Effects of ZrW2O8 Content on the Microstructures and Properties of Composite Coatings Produced by Laser Cladding
Abstract
1. Introduction
2. Materials and Methods
3. Results
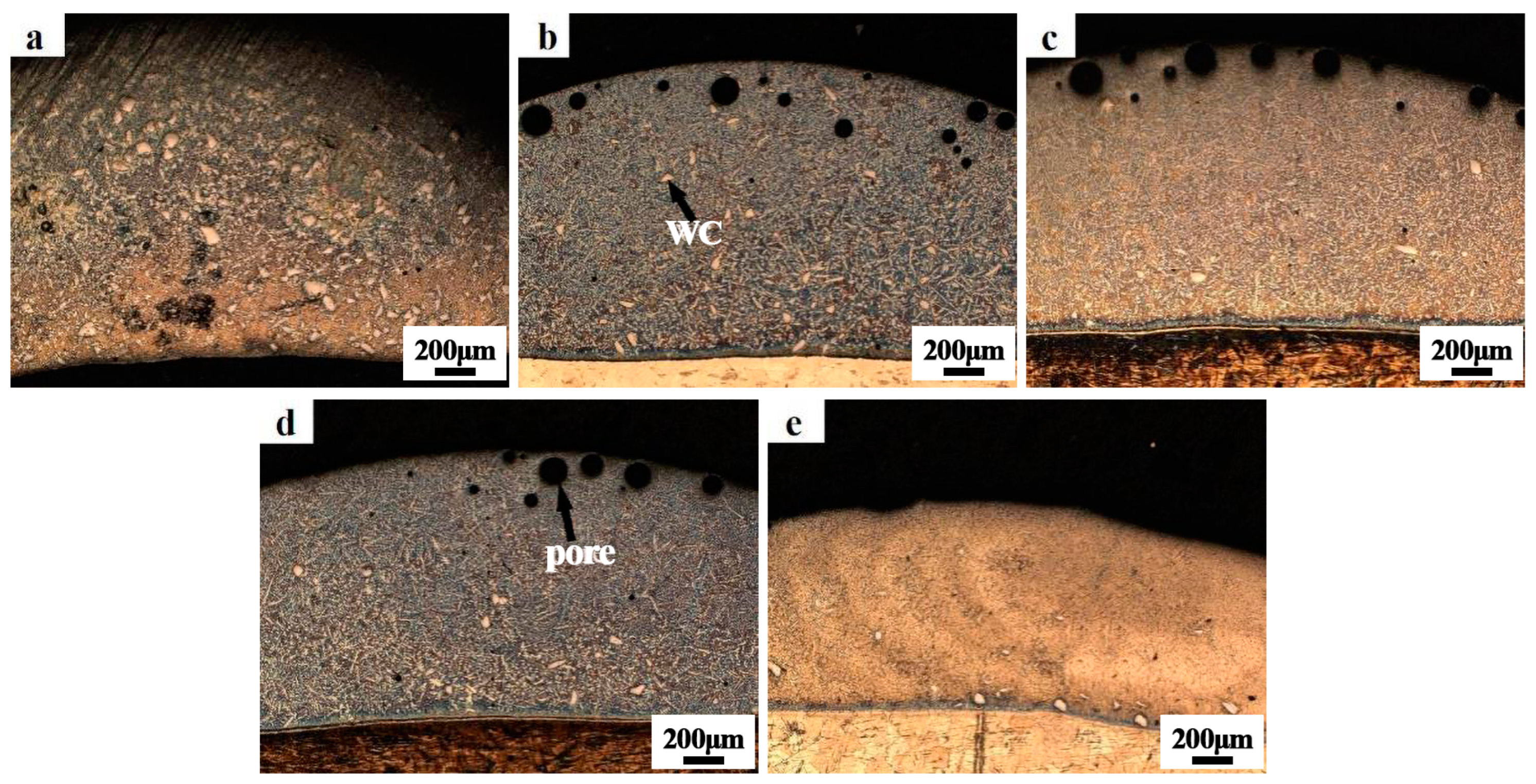
3.1. Macroscopic Forming and Crack Sensitivity of the Coating
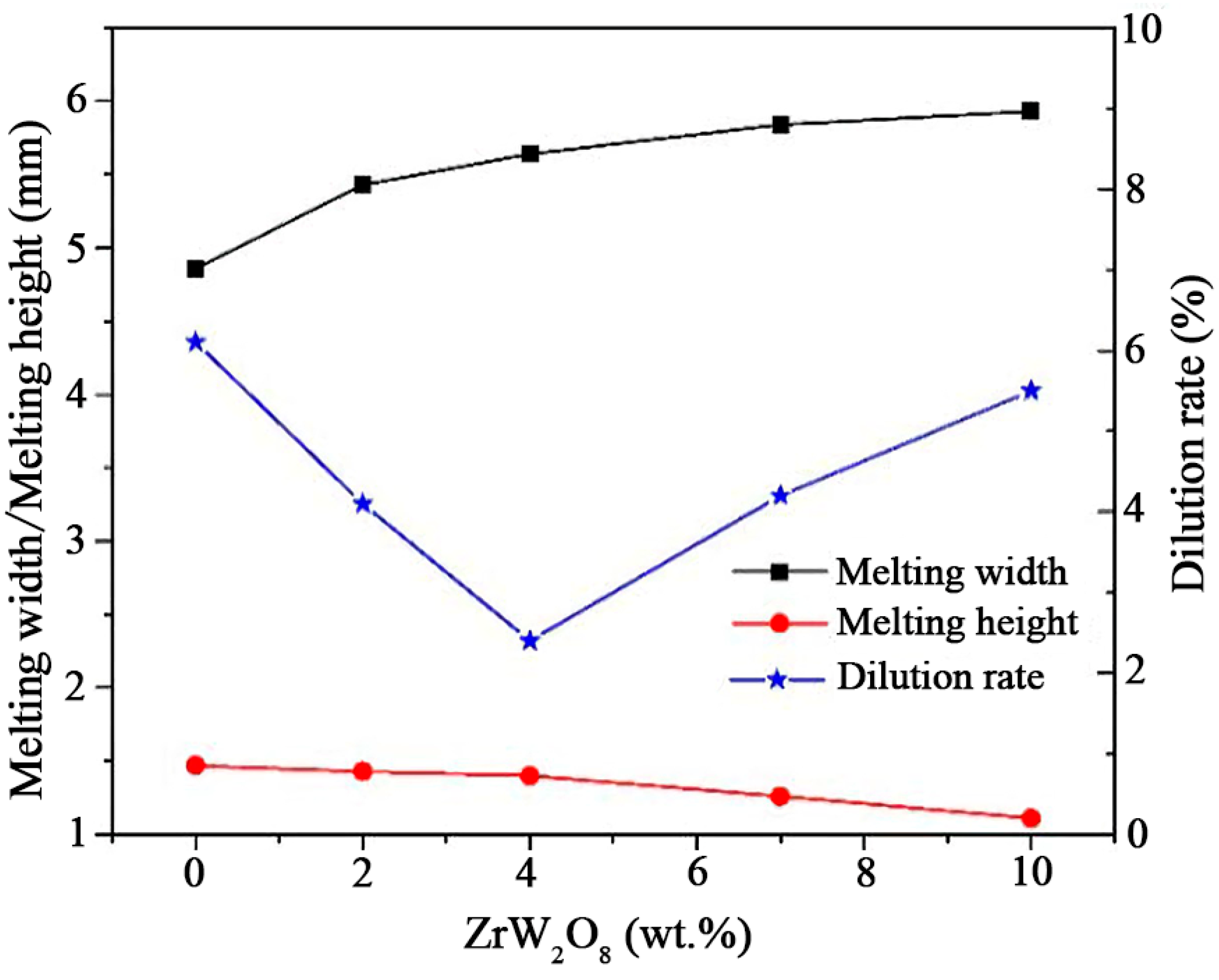
3.2. Melting Width, Melting Height, and Dilution Rate of Coating
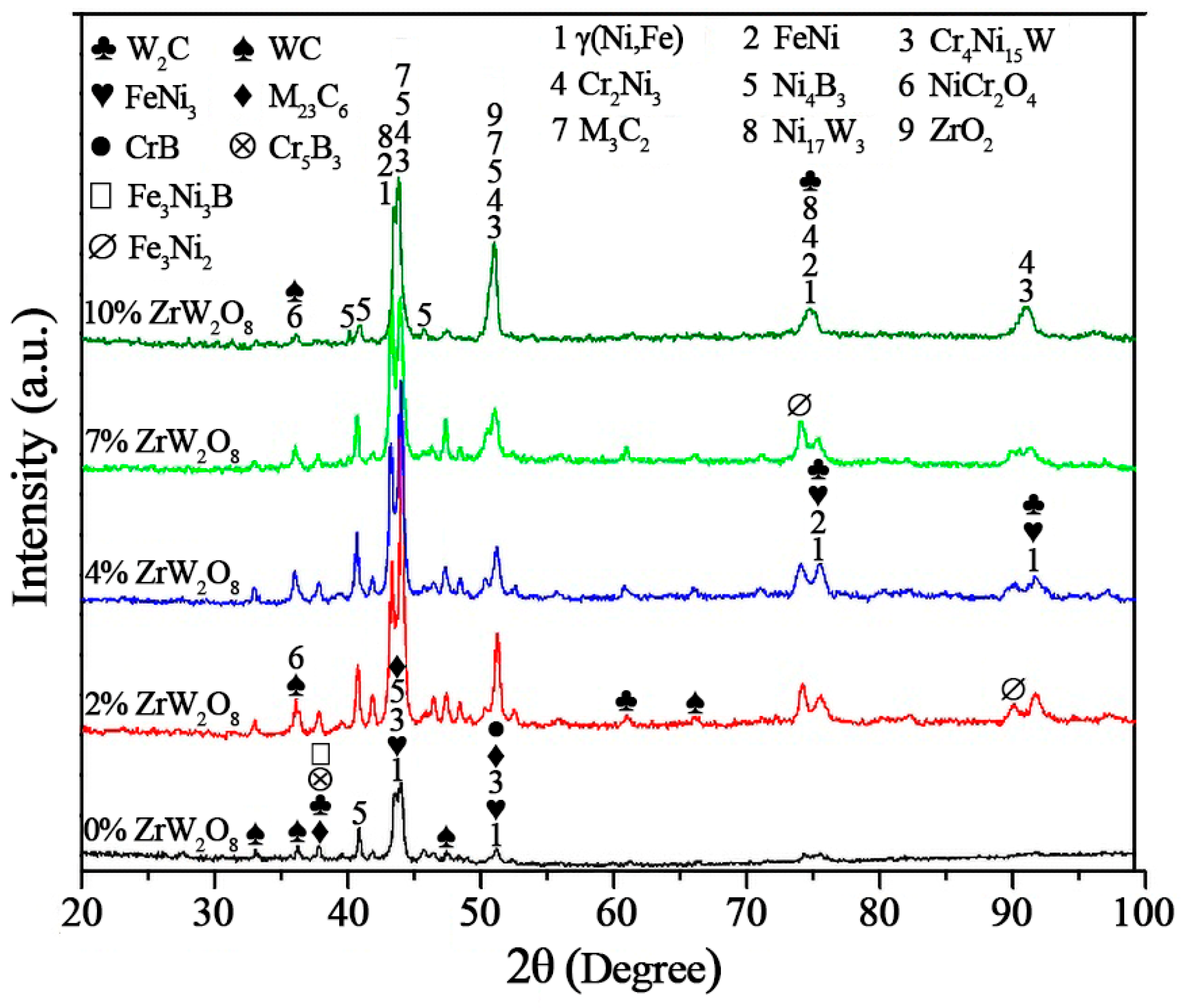
3.3. Phase Analysis of the Coating
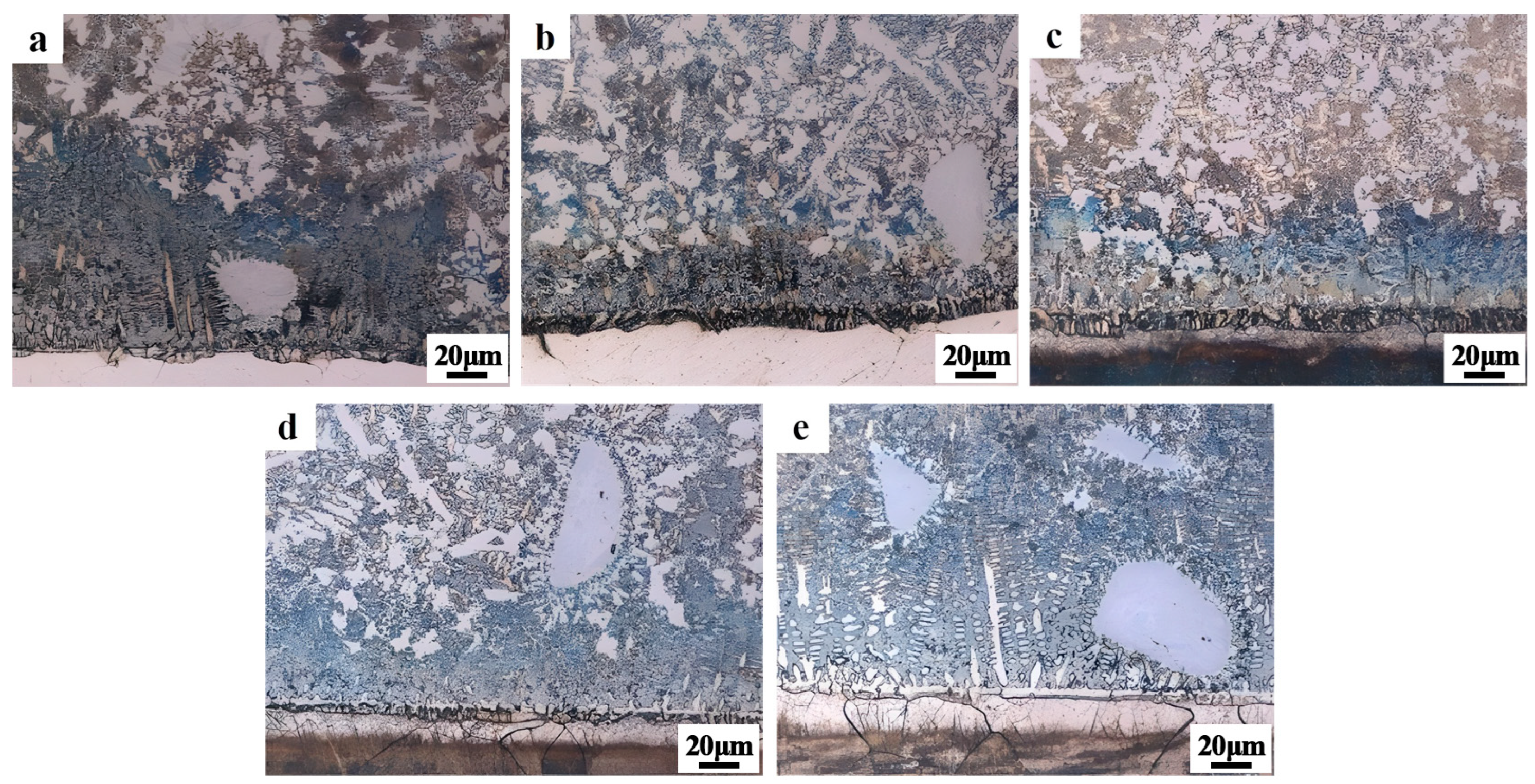
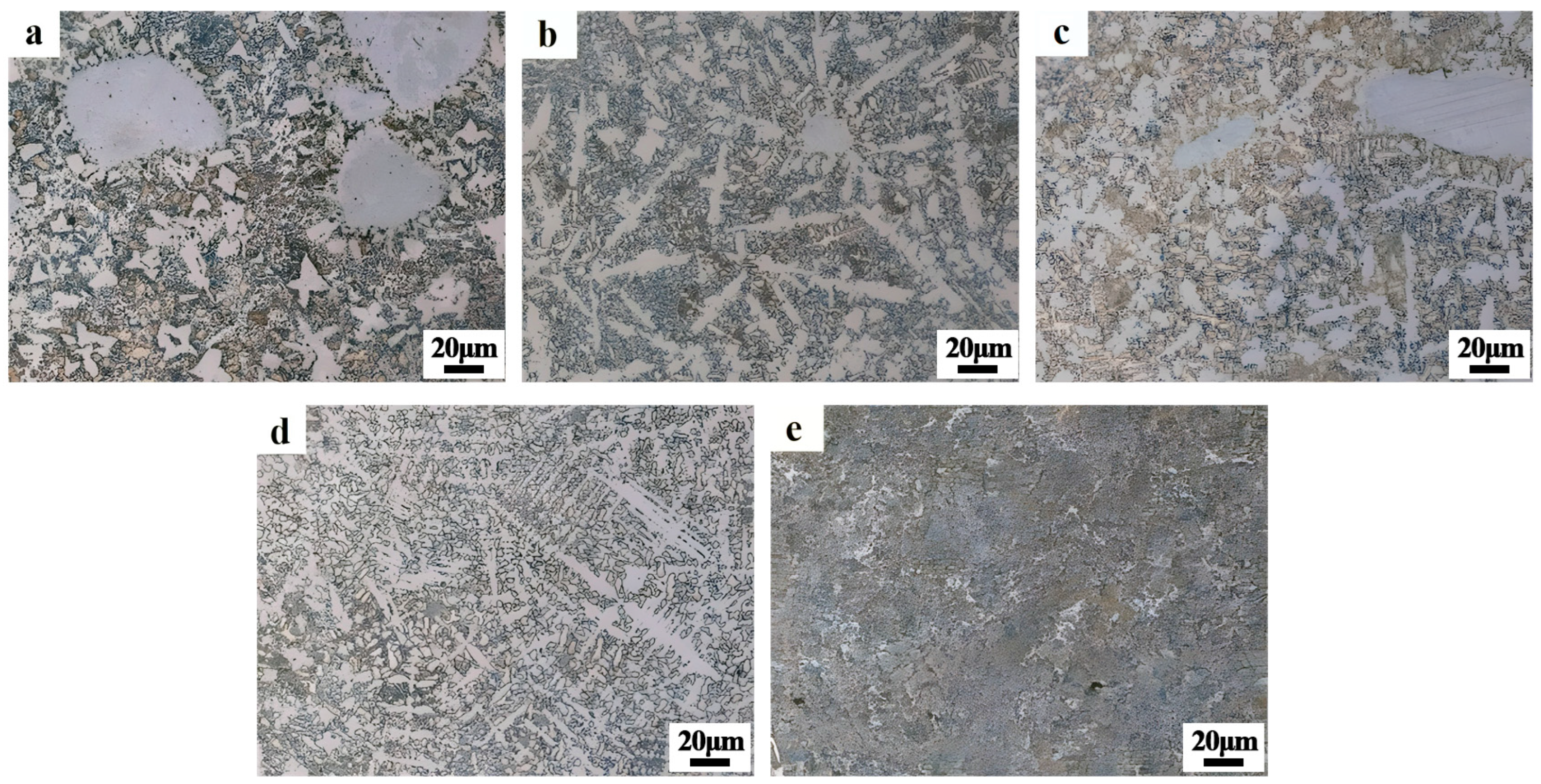
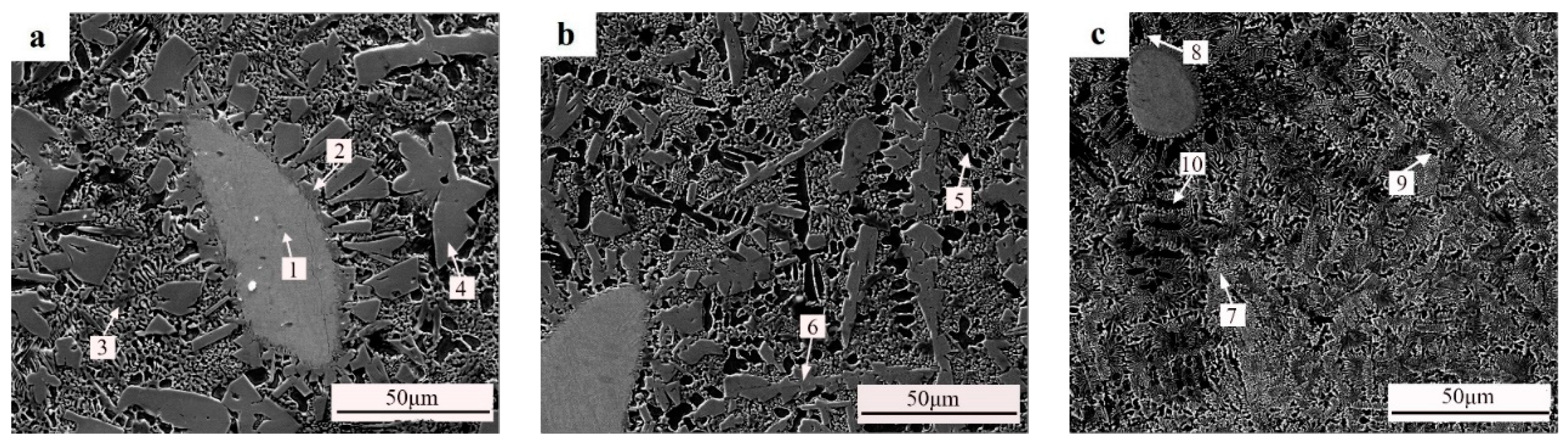
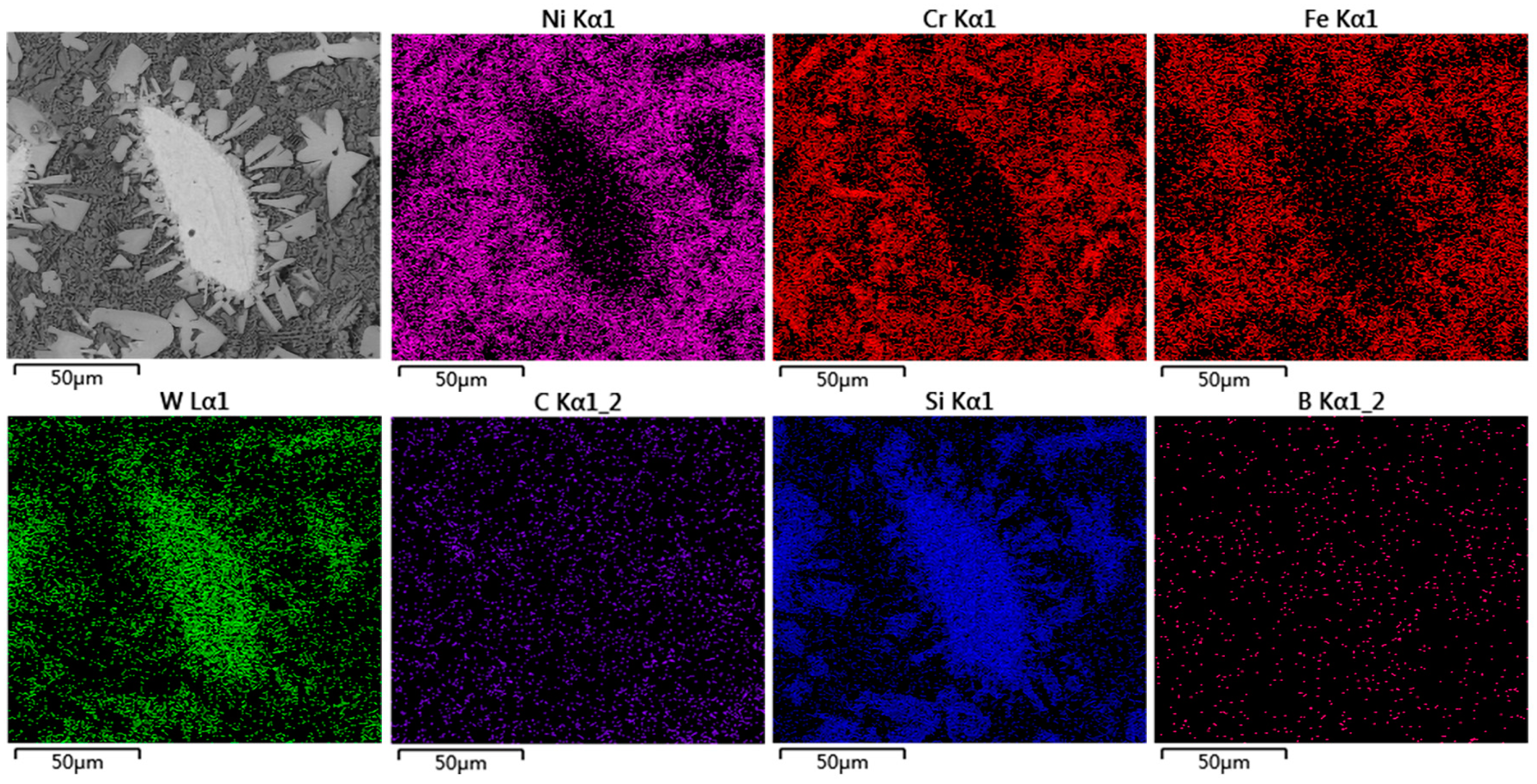
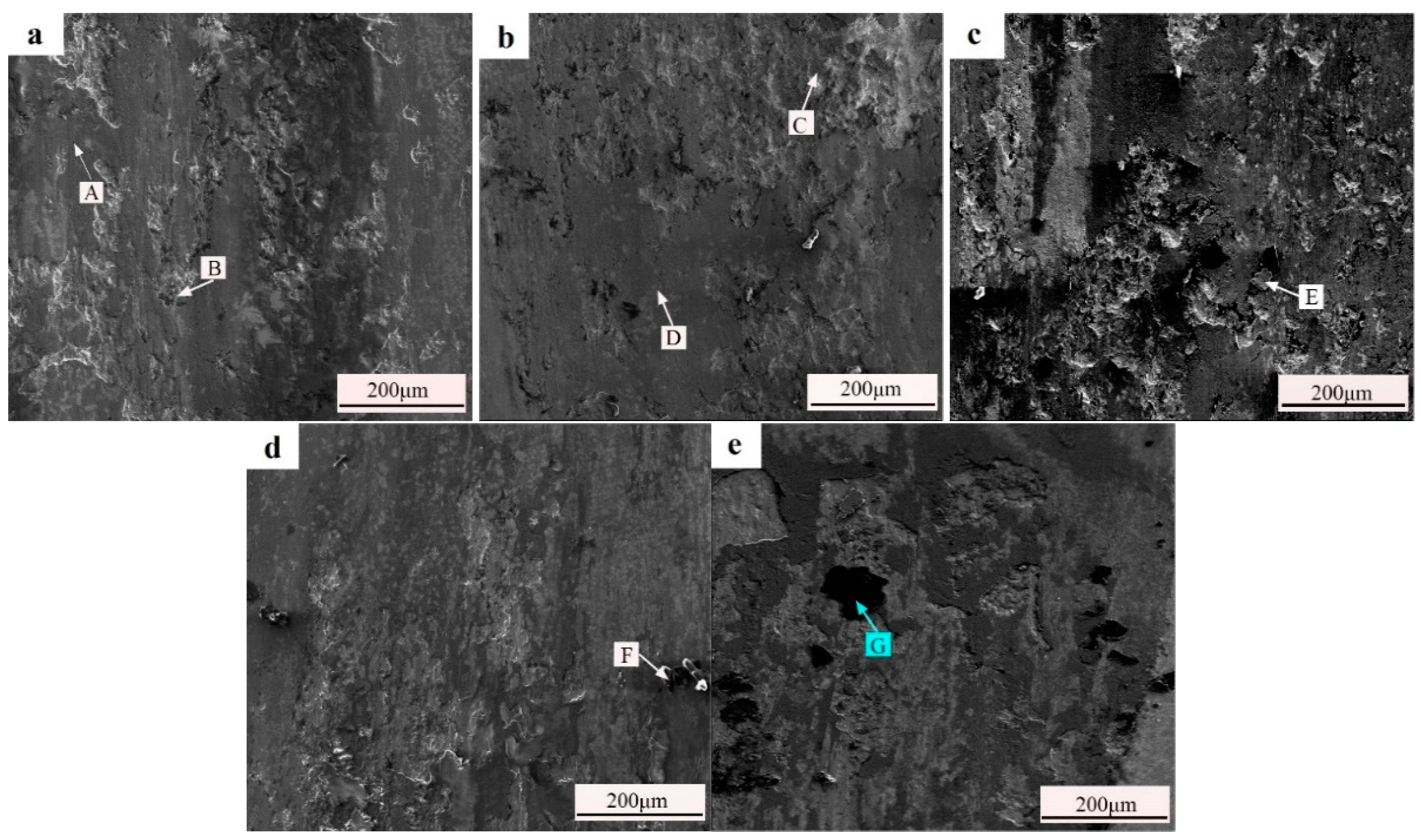
3.4. Microstructure of the Coating
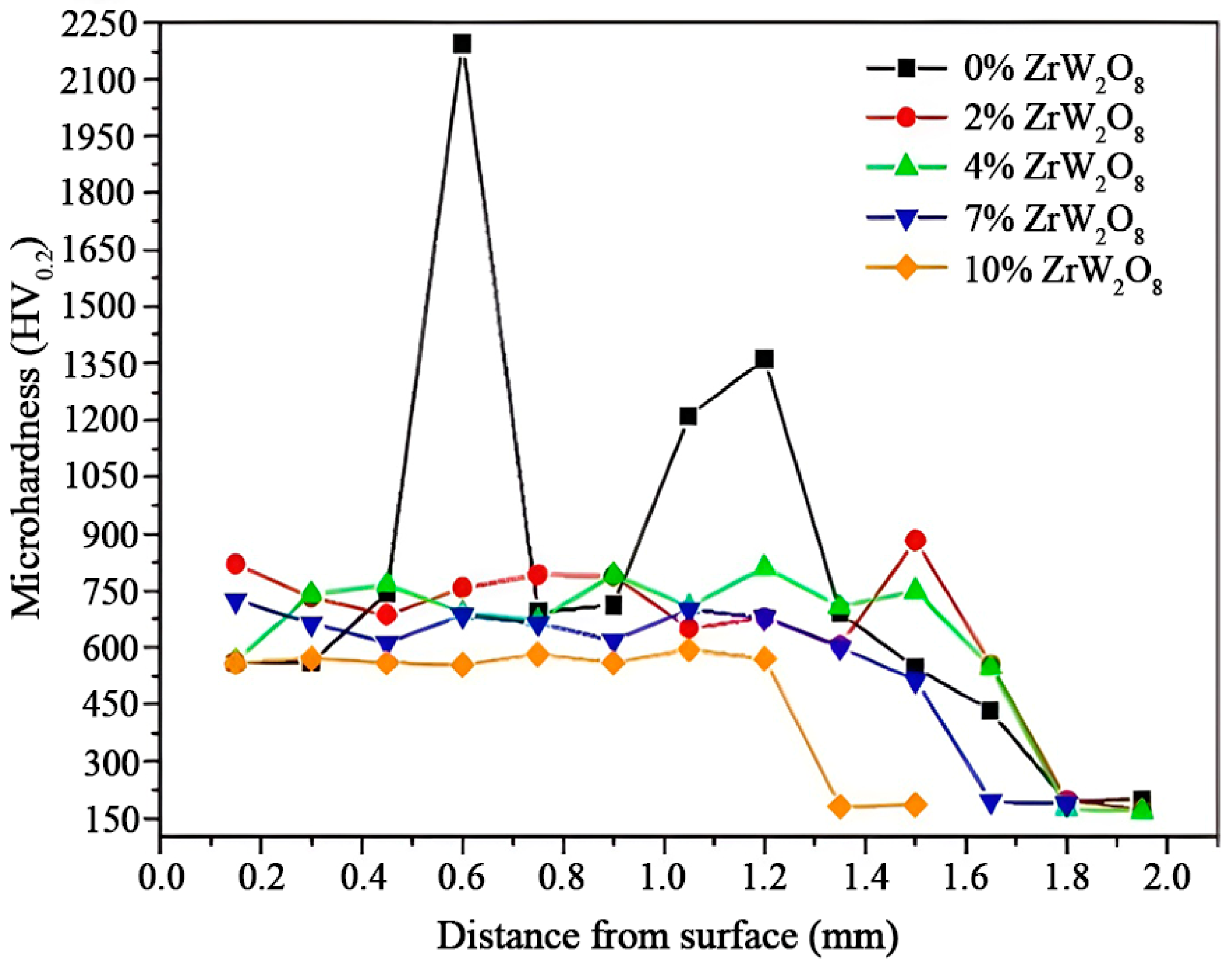
3.5. Microhardness of Coatings
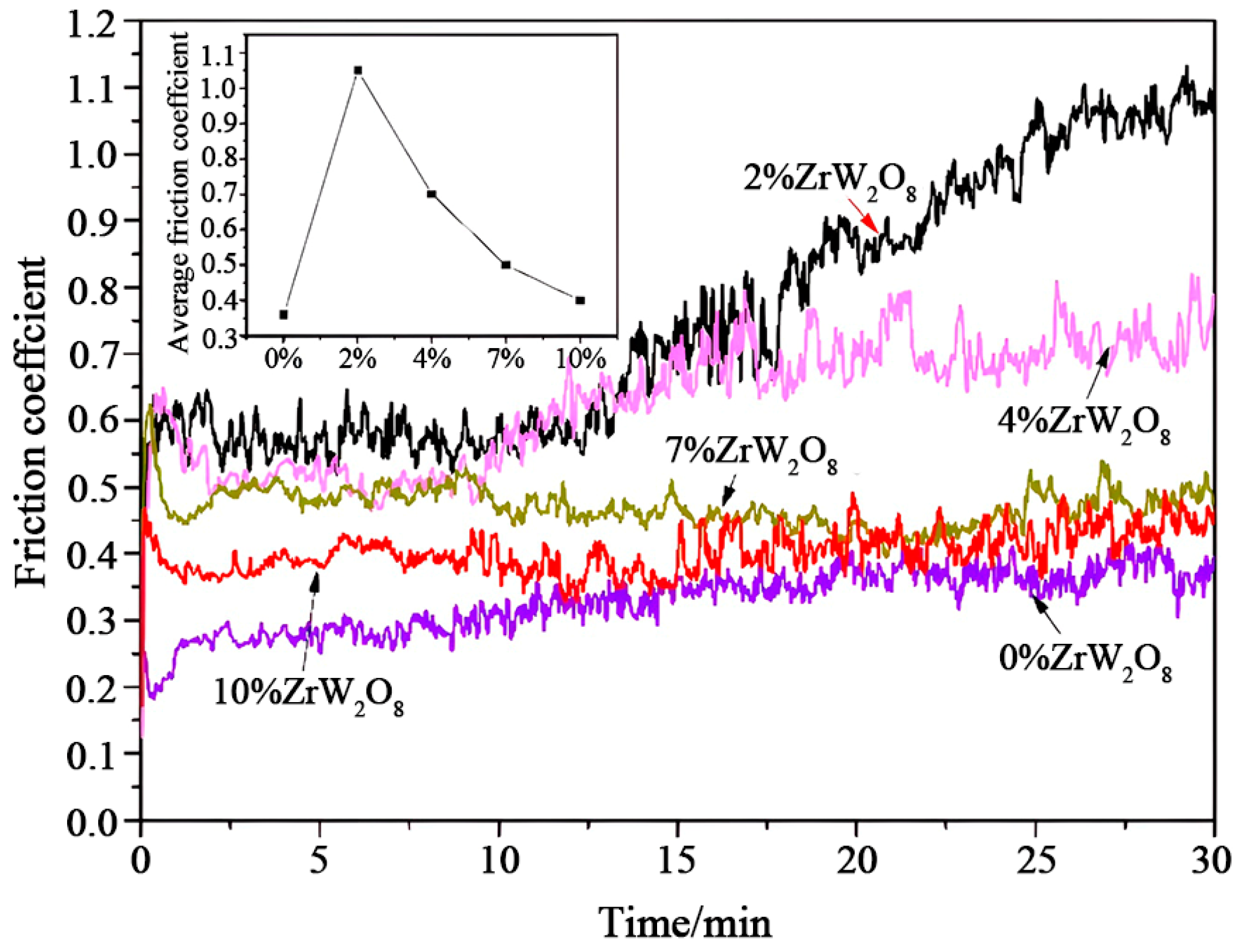
3.6. Wear Behaviors of Coatings
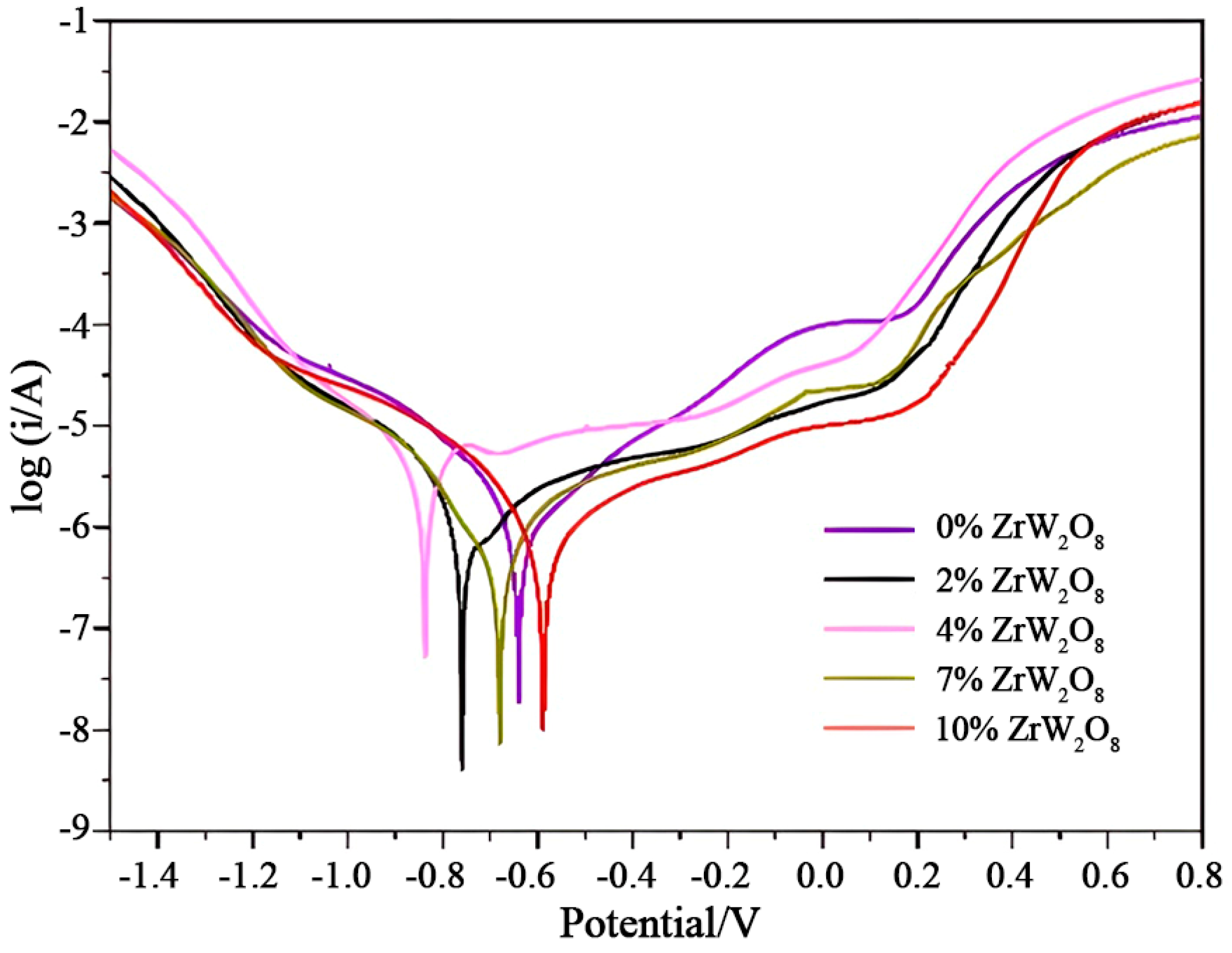
3.7. Electrochemical Measurements of Coatings
4. Conclusions
- (1)
- It is feasible to prepare crack-free laser cladding layers by adding the negative thermal expansion coefficient powder material ZrW2O8 to the Ni60/WC mixed powder.
- (2)
- When the mass fractions of ZrW2O8 in the cladding layer are 2% and 4%, the degree of negative thermal expansion effect accumulation is small, and the in situ generated ZrO2 is insufficient to fully suppress and close the cracks. However, adding ZrW2O8 with mass fractions of 7% and 10% can achieve crack-free cladding layers. Nonetheless, as the ZrW2O8 content continues to increase, it leads to a decrease in the wettability of the composite powder, resulting in more powder losses due to spattering, which is detrimental to the preparation of the cladding layer. Therefore, as a crack inhibitor, the amount of ZrW2O8 powder should be minimized as much as possible without causing cracks, with 10% being the optimal amount in this study. During the laser cladding process, ZrW2O8 decomposes in situ to form ZrO2. The phase transformation of ZrO2 particles exerts compressive stress on the main phase of the cladding layer. This compressive effect on cracks is the primary reason for the suppression and self-healing of cracks.
- (3)
- In the process of preparing laser cladding coatings, incorporating in situ phase-transformable negative thermal expansion materials to achieve crack self-healing is a promising research direction for addressing the issue of cracking in cladding coatings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Riquelme, A.; Rodrigo, P.; Escalera-Rodriguez, M.D.; Rams, J. Evaluation of the Wear Resistance and Corrosion Behavior of Laser Cladding Al/SiC Metal Matrix Composite Coatings on ZE41 Magnesium Alloy. Coatings 2021, 11, 639. [Google Scholar] [CrossRef]
- Zhang, Z.; Yu, T.; Kovacevic, R. Erosion and corrosion resistance of laser cladded AISI 420 stainless steel reinforced with VC. Appl. Surf. Sci. 2017, 410, 225–240. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, M.; Fan, Z.; Huang, S.; Huang, H. Laser deposition of wear-resistant titanium oxynitride/titanium composite coatings on Ti-6Al-4V alloy. Appl. Surf. Sci. 2020, 531, 147–212. [Google Scholar] [CrossRef]
- Zhang, P.; Pang, Y.; Yu, M. Effects of WC Particle Types on the Microstructures and Properties of WC-Reinforced Ni60 Composite Coatings Produced by Laser Cladding. Metals 2019, 9, 583. [Google Scholar] [CrossRef]
- Guo, C.; Chen, J.; Zhou, J.; Zhao, J.; Wang, L.; Yu, Y.; Zhou, H. Effects of WC–Ni content on microstructure and wear resistance of laser cladding Ni-based alloys coating. Surf. Coat. Technol. 2012, 206, 2064–2071. [Google Scholar] [CrossRef]
- Karmakar, D.P.; Muvvala, G.; Nath, A.K. High-temperature abrasive wear characteristics of H13 steel modified by laser remelting and cladded with Stellite 6 and Stellite 6/30% WC. Surf. Coat. Technol. 2021, 422, 127498. [Google Scholar] [CrossRef]
- Chen, C.; Feng, A.; Wei, Y.; Wang, Y.; Pan, X.; Song, X. Effects of WC particles on microstructure and wear behavior of laser cladding Ni60 composite coatings. Opt. Laser Technol. 2023, 163, 109425. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.; Xiao, J.; Hou, Q. Effect of WC mass fraction on the microstructure and friction properties of WC/Ni60 laser cladding layer of brake discs. Ceram. Int. 2021, 47, 28754–28763. [Google Scholar] [CrossRef]
- Lee, C.; Park, H.; Yoo, J.; Lee, C.; Woo, W.; Park, S. Residual stress and crack initiation in laser clad composite layer with Co-based alloy and WC+NiCr. Appl. Surf. Sci. 2015, 345, 286–294. [Google Scholar] [CrossRef]
- Shen, X.; Peng, H.; Xue, Y.; Wang, B.; Su, G.; Zhu, J.; Li, A. Microstructure and Properties of WC/Ni-Based Laser-Clad Coatings with Different WC Content Values. Materials 2022, 15, 6309. [Google Scholar] [CrossRef]
- Alidokht, S.A.; Yue, S.; Chromik, R.R. Effect of WC morphology on dry sliding wear behavior of cold-sprayed Ni-WC composite coatings. Surf. Coat. Technol. 2019, 357, 849–863. [Google Scholar] [CrossRef]
- Fernández, M.; García, A.; Cuetos, J.; González, R.; Noriega, A.; Cadenas, M. Effect of actual WC content on the reciprocating wear of a laser cladding NiCrBSi alloy reinforced with WC. Wear 2015, 324–325, 80–89. [Google Scholar] [CrossRef]
- Wang, X.; Rong, J.; Yao, Y.; Zhang, Y.; Zhong, Y.; Feng, J.; Yu, X.; Zhan, Z. La doping inhibits stress production at the grain boundaries in Ni–WC coating. J. Alloys Compd. 2018, 753, 688–694. [Google Scholar] [CrossRef]
- Qunshuang, M.; Yajiang, L.; Juan, W.; Kun, L. Microstructure evolution and growth control of ceramic particles in wide-band laser clad Ni60/WC composite coatings. Mater. Des. 2016, 92, 897–905. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, X.; Yan, X.; Yang, J.; Fu, T.; Qiu, J. Synthesis of ZrO2/ZrW2O8 composites with low thermal expansion. Compos. Sci. Technol. 2007, 67, 1167–1171. [Google Scholar] [CrossRef]
- Peng, Z.; Sun, Y.; Peng, L. Hydrothermal synthesis of ZrW2O8 nanorods and its application in ZrW2O8/Cu composites with controllable thermal expansion coefficients. Mater. Des. 2014, 54, 989–994. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Q.; Liu, S.; Zhou, T.; Jokisaari, J.R.; Wu, G. Microstructure and thermal expansion analysis of porous ZrW2O8/Al composite. J. Alloys Compd. 2016, 670, 182–187. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.; Zhang, Y.; Zhang, J.; Lu, F.; Wang, X. Synthesis and thermal expansion of 4J36/ZrW2O8 composites. Rare Met. 2010, 29, 371–375. [Google Scholar] [CrossRef]
- Islam, M.S.; Chang, W.; Sha, Z.; Wang, J.; Wu, S.; Rose, L.F.; Kinloch, A.J.; Wang, C.H. Mitigating cryogenic microcracking in carbon-fibre reinforced polymer composites using negative thermal-expansion nanoparticles functionalized by a polydopamine coating. Compos. Part B Eng. 2023, 257, 110676. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, M.; Chen, Z.; Ma, N.; Wang, H. The effect of phase transformation on the thermal expansion property in Al/ZrW2O8 composites. J. Mater. Sci. 2013, 48, 2928–2933. [Google Scholar] [CrossRef]
- Qi, K.; Yang, Y.; Hu, G.; Lu, X.; Li, J. Thermal expansion control of composite coatings on 42CrMo by laser cladding. Surf. Coat. Technol. 2020, 397, 125983. [Google Scholar] [CrossRef]
- Gustafson, P. A thermodynamic evaluation of the C–Fe–W system. Metall. Mater. Trans. A 1987, 18, 175–188. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Yang, Q.; Wei, H.; Fu, X.; Li, W. Effects of WC on microstructure and corrosion resistance of directional structure Ni60 coatings. Surf. Coat. Technol. 2020, 385, 125359. [Google Scholar] [CrossRef]
- Mary, T.A.; Evans, J.S.O.; Vogt, T.; Sleight, A.W. Negative Thermal Expansion from 0.3 to 1050 Kelvin in ZrW2O8. Science 1996, 272, 90–92. [Google Scholar] [CrossRef]
- Zhou, C.; Zhang, Q.; Zhang, M.; Wu, G. In-situ Raman spectroscopy study of thermal mismatch stress and negative thermal expansion behaviours of ZrW2O8 in ZrW2O8/Al composite. J. Alloys Compd. 2017, 718, 356–360. [Google Scholar] [CrossRef]
- Wang, H.; Lu, H.; Song, X.; Yan, X.; Liu, X.; Nie, Z. Corrosion resistance enhancement of WC cermet coating by carbides alloying. Corros. Sci. 2019, 147, 372–383. [Google Scholar] [CrossRef]
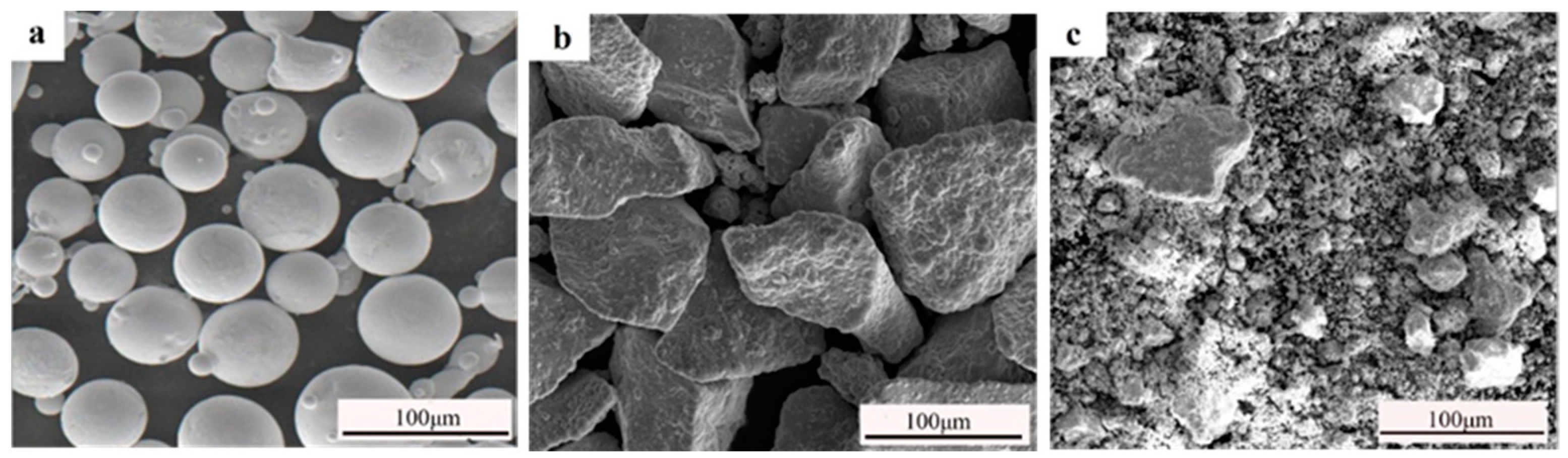

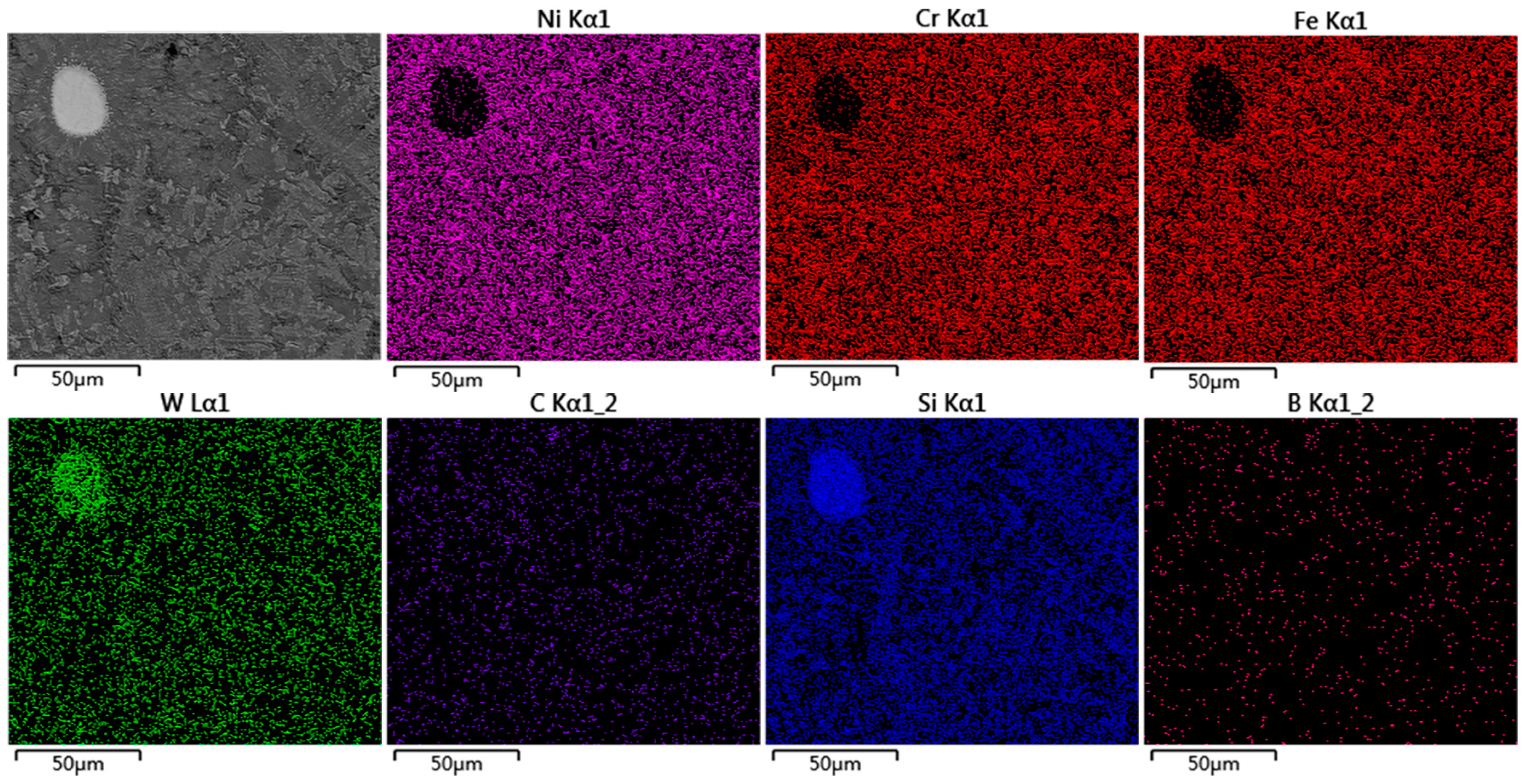

| S | P | C | Si | Mn | Ni | Cr | Fe |
|---|---|---|---|---|---|---|---|
| 0.03 | 0.045 | 0.07–0.08 | 0.075–1 | 2 | 8–11 | 18–20 | Bal |
| C | W | B | Si | Fe | Cr | Ni |
|---|---|---|---|---|---|---|
| 0.80 | 3.00 | 3.50 | 4.00 | 15.00 | 15.00 | Bal |
| Sample | Composition (wt%) | Parameters for the Laser Cladding Process | |||||
|---|---|---|---|---|---|---|---|
| Ni60 | WC | ZrW2O8 | Laser Power (w) | Scanning Speed (mm/s) | Powder Feeding Voltage (v) | ΔZ (mm) | |
| 1 | 70 | 30 | 0 | 1500 | 120 | 7 | 19 |
| 2 | 68.6 | 29.4 | 2 | ||||
| 3 | 66.2 | 29.8 | 4 | ||||
| 4 | 63.1 | 29.9 | 7 | ||||
| 5 | 60.7 | 29.3 | 10 | ||||
| Point | W | Fe | Cr | Ni | B | Si | C | Zr | O |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 91.1 | - | - | - | - | - | 8.9 | - | - |
| 2 | 56.4 | 3.9 | 13.1 | 15.4 | 2.1 | 1.8 | 7.3 | - | - |
| 3 | 1.6 | 16.4 | 5.1 | 60.4 | 2.0 | 3.5 | 11.1 | - | - |
| 4 | 57.8 | 5.6 | 18.9 | 4.6 | 7.6 | - | 5.5 | - | - |
| 5 | 2.9 | 19.7 | 7.3 | 62.0 | 0.5 | 2.3 | 4.4 | - | 0.7 |
| 6 | 56.0 | 3.4 | 11.4 | 19.9 | - | 2.6 | 5.7 | 0.6 | 0.4 |
| 7 | 31.6 | 14.4 | 14.8 | 24.4 | 2.8 | 2.0 | 7.9 | 0.8 | 1.3 |
| 8 | 63.0 | 4.9 | 18.1 | 6.6 | - | - | 6.8 | 0.6 | - |
| 9 | 2.8 | 27.7 | 5.8 | 56.1 | 1.8 | 0.3 | 4.4 | - | 1.1 |
| 10 | 7.5 | 27.8 | 9.2 | 48.9 | - | 1.9 | 3.6 | 0.2 | 0.9 |
| Point | W | Fe | Cr | Ni | B | Si | C | Zr | O |
|---|---|---|---|---|---|---|---|---|---|
| A | 14.3 | 14.2 | 3.0 | 34.6 | - | 2.7 | 8.9 | - | 20.1 |
| B | 8.0 | 17.7 | 12.9 | 38.6 | - | 1.7 | 17.4 | - | 3.9 |
| C | 7.7 | 7.6 | 5.2 | 74.5 | - | - | 1.8 | - | 3.2 |
| D | 25.1 | 7.4 | 7.2 | 32.3 | - | - | 9.9 | - | 18.1 |
| E | - | 13.9 | 6.0 | 50.2 | 20.3 | 2.1 | 5.3 | - | 2.2 |
| F | - | 1.1 | 0.7 | 1.6 | 25.5 | 0.5 | 63.7 | - | 6.8 |
| G | - | 5.0 | 3.0 | 7.6 | 22.4 | 2.4 | 56.6 | 0.2 | 2.8 |
| Sample | Ecorr/V | Icorr × 10−6/(A·cm−2) | Rp/(Ω·cm−2) |
|---|---|---|---|
| 0% ZrW2O8 | −0.641 | 2.22 | 30,604.4 |
| 2% ZrW2O8 | −0.759 | 1.24 | 28,923.6 |
| 4% ZrW2O8 | −0.893 | 9.72 | 9991.8 |
| 7% ZrW2O8 | −0.683 | 1.76 | 62,437.6 |
| 10% ZrW2O8 | −0.588 | 1.72 | 52,495.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Liu, C.; Pang, Y. Effects of ZrW2O8 Content on the Microstructures and Properties of Composite Coatings Produced by Laser Cladding. Coatings 2024, 14, 649. https://doi.org/10.3390/coatings14050649
Zhang P, Liu C, Pang Y. Effects of ZrW2O8 Content on the Microstructures and Properties of Composite Coatings Produced by Laser Cladding. Coatings. 2024; 14(5):649. https://doi.org/10.3390/coatings14050649
Chicago/Turabian StyleZhang, Pengxian, Chang Liu, and Yibin Pang. 2024. "Effects of ZrW2O8 Content on the Microstructures and Properties of Composite Coatings Produced by Laser Cladding" Coatings 14, no. 5: 649. https://doi.org/10.3390/coatings14050649
APA StyleZhang, P., Liu, C., & Pang, Y. (2024). Effects of ZrW2O8 Content on the Microstructures and Properties of Composite Coatings Produced by Laser Cladding. Coatings, 14(5), 649. https://doi.org/10.3390/coatings14050649






