Effect of Electrolytic Plasma Polishing on Surface Properties of Titanium Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
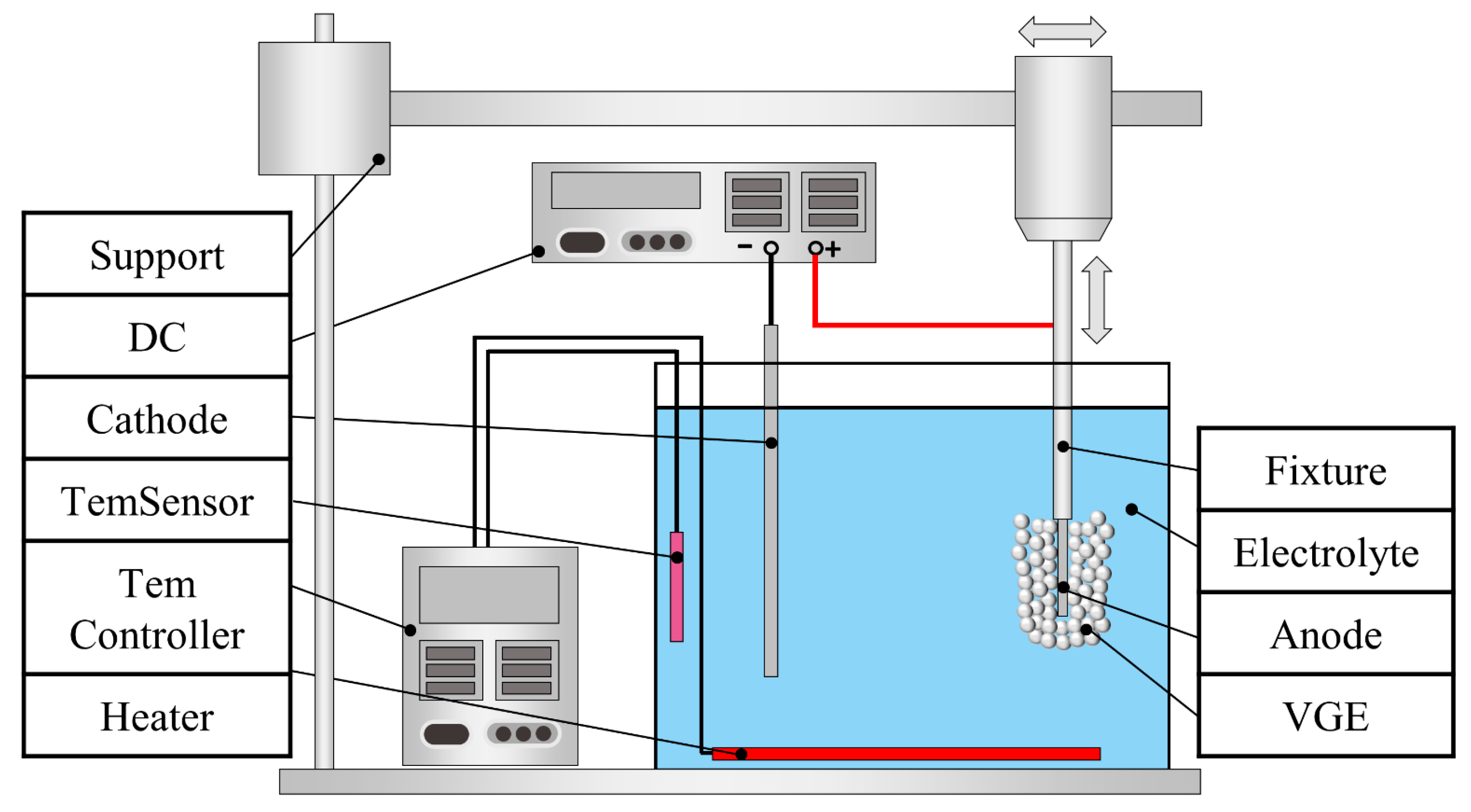
2.2. EPPo Process
2.3. Characterization
2.4. Performance Measurement
3. Results and Discussion
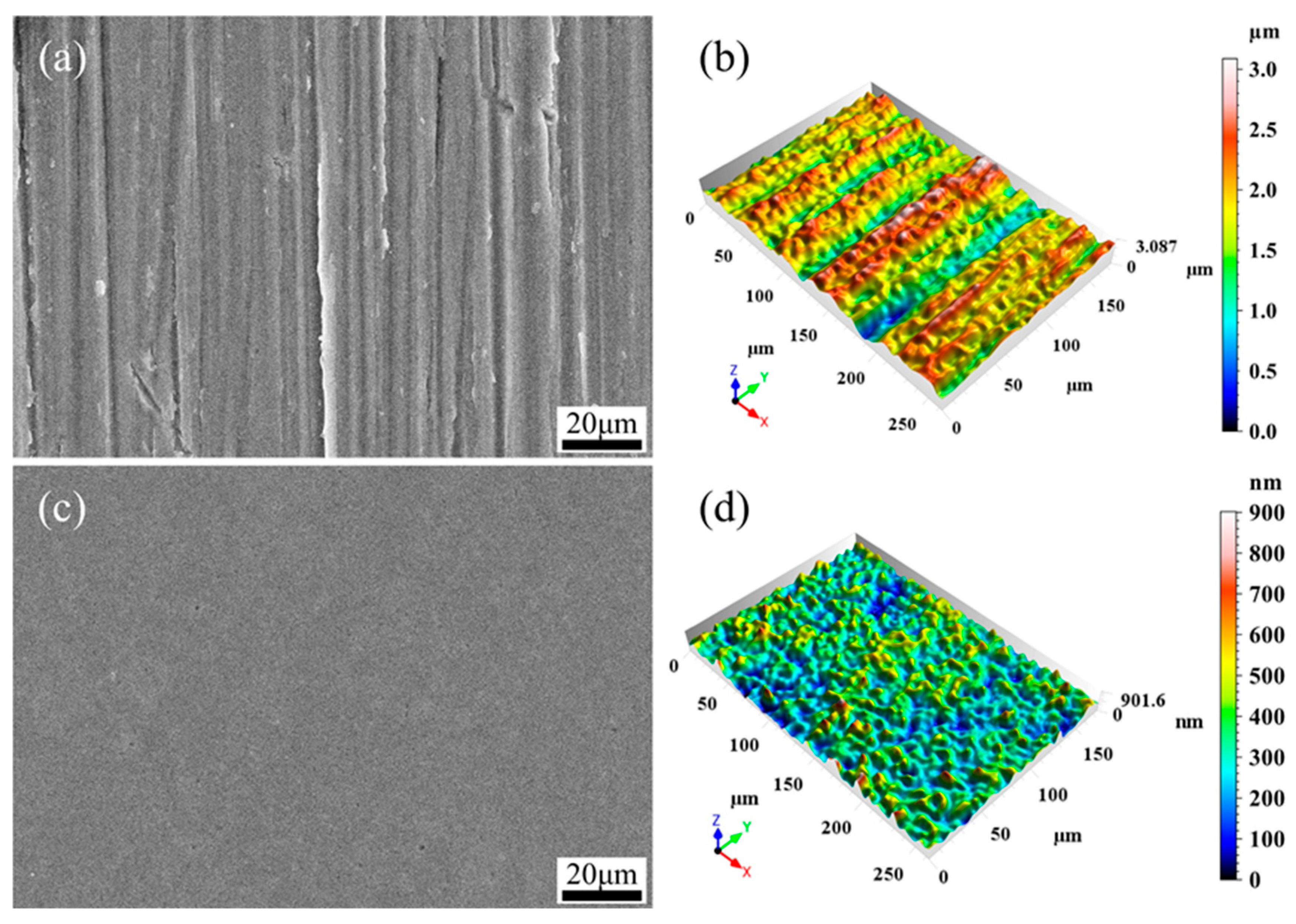
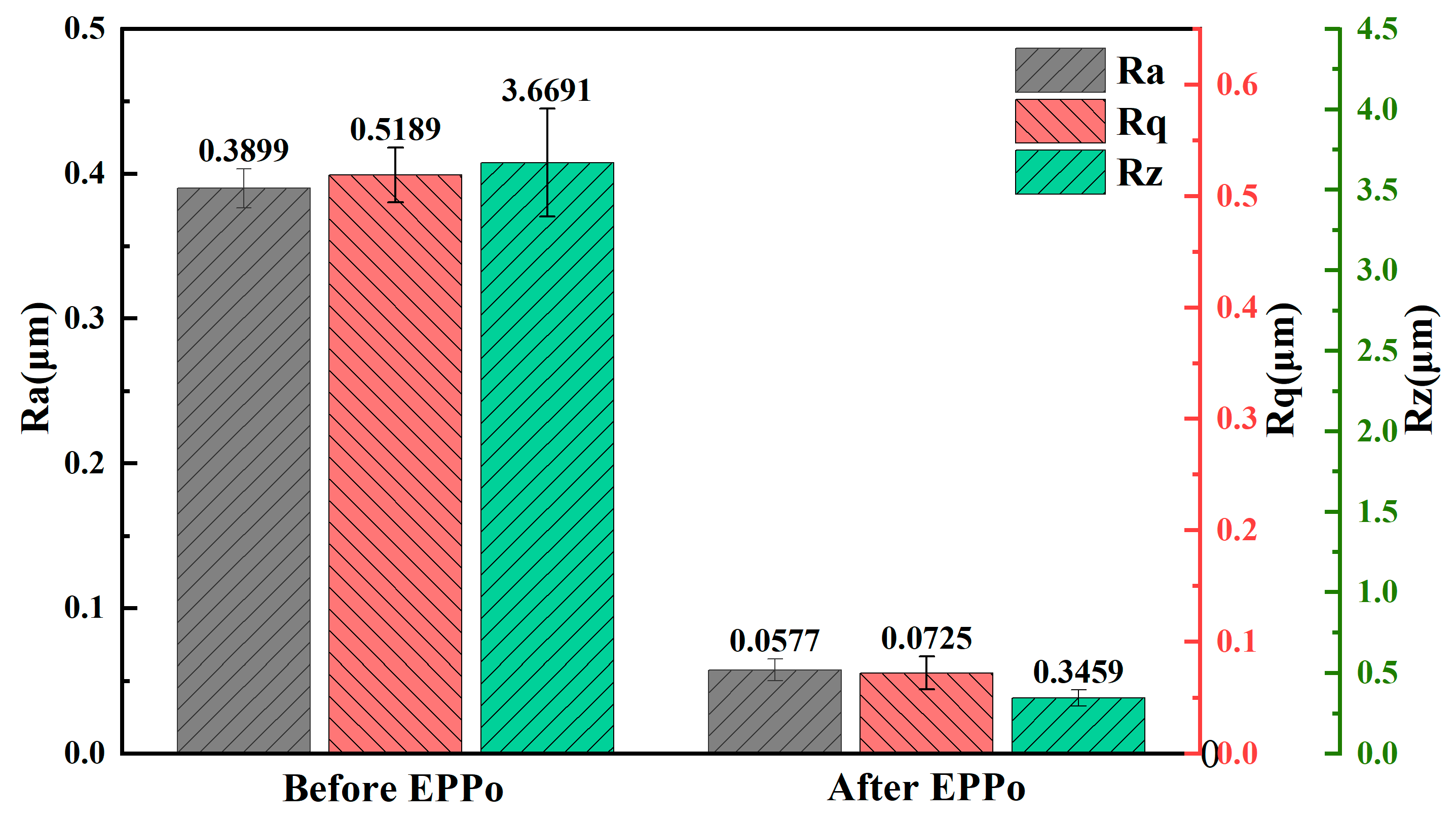
3.1. Surface Roughness and Morphology
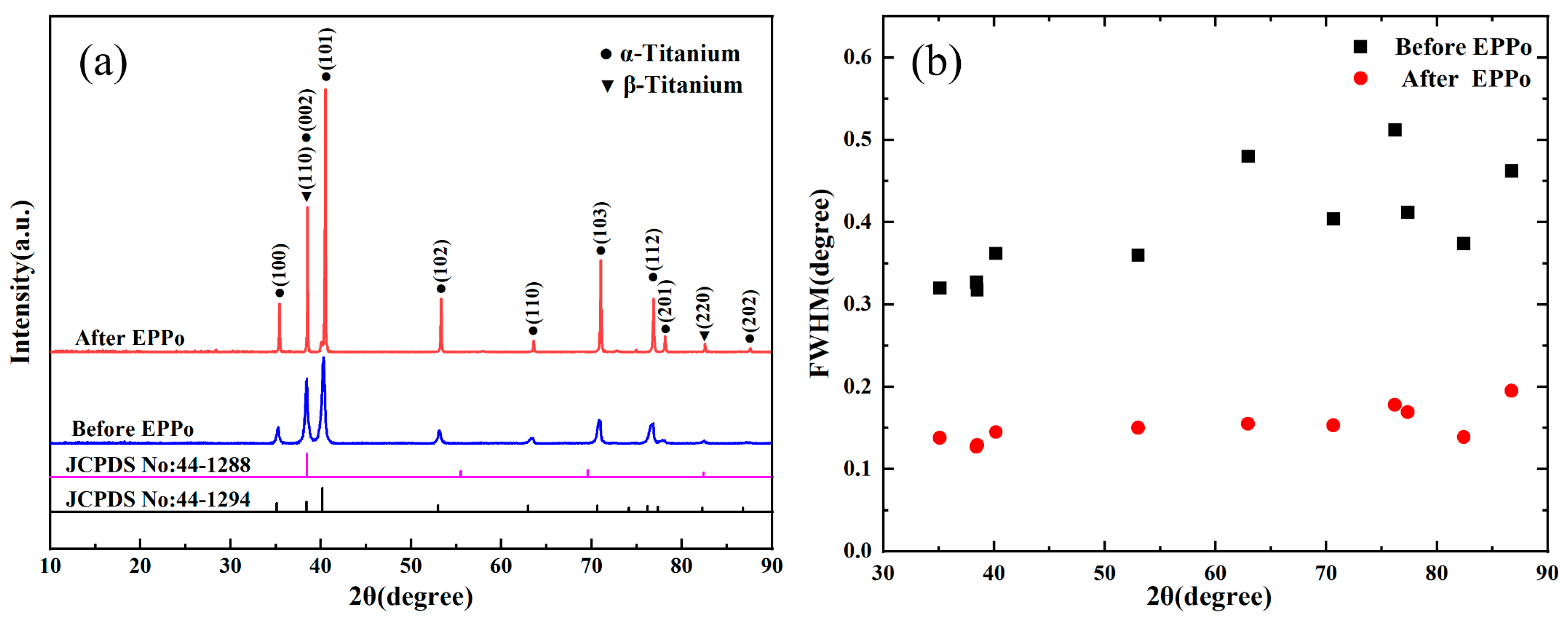
3.2. Microstructure Analysis
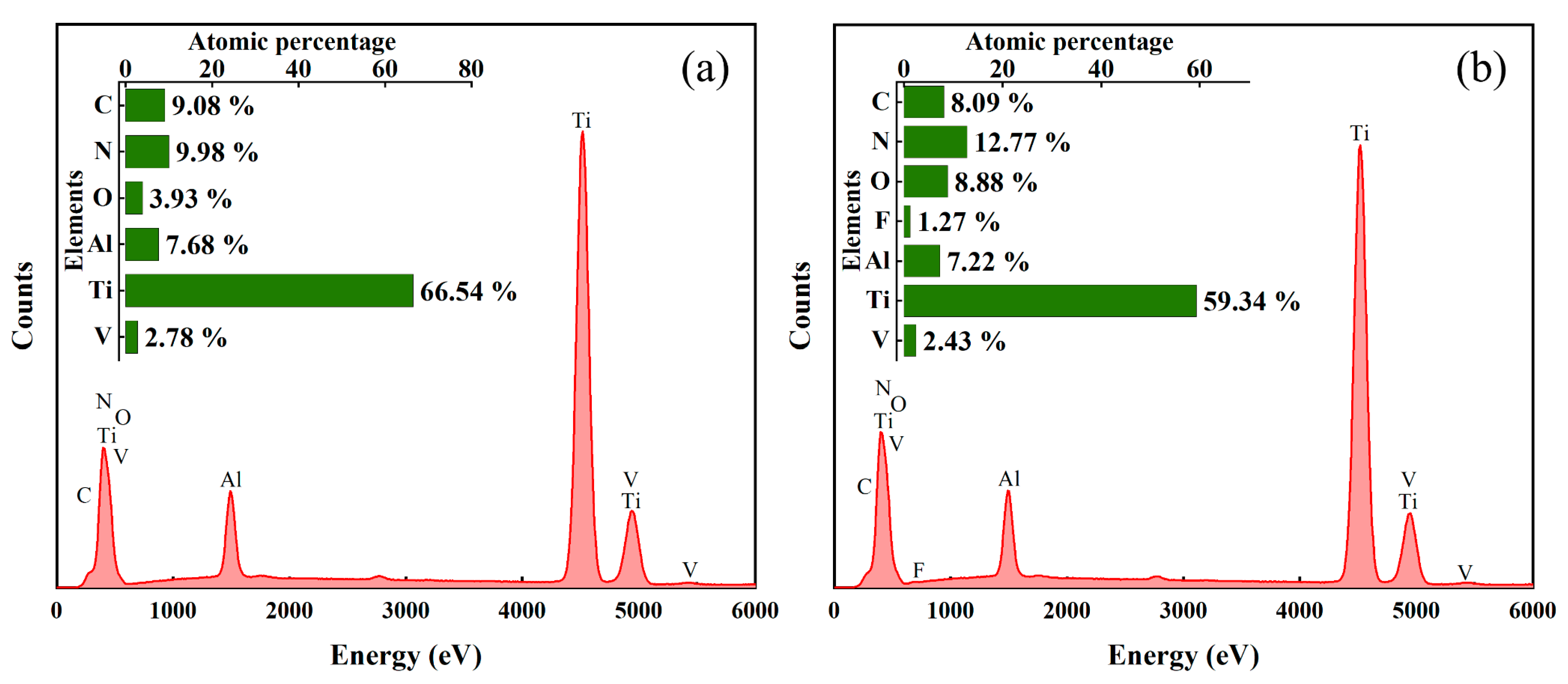
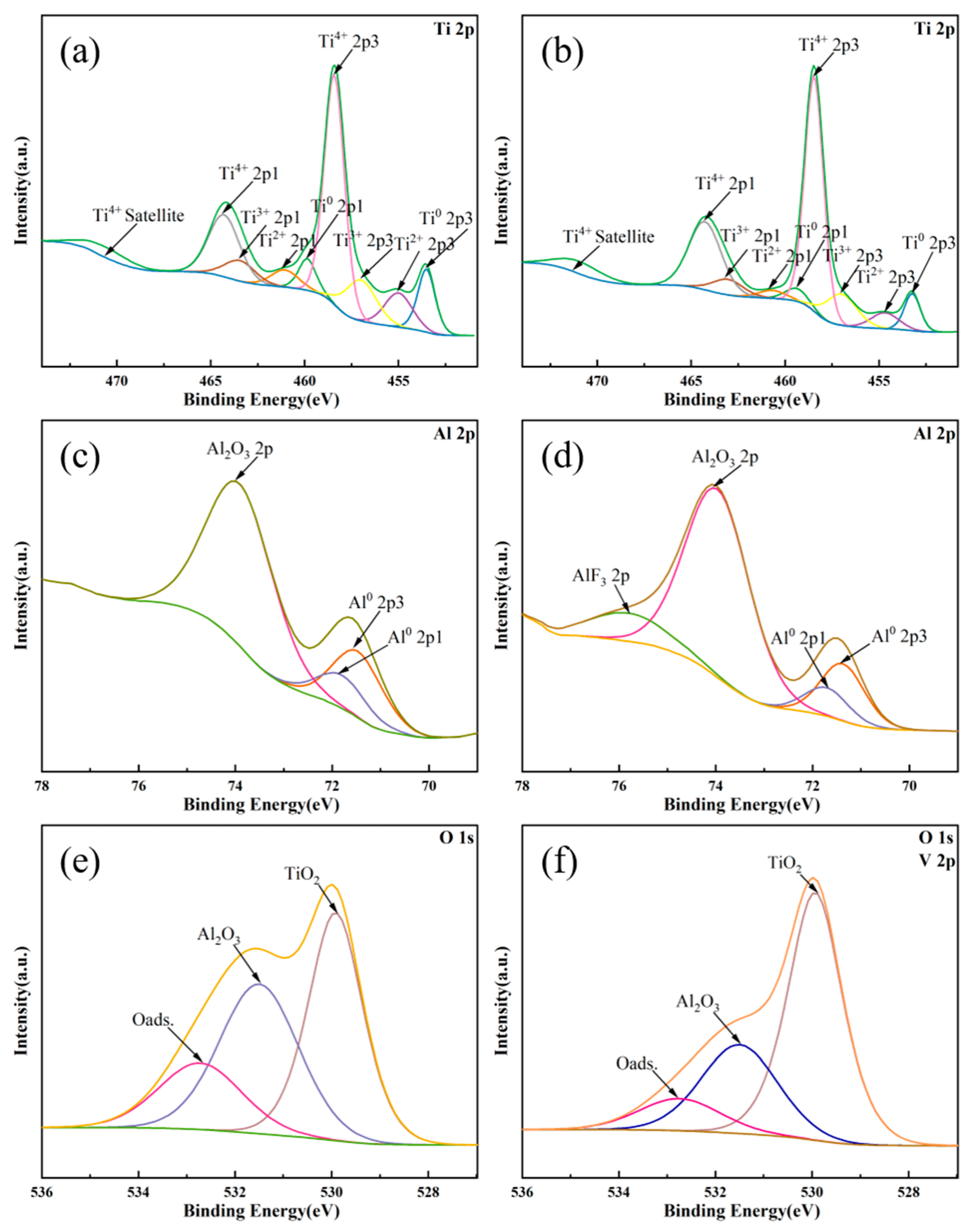
3.3. Surface Elements Analysis
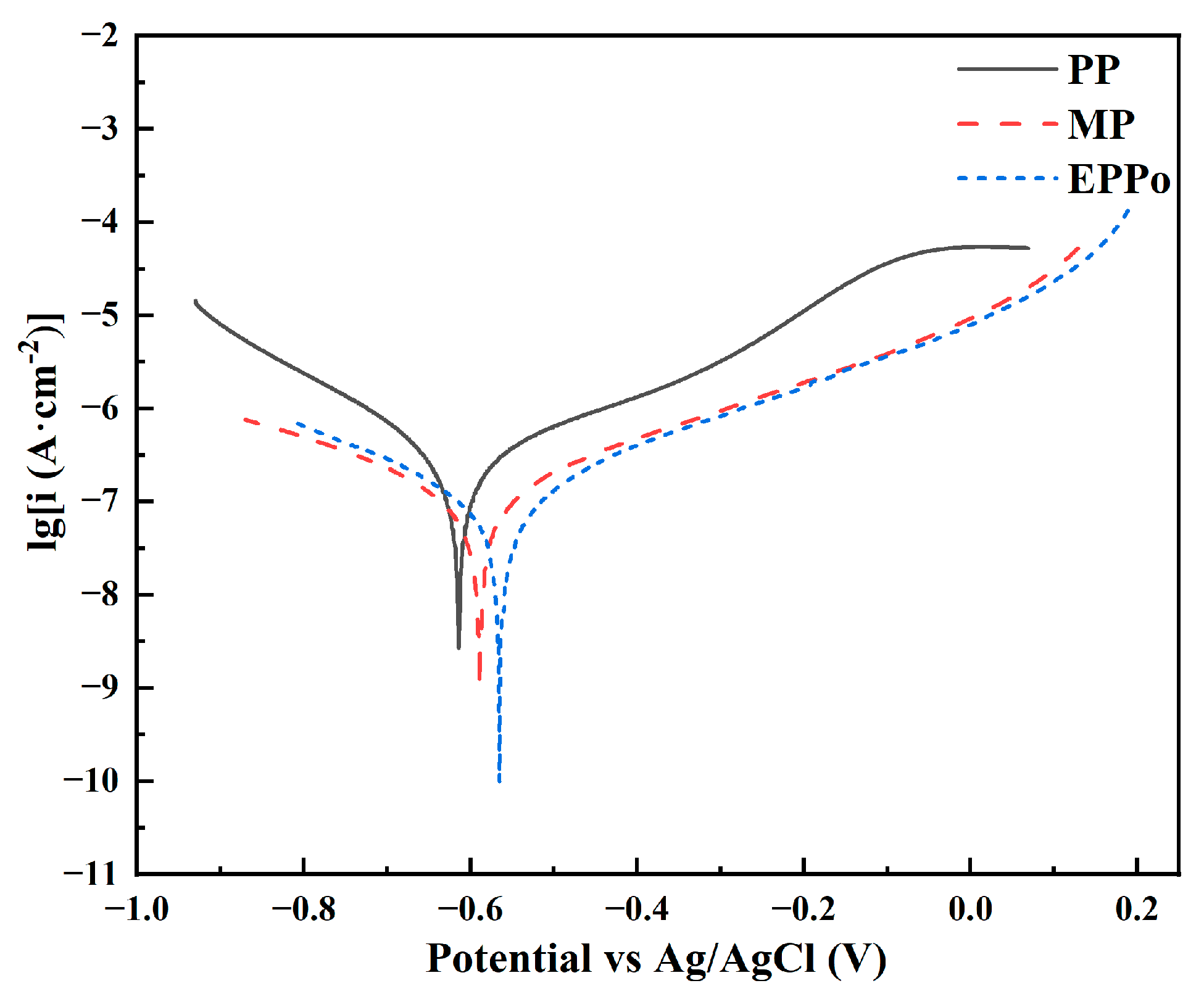
3.4. Corrosion Properties
3.5. Wettability
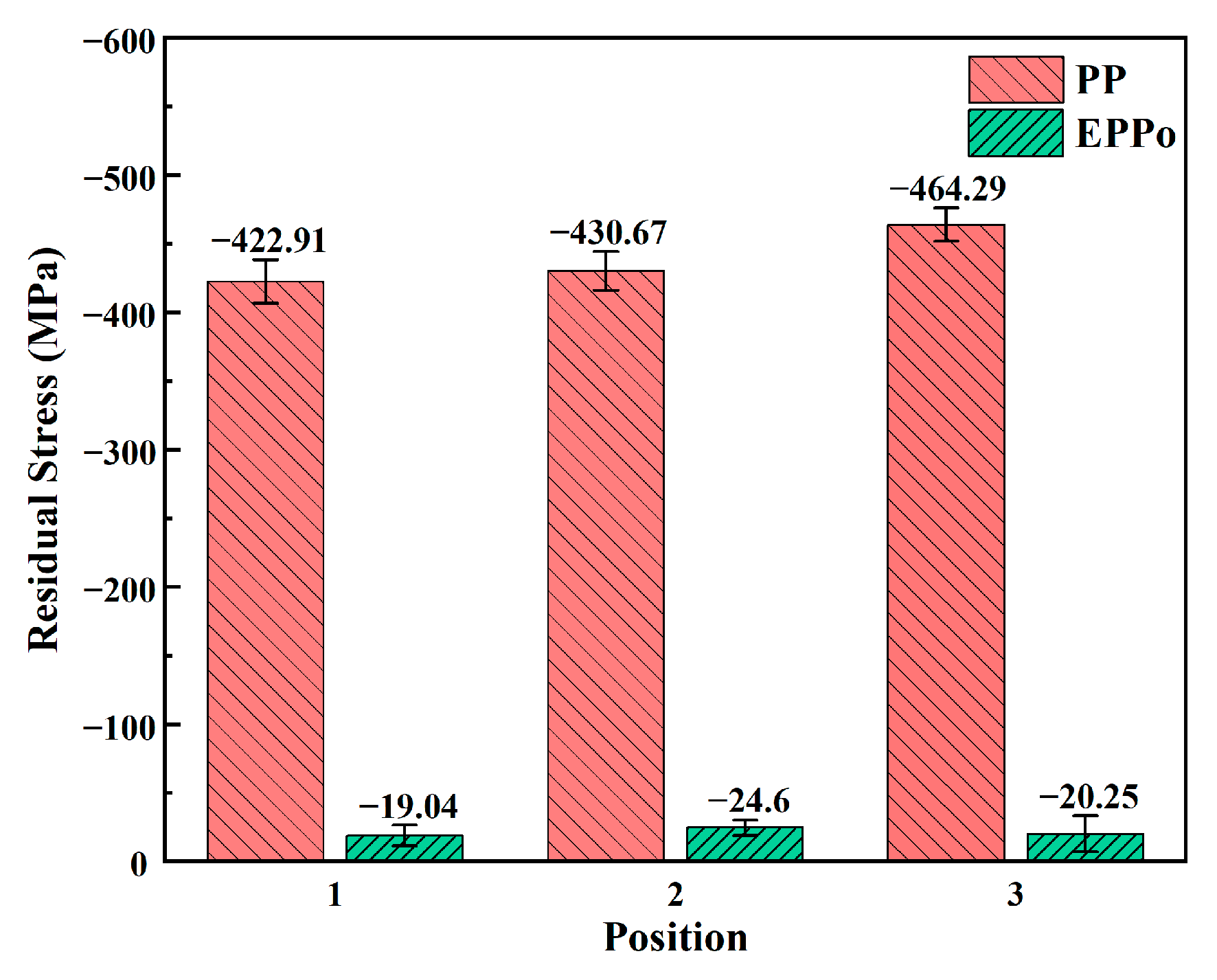
3.6. Residual Stress Variation
4. Conclusions
- (1)
- EPPo can significantly reduce the surface roughness of Ti6Al4V alloy and improve the surface morphology. After EPPo, the sample surface crystallinity and average grain size increased. The surface oxygen content of the sample was significantly increased, and an oxide layer with higher TiO2 content was formed.
- (2)
- After EPPo treatment, the corrosion current of Ti6Al4V decreased from 0.266 to 0.07 6 μA·cm−2, the corrosion potential increased from −0.614 to −0.556 V, and the polarization resistance increased from 149.896 to 523.439 kΩ, indicating that the corrosion resistance of the Ti6Al4V sample increased significantly. The improvement of corrosion resistance by EPPo is related to the improvement of surface flatness, crystal microstructure, and the formation of the oxide film on the surface.
- (3)
- EPPo could improve the hydrophilicity of Ti6Al4V surface, and the contact angles of distilled water and ethanediol droplets on the surface were reduced from 81.14 and 55.17° to 69.78 and 42.78°. The improvement of hydrophilicity by EPPo is related to the decrease in surface roughness, the increase in surface energy, and the alteration of surface structure and chemical composition.
- (4)
- The Ti6Al4V surface shows residual stress relief after EPPo treatment, which may be related to the annealing effect formed by the constant alternating increase and decrease in the surface temperature of the samples during the EPPo process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biffi, C.A.; Fiocchi, J.; Ferrario, E.; Fornaci, A.; Riccio, M.; Romeo, M.; Tuissi, A. Effects of the Scanning Strategy on the Microstructure and Mechanical Properties of a TiAl6V4 Alloy Produced by Electron Beam Additive Manufacturing. Int. J. Adv. Manuf. Technol. 2020, 107, 4913–4924. [Google Scholar] [CrossRef]
- Alessandra Gobbo, V.; Lallukka, M.; Gamna, F.; Prato, M.; Vitale, A.; Ferraris, S.; Najmi, Z.; Cochis, A.; Rimondini, L.; Massera, J.; et al. Functionalization of a Chemically Treated Ti6Al4V-ELI Alloy with Nisin for Antibacterial Purposes. Appl. Surf. Sci. 2023, 620, 156820. [Google Scholar] [CrossRef]
- Rotella, G.; Del Prete, A. Development of Customized Physics-Based Predictive Models for Improved Performance in Turning of Ti6Al4V. J. Manuf. Process. 2022, 81, 727–737. [Google Scholar] [CrossRef]
- Rominiyi, A.L.; Mashinini, P.M. Continuous Wave Laser Welding of Ti6Al4V Alloy Joints: Microstructure and Mechanical Properties. Mater. Lett. 2023, 336, 133934. [Google Scholar] [CrossRef]
- Siti Nur Hazwani, M.R.; Lim, L.X.; Lockman, Z.; Zuhailawati, H. Fabrication of Titanium-Based Alloys with Bioactive Surface Oxide Layer as Biomedical Implants: Opportunity and Challenges. Trans. Nonferrous Met. Soc. China 2022, 32, 1–44. [Google Scholar] [CrossRef]
- Zeidler, H.; Boettger-Hiller, F.; Edelmann, J.; Schubert, A. Surface Finish Machining of Medical Parts Using Plasma Electrolytic Polishing. Procedia CIRP 2016, 49, 83–87. [Google Scholar] [CrossRef]
- Yang, D.; Sun, H.; Wang, J.; Ji, G.; Duan, H.; Xiang, Y.; Fan, Y. The Formation and Stripping Mechanism of Oxide Film on Ti6Al4V Alloy Surface during Electrolytic Plasma Polishing. Surf. Coat. Technol. 2024, 478, 130469. [Google Scholar] [CrossRef]
- Ke, X.; Wu, W.; Wang, C.; Yu, Y.; Zhong, B.; Wang, Z.; Wang, T.; Fu, J.; Guo, J. Material Removal and Surface Integrity Analysis of Ti6Al4V Alloy after Polishing by Flexible Tools with Different Rigidity. Materials 2022, 15, 1642. [Google Scholar] [CrossRef]
- Bordatchev, E.V.; Hafiz, A.M.K.; Tutunea-Fatan, O.R. Performance of Laser Polishing in Finishing of Metallic Surfaces. Int. J. Adv. Manuf. Technol. 2014, 73, 35–52. [Google Scholar] [CrossRef]
- Bezuidenhout, M.; Ter Haar, G.; Becker, T.; Rudolph, S.; Damm, O.; Sacks, N. The Effect of HF-HNO3 Chemical Polishing on the Surface Roughness and Fatigue Life of Laser Powder Bed Fusion Produced Ti6Al4V. Mater. Today Commun. 2020, 25, 101396. [Google Scholar] [CrossRef]
- Zaborski, S.; Sudzik, A.; Wolyniec, A. Electrochemical Polishing of Total Hip Prostheses. Arch. Civ. Mech. Eng. 2011, 11, 1053–1062. [Google Scholar] [CrossRef]
- Aliakseyeu, Y.G.; Korolyov, A.Y.; Niss, V.S. Electrolytic-plasma polishing of cobalt-chromium alloys for medical products. Vescì Akad. Navuk Belarusì. Seryâ Fiz. Teh. Navuk 2019, 64, 296–303. [Google Scholar] [CrossRef]
- Jagadeeshanayaka, N.; Awasthi, S.; Jambagi, S.C.; Srivastava, C. Bioactive Surface Modifications through Thermally Sprayed Hydroxyapatite Composite Coatings: A Review of Selective Reinforcements. Biomater. Sci. 2022, 10, 2484–2523. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Luo, Q.; Zhang, X.; Qiu, J.; Qian, S.; Liu, X. Co-Implantation of Magnesium and Zinc Ions into Titanium Regulates the Behaviors of Human Gingival Fibroblasts. Bioact. Mater. 2021, 6, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Gabor, R.; Cvrček, L.; Doubková, M.; Nehasil, V.; Hlinka, J.; Unucka, P.; Buřil, M.; Podepřelová, A.; Seidlerová, J.; Bačáková, L. Hybrid Coatings for Orthopaedic Implants Formed by Physical Vapour Deposition and Microarc Oxidation. Mater. Des. 2022, 219, 110811. [Google Scholar] [CrossRef]
- Radtke, A.; Grodzicka, M.; Ehlert, M.; Muzioł, T.; Szkodo, M.; Bartmański, M.; Piszczek, P. Studies on Silver Ions Releasing Processes and Mechanical Properties of Surface-Modified Titanium Alloy Implants. IJMS 2018, 19, 3962. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Wang, S.; Chen, G.; Wang, Y.; Zhang, K.; Zhang, C.; Wei, D.; Ouyang, J.; Jia, D.; Zhou, Y. Optimization and Mechanism of Precise Finishing of TC4 Alloy by Plasma Electrolytic Polishing. Surf. Coat. Technol. 2023, 467, 129696. [Google Scholar] [CrossRef]
- Smyslova, M.K.; Tamindarov, D.R.; Plotnikov, N.V.; Modina, I.M.; Semenova, I.P. Surface Electrolytic-Plasma Polishing of Ti-6Al-4V Alloy with Ultrafine-Grained Structure Produced by Severe Plastic Deformation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 461, 012079. [Google Scholar] [CrossRef]
- Navickaitė, K.; Nestler, K.; Böttger-Hiller, F.; Matias, C.; Diskin, A.; Golan, O.; Garkun, A.; Strokin, E.; Biletskiy, R.; Safranchik, D.; et al. Efficient Polishing of Additive Manufactured Titanium Alloys. Procedia CIRP 2022, 108, 346–351. [Google Scholar] [CrossRef]
- Li, H.; Ma, G.; Wang, Z. Corrosion Behavior of ZrO2-TiO2 Composite Coatings Produced on Titanium Alloy via Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2023, 469, 129814. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Rameshbabu, N.; Chandra Bose, A.; Muthupandi, V.; Subramanian, S.; MubarakAli, D.; Thajuddin, N. Fabrication of Corrosion Resistant, Bioactive and Antibacterial Silver Substituted Hydroxyapatite/Titania Composite Coating on Cp Ti. Ceram. Int. 2012, 38, 731–740. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Rameshbabu, N.; Sreekanth, D.; Bose, A.C.; Muthupandi, V.; Babu, N.K.; Subramanian, S. Role of Electrolyte Additives on In-Vitro Electrochemical Behavior of Micro Arc Oxidized Titania Films on Cp Ti. Appl. Surf. Sci. 2012, 258, 6853–6863. [Google Scholar] [CrossRef]
- Jiang, N.; Zhu, S.; Li, J.; Zhang, L.; Liao, Y.; Hu, J. Development of a Novel Biomimetic Micro/Nano-Hierarchical Interface for Enhancement of Osseointegration. RSC Adv. 2016, 6, 49954–49965. [Google Scholar] [CrossRef]
- Chu, Y.; Liu, P.; Chen, Y.; Li, X. Influence of Applied Voltage on Surface Morphology and Wettability of Biological Coatings on Ti6-Al-4V by Micro-Arc Oxidation Treatment. Mat. Res. 2020, 23, e20200002. [Google Scholar] [CrossRef]
- Fan, K.; Liu, D.; Yang, J.; Zhang, X.; Liu, D.; Li, M.; Xiang, J.; Wang, C.; Abdel Wahab, M. Thermal Relaxation of Compressive Residual Stresses in Surface Gradient Nanostructure of TC11 Titanium Alloy. J. Alloys Compd. 2024, 970, 172549. [Google Scholar] [CrossRef]
- Yang, C.; Meng, X.; Li, X.; Li, Z.; Yan, H.; Wu, L.; Cao, F. Effect of Electrolyte Composition on Corrosion Behavior and Tribological Performance of Plasma Electrolytic Oxidized TC4 Alloy. Trans. Nonferrous Met. Soc. China 2023, 33, 141–156. [Google Scholar] [CrossRef]
- Yao, J.; Wang, Y.; Wu, G.; Sun, M.; Wang, M.; Zhang, Q. Growth Characteristics and Properties of Micro-Arc Oxidation Coating on SLM-Produced TC4 Alloy for Biomedical Applications. Appl. Surf. Sci. 2019, 479, 727–737. [Google Scholar] [CrossRef]
- Gittens, R.A.; Scheideler, L.; Rupp, F.; Hyzy, S.L.; Geis-Gerstorfer, J.; Schwartz, Z.; Boyan, B.D. A Review on the Wettability of Dental Implant Surfaces II: Biological and Clinical Aspects. Acta Biomater. 2014, 10, 2907–2918. [Google Scholar] [CrossRef]
- Ji, G.; Sun, H.; Duan, H.; Yang, D.; Sun, J. Effect of Electrolytic Plasma Polishing on Microstructural Evolution and Tensile Properties of 316L Stainless Steel. Surf. Coat. Technol. 2021, 420, 127330. [Google Scholar] [CrossRef]
- GB/T13810-2017; Wrought Titanium and Titanium Alloy for Surgical Implants. Standardization Administration Committee: Beijing, China, 2017.
- ISO 4287:1997; Geometrical Product Specifications (GPS) Surface Texture: Profile Method. Terms, Definitions and Surface Texture Parameters. ISO Publishing: Geneve, Switzerland, 1997.
- Belkin, P.N.; Kusmanov, S.A.; Parfenov, E.V. Mechanism and Technological Opportunity of Plasma Electrolytic Polishing of Metals and Alloys Surfaces. Appl. Surf. Sci. Adv. 2020, 1, 100016. [Google Scholar] [CrossRef]
- Парфенoв, Е.В.; Мукаева, В.Р.; Фарракхoв, Р.Г. Plasma Electrolytic Treatments for Advanced Surface Finishing Technologies. Mater. Technol. Des. 2019, 1, 34–41. [Google Scholar]
- Zhou, C.; Su, H.; Qian, N.; Zhang, Z.; Xu, J. Characteristics and Function of Vapour Gaseous Envelope Fluctuation in Plasma Electrolytic Polishing. Int. J. Adv. Manuf. Technol. 2022, 119, 7815–7825. [Google Scholar] [CrossRef]
- Hierro-Oliva, M.; Gallardo-Moreno, A.M.; González-Martín, M.L. XPS Analysis of Ti6Al4V Oxidation Under UHV Conditions. Metall. Mater. Trans. A 2014, 45, 6285–6290. [Google Scholar] [CrossRef]
- Hedberg, Y.S.; Žnidaršič, M.; Herting, G.; Milošev, I.; Odnevall Wallinder, I. Mechanistic Insight on the Combined Effect of Albumin and Hydrogen Peroxide on Surface Oxide Composition and Extent of Metal Release from Ti6Al4V: Effect of Albumin and Hydrogen Peroxide. J. Biomed. Mater. Res. 2019, 107, 858–867. [Google Scholar] [CrossRef] [PubMed]
- Shirkhanzadeh, M. XRD and XPS Characterization of Superplastic TiO2 Coatings Prepared on Ti6Al4V Surgical Alloy by an Electrochemical Method. J. Mater. Sci. Mater. Med. 1995, 6, 206–210. [Google Scholar] [CrossRef]
- Chávez-Díaz, M.P.; Luna-Sánchez, R.M.; Vazquez-Arenas, J.; Lartundo-Rojas, L.; Hallen, J.M.; Cabrera-Sierra, R. XPS and EIS Studies to Account for the Passive Behavior of the Alloy Ti-6Al-4V in Hank’s Solution. J. Solid State Electrochem. 2019, 23, 3187–3196. [Google Scholar] [CrossRef]
- Böse, O.; Kemnitz, E.; Lippitz, A.; Unger, W.E. C 1s and Au 4f 7/2 Referenced XPS Binding Energy Data Obtained with Different Aluminium Oxides, -Hydroxides and -Fluorides. Fresenius’ J. Anal. Chem. 1997, 358, 175–179. [Google Scholar] [CrossRef]
- Yazdi, R.; Ghasemi, H.M.; Abedini, M.; Wang, C.; Neville, A. Mechanism of Tribofilm Formation on Ti6Al4V Oxygen Diffusion Layer in a Simulated Body Fluid. J. Mech. Behav. Biomed. Mater. 2018, 77, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Abdun Nafi, M.; Anjir Karim, M.; Lalvani, S.; James, P.F.; Sommers, A.; Jahan, M.P. Investigating Wettability and Corrosion Resistance of the Titanium Alloy Surface Engineered by the WEDM Process. Manuf. Lett. 2023, 35, 450–459. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Zhang, H.-Y.; Zheng, C.; Yang, H.-Y.; Qin, P.; Zhao, C.; Lu, S.; Liang, S.-X.; Chai, L.; Zhang, L.-C. Corrosion Behavior and Characteristics of Passive Films of Laser Powder Bed Fusion Produced Ti–6Al–4V in Dynamic Hank’s Solution. Mater. Des. 2021, 208, 109907. [Google Scholar] [CrossRef]
- Chen, X.; Liao, Q.; Gong, M.; Fu, Q. Corrosion Performances of Selective Laser Melting Ti6Al4V Alloy in Different Solutions. Metals 2023, 13, 192. [Google Scholar] [CrossRef]
- Asgar, H.; Deen, K.M.; Rahman, Z.U.; Shah, U.H.; Raza, M.A.; Haider, W. Functionalized Graphene Oxide Coating on Ti6Al4V Alloy for Improved Biocompatibility and Corrosion Resistance. Mater. Sci. Eng. C 2019, 94, 920–928. [Google Scholar] [CrossRef] [PubMed]
- Lodhi, M.J.K.; Deen, K.M.; Greenlee-Wacker, M.C.; Haider, W. Additively Manufactured 316L Stainless Steel with Improved Corrosion Resistance and Biological Response for Biomedical Applications. Addit. Manuf. 2019, 27, 8–19. [Google Scholar] [CrossRef]
- Ji, G.; Sun, H.; Duan, H.; Yang, D.; Sun, J. Enhancement of Corrosion Resistance for Medical Grade 316L Stainless Steel by Electrolytic Plasma Polishing. J. Mater. Eng Perform 2023, 32, 1498–1507. [Google Scholar] [CrossRef]
- Hao, Y.; Yang, S.; Li, D.; Li, W.; Li, X. Vibratory Finishing for the Cavity of Aero-Engine Integral Casting Casing: Mechanism Analysis and Performance Evaluation. Int. J. Adv. Manuf. Technol. 2022, 125, 713–729. [Google Scholar] [CrossRef]
- Winiarski, J.; Tylus, W.; Szczygieł, B. EIS and XPS Investigations on the Corrosion Mechanism of Ternary Zn–Co–Mo Alloy Coatings in NaCl Solution. Appl. Surf. Sci. 2016, 364, 455–466. [Google Scholar] [CrossRef]
- Liu, C.; Tong, S.; Yue, Y.; Wang, H.; Song, J.; Li, Y.; Wang, Q.; Wang, Z. Laser-Based Fabrication of Superwetting Titanium Alloy with Enhanced Corrosion and Erosion-Corrosion Resistance. Colloids Surf. A Physicochem. Eng. Asp. 2024, 688, 133648. [Google Scholar] [CrossRef]
- Nelson, B.; Huang, W.; Shen, N.; Shaw, S.; Lamuta, C.; Ding, H. Evolving Surface Wettability in Laser-Powder Bed Fusion Printed Metal Parts. Manuf. Lett. 2024, 40, 45–49. [Google Scholar] [CrossRef]
- Chen, S.; Ma, J.; Gao, H.; Wang, Y.; Chen, X. Research on Residual Stresses and Microstructures of Selective Laser Melted Ti6Al4V Treated by Thermal Vibration Stress Relief. Micromachines 2023, 14, 354. [Google Scholar] [CrossRef]
- Singh, G.; Sharma, S.; Mittal, M.; Singh, G.; Singh, J.; Changhe, L.; Khan, A.M.; Dwivedi, S.P.; Tahir Mushtaq, R.; Singh, S. Impact of Post-Heat-Treatment on the Surface-Roughness, Residual Stresses, and Micromorphology Characteristics of Plasma-Sprayed Pure Hydroxyapatite and 7%-Aloxite Reinforced Hydroxyapatite Coatings Deposited on Titanium Alloy-Based Biomedical Implants. J. Mater. Res. Technol. 2022, 18, 1358–1380. [Google Scholar] [CrossRef]
- Sanjuán, M.; Brizuela-Velasco, A.; Gil, J.; Cerrolaza, M.; Montalvillo, E.; Fernández-Hernández, S.; Robles, D. Hybrid Surface Implants: Influence of Residual Stress on Mechanical Behavior, Evaluated by Finite Element Analysis and Validation by Fatigue Tests. Dent. Mater. 2024, 40, 9–18. [Google Scholar] [CrossRef] [PubMed]



| Element | V | Al | Fe | N | C | O | H | Ti |
|---|---|---|---|---|---|---|---|---|
| wt.% | 4.1 | 6.33 | 0.10 | 0.01 | 0.01 | 0.112 | 0.001 | Base |
| Parameter | Electrolyte | Temperature | Voltage | Time | Immersion Depth | Interelectrode Distance |
|---|---|---|---|---|---|---|
| Value | 3.53 | 75 | 350 | 7 | 15 | 5 |
| Unit | wt.% NH4F and KF·2H2O | °C | V | min | cm | cm |
| Test Liquids | γld (mJ/m2) | γlp (mJ/m2) | γl (mJ/m2) |
|---|---|---|---|
| Distilled water | 51.0 | 21.8 | 72.8 |
| Ethanediol | 19.0 | 29.3 | 48.3 |
| Indices of Crystal Face (h k l) | Crystallite Size before EPPo (Å) | Crystallite Size after EPPo (Å) |
|---|---|---|
| (1 0 0) | 274 | 881 |
| (0 0 2) | 270 | 943 |
| (1 1 0) | 279 | 941 |
| (1 0 1) | 243 | 805 |
| (1 0 2) | 257 | 799 |
| (1 1 0) | 199 | 792 |
| (1 0 3) | 249 | 839 |
| (1 1 2) | 202 | 686 |
| (2 0 1) | 256 | 748 |
| (2 2 0) | 293 | >1000 |
| (2 0 2) | 243 | 658 |
| Atomic (%) | Ti0 | Ti2+ | Ti3+ | Ti4+ | Al0 | Al2O3 | Al-F | TiO2 | Al2O3 | Oads. |
|---|---|---|---|---|---|---|---|---|---|---|
| Before EPPo | 14.52 | 12.25 | 16.34 | 56.89 | 31.55 | 68.45 | 0 | 42.44 | 39.19 | 18.37 |
| After EPPo | 9.29 | 6.87 | 15.01 | 68.82 | 20.83 | 66.87 | 12.3 | 60.12 | 28.72 | 11.16 |
| Samples | Ecorr (V) | Icorr (μA·cm−2) | Cathodic Tafel Slop | Anodic Tafel Slop | Rp (kΩ) |
|---|---|---|---|---|---|
| PP | −0.614 ± 0.010 | 0.266 ± 0.036 | 6.398 ± 0.218 | 4.491 ± 0.155 | 149.896 ± 11.526 |
| MP | −0.589 ± 0.008 | 0.125 ± 0.058 | 4.281 ± 0.143 | 4.838 ± 0.097 | 380.792 ± 9.347 |
| EPPo | −0.556 ± 0.012 | 0.076 ± 0.021 | 5.330 ± 0.262 | 5.548 ± 0.214 | 523.439 ± 21.763 |
| Samples | Rs (Ω·cm2) | T × 10−6 (Ω−1·cm−2·S-P) | P | Rct (kΩ·cm2) | C (μF·cm−2) |
|---|---|---|---|---|---|
| PP | 104.30 | 34.351 | 0.9072 | 179.89 | 41.38 |
| MP | 103.20 | 25.816 | 0.8799 | 381.18 | 35.27 |
| EPPo | 102.20 | 21.804 | 0.9094 | 1109.10 | 29.95 |
| Samples | γsd (mJ·m−2) | γsp (mJ·m−2) | γs (mJ·m−2) |
|---|---|---|---|
| PP | 7.5268 | 23.0457 | 30.5725 |
| MP | 34.4767 | 10.2355 | 44.7122 |
| EPPo | 14.5005 | 21.7702 | 36.2707 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Sun, H.; Ji, G.; Xiang, Y.; Wang, J. Effect of Electrolytic Plasma Polishing on Surface Properties of Titanium Alloy. Coatings 2024, 14, 615. https://doi.org/10.3390/coatings14050615
Yang D, Sun H, Ji G, Xiang Y, Wang J. Effect of Electrolytic Plasma Polishing on Surface Properties of Titanium Alloy. Coatings. 2024; 14(5):615. https://doi.org/10.3390/coatings14050615
Chicago/Turabian StyleYang, Dongliang, Huanwu Sun, Gangqiang Ji, Yuxia Xiang, and Juan Wang. 2024. "Effect of Electrolytic Plasma Polishing on Surface Properties of Titanium Alloy" Coatings 14, no. 5: 615. https://doi.org/10.3390/coatings14050615
APA StyleYang, D., Sun, H., Ji, G., Xiang, Y., & Wang, J. (2024). Effect of Electrolytic Plasma Polishing on Surface Properties of Titanium Alloy. Coatings, 14(5), 615. https://doi.org/10.3390/coatings14050615






