Abstract
The high-temperature heat treatment of electroless nickel–phosphorus (Ni-P) coatings in an air environment, and its consequences have scarcely been investigated. This work investigated tribological characteristics of the high-temperature, heat-treated, electroless Ni-P coatings on steel substrates with low-, mid-, and high-phosphorus content for which the average phosphorus content was 2.4 wt.%, 7.1 wt.%, and 10.3 wt.%, respectively. X-ray fluorescence and energy dispersive spectroscopy were implemented to determine the phosphorus content of the coatings. The oxidation of Ni and the formation of the NiO layer on the coating surface was confirmed by the X-ray diffraction technique. A reciprocating sliding method on a ball-on-flat system was utilized to evaluate the coating’s friction and wear behavior. Among the coatings with varying phosphorus content, a high hardness of 1086 HV was found for high-phosphorus coating when heat-treated at 400 °C in an air environment, and that was decreased to 691 HV when heat-treated at 650 °C. The oxidation of nickel in the electroless Ni-P coating occurred when heat-treated at 400 °C in an air environment, and this phenomenon was increased more when the temperature was increased to 650 °C. The characteristics of the NiO layer that formed on the surface of the heat-treated electroless Ni-P coating were influenced by the concentration of phosphorus, which caused different colors of NiO to be seen on the Ni-P coating surface. A greenish black NiO layer on the low-phosphorus and black NiO layer on the mid- and high-phosphorus Ni-P coating was developed during heat treatment at 650 °C in an air atmosphere. The adhesion and tribological characteristics of the Ni-P coatings were affected by the NiO layer developed on the heat-treated Ni-P coating surfaces. The Ni-P coatings with mid- and high-phosphorus content showed enhanced wear-resistance characteristics when they underwent heat treatment in an air atmosphere at the high temperature of 650 °C. The wear volume obtained for as-plated mid-phosphorus and high-phosphorus Ni-P coatings was 0.111 mm3 and 0.128 mm3, respectively, and that was reduced to 0.031 mm3 and 0.051 mm3, respectively, after the high-temperature heat treatment.
1. Introduction
Electroless Ni-P coatings show diverse mechanical and chemical characteristics when the phosphorus content of the coating is varied. A high-phosphorus (>10 wt.% P) Ni-P coating carries a prominent ability to protect materials from corrosion, whereas low-phosphorus (<5 wt.% P) Ni-P coating demonstrates high hardness, wear resistance, and excellent mechanical and engineering properties [1]. The phosphorus content in the electroless Ni-based coatings can be varied with suitable bath formulations. Another critical factor in controlling the co-deposition of phosphorus and coating rate is pH, on which bath stability also depends. The microstructure of as-plated Ni-P coatings can range from nanocrystalline to amorphous, depending on the amount of phosphorus in the coating. However, it suffers microstructural alteration by precipitating nickel-phosphide (Ni3P) grains when treated at a certain temperature for a specific time. Heat treatment of the Ni-P coating is found to be effective in improving its mechanical and electrochemical properties. These coatings can be heat-treated in both inert (vacuum, Ar, N2, etc.) and reactive (O2 or air) environments [2,3,4].
Many studies conducted high-temperature heat treatment in an inert or vacuum environment. They focused their studies on microstructural transformations, mechanical properties, and some other applications of Ni-based coatings [5,6,7]. However, the study of the coating behavior when heat-treated at high temperatures in the presence of oxygen is still lacking. Weiss reported the diffusion of Fe into the Ni-P layer in his study of high-temperature oxidation of iron and nickel in electroless Ni-P coatings on steel substrates [8]. Farrokhzad [9] studied the high-temperature oxidation behavior of electroless Ni-P-hBN composite coating on a carbon steel substrate, where he observed the oxidation behavior at 500, 600, and 700 °C for an extended period (up to 96 h). He reported that the two-layer system of the Ni-P-hBN layer coated on the Ni-P layer had shown enhanced adhesive behavior of NiO to the coating surface. Despite the study of the oxidation behavior of electroless Ni-P coatings at high temperatures, very few studies have reported the tribological properties of electroless Ni-P coatings annealed at high temperatures in the air environment. Arora et al. reported an enhancement of wear-resistance behavior of mid-phosphorus Ni-P coatings on steel substrates when heat-treated at 400 °C in the atmospheric air environment [10]. Cheng et al. [11] treated Ni-P coatings in an air-filled atmosphere at different temperatures (200 °C to 800 °C). They investigated the microstructural transformation and wear-resistance behavior of the heat-treated Ni-P coatings without insisting on nickel oxidation and its consequences on tribological behavior. When heat-treated at 600 °C in an air environment, Dong et al. [12] observed a deterioration of the wear-resistance properties of the electroless Ni-P-SiO2 composite coating. However, they did not establish a correlation between the wear characteristics and the NiO that was subsequently developed after the heat treatment in the air. Biswas et al. [13] studied the oxidation effect on the tribological properties of the Ni-P-Cu alloy heat-treated at various temperatures (up to 800 °C) for which the phosphorus content was around 9 wt.%. However, they did not mention the oxidation behavior of pure Ni-P alloys with varying phosphorus content. For a high-temperature application of the Ni-P coatings, such as heat exchanger’s parts (tubes, screws, nuts, bolts, etc.), there is still a lack of studies that describe nickel oxidation and the corresponding wear-resistance behavior. This led to the realization that further research into the mechanical and tribological properties of high-temperature heat-treated Ni-P coatings with varying phosphorus contents in the air environment was still necessary.
In this work, we have investigated the consequences of high-temperature (650 °C) heat treatment in the air environment of the low-, mid-, and high-phosphorus Ni-P coatings on its surface, mechanical, and tribological characteristics. The Ellingham diagram suggests that the formation of NiO in the presence of atmospheric oxygen can be expected at the temperature of 500 °C [14,15]. However, NiO is formed in trace amounts when the Ni-P alloy coatings are heat-treated at 400 °C in the presence of air. The adhesion properties of the NiO layer formation, especially after the heat treatment at 650 °C, depend on the coatings’ phosphorus content. On the low-phosphorus coating, adhesive NiO is visible, but a brittle and less adhesive NiO layer is developed on the mid- and high-phosphorus coatings. The adhesive NiO layer on the low-phosphorus coating has played a role in obtaining a stable COF curve in the heat-treated Ni-P coatings. However, the wear-resistance behavior of low-phosphorus coating is found to have deteriorated a lot after the heat treatment due to microstructural changes, such as a decreased formation of the Ni3P phase that resulted in the reduction of coating hardness. On the other hand, the sufficient evolution of the Ni3P phase in mid- and high-phosphorus coatings, along with the NiO layer developed on their surfaces, has led to enhanced wear-resistance properties after the high-temperature heat treatment.
2. Materials and Methods
2.1. Electroless Ni Plating Process
The austenite stainless-steel (ASTM 304SS) substrates 70 × 50 × 4 mm3 were prepared for the electroless Ni-P plating. The substrates were sandblasted (having a mesh size of 180), cleaned with acetone, and rinsed with DI water. Furthermore, the clean substrates were subjected to electrochemical degreasing in a hot alkaline solution (Clean-L20, Maru Enhanced Materials, Gangneung, Republic of Korea) for 2 min, followed by mixed acid (HCl, HNO3, and HF) etching for 5 min. The HCl allowed the etched substrates to be activated for electroless plating. Ni-P coatings with low-, mid-, and high-phosphorus content were prepared using separate plating baths. Nickel sulfate hexahydrate and sodium hypophosphite monohydrate were used as the Ni source and reducing agent, respectively. Similarly, lactic acid and sodium citrate dihydrate were used as complexing and buffering agents. In this work, coating specimens were produced using a dual-complexing agent: low-phosphorus (LP) and mid-phosphorus (MP) coatings were produced using sodium citrate and sodium acetate. In mid-phosphorus coating, the bath was prepared with a high concentration of sodium citrate and a low concentration of sodium acetate as compared to the low-phosphorus coating bath without altering the Ni-to-hypophosphite ratio. The higher sodium citrate concentration in the bath led to an increase in the phosphorus content of the Ni-P coatings from the low-phosphorus range to the mid-phosphorus range [16]. However, high-phosphorus (HP) coatings were produced using sodium citrate and lactic acid. Thiourea was used to stabilize the bath and prevent decomposition. The details of the bath composition are provided in Table 1 below. The loading factor, i.e., the ratio of the plating surface to the bath volume for each coating, was maintained at 0.8 dm2/L.

Table 1.
Bath composition of low-, mid-, and high-phosphorus electroless Ni-P bath.
2.2. Heat Treatment Process
The plated specimens were subjected to heat treatment in a muffle furnace in the air environment at atmospheric pressure. The heat treatment of the coatings was performed in two ways. The first type of heat treatment was conducted at 400 °C for 1 h, whereas the second type was conducted in two stages: 400 °C for 1 h, followed by 650 °C for 4 h. The first and second types of heat-treated coating specimens are represented as HT400 and HT650, respectively. To make a more straightforward representation of the coating specimens with heat treatment and phosphorus content conditions, the Ni-P coating specimens are recognized by their specific name, as provided in Table 2.

Table 2.
Representation of coating specimens with their heat-treatment and phosphorus-content conditions.
2.3. Characterization of Coatings
Coating surface morphology and cross-section imaging of coatings specimens were performed by confocal microscope (Olympus, LEXT OLS4100, Tokyo, Japan) and scanning electron microscope (SEM-AIS2300C, Seron Technologies, Uiwang-si, Republic of Korea). The chemical composition was analyzed via an energy dispersive X-ray spectroscope (EDS, Oxford Instruments, Abingdon, UK) and X-ray fluorescence spectroscopy (XRF, DPO-200, Olympus). The microstructural phase of the heat-treated coating specimens was analyzed via X-ray diffraction (XRD, Bruker D8 Advance A25 Plus, Billerica, MA, USA) with a Cu-Kα source (1.542 Å) and in an operating voltage of 40 kV and current 30 mA. The hardness test was conducted in a Vickers microhardness tester (Leeb Testing Instrument, LHVS-1000Z, Chongqing, China) using a 100 g load and 10 s indentation time.
2.4. Adhesion Test
The adhesion test of the coating specimens was conducted by making 10 vertical and 10 horizontal crosscuts a millimeter apart using a sharp steel blade. After that, an adhesive tape was pasted on the coating surface, covering the cutting marks, and abruptly pulled out perpendicularly. The adhesion test areas were inspected visually to determine whether any coating flakes had detached. Scratch test was also performed using Rockwell D-275 indenter (CSM, Revetest, Peseux, Switzerland) by applying a progressive load of 1 to 40 N at a room temperature of 22 °C. The relative humidity at the time of measurement was 35%.
2.5. Tribology and Wear Test
A ball-on-flat method consisting of a liner reciprocating sliding system was implemented using a tribometer (CSM Instrument, Peseux, Switzerland) to evaluate the friction and wear behavior of the coatings according to the ASTM-G133-05 test standard [17]. The tests were conducted in dry condition (without lubrication) using a SAE52100 bearing steel ball (diameter 12.7 mm, Vickers hardness 848 HV) as the counter sliding surface in 5 and 10 N normal loads. For all the coating specimens, other testing conditions, such as stroke length, sliding velocity, and sliding time, were fixed to 4 mm, 2.5 cm/s, and 30 min, respectively. Similarly, temperature and relative humidity were 22 °C and 32% during the test time, respectively. The weight-loss method determined the wear damage of sliding surfaces (coating and ball) after performing at least three sliding tests using a weighing machine (with the least count of 0.1 mg). The kinetic friction coefficient (COF) as a function of time was recorded by an in-built transducer during the sliding process. The wear scars imprinted on the coating and ball surfaces were analyzed via a confocal microscope.
3. Results and Discussion
3.1. Surface and Cross-Sectional Observation before and after the Heat Treatment
The electroless Ni-based coating follows the substrate surface asperity. As a result, the coating seems rough (or smooth) if the substrate is rough (or smooth). The austenite stainless-steel (ASTM 304SS) substrates were sandblasted, as was mentioned in the materials and method section above, and as a result, their surfaces were rougher than those of the finely polished substrate. The craters and kinks that developed during the sandblast of the substrates persisted even after the Ni-P coating (Figure 1). Moreover, the appearance of nodules on the coating surface was due to the characteristic features of the electroless Ni-based coatings. The nodule size somewhat alters the coating-surface roughness. Large nodules lead to an increase in the surface roughness to some extent. In this study, slightly small-sized nodules were formed on the low-phosphorus AP-LP coating surface (Figure 1a) in comparison with mid- and high-phosphorus AP-MP and AP-HP coating surfaces (Figure 1b,c) despite the same substrate pre-treatment conditions. Because of the larger nodule size, the AP-MP and AP-HP coatings were anticipated to have a slightly rougher surface than the AP-LP coating. The nodular growth phenomenon was not observed clearly in the HT400 specimens, although the initiation of nickel oxidation changed the appearance (Figure 1d–f).
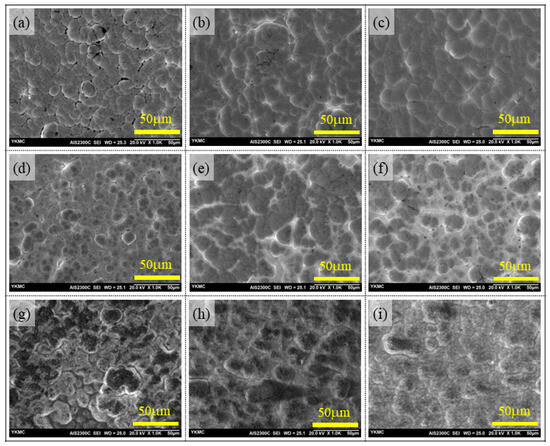
Figure 1.
Upper-row images are the surface morphology of as-plated Ni-P coatings observed via confocal microscope for (a) AP-LP, (b) AP-MP, and (c) AP-HP specimens. Similarly, the middle-row images are the morphologies of 400 °C heat-treated (d) HT400-LP, (e) HT400-MP, and (f) HT400-HP specimens. The lower row images are the morphologies of 650 °C heat-treated (g) HT650-LP, (h) HT650-MP, and (i) HT650-HP specimens. The scale bar on the images represents 50 μm.
However, the HT650 samples’ morphologies were modified by the high-temperature (650 °C) heat treatment in an air atmosphere (Figure 1g–i). The high-temperature treatment technique was responsible for this alteration since it formed nickel oxide (NiO) layers on the top surface and Ni diffusion. The HT650 specimens had a darker surface look than the as-plated specimens because nickel oxidation mainly affected the top surface of the specimens. Apart from nickel oxidation, the HT650-LP and HT650-MP specimens (Figure 1g,h) experienced nodule growth in contrast to their as-plated counterparts (Figure 1a,b). Nevertheless, following the 650 °C heat treatment, no discernible nodular development was seen in the HT650-HP coatings (Figure 1i). The high-temperature, heat-treated HT650 specimens showed a difference in surface color as well: the low-phosphorus (HT650-LP) specimens had a greenish-black color, while the mid- and high-phosphorus (HT650-MP & HT650-HP) specimens had a black color (the surface color images are provided in the Supplementary Materials, Figures S1 and S2). The color difference seen in HT650 specimens may have resulted from variations in the phosphorus content of the coating, which could have impacted the growth of the NiO phase. The color formation in NiO was related to the layer thickness and the defects created on NiO crystal [18,19].
Three different plating baths were utilized to produce electroless Ni-P coating specimens with low-, mid-, and high-phosphorus. However, all coating specimens had the same coating parameters, such as the coating temperature (85 °C) and duration (1 h). The findings from the cross-sectional pictures (Figure 2 and Table 1) showed that the AP-HP coating had a coating thickness of approximately 16 μm, more than the coating thickness of AP-LP and AP-MP coatings (~13 μm). The concentration ratio of the nickel source to the reducing agent and the complexing and buffering capabilities of the complexing agents influenced the variation in the coating rate among the various phosphorus-content specimens. The main reason for the increase in the coating rate in the AP-HP specimen was attributed to the complexing and buffering capabilities of sodium citrate and lactic acid. Properly controlling the amount of free Ni2+ ions in the bath, it was found that the combination of sodium citrate and lactic acid could effectively increase the coating rate [20]. The complexing agent in the electroless Ni-P bath controlled the generation of nickel salts, which prevented the bath from decomposing in addition to controlling free Ni2+ ions [20,21].
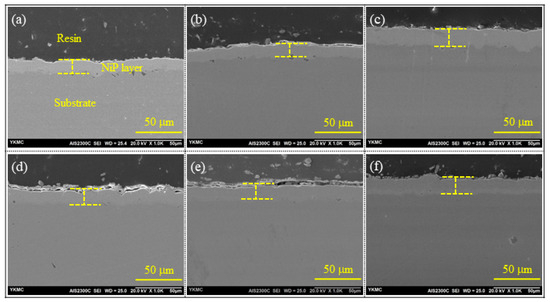
Figure 2.
SEM images of coating cross-section: in upper row as-plated (a) AP-LP, (b) AP-MP, and (c) AP-HP specimens. Similarly, in the lower row, the cross-section of 650 °C heat-treated (d) HT650-LP, (e) HT650-MP, and (f) HT650-HP specimens.
Furthermore, the coating thickness of the heat-treated HT650-LP and HT650-MP coatings was found to be increased slightly than that of their as-plated counterpart specimens (Figure 2d,e). The coating thickness increment was related to the formation of the NiO layer on the top surface of HT650. However, this phenomenon was not observed in the HT650-HP specimen (Table 3). The coating thickness almost remained the same after the high-temperature heat treatment of high-phosphorus coating. This might have to do with phase transition and porosity elimination.

Table 3.
Coating characteristics (phosphorus content, coating thickness, and Vicker’s microhardness) before and after the heat treatment of low-, mid-, and high-phosphorus electroless Ni-P coatings.
3.2. Phosphorus Content, Hardness, and Adhesion Behavior
The results of the EDS (Table 3) confirm that the coatings under examination had phosphorus contents that fall into three categories: low-phosphorus (below 5 wt.% P), mid-phosphorus (6–9 wt.% P), and high-phosphorus (10 wt.% P and beyond) coatings. In the as-plated specimen, the phosphorus distribution was uniform throughout the coating layer (Figure 3a). However, after the heat treatment at 650 °C, phosphorus segregation occurred near the top coating surface (Figure 3b). The phenomenon of phosphorus segregation was observed for all coating specimens regardless of their phosphorus content (Figure S3, Supplementary Materials). Additionally, the high-temperature (650 °C) treatment of the Ni-P coatings caused Ni and Fe atom diffusion near the interfacial region. Biswas et al. [13] observed similar phenomena of Fe diffusion from AISI 1040 low-carbon steel substrate to Ni-P-Cu coating interfacial region that led to the formation of intermetallic compounds when heat-treated at 600 °C. The interfacial diffusion of Ni and Fe could have increased the adhesion of coating with the substrate in HT650 specimens as observed by Singh et al. [22].
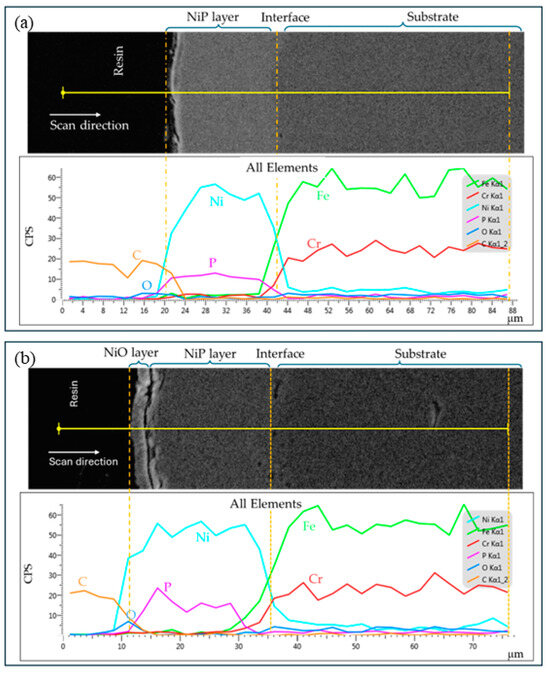
Figure 3.
EDS elemental line profile spectra of (a) AP-HP and (b) HT650-HP specimens. Phosphorus segregation (spiked P-peak) near the top coating surface is observed after the heat treatment at 650 °C in the air environment. Also, Ni and Fe diffusion is observed near the interface region.
The Vickers microhardness data for the coatings, both before and after the heat treatment, are listed in Table 3. It was found that the as-plated Ni-P coating with low-phosphorus content (AP-LP) was harder than that with mid-phosphorus (AP-MP) and high-phosphorus (AP-HP), which was consistent with the previous studies [21,23,24]. The hardness was increased further when the coatings underwent heat treatment at 400 °C. The high-phosphorus coating specimen, HT400-HP, was turned into harder coating (~1086 HV) among all the specimens. Many studies have reported that the electroless Ni-P coatings receive optimum hardness when heat-treated at a temperature near 400 °C [21,25,26,27]. It is worth noting that not all coatings became hard with similar proportions, even if they were heat-treated at 400 °C. The phosphorus content might have played a crucial role in the phase transformation of the heat-treated coatings, as the evolution of the hard Ni3P phase depends on the amount of phosphorus.
Furthermore, the high temperature (650 °C) heat treatment deteriorated the coating’s hardness. The hardness values of HT650-LP and HT650-MP coatings were less than those of their as-plated specimens. The low-phosphorus specimen HT600-LP had the lowest hardness value of 278 HV. Similarly, the hardness of mid-phosphorus HT650-MP and high-phosphorus HT650-HP specimens was reduced to 532 HV and 691 HV, respectively, which were far less than the hardness of corresponding coatings heat-treated at 400 °C (i.e., HT400-MP and HT400-HP specimens). The deterioration of the hardness of HT650 specimens could be related to grain coarsening and nickel oxidation. It is interesting to note that the hardness of the HT650-HP specimen is higher than that of HT650-MP. This could be associated with the amount of Ni3P hard-phase formation on those specimens (refer to XRD results).
The crosscut adhesion test was conducted to evaluate the adhesion of the coatings before and after the heat treatment. Both as-plated and HT400 coating specimens demonstrated excellent adhesion to the ASTM 304SS steel substrates regardless of the phosphorus content (Figure S4, Supplementary Materials). However, the HT650 specimens showed mixed results for adhesion behavior, primarily because of the NiO layer formed on the top surface because of nickel oxidation. After the adhesion test, the NiO layer in the HT650-MP and HT650-HP specimens was detached rather than the whole coating layer. In contrast, there was no evidence of flaking off and detachment of the NiO layer from the HT650-LP specimen. The difference in the adhesion characteristics of NiO may be linked to the phase that emerged in the 650 °C heat-treated coatings because of the phosphorus content. Additionally, the findings of the scratch test (Figures S5 and S6, Supplementary Materials) revealed similar adhesion behavior between the NiO layer and the Ni-P coating layer beneath the NiO layer. Nevertheless, after the scratch test on both the as-plated and the HT650 specimens, there was no evidence of the Ni-P layer’s separation from the steel substrate.
3.3. X-ray Diffraction Analysis of Heat-Treated Coatings
The microstructural behavior of Ni-P coatings with varying phosphorus contents that were heat-treated in an air environment has been studied by comparing the phase evolution that occurred at 400 °C and 650 °C using the results of the XRD analysis. Depending on the phosphorus content, the Ni-P coating consists of nanocrystalline to amorphous phase microstructures in its as-plated condition. However, when they were allowed to heat at 400 °C for a specific time, the Ni and Ni3P phases were precipitated [28,29,30]. The XRD spectra (Figure 4a) indicated that the heat treatment of Ni-P coating at 400 °C in an air environment at atmospheric pressure for 1 h led to develop not only Ni and Ni3P phases but also a trace quantity of bunsenite (NiO) phase, which was hard to quantify. The HT400-HP specimen exhibited NiO (111) reflection, as indicated by the peak situated at 37.3 degrees in the magnified image displayed in the inset of Figure 4a. Other HT400 specimens’ XRD spectra likewise showed the same peak, indicating the presence of NiO (111) reflection; thus, the color of HT400 specimens differed from the as-plated specimens (Figure S1, Supplementary Materials). The Ellingham diagram predicts that unalloyed Ni oxidation begins at 500 °C at atmospheric pressure, but these results suggest that Ni oxidation in Ni-P alloy coatings can occur even at low temperatures.
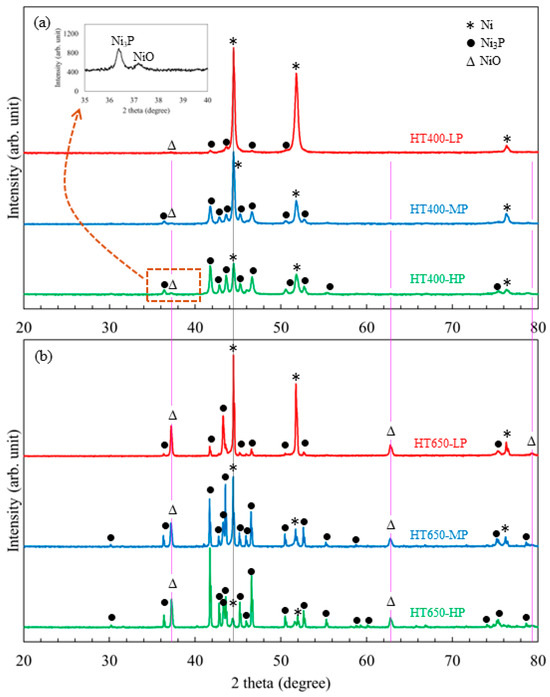
Figure 4.
XRD spectra of low-, mid-, and high-phosphorus Ni-P coatings that are heat-treated at (a) 400 °C (HT400 specimens) and (b) 650 °C (HT650 specimens) in an air environment. The inset image in (a) is a magnified view of the XRD spectra from the HT400-HP specimen, where a faint peak at 37.3 degrees belongs to NiO (111) reflection.
The phase composition analysis (Table 4) showed that the Ni3P crystallite phase was predominantly observed in the high-phosphorus HT400-HP specimen. In contrast, the Ni crystallite phase was predominantly presented in the low-phosphorus HT400-LP specimen. After heat-treating, the high-phosphorus Ni-P coating at 400 °C in air, enough hard Ni3P phase precipitated, making it the hardest specimen of all the coatings. It is evident that the Ni further oxidizes at a higher temperature than 400 °C and in an increasing treatment time. The XRD data showed that the low-phosphorus HT650-LP specimen had higher NiO phase concentrations (~35%) than mid- and high-phosphorus specimens (Figure 4b and Table 4). Hence, a thicker NiO layer was developed on the surface of the HT650-LP specimen compared to the HT650-MP and HT650-HP specimens. As a result, the color differences appeared in NiO layers developed on those coatings.

Table 4.
Phase composition of the heat-treated Ni-P coatings obtained via XRD analysis.
Moreover, the diffraction peaks associated with NiO are well matched with the PDF 00-047-1049, for which the crystal structure is cubic and space group Fm-3m (225). Also, the Ni crystal has a cubic structure with the same space group, and its spectra matched well with PDF 00-004-0850. However, Ni3P has a tetragonal structure with space group I-4 (82), and its spectra match PDF 04-015-7502. The extra peaks associated with Ni3P phases are distinctly observed in the HT650-HP specimen. It is noted that the Ni3P phase is observed to be in higher concentration in HT650-HP, which makes the coating harder even after heat treatment at 650 °C. As both Ni and NiO have cubic structures, the adhesion behavior of the NiO layer might be better with Ni than with Ni3P. Owing to these outcomes, improved adhesion behavior of the NiO layer is observed in low-phosphorus HT650-LP rather than mid-phosphorus HT650-MP and high-phosphorus HT650-HP specimens.
3.4. Friction and Wear Behavior
Friction is an opposing force that resists the movement of surfaces when they are in contact. The COF is a parameter that indicates how hard the surfaces are moving one over another. Likewise, wear is “the progressive loss of substance from the operating surface of a body as a result of relative motion of the surface to another body” [31]. This study presents a qualitative and quantitative aspect of the wear behavior of coating. The wear scar imprinted on the coating and ball surfaces after the reciprocating sliding test and the corresponding friction coefficient recorded during the tests are analyzed to evaluate the wear-resistant behavior of the coatings. The effect of heat treatment on the wear-resistance behavior of the coatings is explained in terms of the advancement of the surface and microstructural characteristics of the heat-treated coatings.
3.4.1. Friction Curve and Wear Scar Analysis
Figure 5a displays the coefficient of friction (COF) versus sliding cycle of the as-plated coatings acquired during the wear test with a 5 N applied load. Before 1000 sliding cycles, every as-plated specimen displayed a comparable COF trend. The COF curves began to vary as the sliding continued over 1000 cycles. The COF curves emerged rising and falling until the sliding process concluded, showing the presence of coating surface damage. The debris that developed due to the coating degradation and adhered to the sliding track caused the COF curves to rise and fall. Regardless of phosphorus content, all as-plated (AP-LP, AP-MP, and AP-HP) coating specimens exhibited the rising and falling COF curve phenomenon.
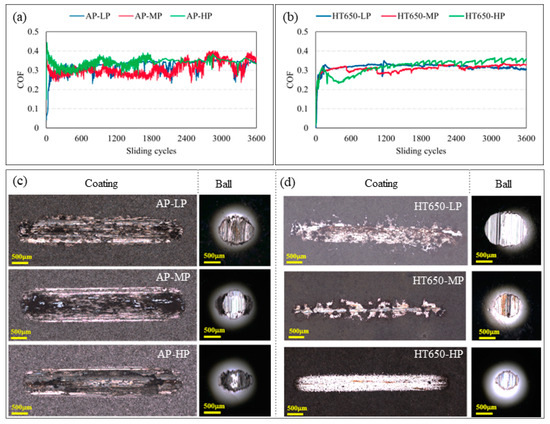
Figure 5.
The COF curves recorded during the sliding wear test of (a) as-plated and (b) HT650 coating specimens. The images in (c,d) are the corresponding wear scars imprinted on the coatings and balls obtained from a confocal microscope. The scale bar embedded on the wear scar images represents 500 μm. (Applied load = 5 N and sliding velocity = 2.5 cm/s).
When coatings were heat-treated to 650 °C, noticeable surface properties were introduced, resulting in relatively steady COF curves for HT650 specimens (Figure 5b). In contrast to the as-plated counterpart, the frictional phenomenon was decreased with the HT650 specimens because the steady COF curves were visible during the entire sliding process with a slight reduction in COF value. However, there was some fluctuation in the COF values of the HT650-MP and HT650-HP specimens. The HT650 specimens’ varying frictional behaviors can be explained by the nickel oxide layer’s lubricity, brittleness, thickness, and adhesive qualities, among other characteristics. The adhesion test findings (Figure S4, Supplementary Materials) indicated that the NiO layer created on the surface of the HT650-MP and HT650-HP specimens exhibited less adhesive and brittle character than the NiO layer formed on the HT650-LP specimen. The adhesive NiO layer of the HT650-LP specimen consequently resulted in a constant COF curve, in contrast to the HT650-MP and HT650-HP specimens.
Additionally, surface roughness is another parameter that plays a significant role in the frictional behavior of the coatings. As mentioned in the experimental section, the substrates were subjected to sandblasting just before the chemical treatments and coating process. Therefore, it is customary to state that all substrates had the same level of surface roughness before applying the Ni-P coatings. However, a slight change in roughness might be anticipated because of the nodular growth phenomena in the coatings. Furthermore, the specimens having mid- and high-phosphorus content (HT650-MP and HT650-HP) showed higher hardness than the low-phosphorus HT650-LP specimen after the heat treatment. The interplay of hard and brittle NiO debris within the sliding track might be the reason for the fluctuating COF curves in the HT650-MP and HT650-HP specimens.
The degree of damage caused by sliding surfaces is a well-known way to evaluate the materials’ wear characteristics. The wear scars that formed on the coating surfaces and the counter sliding surfaces (steel ball) were examined to comprehend the wear mechanism of the coatings with various phosphorus content in their as-plated and heat-treated conditions. Based on scar width, depth, worn-out volume, and damage on the counter ball surface, it was found that HT650 specimens demonstrated improved wear-resistance behavior in comparison to as-plated specimens (Figure 5c,d). As-plated specimens exhibited a more severe deformation of the coating surface than the HT650 specimens. The wear debris smeared on the wear tracks was high in the as-plated specimens, indicating massive damage to the coatings. It was observed that among the HT650 specimens, considerable damage was seen on the HT650-LP coating surface together with its pair (ball) surface. However, the coating material was not delaminated much; hence, comparably little damage was seen on the HT650-MP and HT650-HP specimen surfaces and their pair surfaces when the sliding tests were conducted at the load of 5 N, although the abrasion wear was predominant to deform the coatings. A similar trend of wear phenomena was observed in the low-, mid-, and high-phosphorus coating specimens heat-treated at 400 °C (Figure S7, Supplementary Materials). Among the 400 °C heat-treated specimens, HT400-MP and HT400-HP showed improved wear-resistance properties compared to HT400-LP. The weight-loss and wear-volume measurements after the wear test of HT400-MP and HT400HP specimens could not be conducted as they were beyond the detection limit of the available resources. Therefore, the sliding wear tests were conducted further by increasing the applied load to 10 N.
The same pattern of rise and decline in COF was seen in the as-plated specimens’ frictional properties under increasing applied load (Figure 6a), as it was under 5 N load (Figure 5a). Nevertheless, in the HT650 specimens, a slight rise in COF was noted with a 10 N load (Figure 6b) compared to a 5 N load. The COF curves in this instance were found to be more stable with relatively low values in the HT650-MP and HT650-HP specimens than in the HT650-LP and all as-plated specimens. Following the completion of 3600 sliding cycles under a 10 N load, the wear scar that developed on the surfaces of the as-plated coating specimens, AP-LP, AP-MP, and AP-HP, revealed significant damage. The wear scar size was almost the same in all as-plated specimens, regardless of their hardness value (Figure 6c). Similarly, considerable damage was visible in the wear scars formed on the steel balls used to test the wear properties of as-plated specimens. Additionally, the heat-treated HT650-LP specimen with low-phosphorus content had the same degree of wear damage observed in the as-plated specimens (Figure 6d). In contrast to the low-phosphorus HT650-LP, the mid- and high-phosphorus HT650-MP and HT650-HP specimens demonstrated reduced wear damage.
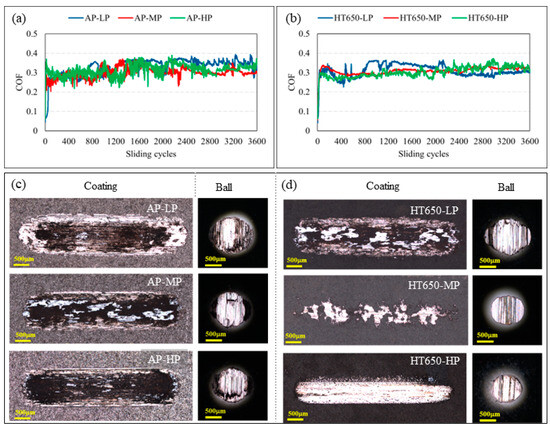
Figure 6.
The COF curves recorded during the sliding wear test of (a) as-plated and (b) HT650 coating specimens. The images in (c,d) are the corresponding wear scars imprinted on the coatings and balls obtained from a confocal microscope. The scale bar embedded on the wear-scar images represents 500 μm (Applied load = 10 N and sliding velocity = 2.5 cm/s).
The sliding surfaces’ contact area determines the wear scar size that grows when wear wipes off one of the sliding surfaces, resulting in an increasing contact area. Therefore, it might not be enough to examine the wear behavior of the coatings using a qualitative observation of the wear-scar size. The coatings’ weight loss and wear volume can be measured to provide a quantitative analysis by evaluating the specimens’ weight change or measuring the wear track’s depth profile after the wear test. The wear rate of a material is directly proportional to its worn surface volume, which is provided by the following Equation (1):
where V (mm3) is the worn surface volume, F (N) is the applied normal load, and d (m) is the sliding distance. Thus, Equation (1) indicates that the larger the wear volume, the greater the wear rate of the materials.
3.4.2. Wear-Volume Analysis
The wear-volume data of the coating specimens and steel balls acquired after the sliding wear test are shown in Figure 7. The wear volume of coatings and balls was determined based on the average wear volume acquired from three sliding test results. Considering the wear-volume data (Figure 7a) and Equation (1), regardless of the wear-scar size imprinted on the coated surface, the low-phosphorus AP-LP specimen exhibited the best wear-resistance behavior among the as-plated specimens. The wear behavior of AP-MP and AP-HP did not differ much from the 304SS substrate’s wear behavior (Figure S8, Supplementary Materials). The substrate’s wear volume was measured to be 0.135 mm3, whereas the AP-LP coating demonstrated a drastically reduced wear volume of 0.056 mm3. In contrast, AP-MP and AP-HP coating specimens exhibited marginally reduced wear volumes of 0.111 mm3 and 0.128 mm3, respectively.
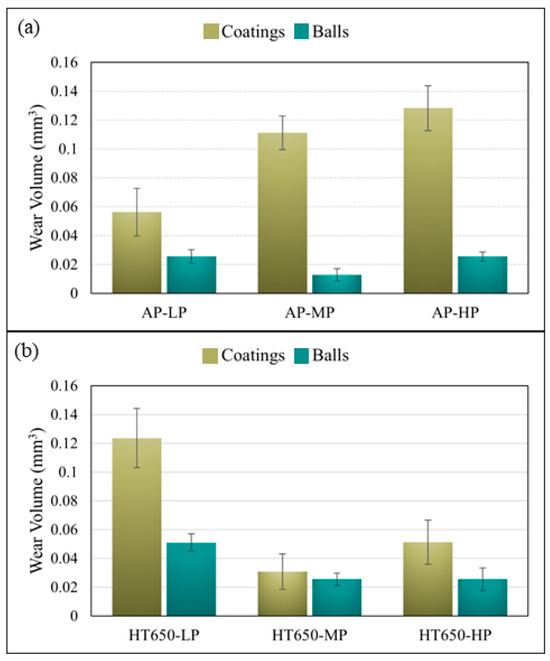
Figure 7.
The wear volume of coatings and steel balls was obtained when the reciprocating sliding wear test was conducted using a 10 N load on the (a) as-plated and (b) HT650 specimen surfaces.
Nevertheless, the low phosphorus specimen (HT650-LP) displayed a decline in wear-resistance behavior after the heat treatment at 650 °C (Figure 7b) for which the wear volume was 0.124 mm3. However, out of all the specimens put through the wear testing, the mid-phosphorus (HT650-MP) specimen showed the predominantly highest wear-resistance behavior since it had the lowest wear volume of 0.031 mm3. The heat-treated high-phosphorus (HT650-HP) specimen displayed a reduced wear volume of 0.051 mm3, equivalent to the wear volume of HT650-MP, in comparison to the wear volume (0.128 mm3) of its as-plated (AP-HP) counterpart. Despite the formation of a thick adhesive NiO layer on the surface of the low-phosphorus (HT650-LP) specimen, the deterioration in its wear-resistance behavior was associated with the decreasing coating hardness after the heat treatment at 650 °C. The NiO layer formed on the top surface of comparably harder HT650-MP and HT650-HP coating was found to reduce the wear damage effectively. It could be possible that the debris from the brittle NiO on them functioned as a dry lubricant to prevent friction from rising when sliding the surfaces and shield the coated surfaces from extreme abrasion.
4. Conclusions
Based on the findings, the following conclusions have been drawn in the low-, mid-, and high-phosphorus electroless Ni-P coatings heat-treated at high-temperature in the air atmosphere:
- Nickel oxidation in electroless Ni-P coating occurred at 400 °C, increasing to a high temperature of 650 °C. Phosphorus concentration affected NiO layer characteristics, causing varying NiO colors on the surface.
- After heat treatment at 650 °C, the low-phosphorus electroless Ni-P (HT650-LP) coating produced a greenish-black NiO layer that was found to be more adhesive than the black NiO layer developed on the Ni-P coatings containing mid- (HT650-MP) and high-phosphorus (HT650-HP).
- However, the scratch test demonstrated that the Ni-P layer firmly adhered to the steel substrate in their as-plated and 650 °C heat-treated conditions.
- When heated to 400 °C, high-phosphorus coatings had a hardness of 1086 HV, which was higher than any other coating under examination, but this hardness dropped to 691 HV at 650 °C.
- The NiO layer on the top surface of the harder HT650-MP and HT650-HP coatings effectively reduced wear damage by providing the benefit of dry lubrication to the sliding surfaces. However, the adhesive NiO layer on the top surface of HT650-LP could not show improvement in wear due to the low hardness of the coating.
- The HT650-HP specimen showed a slightly higher wear volume (0.051 mm3) than the wear volume of the HT650-MP coating (0.031 mm3), but which was far less than the wear volume (0.128 mm3) of its as-plated AP-HP coating.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/coatings14050648/s1, Figure S1. Coating appearance before and after heat treatment. The color of coatings changes due to the oxidation of Ni when the NiP coatings are heat-treated in the presence of oxygen. Figure S2. Upper row images are the surface morphology of as-plated Ni-P coatings observed via confocal microscope for (a) AP-LP, (b) AP-MP, and (c) AP-HP specimens. Similarly, the lower row images are the morphologies of 650 °C heat-treated (g) HT650-LP, (h) HT650-MP, and (i) HT650-HP specimens. The scale bar on the images represents 50 μm. Figure S3. EDS elemental line profile spectra of (a) HT650-LP and (b) HT650-MP specimens. Phosphorus segregation (spiked P spectra) near the top coating surface is observed after the heat treatment at 650 °C. Also, Ni and Fe diffusion near the interface region has been noticed in specimens regardless of P-content. Figure S4. Cross-cut adhesion test of low-, mid-, and high-phosphorus electroless Ni-P coatings in as-plated (AP), heat-treated at 400 °C (HT400), and heat-treated at 650 °C (HT650) conditions. Figure S5. The first column consists of the scratch images of the as-plated coatings (a) AP-LP, (b) AP-MP, and (c) AP-HP. Similarly, the second column consists of the scratch images of the 650 °C heat-treated coatings (d) HT650-LP, (b) HT650-MP, and (c) HT650-HP obtained via SEM after the scratch test using Rockwell (D-275) indenter. A progressive load from 1 to 40 N was applied, and the scratch length was fixed to 4 mm for all the coatings. The scale bar shown in the images represents 500 μm. Figure S6. EDS elemental analysis was performed on the extreme end (indicated by a rectangle) of the scratch mark on the HT650-HP specimen where the applied load was maximum. The absence of Fe signals indicates that Ni-P coating was not detached completely from the steel substrate, and the debris around the scratch mark was only from the top (NiO) layer of the coating. Figure S7. shows (a) the coefficient of friction curve and (b) confocal microscope images of wear scars observed on the HT400 coating sample and steel ball surfaces after the reciprocating sliding wear test using a 5N load. Figure S8. (a) Wear- scar images and (b) wear volume of the 304SS substrate and as-plated coatings obtained when the applied load was 10 N. The wear volume of the 304SS substrate was found to be higher in comparison with the wear volume of the as-plated coatings. Low-phosphorus Ni-P coating in as-plated condition, AP-LP, had shown enhanced wear- resistance properties among the as-plated coatings. The scale bar in the wear images represents 500 μm. Refs. [18,19] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, D.R.D. and Y.U.H.; methodology, D.R.D.; software, D.R.D.; validation, D.R.D., Y.U.H., B.G.L. and S.Y.C.; formal analysis, D.R.D., Y.U.H., and B.G.L.; investigation, D.R.D.; resources, T.H.K., S.Y.C.; data curation, D.R.D.; writing—original draft preparation, D.R.D.; writing—review and editing, D.R.D., Y.U.H., T.H.K., G.B.J. and S.Y.C.; visualization, D.R.D.; supervision, S.Y.C.; project administration, Y.U.H. and B.G.L.; funding acquisition, G.B.J. and S.Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The research work was conducted using internal resources of Young Kwang YKMC Inc.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available from authors upon reasonable request.
Acknowledgments
The authors would like to acknowledge the technical advice of Soo Wohn Lee, Yuwaraj Khatri Kshetri, and Jin-Hyuk Choi of Sun Moon University, Asan, Republic of Korea.
Conflicts of Interest
Authors Dhani Ram Dhakal, Young Uk Han, Byung Geon Lee, Gi Bum Jang and Sung Youl Cho were employed by the company Young Kwang YKMC Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Zhang, Y.Z.; Yao, M. Studies of Electroless Nickel Deposits with Low Phosphorus Content. Trans. Inst. Met. Finish. 1999, 77, 78–83. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Allahkaram, S.R.; Zarebidaki, A. An Investigation on Effects of Heat Treatment on Corrosion Properties of Ni-P Electroless Nano-Coatings. Mater. Des. 2010, 31, 3174–3179. [Google Scholar] [CrossRef]
- Vitry, V.; Yunacti, M.; Mégret, A.; Khalid, H.A.; Staia, M.H.; Montagne, A. Selection of New Heat Treatment Conditions for Novel Electroless Nickel-Boron Deposits and Characterization of Heat-Treated Coatings. Coatings 2023, 13, 1. [Google Scholar] [CrossRef]
- Khodaei, M.; Gholizadeh, A.M. Surfactant-Free Commercial Electroless Bath with Low Concentration of SiC Nanoparticles to Prepare the NiP-SiC Nanocomposite Coatings. Mater. Res. Express 2021, 8, 055009. [Google Scholar] [CrossRef]
- Keong, K.G.; Sha, W.; Malinov, S. Crystallisation Kinetics and Phase Transformation Behaviour of Electroless Nickel–Phosphorus Deposits with High Phosphorus Content. J. Alloys Compd. 2002, 334, 192–199. [Google Scholar] [CrossRef]
- Palaniappa, M.; Seshadri, S.K. Structural and Phase Transformation Behaviour of Electroless Ni-P and Ni-W-P Deposits. Mater. Sci. Eng. A 2007, 460–461, 638–644. [Google Scholar] [CrossRef]
- León-Patiño, C.A.; García-Guerra, J.; Aguilar-Reyes, E.A. Tribological Characterization of Heat-Treated Ni-P and Ni-P-Al2O3 Composite Coatings by Reciprocating Sliding Tests. Wear 2019, 426–427, 330–340. [Google Scholar] [CrossRef]
- Weiss, Z. High-temperature Oxidation of Iron Covered by Electroless Ni–P Coating: A GDOS Depth Profiling Study. Surf. Interface Anal. 1992, 18, 691–694. [Google Scholar] [CrossRef]
- Farrokhzad, M.A. High Temperature Oxidation Behaviour of Autocatalytic Ni-P-BN(h) Coatings. Surf. Coat. Technol. 2017, 309, 390–400. [Google Scholar] [CrossRef]
- Arora, A.; Kiran, K.U.V.; Ramakrishnan, B.; Sunil, B.R.; Dumpala, R. Effect of Heat Treatment on Mechanical and Tribological Characteristics of Electroless Ni-P Deposits. J. Phys. Conf. Ser. 2019, 1355, 012032. [Google Scholar] [CrossRef]
- Cheng, K.; Wu, Z. Effect of Heat Treatment on the Microstructure and Mechanical Properties of Electroless Nickel-Phosphorus Coatings. J. Phys. Conf. Ser. 2020, 1520, 012002. [Google Scholar] [CrossRef]
- Dong, D.; Chen, X.H.; Xiao, W.T.; Yang, G.B.; Zhang, P.Y. Preparation and Properties of Electroless Ni–P–SiO2 Composite Coatings. Appl. Surf. Sci. 2009, 255, 7051–7055. [Google Scholar] [CrossRef]
- Biswas, A.; Das, S.K.; Sahoo, P. Oxidation Issues during Heat Treatment and Effect on the Tribo-Mechanical Performance of Electroless Ni-P–Cu Deposits. Proc. Inst. Mech. Eng. Part L J. Mater. Des. Appl. 2021, 235, 1665–1685. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Pettit, F.S. Introduction to the High Temperature Oxidation of Metals, 2nd ed.; Cambridge University Press: Cambridge, UK, 2006; ISBN 9780521480420. [Google Scholar]
- Haugsrud, R. On the High-Temperature Oxidation of Nickel. Corros. Sci. 2003, 45, 211–235. [Google Scholar] [CrossRef]
- Omar, R.; Aboraia, M.S.; Oraby, E.A.; Gubner, R.; Rizk, A.E. The Effect of Sodium Citrate as a Complex Agent on the Corrosion Properties of the Electroless Ni-P Coating. Mater. Res. Express 2018, 5, 126511. [Google Scholar] [CrossRef]
- ASTM G133-05; Standard Test Method for Linearly Reciprocating Ball-on-Flat Sliding Wear. ASTM International: West Conshohocken, PA, USA, 2016. [CrossRef]
- Mohseni Meybodi, S.; Hosseini, S.A.; Rezaee, M.; Sadrnezhaad, S.K.; Mohammadyani, D. Synthesis of Wide Band Gap Nanocrystalline NiO Powder via a Sonochemical Method. Ultrason. Sonochem. 2012, 19, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Renaud, A.; Chavillon, B.; Cario, L.; Pleux, L.L.; Szuwarski, N.; Pellegrin, Y.; Blart, E.; Gautron, E.; Odobel, F.; Jobic, S. Origin of the Black Color of NiO Used as Photocathode in P-Type Dye-Sensitized Solar Cells. J. Phys. Chem. C 2013, 117, 22478–22483. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Cai, H.; Wang, A.; Zhang, J. Competitive Deposition of Electroless Ni-W-P Coatings on Mild Steel via a Dual-Complexant Plating Bath Composed of Sodium Citrate and Lactic Acid. Surf. Coat. Technol. 2015, 279, 9–15. [Google Scholar] [CrossRef]
- Sudagar, J.; Lian, J.; Sha, W. Electroless Nickel, Alloy, Composite and Nano Coatings—A Critical Review. J. Alloys Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef]
- Singh, V.; Marya, M. Thermally Assisted Adhesion Enhancement in High-Phosphorous Electroless Nickel Plating. J. Mater. Eng. Perform. 2019, 28, 2147–2157. [Google Scholar] [CrossRef]
- Czagány, M.; Baumli, P.; Kaptay, G. The Influence of the Phosphorous Content and Heat Treatment on the Nano-Micro-Structure, Thickness and Micro-Hardness of Electroless Ni-P Coatings on Steel. Appl. Surf. Sci. 2017, 423, 160–169. [Google Scholar] [CrossRef]
- Genova, V.; Paglia, L.; Pulci, G.; Pedrizzetti, G.; Pranzetti, A.; Romanelli, M.; Marra, F. Medium and High Phosphorous Ni-P Coatings Obtained via an Electroless Approach: Optimization of Solution Formulation and Characterization of Coatings. Coatings 2023, 13, 1490. [Google Scholar] [CrossRef]
- Palaniappa, M.; Seshadri, S.K. Friction and Wear Behavior of Electroless Ni–P and Ni–W–P Alloy Coatings. Wear 2008, 265, 735–740. [Google Scholar] [CrossRef]
- Yan, M.; Ying, H.G.; Ma, T.Y. Improved Microhardness and Wear Resistance of the As-Deposited Electroless Ni–P Coating. Surf. Coat. Technol. 2008, 202, 5909–5913. [Google Scholar] [CrossRef]
- Dhakal, D.R.; Kshetri, Y.K.; Chaudhary, B.; Kim, T.-H.; Lee, S.W.; Kim, B.S.; Song, Y.; Kim, H.S.; Kim, H.H. Particle-Size-Dependent Anticorrosion Performance of the Si3N4-Nanoparticle-Incorporated Electroless Ni-P Coating. Coatings 2021, 12, 9. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Seshadri, S.K. Preparation and Characterization of Electroless Ni-P and Ni-P-Si3N4 Composite Coatings. Trans. Inst. Met. Finish. 1999, 77, 84–86. [Google Scholar] [CrossRef]
- Keong, K.G.; Sha, W.; Malinov, S. Hardness Evolution of Electroless Nickel–Phosphorus Deposits with Thermal Processing. Surf. Coat. Technol. 2003, 168, 263–274. [Google Scholar] [CrossRef]
- Buchtík, M.; Krystỳnová, M.; Másilko, J.; Wasserbauer, J. The Effect of Heat Treatment on Properties of Ni-P Coatings Deposited on a AZ91 Magnesium Alloy. Coatings 2019, 9, 461. [Google Scholar] [CrossRef]
- Kloss, H.; Wäsche, R. Analytical Approach for Wear Prediction of Metallic and Ceramic Materials in Tribological Applications. Wear 2009, 266, 476–481. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).