Al2O3 Coatings for Protection of Stainless Steel 316L against Corrosion in Zn-Al and Zn-Al-Mg
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Static Immersion Testing
2.3. Material Characterisation
3. Results and Discussion
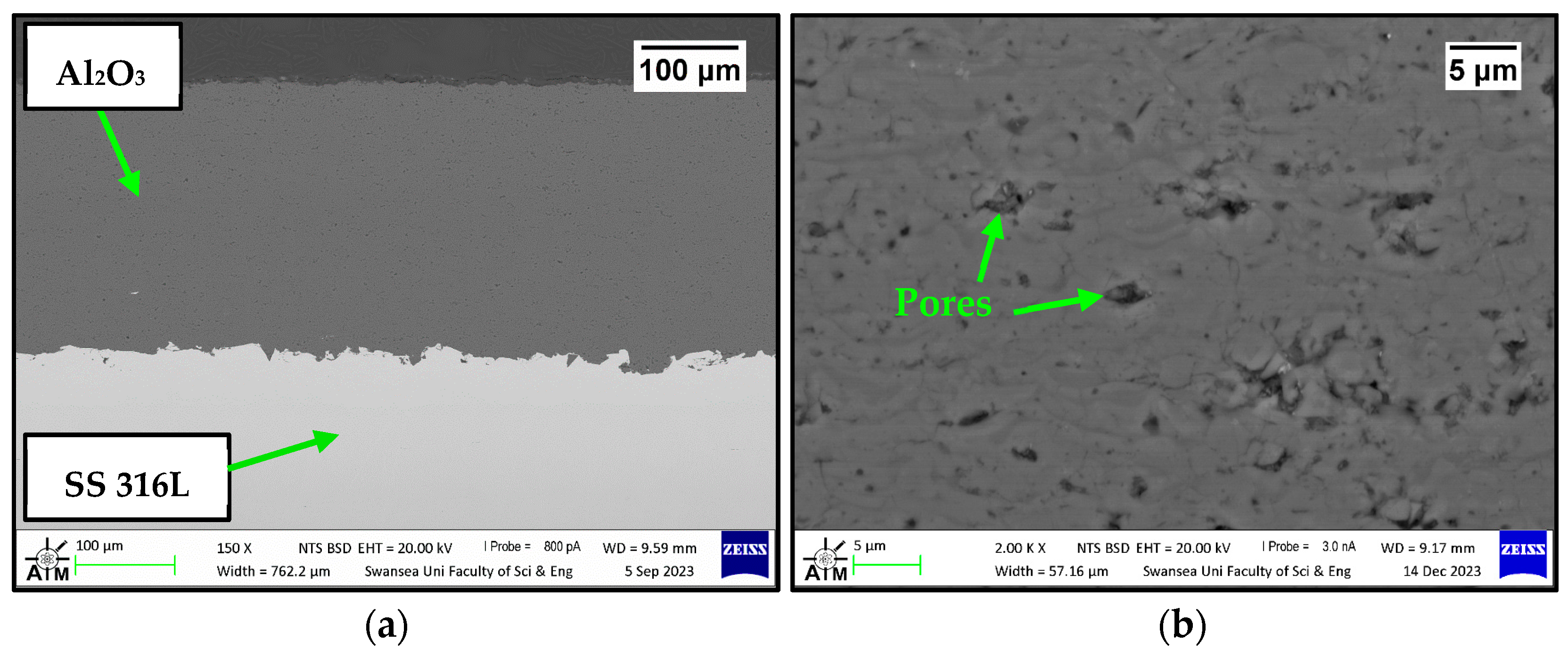
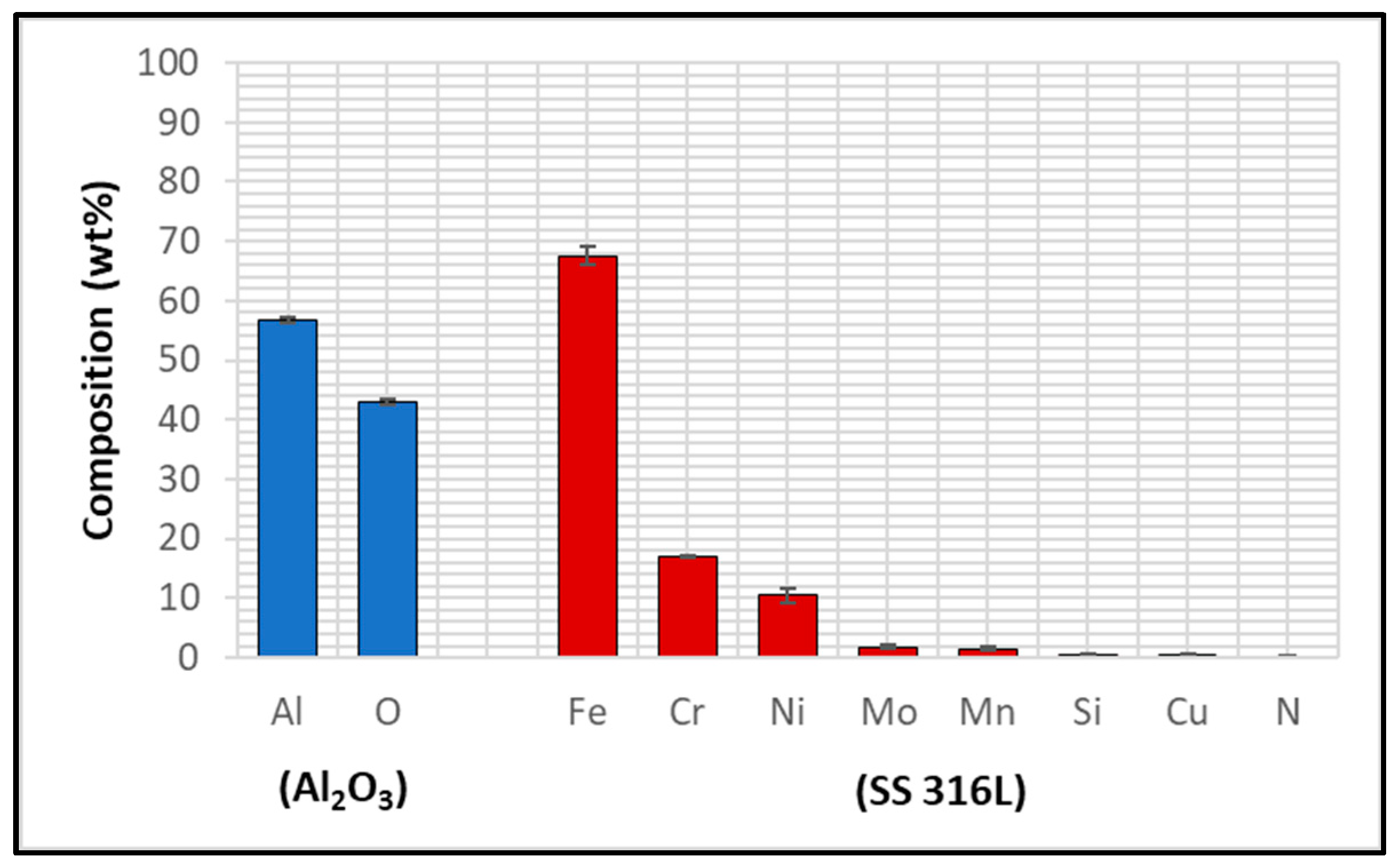
3.1. Characterisation of Untested Al2O3 Coatings
3.2. Static Testing
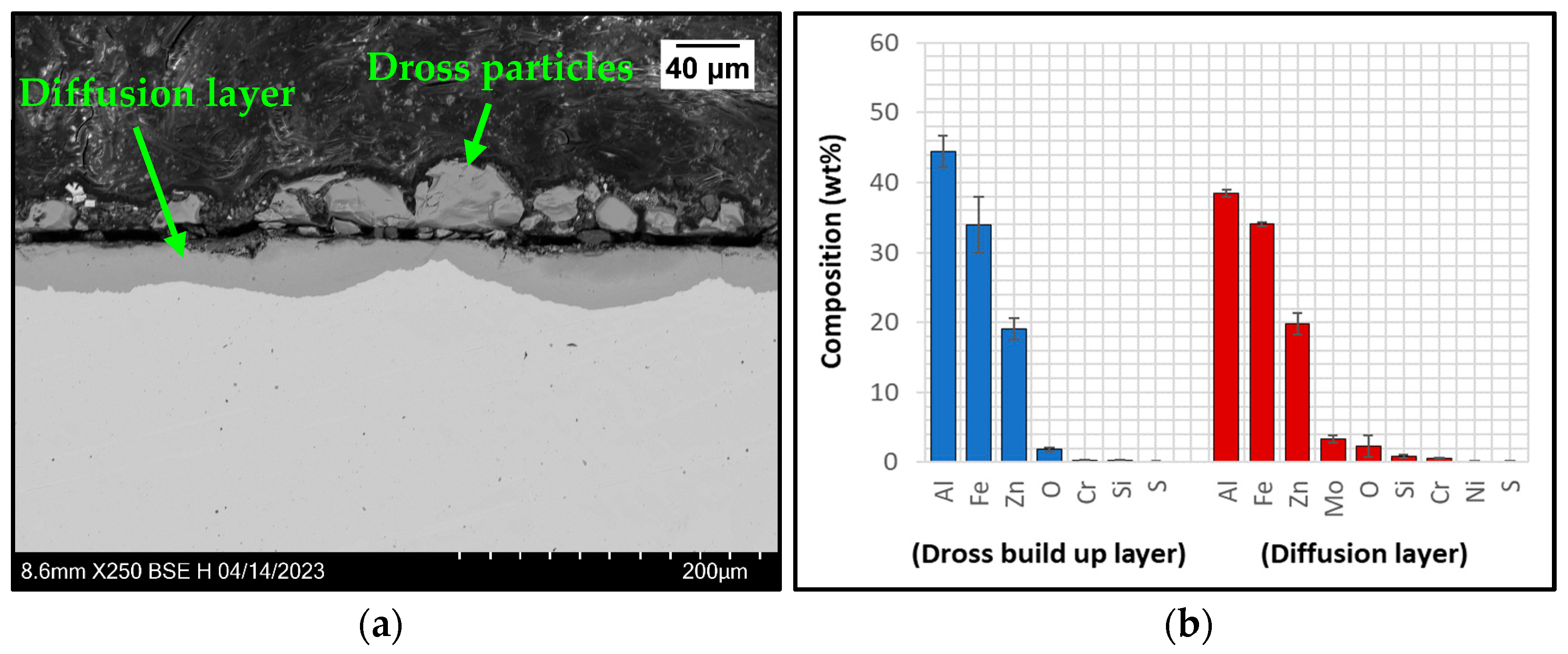
3.2.1. Immersion Tests in Zn-Al
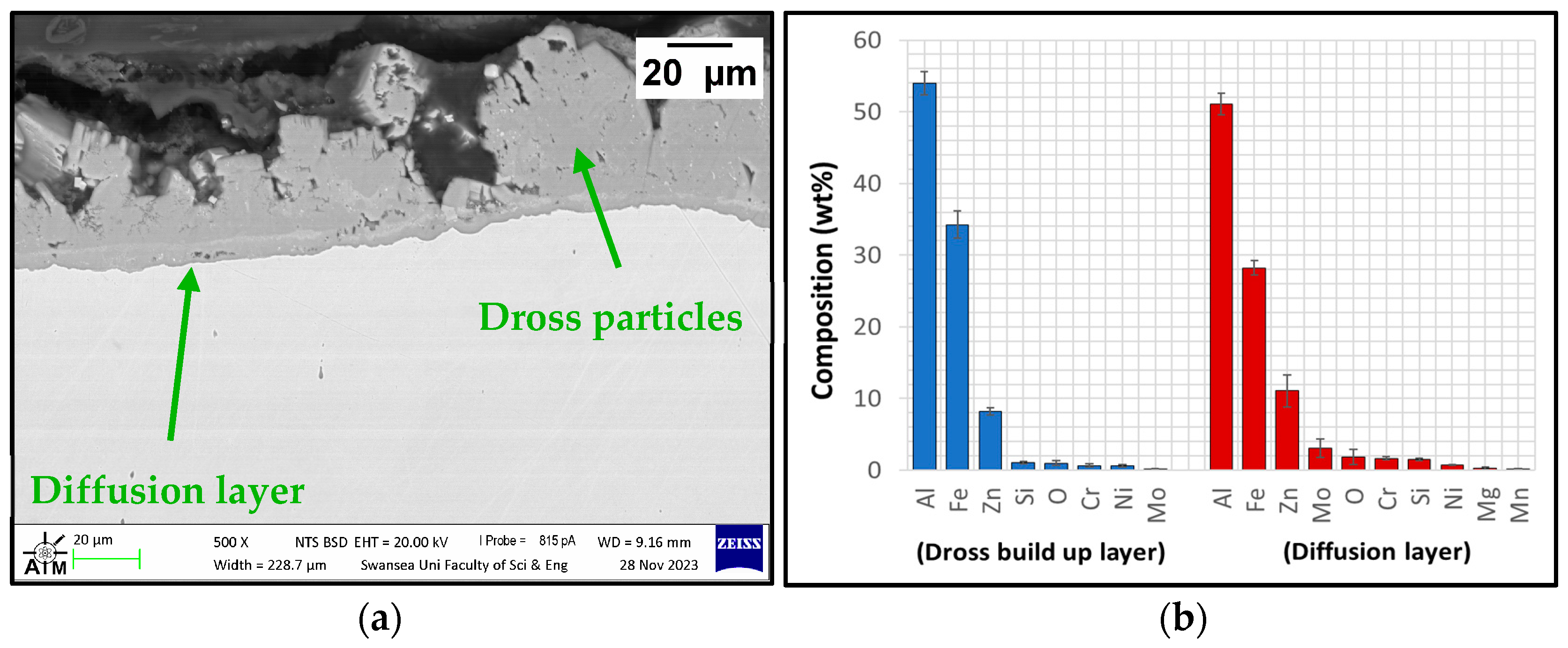
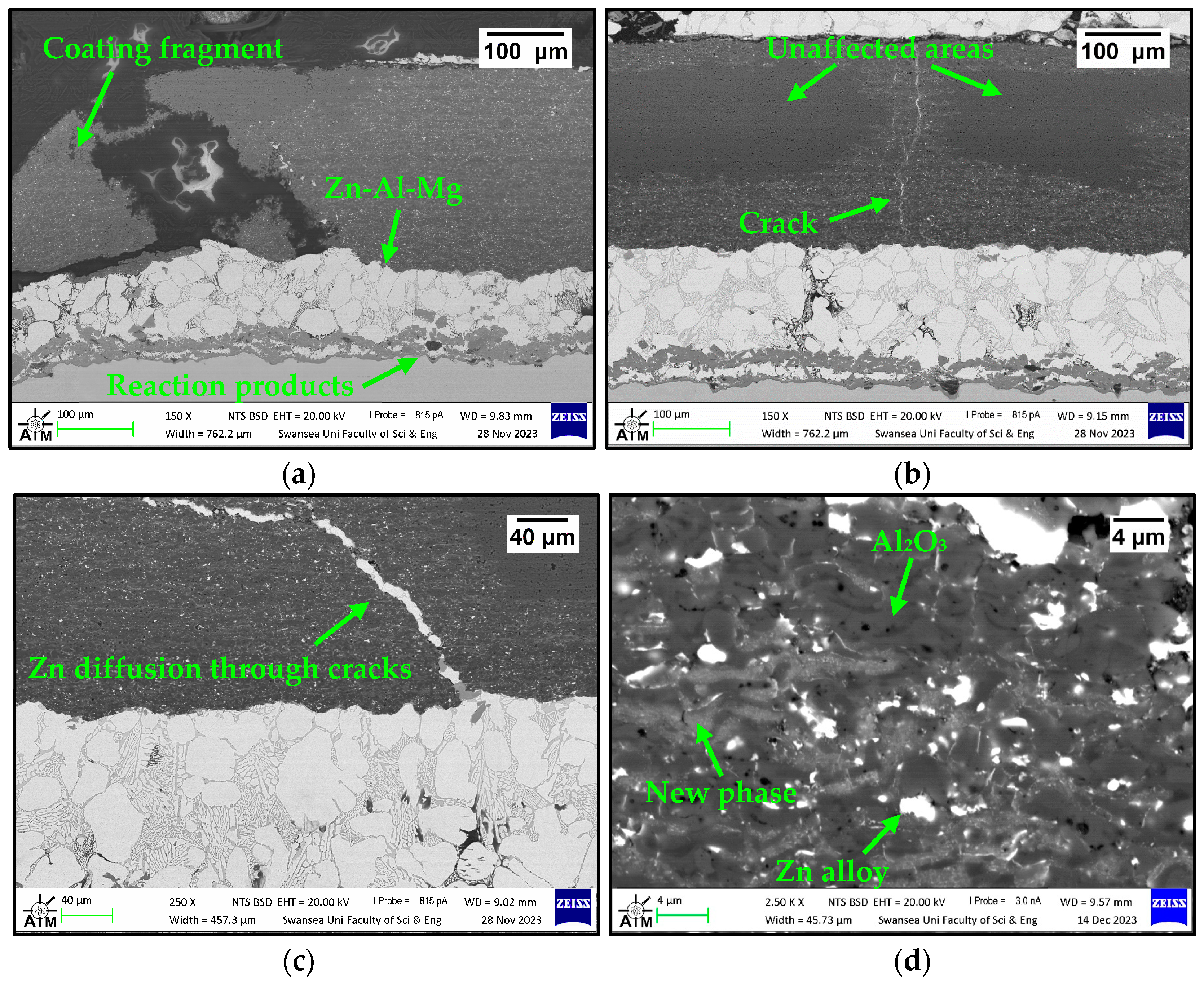
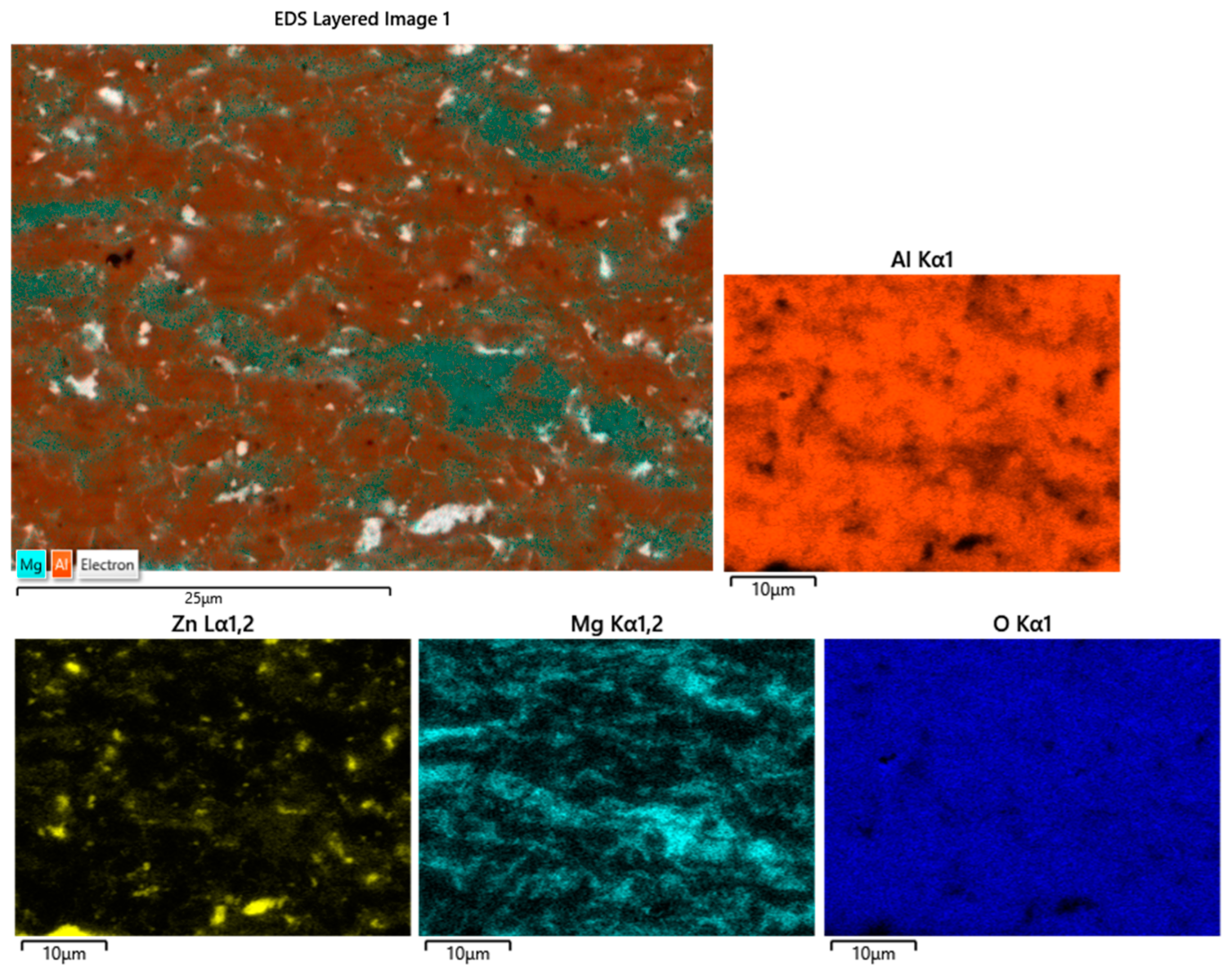
3.2.2. Immersion Tests in Zn-Al-Mg
3.2.3. Analysis of Coating Failure
4. Conclusions
- Uncoated SS 316L specimens reacted during exposure to Zn-Al and Zn-Al-Mg as diffusion layers were detected at the steel-Zn interface, confirming that the use of uncoated SS 316L hardware in the hot-dip galvanising bath is not recommended. In the Zn-Al-Mg bath, the corrosion of SS 316L was less severe than in the Zn-Al bath due to the higher Al content of approximately 10%.
- The Al2O3 coatings were damaged following exposure to molten metal at high temperatures. The difference between the CTEs of Al2O3 and SS 316L was approximately 13 × 10−6 K−1 and led to stress-induced cracking, which could be detrimental to the service life of the bearings, as they provided paths for the liquid Zn alloy to permeate and react with the SS 316L. As a result, spallation and breakdown of the Al2O3 coatings occurred.
- Unlike SS 316L, the Al2O3 coatings showed excellent corrosion resistance after immersion in Zn-Al; however, examination of the coatings immersed in Zn-Al-Mg revealed possible interactions with the molten metal bath due to the reduction of Al2O3 by molten Mg. Therefore, the use of bearings coated with Al2O3 should be limited to hot-dip galvanising baths containing Zn-Al composition in order to avoid coating degradation and the risk of incurring frequent line stops.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gossuin, T.; Moreas, G. Cleanliness Measurement by Innovative Libs Method. In Proceedings of the 12th International Conference on Zinc and Zinc Alloy Coated Steel Sheet (Galvatech), Virtual Conference, Online, 21–23 June 2021. [Google Scholar]
- Beentjes, P.; Bottema, J.; Salgin, B.; Vrenken, J. An Innovative GI with Improved Galling and Surface Properties for Exposed Automotive Applications. In Proceedings of the 12th International Conference on Zinc and Zinc Alloy Coated Steel Sheet, Virtual Conference, Online, 21–23 June 2021. [Google Scholar]
- Tang, N.Y.; Daniel, L.; Zhang, K. Performance of Submerged Hardware in Continuous Galvanizing. Corros. Sci. Technol. 2010, 9, 116–121. [Google Scholar]
- Zhang, K.; Tang, N.Y.; Goodwin, F. Research and development of pot bearings in continuous galvanizing. In Proceedings of the 6th International Conference on Zinc and Zinc Alloy Coated Steel Sheet, Chicago, IL, USA, 4–7 April 2004. [Google Scholar]
- Zhang, K. Effects of test conditions on the tribological behaviour of a journal bearing in molten zinc. Wear 2005, 259, 1248–1253. [Google Scholar] [CrossRef]
- Marder, A.; Goodwin, F. The Metallurgy of Zinc Coated Steels; Elsevier: Cambridge, MA, USA, 2023. [Google Scholar]
- Escott, L.J.; Penney, D.J.; Das, A.; Thomas, D. Effects of Heat Treatments on the Morphology and Mechanical Properties of a CoCrW Alloy for Hot Dip Galvanising Applications. In Proceedings of the 12th International Conference on Zinc and Zinc Alloy Coated Steel Sheet, Virtual Conference, Online, 21–23 June 2021. [Google Scholar]
- Faulkner, R.; Penney, D.; Bright, M. Offline Simulation of Galvanising Bath Journal Bearings as a Cost Effective Solution to Improve Line Performance and Mitigate Risk. In Proceedings of the 12th International Conference on Zinc & Zinc Alloy Coated Steel Sheet, Virtual Conference, Online, 21–23 June 2021. [Google Scholar]
- Matthews, S.J.; James, B. Review of Thermal Spray Coating Applications in the Steel Industry: Part 2—Zinc Pot Hardware in the Continuous Galvanizing Line. J. Therm. Spray Technol. 2010, 19, 1277–1286. [Google Scholar] [CrossRef]
- Shi, Y.; Tong, J.; Zheng, Q.; Rao, S.; Jin, H.; Rao, S. Analysis About the Failure Mechanism of the Sleeves in Sink Roll System. J. Fail. Anal. Prev. 2018, 18, 183–188. [Google Scholar] [CrossRef]
- Nag, A.; Bhadu, M.K.; Bijalwan, P.K.; Pathak, A.S. Investigation of selected HVOF and plasma sprayed coatings for sustained performance in molten zinc. Corros. Sci. 2021, 180, 109177. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, D.; He, J.; Zhang, J.; Li, X. Degradation behaviour of ZrO2–Ni/Al gradient coatings in molten Zn. Surf. Coat. Technol. 2006, 201, 2455–2459. [Google Scholar] [CrossRef]
- Jarosinski, W.; Quets, J.; Wang, D.; Belov, V.; Klryman, A.S. Thermal Spray Coated Rolls for Molten Metal Baths. U.S. Patent 8,507,105 B2, 13 August 2013. [Google Scholar]
- Liu, X.; Zhao, X.; Yang, F. Room-Temperature and High-Temperature Wear Behaviors of As-Sprayed and Annealed Cr3C2-25NiCr Coatings Prepared by High Velocity Air-Fuel Spraying. Coatings 2020, 10, 1090. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.W.; Zhang, L.; Liu, H.J.; Zeng, C.L. Stability investigation of electrodeposited zirconium diboride ceramic coatings in molten zinc. Mater. Corros. 2019, 70, 492–502. [Google Scholar] [CrossRef]
- Chakraborty, A.; Ghassemi-Armaki, H. Evaluation of Initiation and Propagation of LME Cracks on the Galvanised 3G-AHSS Using Interrupted Resistance Spot-Welding Method. In Proceedings of the 12th International Conference on Zinc and Zinc Alloy Coated Steel Sheet, Virtual Conference, Online, 21–23 June 2021. [Google Scholar]
- Gražulis, S.; Chateigner, D.; Downs, R.; Yokochi, A.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography Open Database—An Open-Access Collection of Crystal Structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef]
- Huang, W.; Qiu, H.; Zhang, Y.; Zhang, F.; Gao, L.; Omran, M.; Chen, G. Microstructure and phase transformation behavior of Al2O3–ZrO2 under microwave sintering. Ceram. Int. 2023, 49, 4855–4862. [Google Scholar] [CrossRef]
- Kaunisto, K.; Lagerbom, J.; Honkanen, M.; Varis, T.; Lambai, A.; Mohanty, G.; Levänen, E.; Kivikytö-Reponen, P.; Frankberg, E. Evolution of alumina phase structure in thermal plasma processing. Ceram. Int. 2023, 49, 21346–21354. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, N.-Y.; Goodwin, F.E.; Sexton, S. Reaction of 316L stainless steel with a galvanizing bath. J. Mater. Sci. 2007, 42, 9736–9745. [Google Scholar] [CrossRef]
- Khaliq, A.; Alghamdi, A.S.; Ramadan, M.; Subhani, T.; Rajhi, W.; Haider, W.; Hasan, M.M. Intermetallic Compounds Formation during 316L Stainless Steel Reaction with Al-Zn-Si Coating Alloy. Crystals 2022, 12, 735. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, M.; Wang, J.; Li, F.; Zhu, S.; Wang, F. Enamel coating for protection of the 316 stainless steel against tribo-corrosion in molten zinc alloy at 460 °C. J. Mater. Sci. Technol. 2021, 65, 126–136. [Google Scholar] [CrossRef]
- Liu, X.; Barbero, E.; Xu, J.; Burris, M.; Chang, K.-M.; Sikka, V. Liquid metal corrosion of 316L, Fe3Al, and FeCrSi in molten Zn-Al baths. Metall. Mater. Trans. A 2005, 36, 2049–2058. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, M.; Li, F.; Zhu, S.; Wang, F. Synergistic effect of corrosion and wear of the 316 stainless steel in molten zinc alloy at 460 °C. Corros. Sci. 2020, 165, 108411. [Google Scholar] [CrossRef]
- Kuperus, M. The Delamination Process of the Dross Build-Up Structure on Submerged Hardware in Zn-Al and Zn-Mg-Al Baths: An Empirical Study. Master’s Thesis, Delft University of Technology, Delft, The Netherlands, 2018. [Google Scholar]
- Marder, A.R. The metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Bright, M.A. Dissolution and Diffusion Characteristics of 316L Stainless Steel in Molten Zinc Containing Variable Concentrations of Aluminum. Master’s Thesis, West Virginia University, Morgantown, WV, USA, 2007. [Google Scholar]
- Liu, J.; Suryanarayana, C.; Ghosh, D.; Subhash, G.; An, L. Synthesis of Mg–Al2O3 nanocomposites by mechanical alloying. J. Alloys Compd. 2013, 563, 165–170. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Babamiri, B.B.; Zhou, Y.; Dargusch, M.; Hazeli, K.; Zhang, M.-X. Enhancing specific energy absorption of additively manufactured titanium lattice structures through simultaneous manipulation of architecture and constituent material. Addit. Manuf. 2022, 55, 102887. [Google Scholar] [CrossRef]
- University of Cambridge. Dissemination of IT for the Promotion of Materials Science (DoITPoMS). Available online: https://www.doitpoms.ac.uk/tlplib/ellingham_diagrams/ellingham.php (accessed on 19 March 2024).
- Noda, N.-A.; Yamada, M.; Sano, Y.; Sugiyama, S.; Kobayashi, S. Thermal stress for all-ceramics rolls used in molten metal to produce stable high quality galvanized steel sheet. Eng. Fail. Anal. 2008, 15, 261–274. [Google Scholar] [CrossRef]
- Mizuno, H.; Kitamura, J. MoB/CoCr Cermet Coatings by HVOF Spraying against Erosion by Molten Al-Zn Alloy. J. Therm. Spray Technol. 2007, 16, 404–413. [Google Scholar] [CrossRef]
- Huang, W.; Zhang, Y.; Lu, J.; Gao, L.; Zhang, F.; Chen, J.; Omran, M.; Chen, G. Effect of sintering time on the microstructure and stability of Al2O3–ZrO2 composite powders under microwave-assisted sintering. Ceram. Int. 2023, 49, 8993–8999. [Google Scholar] [CrossRef]
- Mizuno, H.; Kitamura, J. Thermal Spray Coating and Thermal Spray Powder. U.S. Patent 7,862,911 B2, 4 January 2011. [Google Scholar]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alparone, G.P.; Penney, D.; Sullivan, J.; Edy, J.; Mills, C. Al2O3 Coatings for Protection of Stainless Steel 316L against Corrosion in Zn-Al and Zn-Al-Mg. Coatings 2024, 14, 606. https://doi.org/10.3390/coatings14050606
Alparone GP, Penney D, Sullivan J, Edy J, Mills C. Al2O3 Coatings for Protection of Stainless Steel 316L against Corrosion in Zn-Al and Zn-Al-Mg. Coatings. 2024; 14(5):606. https://doi.org/10.3390/coatings14050606
Chicago/Turabian StyleAlparone, Giovanni Paolo, David Penney, James Sullivan, James Edy, and Christopher Mills. 2024. "Al2O3 Coatings for Protection of Stainless Steel 316L against Corrosion in Zn-Al and Zn-Al-Mg" Coatings 14, no. 5: 606. https://doi.org/10.3390/coatings14050606
APA StyleAlparone, G. P., Penney, D., Sullivan, J., Edy, J., & Mills, C. (2024). Al2O3 Coatings for Protection of Stainless Steel 316L against Corrosion in Zn-Al and Zn-Al-Mg. Coatings, 14(5), 606. https://doi.org/10.3390/coatings14050606




