosion Mechanism. J. Jpn. Soc. Tribol. 2022, 67, 484–489. [Google Scholar]
- Group, P. Researchers develop green energy-based electrochemical corrosion protection. Paintindia 2023, 73, 125. [Google Scholar]
- Jin, H.; Ji, R.; Sun, H.; Liu, S.; Dong, T.; Wang, L.; Zhao, L.; Ma, C.; Liu, Y.; Cai, B.; et al. Efficient preparation of Ni-Fe-SiC pipeline internal surface coating by active/inert metal combined anode jet electrodeposition. J. Manuf. Process. 2023, 89, 284–297. [Google Scholar] [CrossRef]
- Noda, K.; Koseki, W.; Hirobe, S.; Shiratori, H.; Yagi, Y. Fundamentals of Corrosion Reaction and Corrosion Protection by Surface Treatment. J. Jpn. Soc. Tribol. 2022, 67, 458–465. [Google Scholar]
- Ouakki, M.; Galai, M.; Cherkaoui, M. Imidazole derivatives as efficient and potential class of corrosion inhibitors for metals and alloys in aqueous electrolytes: A review. J. Mol. Liq. 2022, 345, 117815. [Google Scholar] [CrossRef]
- Galleguillos Madrid, F.M.; Soliz, A.; Cáceres, L.; Bergendahl, M.; Leiva-Guajardo, S.; Portillo, C.; Olivares, D.; Toro, N.; Jimenez-Arevalo, V.; Páez, M. Green Corrosion Inhibitors for Metal and Alloys Protection in Contact with Aqueous Saline. Materials 2024, 17, 3996. [Google Scholar] [CrossRef]
- Privitera, A.; Ruggiero, L.; Venditti, I. One step nanoencapsulation of corrosion inhibitors for gradual release application. Mater. Today Chem. 2022, 24, 100851. [Google Scholar] [CrossRef]
- Auepattana, A.K.; Phakkeeree, T.; Crespy, D. Stimuli-responsive polymeric additives for anticorrosion. J. Appl. Polym. Sci. 2022, 139, 51730. [Google Scholar] [CrossRef]
- Golubina, E.N.; Kizim, N.F. From Hydrophylicity to Surface Hydrophobicity: Variation of the Wettability of a Material on a Substrate Due to Local Vibrational Effects during Its Interface Synthesis. Russ. J. Phys. Chem. 2023, 97, 100–104. [Google Scholar] [CrossRef]
- Guo, M.; Rong, Y.; Huang, Y.; Feng, X.; Hu, H.; Wu, C.; Zhang, G. Ultrafast laser-chemical modification hybrid fabrication of hydrostatic bearings with a superhydrophobicity solid-liquid interface. Sci. China Technol. Sci. 2024, 67, 696–708. [Google Scholar] [CrossRef]
- Li, X.; Ma, C.; Shi, T.; Yang, H.; Zhang, C.; Qi, W.; Li, C.; Liu, R.; He, W.; Liu, Y. Waterborne robust superhydrophobic PFDTES@TiO2-PU coating with stable corrosion resistance, long-term environmental adaptability, and delayed icing functions on Al-Li alloy. J. Mater. Res. Technol. 2024, 32, 3357–3370. [Google Scholar] [CrossRef]
- Abu, M.M.; Hyun, K.S.; Kye-Si, K. Multinozzle electrospray method for high-throughput and uniform coating: Application of superhydrophobic coating. J. Coat. Technol. Res. 2023, 20, 1069–1081. [Google Scholar]
- Piscitelli, F.; Palazzo, S.; Nicola, F.D. Icing Wind Tunnel Test Campaign on a Nacelle Lip-Skin to Assess the Effect of a Superhydrophobic Coating on Ice Accretion. Appl. Sci. 2023, 13, 5183. [Google Scholar] [CrossRef]
- Gallyamova, R.; Safiullin, R.; Dokichev, V.; Musin, F.F. Preparation of TiO2 Coatings on Carbon Fibers. Mater. Sci. Forum 2022, 1049, 117–123. [Google Scholar] [CrossRef]
- Lin, W.T.; Lin, Z.W.; Kuo, T.Y.; Chien, C.S.; Huang, J.W.; Chung, Y.L.; Chang, C.P.; Ibrahim, M.Z.; Lee, H.T. Mechanical and biological properties of atmospheric plasma-sprayed carbon nanotube-reinforced tantalum pentoxide composite coatings on Ti6Al4V alloy. Surf. Coat. Technol. 2022, 437, 128356. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Yuwono, J.A.; Meng, G.; Feng, Z. Developing levodopa-modified sol-gel coating with enhanced anticorrosion performance on Mg alloy AZ31. Anti-Corros. Methods Mater. 2023, 70, 129–138. [Google Scholar] [CrossRef]
- Camalan, M.; Arol, A.H. Preliminary assessment of spray coating, solution-immersion and dip coating to render minerals superhydrophobic. Miner. Eng. 2022, 176, 107357. [Google Scholar] [CrossRef]
- Eren, O.; Kamara, A.M.; Sezer, H.K.; Marimuthu, S. Synthesis of Aluminium Nitride-Based Coatings on Mild Steel Substrates Utilising an Integrated Laser/Sol–Gel Method. Photonics 2024, 11, 382. [Google Scholar] [CrossRef]
- Ada, H.; El Rubaye, A.Q.; Tokeser, E.A.; Mavi, A.; Aksoz, S. Coating of Ti6Al4V Alloys by Physical Vapor Deposition Method and Micro-scratch and Corrosion Test Investigations of Coated Samples. J. Adv. Appl. Sci. 2023, 2, 36–45. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Guo, W.; Zhou, L.; Cui, Q.; Deng, X.; Shu, W.; Shi, T.; Xing, Z.; Wang, H. Effect of TiO2 content on the thermal control properties of Al2O3-xTiO2 composite coatings prepared by supersonic plasma spraying technology. J. Mater. Res. Technol. 2024, 32, 3582–3593. [Google Scholar] [CrossRef]






| Names | Types |
|---|---|
| 2198 Al-Li alloy | 1 mm thick |
| nano-alumina particles | 50 nm, 99.8% |
| PDMS | Sylgard184 |
| silicone rubber curing agent | Sylgard184 |
| n-hexane | 97.0% |
| anhydrous ethanol | 99.7% |
| sodium chloride | 99.5% |
| standard sand | 0.3–0.6 mm |
| deionized water | 99.7% |
| Elements | Signal Type | At % |
|---|---|---|
| C | EDS | 24.65 |
| N | EDS | 0.02 |
| O | EDS | 34.76 |
| Al | EDS | 26.78 |
| Si | EDS | 23.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Li, X. Efficient and Controllable Preparation of Super-Hydrophobic Alumina-Based Ceramics Coating on Aviation Al-Li Alloy Surface for Corrosion Resistance and Anti-Icing Behavior. Coatings 2024, 14, 1223. https://doi.org/10.3390/coatings14091223
Li B, Li X. Efficient and Controllable Preparation of Super-Hydrophobic Alumina-Based Ceramics Coating on Aviation Al-Li Alloy Surface for Corrosion Resistance and Anti-Icing Behavior. Coatings. 2024; 14(9):1223. https://doi.org/10.3390/coatings14091223
Chicago/Turabian StyleLi, Ben, and Xuewu Li. 2024. "Efficient and Controllable Preparation of Super-Hydrophobic Alumina-Based Ceramics Coating on Aviation Al-Li Alloy Surface for Corrosion Resistance and Anti-Icing Behavior" Coatings 14, no. 9: 1223. https://doi.org/10.3390/coatings14091223
APA StyleLi, B., & Li, X. (2024). Efficient and Controllable Preparation of Super-Hydrophobic Alumina-Based Ceramics Coating on Aviation Al-Li Alloy Surface for Corrosion Resistance and Anti-Icing Behavior. Coatings, 14(9), 1223. https://doi.org/10.3390/coatings14091223





