Comparison of the Erosive Wear Resistance of Ductile Cast Iron Following Laser Surface Melting and Alloying
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
- The laser surface melting processes of the EN-GJS-350-22 ductile cast iron in argon and nitrogen shields caused modification of the microstructure of the surface layers that consist of primary austenite dendrites, partly after martensitic transformation, and eutectic austenite and ledeburite in the interdendritic spaces. The use of the nitrogen shield did not affect the microstructure or nitrogen’s presence in the surface layer’s chemical composition.
- The laser surface alloying process of the EN-GJS-350-22 ductile cast iron with titanium allowed the formation of homogeneous in situ metal–matrix composite surface layers. Depending on the shielding gas (argon or nitrogen), cubic or dendritic TiC/TiCN particles precipitated from the liquid metal. The matrix microstructure consisted of primary austenite dendrites, partly after martensitic transformation, and ledeburite in the interdendritic spaces.
- By the selection of the LSA process parameters it is possible to shape the structure of the surface layers, in particular the titanium concentration, precipitate’s morphology, fraction, and chemical composition. The parameters selected for the research allowed surface layers reinforced with 5.5–20.2 vol.% of TiC and 4.8–10.1 vol.% of TiCN to be produced.
- The modification of the microstructure by the laser surface melting of ductile cast iron resulted in the average Vickers hardness increasing by a maximum of 370% and 362% for the argon and nitrogen shields, respectively. The laser-alloyed surface layers showed average Vickers hardness increases of a maximum of 297% and 295% for the argon and nitrogen shields, respectively.
- The laser surface melting and alloying processes caused an erosion resistance increase in the ductile cast iron. The erosion values of the LSM and LSA surface layers for a 30° impingement angle decreased by a maximum of 30% and 48%, respectively. In the case of the 90° impingement angle, the LSM layers showed similar erosion values, and for LSA layers, the erosion values decreased by up to 26% in comparison to the substrate material. The composite microstructure of the LSA surface layers caused a higher increase in the erosion resistance in comparison to LSM layers in addition to the lower hardness.
- The erosion mechanism of the substrate material and laser-melted surface layers is ductile. For the laser-alloyed surface layers, the erosion mechanism is more complex, being ductile for the matrix material and brittle for the TiC(N) precipitate.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elliot, R. Cast Iron Technology, 1st ed.; Butterworth & Co.: London, UK, 1988; pp. 13–20. [Google Scholar]
- Czupryński, A.; Żuk, M. Matrix Composite Coatings Deposited on AISI 4715 Steel by Powder Plasma-Transferred Arc Welding. Part 3. Comparison of the Brittle Fracture Resistance of Wear-Resistant Composite Layers Surfaced Using the PPTAW Method. Materials 2021, 14, 6066. [Google Scholar] [CrossRef] [PubMed]
- Poloczek, T.; Lont, A.; Górka, J. The Structure and Properties of Laser-Cladded Inconel 625/TiC Composite Coatings. Materials 2023, 16, 1265. [Google Scholar] [CrossRef] [PubMed]
- Krella, A. Resistance of PVD Coatings to Erosive and Wear Processes: A Review. Coatings 2020, 10, 921. [Google Scholar] [CrossRef]
- Jonda, E.; Szala, M.; Sroka, M.; Łatka, L.; Walczak, M. Investigations of cavitation erosion and wear resistance of cermet coatings manufactured by HVOF spraying. Appl. Surf. Sci. 2023, 608, 155071. [Google Scholar] [CrossRef]
- Cui, C.; Wu, M.; Miao, X.; Gong, Y.; Zhao, Z. The effect of laser energy density on the geometric characteristics, microstructure and corrosion resistance of Co-based coatings by laser cladding. JMR&T 2021, 15, 2405–2418. [Google Scholar]
- Anas, A.S.; Avanish, K.D. Recent trends in laser cladding and surface alloying. Opt. Laser Technol. 2021, 134, 106619. [Google Scholar]
- Malek Ghaini, F.; Ameri, M.H.; Torkamany, M.J. Surface transformation hardening of ductile cast iron by a 600w fiber laser. Optik 2020, 203, 163758. [Google Scholar] [CrossRef]
- Ion, J.C. Laser Transformation Hardening. Mater. Surf. Eng. 2002, 18, 14–31. [Google Scholar] [CrossRef]
- Kotarska, A.; Janicki, D.; Górka, J.; Poloczek, T. Solid particle erosion of laser surface melted ductile cast iron. Arch. Foundry Eng. 2020, 20, 105–111. [Google Scholar] [CrossRef]
- Murakami, R.; Narita, I.; Miyahara, H. Surface microstructure and properties of nodular cast iron rapidly solidified by laser surface melting. Mater. Trans. 2018, 59, 1465–1470. [Google Scholar] [CrossRef]
- Jeyaprakash, N.; Yang, C.H.; Duraiselvam, M.; Prabu, G. Microstructure and tribological evolution during laser alloying WC-12%Co and Cr3C2-25%NiCr powders on nodular iron surface. Results Phys. 2019, 12, 1610–1620. [Google Scholar] [CrossRef]
- Yan, H.; Wang, A.; Xiong, Z.; Xu, K.; Huang, Z. Microstructure and wear resistance of composite layers on a ductile iron with multicarbide by laser surface alloying. Appl. Surf. Sci. 2010, 256, 7001–7009. [Google Scholar] [CrossRef]
- Paczkowska, M. The Comparison of the Effects of Nodular Cast Iron Laser Alloying with Selected Substances. Materials 2022, 15, 7561. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Cai, K.; Li, X.; Pang, M. Research on Laser Cladding Co-Based Alloy on the Surface of Vermicular Graphite Cast Iron. Coatings 2021, 11, 1241. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; Yan, S.; Liu, X.; He, P.; Xu, B. Microstructure evolution during laser cladding Fe-Cr alloy coatings on ductile cast iron. Opt. Laser Technol. 2018, 108, 255–264. [Google Scholar] [CrossRef]
- Arias-Gonzalez, F.; del Val, J.; Comesana, R.; Penide, J.; Lusquinos, F.; Quintero, F.; Riveiro, A.; Boutinguiza, M.; Pou, J. Fiber laser cladding of nickel-based alloy on cast iron. Appl. Surf. Sci. 2016, 374, 197–205. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; He, P.; Yan, S.; Li, E.; Liu, X.; Xu, B. Microstructure characteristics and mechanical properties of new-type FeNiCr laser cladding alloy coating on nodular cast iron. J. Mater. Process. Technol. 2019, 269, 163–171. [Google Scholar] [CrossRef]
- Grum, J.; Sturm, R. Microstructure analysis of nodular iron 400-12 after laser surface melt hardening. Mater. Charact. 1996, 37, 81–88. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, T.; Wang, Q.; Zhou, H. Effects of Laser Melting Distribution on Wear Resistance and Fatigue Resistance of Gray Cast Iron. Metals 2020, 10, 1257. [Google Scholar] [CrossRef]
- Pagano, N.; Angelini, V.; Ceschini, L.; Campana, G. Laser remelting for enhancing tribological performances of a ductile iron. Proc. CIRP 2016, 41, 987–991. [Google Scholar] [CrossRef]
- Alabeedi, K.F.; Abboud, J.H.; Benyounis, K.Y. Microstructure and erosion resistance enhancement of nodular cast iron by laser melting. Wear 2009, 266, 925–933. [Google Scholar] [CrossRef]
- Paczkowska, M.; Makuch, N.; Kulka, M. The influence of various cooling rates during laser alloying on nodular iron surface layer. Opt. Laser Technol. 2018, 102, 60–67. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, C.; Dong, J.; Zhou, T.; Tong, X.; Zhou, H. The comparative study of W/Cr addition in Fe-base by laser surface alloying on fatigue wear resistance of GCI. Surf. Coat. Technol. 2016, 286, 25–35. [Google Scholar] [CrossRef]
- Yilbas, B.S.; Akhtar, S.S.; Karatas, C.; Boran, K. Laser treatment of dual matrix cast iron with presence of WC particles at the surface: Influence of self-annealing on stress fields. Opt. Laser Technol. 2016, 76, 6–18. [Google Scholar] [CrossRef]
- Janicki, D. The friction and wear behaviour of in-situ titanium carbide reinforced composite layers manufactured on ductile cast iron by laser surface alloying. Surf. Coat. Technol. 2021, 406, 126634. [Google Scholar] [CrossRef]
- Kotarska, A. The Laser Alloying Process of Ductile Cast Iron Surface with Titanium. Metals 2021, 11, 282. [Google Scholar] [CrossRef]
- Janicki, D. Shaping the Structure and Properties of Surface Layers of Ductile Cast Iron by Laser Alloying, 1st ed.; Wydawnictwo Politechniki Śląskiej: Gliwice, Poland, 2018; p. 29. [Google Scholar]
- Lont, A.; Górka, J.; Janicki, D.; Matus, K. The Laser Alloying Process of Ductile Cast Iron Surface with Titanium Powder in Nitrogen Atmosphere. Coatings 2022, 12, 227. [Google Scholar] [CrossRef]
- ASTM G76-04; Standard Test Method for Conducting Erosion Tests by Solid Particle Impingement Using Gas Jets. ASTM International: West Conshohocken, PA, USA, 2004.
- Kundakcıoğlu, E.; Lazoglu, I.; Poyraz, Ö.; Yasa, E.; Cizicioğlu, N. Thermal and molten pool model on selective laser melting process of Inconel 625. Int. J. Adv. Manuf. Technol. 2018, 95, 3977–3984. [Google Scholar] [CrossRef]
- Frisk, K. A revised thermodynamic description of the Ti-C system. Calphad 2003, 27, 367–373. [Google Scholar] [CrossRef]
- Zhu, H.; Dong, K.; Wang, H.; Huang, J.; Li, J.; Xie, Z. Reaction mechanisms of the TiC/Fe composite fabricated by exothermic dispersion from Fe-Ti-C element system. Powder Technol. 2013, 246, 456–461. [Google Scholar] [CrossRef]
- Jonsson, S. Calculation of the Ti-C-N system. Z. Metallkde 1996, 87, 713–720. [Google Scholar] [CrossRef]
- Dumitrescu, L.F.S.; Hillert, M.; Sundman, B. A reassessment of Ti-C-N based on a critical review of available assessments of Ti-N and Ti-C. Z. Metallkde 1999, 90, 534–541. [Google Scholar] [CrossRef]
- Gorbachev, I.I.; Popov, V.V. Analysis of the solubility of carbides, nitrides and carbonitrides in steels using methods of computer thermodynamics: III. Solubility of carbides, nitrides and carbonitrides in the Fe-Ti-C, Fe-Ti-N and Fe-Ti-C-N systems. Phys. Met. Metallogr. 2009, 108, 484–495. [Google Scholar] [CrossRef]
- Stachowiak, G.W.; Batchelor, A.W. Engineering Tribology, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2014; pp. 551–556. [Google Scholar]



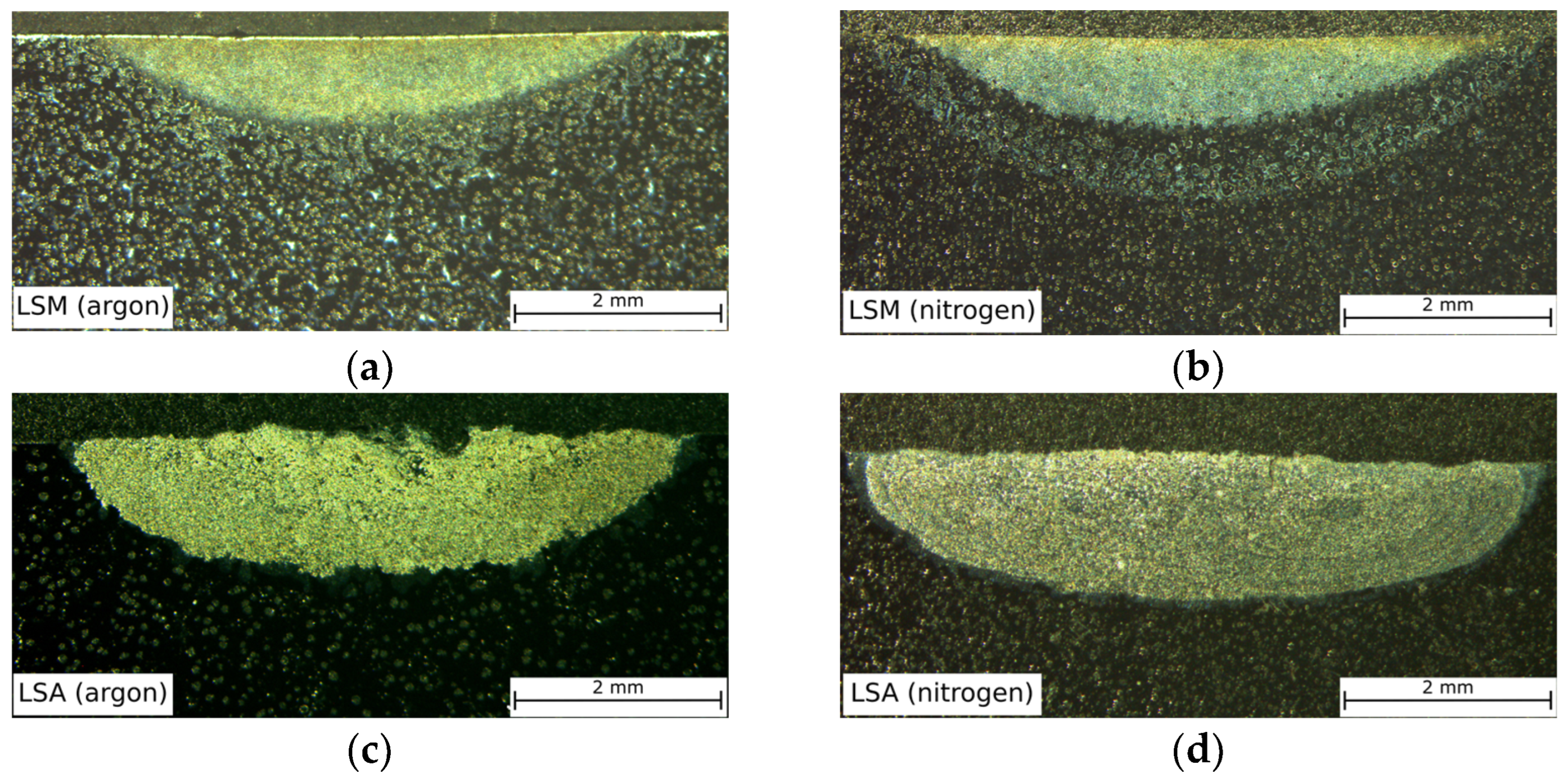

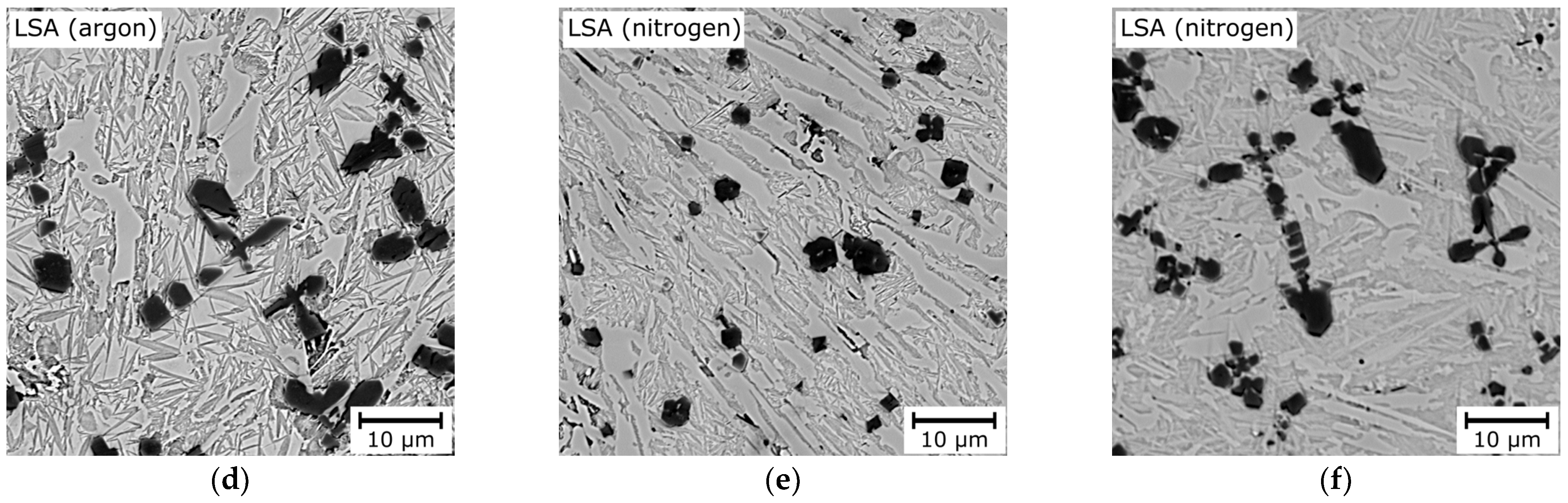



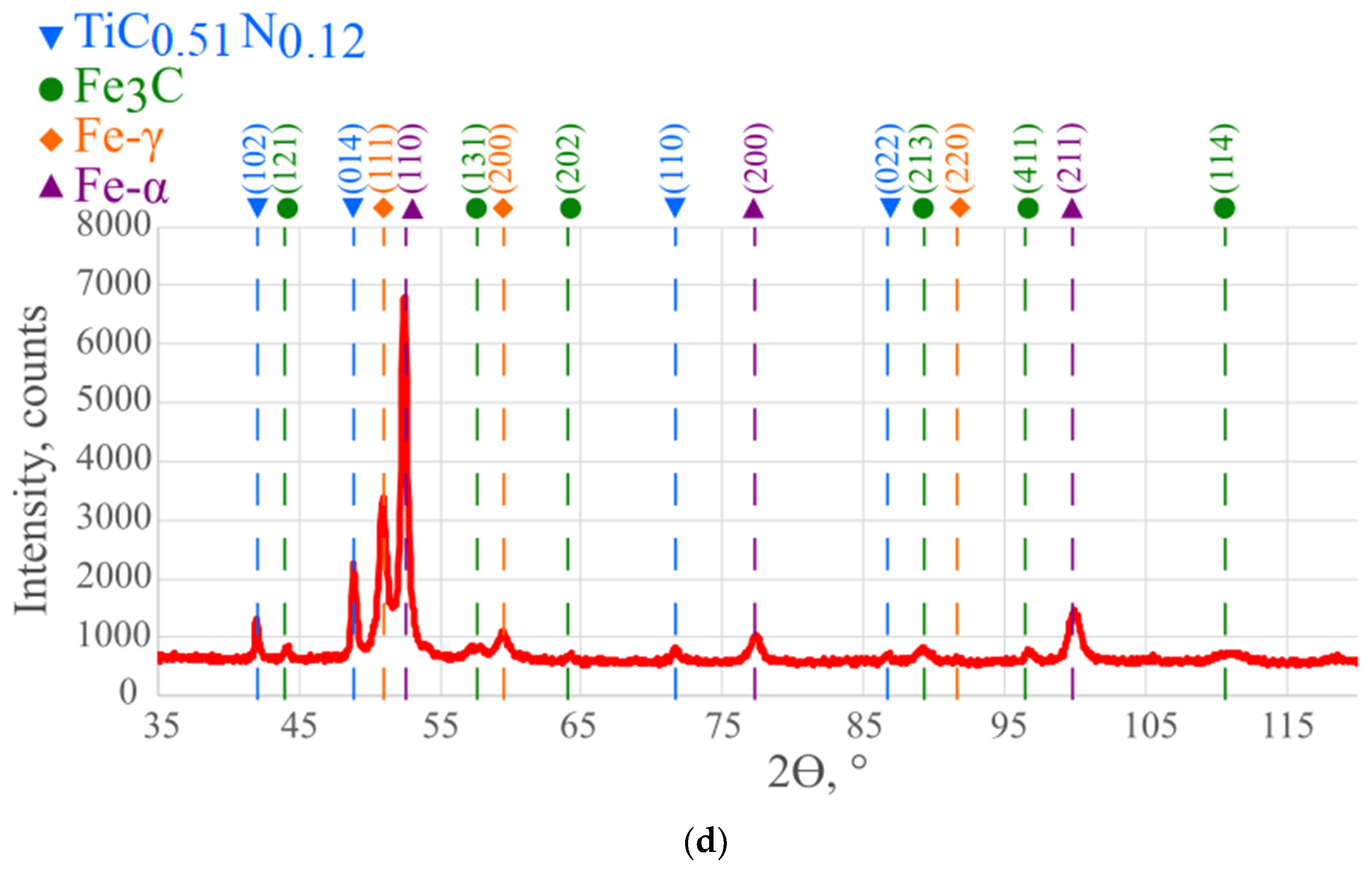


| Chemical Composition, wt.% | ||||||||
|---|---|---|---|---|---|---|---|---|
| C | Si | Mn | P | S | Cu | Ti | Mg | Cr |
| 3.66 | 2.71 | 0.527 | 0.042 | 0.001 | 0.068 | 0.032 | 0.012 | 0.124 |
| Wavelength of the Laser Radiation, nm | 808–940 ± 5 |
| Range of Laser Power, W | 100–2000 |
| Focal Length, mm | 82 |
| Laser Beam Spot Size, mm | 1.5 × 6.6 |
| Range of Laser Power Intensity, W/mm2 | 10.1–202.0 |
| Designation | Process | Laser Beam Power, W | Traverse Speed, m/min | Shielding Gas | Powder-Transporting Gas | Powder Feed Rate, mg/mm |
|---|---|---|---|---|---|---|
| A1 | LSM | 2000 | 0.2 | argon | - | - |
| A2 | LSM | 2000 | 0.4 | argon | - | - |
| A3 | LSM | 1500 | 0.075 | argon | - | - |
| N1 | LSM | 2000 | 0.2 | nitrogen | - | - |
| N2 | LSM | 2000 | 0.4 | nitrogen | - | - |
| N3 | LSM | 1500 | 0.075 | nitrogen | - | - |
| TA1 | LSA | 2000 | 0.075 | argon | argon | 4 |
| TA2 | LSA | 2000 | 0.075 | argon | argon | 8 |
| TA3 | LSA | 2000 | 0.075 | argon | argon | 12 |
| TA4 | LSA | 2000 | 0.075 | argon | argon | 16 |
| TN1 | LSA | 1750 | 0.075 | nitrogen | nitrogen | 5.33 |
| TN2 | LSA | 2000 | 0.075 | nitrogen | nitrogen | 4 |
| TN3 | LSA | 2000 | 0.075 | nitrogen | nitrogen | 5.33 |
| Designation (According to Table 3) | Average Titanium Content, wt.% | Average TiC(N) Fraction, vol.% | Average Fe3C Fraction, vol.% | Average Retained Austenite Fraction, wt.% | Average Fe-α (Martensite) Fraction, wt.% |
|---|---|---|---|---|---|
| A1 | - | - | 55.7 ± 2.86 | 10.9 ± 1.23 | 33.6 ± 1.71 |
| A2 | - | - | 45.0 ± 4.24 | 30.2 ± 1.41 | 19.7 ± 1.83 |
| A3 | - | - | 53.8 ± 1.66 | 6.9 ± 0.91 | 39.3 ± 1.75 |
| N1 | - | - | 48.9 ± 1.17 | 10.4 ± 0.97 | 40.9 ± 1.62 |
| N2 | - | - | 42.1 ± 1.88 | 32.4 ± 1.34 | 26.0 ± 0.94 |
| N3 | - | - | 50.9 ± 1.19 | 8.2 ± 1.15 | 41.1 ±1.14 |
| TA1 | 3.2 ± 0.33 | 5.5 ± 1.13 | 32.7 ± 4.33 | 11.3 ± 0.89 | 50.9 ± 2.05 |
| TA2 | 5.5 ± 1.7 | 9.6 ± 2.29 | 26.6 ± 2.08 | 11.6 ± 1.17 | 53.1 ± 1.62 |
| TA3 | 9.1 ± 2.78 | 14.1 ± 3.91 | 13.9 ± 1.52 | 10.1 ± 1.21 | 62.0 ± 1.15 |
| TA4 | 12.9 ± 1.71 | 20.1 ± 2.29 | 4.7 ± 2.34 | 9.5 ± 1.18 | 66.1 ± 1.39 |
| TN1 | 3.6 ± 0.88 | 6.5 ± 1.71 | 33.4 ± 2.25 | 11.8 ± 1.31 | 48.6 ± 1.81 |
| TN2 | 3.2 ± 1.26 | 4.8 ± 1.57 | 34.4 ± 5.76 | 12.4 ± 1.05 | 48.7 ± 1.37 |
| TN3 | 5.8 ± 1.35 | 10.1 ± 2.23 | 27.1 ± 3.93 | 13.3 ± 0.88 | 49.5 ± 1.42 |
| Designation (According to Table 3) | Average Vickers Hardness, HV0.2 | Average Erosion Value, mm3/g | Average Steady-State Erosion Rate, mg/min | ||
|---|---|---|---|---|---|
| 30° | 90° | 30° | 90° | ||
| A1 | 875 ± 19.6 | 0.047 ± 0.004 | 0.038 ± 0.006 | 0.69 ± 0.05 | 0.57 ± 0.1 |
| A2 | 843 ± 17.2 | 0.047 ± 0.002 | 0.043 ± 0.007 | 0.7 ± 0.03 | 0.64 ± 0.1 |
| A3 | 792 ± 21.7 | 0.050 ± 0.003 | 0.039 ± 0.002 | 0.73 ± 0.05 | 0.58 ± 0.04 |
| N1 | 854 ± 19.0 | 0.046 ± 0.008 | 0.041 ± 0.002 | 0.69 ± 0.11 | 0.61 ± 0.03 |
| N2 | 833 ± 57.3 | 0.043 ± 0.009 | 0.048 ± 0.004 | 0.63 ± 0.14 | 0.71 ± 0.06 |
| N3 | 832 ± 33.3 | 0.048 ± 0.005 | 0.039 ± 0.004 | 0.72 ± 0.07 | 0.57 ± 0.06 |
| TA1 | 695 ± 39.5 | 0.036 ± 0.001 | 0.025 ± 0.003 | 0.52 ± 0.02 | 0.37 ± 0.04 |
| TA2 | 702 ± 33.0 | 0.032 ± 0.001 | 0.025 ± 0.004 | 0.45 ± 0.02 | 0.36 ± 0.04 |
| TA3 | 694 ± 62.7 | 0.039 ± 0.001 | 0.028 ± 0.002 | 0.56 ± 0.02 | 0.39 ± 0.02 |
| TA4 | 701 ± 43.7 | 0.039 ± 0.001 | 0.031 ± 0.005 | 0.54 ± 0.01 | 0.43 ± 0.06 |
| TN1 | 663 ± 54.1 | 0.041 ± 0.003 | 0.027 ± 0.002 | 0.6 ± 0.05 | 0.39 ± 0.03 |
| TN2 | 696 ± 30.5 | 0.037 ± 0.004 | 0.029 ± 0.004 | 0.55 ± 0.04 | 0.43 ± 0.04 |
| TN3 | 627 ± 40.8 | 0.041 ± 0.002 | 0.026 ± 0.003 | 0.6 ± 0.03 | 0.38 ± 0.04 |
| Substrate | 236 ± 36.4 | 0.0617 ± 0.0004 | 0.034 ± 0.0032 | 0.88 ± 0.01 | 0.48 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górka, J.; Lont, A.; Janicki, D.; Poloczek, T.; Rzeźnikiewicz, A. Comparison of the Erosive Wear Resistance of Ductile Cast Iron Following Laser Surface Melting and Alloying. Coatings 2024, 14, 646. https://doi.org/10.3390/coatings14050646
Górka J, Lont A, Janicki D, Poloczek T, Rzeźnikiewicz A. Comparison of the Erosive Wear Resistance of Ductile Cast Iron Following Laser Surface Melting and Alloying. Coatings. 2024; 14(5):646. https://doi.org/10.3390/coatings14050646
Chicago/Turabian StyleGórka, Jacek, Aleksandra Lont, Damian Janicki, Tomasz Poloczek, and Agnieszka Rzeźnikiewicz. 2024. "Comparison of the Erosive Wear Resistance of Ductile Cast Iron Following Laser Surface Melting and Alloying" Coatings 14, no. 5: 646. https://doi.org/10.3390/coatings14050646
APA StyleGórka, J., Lont, A., Janicki, D., Poloczek, T., & Rzeźnikiewicz, A. (2024). Comparison of the Erosive Wear Resistance of Ductile Cast Iron Following Laser Surface Melting and Alloying. Coatings, 14(5), 646. https://doi.org/10.3390/coatings14050646









