Enhancing Uptake Capability of Green Carbon Black Recycled from Scrap Tires for Water Purification
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of GCB
2.3. Adsorption Test for CB, GCB, and Acid Treated GCB
2.4. Characterization
3. Results
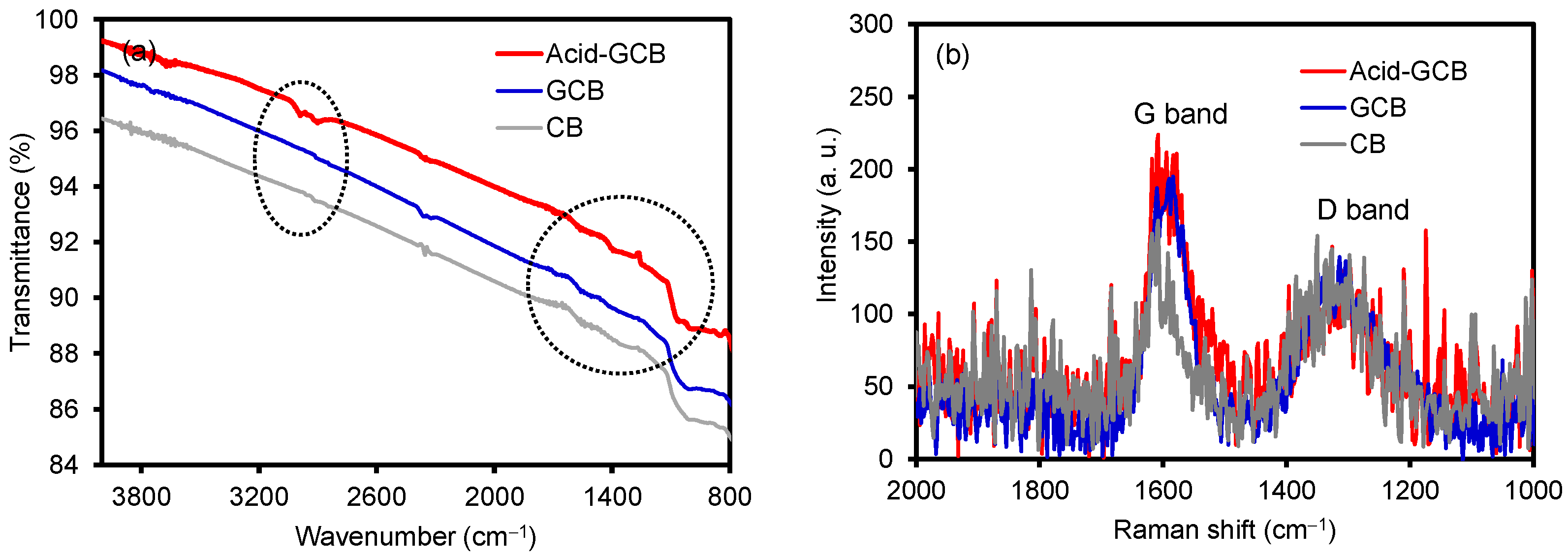
3.1. Structural and Compositional Properties of CB, GCB, and Acid-Treated GCB
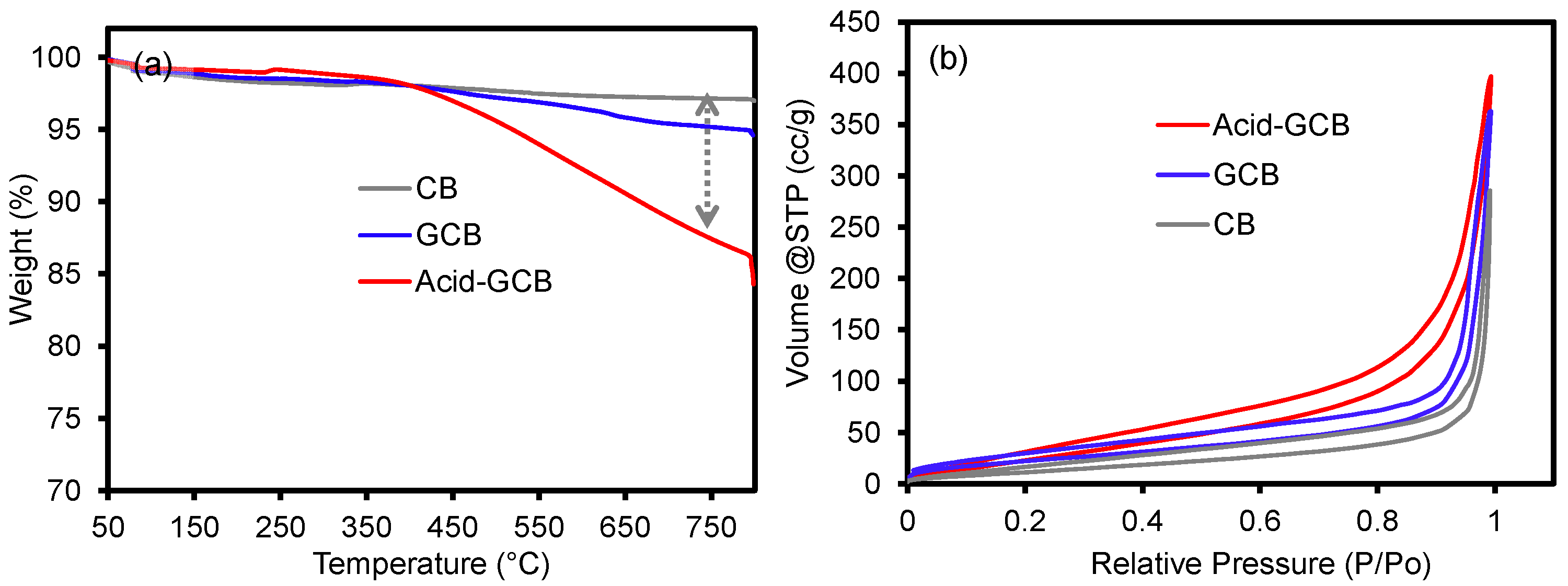
3.2. Thermal and Adsorption Properties of CB, GCB, and Acid-Treated GCB
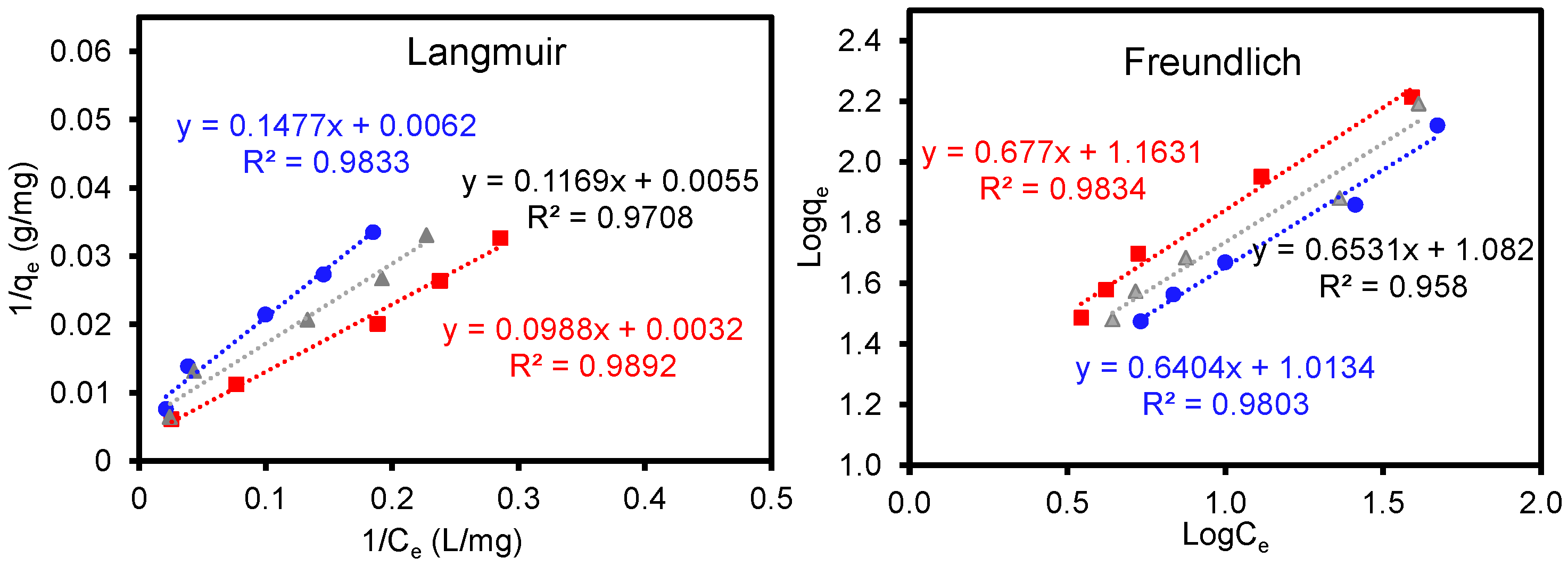
3.3. Removal of Organic Dyes and Metal Ions Using CB, GCB, and Acid-Treated GCB (0.5 M Acetic Acid)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burgaz, E.; Gencoglu, O.; Göksüzoğlu, M. Carbon black reinforced natural rubber/butadiene rubber and natural rubber/butadiene rubber/styrene-butadiene rubber composites: Part II. Dynamic mechanical properties and fatigue behavior. Res. Eng. Struct. Mater. 2019, 5, 233–247. [Google Scholar] [CrossRef]
- Khudoynazarov, F.; Nurmanov, S.; Yakubo, Y. The composition and thermodynamic properties of pyrolytic carbon black. Int. J. Mater. Chem. 2022, 12, 32–38. [Google Scholar]
- Saini, D.; Gunture; Kaushik, J.; Aggarwal, R.; Tripathi, K.M.; Sonkar, S.K. Carbon nanomaterials derived from black carbon soot: A review of materials and applications. ACS Appl. Nano Mater. 2021, 4, 12825–12844. [Google Scholar] [CrossRef]
- Toth, P.; Vikström, T.; Molinder, R.; Wiinikka, H. Structure of carbon black continuously produced from biomass pyrolysis oil. Green Chem. 2018, 20, 3981–3992. [Google Scholar] [CrossRef]
- Wang, J.; Man, H.; Sun, L.; Zang, S. Carbon black: A good adsorbent for triclosan removal from water. Water 2022, 14, 576. [Google Scholar] [CrossRef]
- Costa, S.M.R.; Fowler, D.; Carreira, G.A.; Portugal, I.; Silva, C.M. Production and upgrading of recovered carbon black from the pyrolysis of end-of-life tires. Materials 2022, 15, 2030. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, C.; Manjare, S.; Rajan, S.K. Recycling of waste tire by pyrolysis to recover carbon black: Alternative & environment-friendly reinforcing filler for natural rubber compounds. Compos. Part B 2020, 200, 108346. [Google Scholar]
- González-González, R.B.; González, L.T.; Iglesias-González, S.; González-González, E.; Martinez-Chapa, S.O.; Madou, M.; Alvarez, M.M.; Mendoza, A. Characterization of chemically activated pyrolytic carbon black derived from waste tires as a candidate for nanomaterial precursor. Nanomaterials 2020, 10, 2213. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Kim, J.-H.; Park, H.-H. Upcycling green carbon black as a reinforcing agent for styrene–butadiene rubber materials. RSC Adv. 2022, 12, 30480–30486. [Google Scholar] [CrossRef]
- Yang, F.; Liang, S.; Wu, H.; Yue, C.; Yan, H.; Wu, H.; Chen, X.; Zhang, J.; Yan, S.; Duan, Y. Upgrading the pyrolysis carbon black from waste tire by hybridization with cellulose. Ind. Eng. Chem. Res. 2022, 61, 6512–6520. [Google Scholar] [CrossRef]
- Alkhabbas, M.; Al-Ma’abreh, A.M.; Edris, G.; Saleh, T.; Alhmood, H. Adsorption of anionic and cationic dyes on activated carbon prepared from oak cupules: Kinetics and thermodynamics studies. Int. J. Environ. Res. Public Health 2023, 20, 3280. [Google Scholar] [CrossRef] [PubMed]
- Suhas; Kushwaha, S.; Tyagi, I.; Ahmed, J.; Chaudhary, S.; Chaudhary, M.; Inbaraj, B.S.; Goscianska, J.; Karri, R.R.; Sridhar, K. Adsorptive analysis of azo dyes on activated carbon prepared from Phyllanthus emblica fruit stone sequentially via hydrothermal treatment. Agronomy 2022, 12, 2134. [Google Scholar] [CrossRef]
- Valdivia, A.E.O.; Osorio, C.M.; Rodríguez, Y.M.V. Preparation of activated carbon from coffee waste as an adsorbent for the removal of chromium (III) from water. Optimization for an experimental Box-Behnken design. Chemistry 2020, 2, 2–10. [Google Scholar] [CrossRef]
- Wu, H.; Sun, W.; Wei, H.; Zhao, Y.; Jin, C.; Yang, X.; Rong, X.; Sun, C. Efficient removal of acetic acid by a regenerable resin-based spherical activated carbon. Water Sci. Technol. 2021, 84, 679–711. [Google Scholar] [CrossRef] [PubMed]
- El-Maadawy, M.M.; Elzoghby, A.A.; Masoud, A.M.; El-Deeb, Z.M.; El Naggar, A.M.A.; Taha, M.H. Conversion of carbon black recovered from waste tires into activated carbon via chemical/microwave methods for efficient removal of heavy metal ions from wastewater. RSC Adv. 2024, 14, 6324–6338. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.C.K.; Mui, E.L.K.; Lau, K.S.T.; McKay, G. Production of activated carbons from waste tire—Process design and economical analysis. Waste Manag. 2004, 24, 875–888. [Google Scholar] [CrossRef] [PubMed]
- Acocella, M.R.; Maggio, M.; Ambrosio, C.; Aprea, N.; Guerra, G. Oxidized carbon black as an activator of transesterification reactions under solvent-free conditions. ACS Omega 2017, 2, 7862–7867. [Google Scholar] [CrossRef]
- Dong, P.; Maneerung, T.; Ng, W.C.; Zhen, X.; Dai, Y.; Tong, Y.W.; Ting, Y.-P.; Koh, S.N.; Wang, C.-H.; Neoh, K.G. Chemically treated carbon black waste and its potential applications. J. Hazard. Mater. 2017, 321, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Park, M.; Park, S.-J. Current progress on the surface chemical modification of carbonaceous materials. Coatings 2019, 9, 103. [Google Scholar] [CrossRef]
- Bakr, A.M.; McBain, J.W. The sorption of toluene and acetic acid and their mixtures by carbon. J. Am. Chem. Soc. 1924, 46, 2718–2725. [Google Scholar] [CrossRef]
- Ghosh, D.; Singha, P.S.; Firdaus, S.B.; Ghosh, S. Metanil yellow: The toxic food colorant. Asian Pac. J. Health Sci. 2017, 4, 65–66. [Google Scholar] [CrossRef]
- Mani, S.; Bharagava, R.N. Exposure to crystal violet, its toxic, genotoxic and carcinogenic effects on environment and its degradation and detoxification for environmental safety. In Reviews of Environmental Contamination and Toxicology; de Voogt, W.P., Ed.; Springer: Cham, Switzerland, 2016; Volume 237, pp. 71–104. [Google Scholar]
- Yuan, D.; Yang, K.; Zhu, E.; Li, X.; Sun, M.; Xiao, L.; Hari, Q.; Tang, S. Peracetic acid activated with electro-Fe2+ process for dye removal in water. Coatings 2022, 12, 466. [Google Scholar] [CrossRef]
- Matthews, T.; Majoni, S.; Nyoni, B.; Naidoo, B.; Chiririwa, H. Adsorption of lead and copper by a carbon black and sodium bentonite composite material: Study on adsorption isotherms and kinetics. Iran. J. Chem. Chem. Eng. 2019, 38, 101–109. [Google Scholar]
- Williams, J.H.; Gbadomosi, M.; Greytak, A.B.; Myrick, M.L. Measuring the surface area of carbon black using BET isotherms: An experiment in physical chemistry. J. Chem. Educ. 2023, 100, 4838–4844. [Google Scholar] [CrossRef]
- Zhai, Q.-Z. Studies of adsorption of crystal violet from aqueous solution by nano mesocellular foam silica: Process equilibrium, kinetic, isotherm, and thermodynamic studies. Water Sci. Technol. 2020, 81, 2092–2108. [Google Scholar] [CrossRef]
- da Silva, E.L.; Vega, M.R.O.; dos Santos Correa, P.; Cuna, A.; Tancredi, N.; de Fraga Malfatti, C. Influence of activated carbon porous texture on catalyst activity for ethanol electro-oxidation. Int. J. Hydrogen Energy 2014, 39, 14760–14767. [Google Scholar] [CrossRef]
- Guo, Y.; Zheng, Y.; Huang, M. Enhanced activity of PtSn/C anodic electrocatalyst prepared by formic acid reduction for direct ethanol fuel cells. Electrochim. Acta 2008, 53, 3102–3108. [Google Scholar] [CrossRef]
- Sugatri, R.I.; Wirasadewa, Y.C.; Saputro, K.E.; Muslih, E.Y.; Ikono, R.; Nasir, M. Recycled carbon black from waste of tire industry: Thermal study. Microsyst. Technol. 2018, 24, 749–755. [Google Scholar] [CrossRef]
- Colomer, M.T. Straightforward synthesis of Ti-doped YSZ gels by chemical modification of the precursors alkoxides. J. Sol-Gel. Sci. Technol. 2013, 67, 135–144. [Google Scholar] [CrossRef]
- Gillibert, R.; Magazzù, A.; Callegari, A.; Bronte-Ciriza, D.; Foti, A.; Donato, M.G.; Maragò, O.M.; Volpe, G.; de La Chapelle, M.L.; Lagarde, F.; et al. Raman tweezers for tire and road wear micro- and nanoparticles analysis. Environ. Sci. Nano 2022, 9, 145–161. [Google Scholar] [CrossRef]
- Jankovská, Z.; Vecer, M.; Koutník, I.; Matejová, L. A case study of waste scrap tyre-derived carbon black tested for nitrogen, carbon dioxide, and cyclohexane adsorption. Molecules 2020, 25, 4445. [Google Scholar] [CrossRef]
- Kamran, U.; Park, S.-J. Acetic acid-mediated cellulose-based carbons: Influence of activation conditions on textural features and carbon dioxide uptakes. J. Colloid Interface Sci. 2021, 594, 745–758. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Ganesan, M.; Ambalavanan, S. An in situ generated carbon as integrated conductive additive for hierarchical negative plate of lead-acid battery. J. Power Sources 2014, 251, 20–29. [Google Scholar] [CrossRef]
- Kirakosyan, A.; Lee, D.; Choi, Y.; Jung, N.; Choi, J. Poly(styrene sulfonic acid)-grafted carbon black synthesized by surface-initiated atom transfer radical polymerization. Molecules 2023, 28, 4168. [Google Scholar] [CrossRef] [PubMed]
- Pantea, D.; Darmstadt, H.; Kaliaguine, S.; Roy, C. Electrical conductivity of conductive carbon blacks: Influence of surface chemistry and topology. Appl. Surf. Sci. 2003, 217, 181–193. [Google Scholar] [CrossRef]
- Blanco, Y.S.; Topel, O.; Bajnoczi, E.G.; Werner, J.; Bjorneholm, O.; Persson, I. Chemical equilibria of aqueous ammonium–carboxylate systems in aqueous bulk, close to and at the water–air interface. Phys. Chem. Chem. Phys. 2019, 21, 12434–12445. [Google Scholar] [CrossRef]
- Nguyen, H.K.K.; Addou, R.; Chukwu, K.C.; Herman, G.S.; Árnadóttir, L. Ambient-pressure X-ray photoelectron spectroscopy study of acetic acid thermal decomposition on Pd (111). J. Phys. Chem. C 2023, 127, 11472–11480. [Google Scholar] [CrossRef]
- Ahn, D.; Choi, H.-J.; Kim, H.-D.; Yeo, S.Y. Properties of conductive polyacrylonitrile fibers prepared by using benzoxazine modified carbon black. Polymers 2020, 12, 179. [Google Scholar] [CrossRef]
- Kim, M.I.; Cho, J.H.; Bai, B.C.; Im, J.S. The control of volume expansion and porosity in carbon block by carbon black (CB) addition for increasing thermal conductivity. Appl. Sci. 2020, 10, 6068. [Google Scholar] [CrossRef]
- Thonglhueng, N.; Sirisangsawang, R.; Sukpancharoen, S.; Phetyim, N. Optimization of iodine number of carbon black obtained from waste tire pyrolysis plant via response surface methodology. Heliyon 2022, 8, e11971. [Google Scholar] [CrossRef]
- Gomez-Hernandez, R.; Panecatl-Bernal, Y.; Mendez-Rojas, M.A. High yield and simple one-step production of carbon black nanoparticles from waste tires. Heliyon 2019, 5, e02139. [Google Scholar] [CrossRef]
- Bernal, R.A.O.; Olekhnovich, R.O.; Uspenskaya, M.V. Influence of thermal treatment and acetic acid concentration on the electroactive properties of chitosan/PVA-based micro- and nanofibers. Polymers 2023, 15, 3719. [Google Scholar] [CrossRef]
- Rađenović, A.; Malina, J. Adsorption ability of carbon black for nickel ions uptake from aqueous solution. Hem. Ind. 2013, 67, 51–58. [Google Scholar] [CrossRef]
- Nath, P.P.; Sarkar, K.; Tarafder, P.; Paul, G. Development of a visible spectrophotometric method for the quantitative determination of metanil yellow in different food samples. Int. J. Pharma Bio. Sci. 2013, 4, 685–692. [Google Scholar]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Jia, R.-L.; Wang, C.-Y. Adsorption of PtCl62− anions on the surface of carbon black. React. Kinet. Catal. Lett. 2006, 88, 51–56. [Google Scholar] [CrossRef]
- Legocka, I.; Kusmierek, K.; Swiatkowski, A.; Wierzbicka, E. Adsorption of 2,4-D and MCPA herbicides on carbon black modified with hydrogen peroxide and aminopropyltriethoxysilane. Materials 2022, 15, 8433. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Kang, J.; Yang, H.; Yoon, S.; Kim, J.-H.; Park, H.-H. Enhancing Uptake Capability of Green Carbon Black Recycled from Scrap Tires for Water Purification. Coatings 2024, 14, 389. https://doi.org/10.3390/coatings14040389
Choi J, Kang J, Yang H, Yoon S, Kim J-H, Park H-H. Enhancing Uptake Capability of Green Carbon Black Recycled from Scrap Tires for Water Purification. Coatings. 2024; 14(4):389. https://doi.org/10.3390/coatings14040389
Chicago/Turabian StyleChoi, Jiho, Jihyun Kang, Huiseong Yang, Sangin Yoon, Jun-Hyun Kim, and Hyun-Ho Park. 2024. "Enhancing Uptake Capability of Green Carbon Black Recycled from Scrap Tires for Water Purification" Coatings 14, no. 4: 389. https://doi.org/10.3390/coatings14040389
APA StyleChoi, J., Kang, J., Yang, H., Yoon, S., Kim, J.-H., & Park, H.-H. (2024). Enhancing Uptake Capability of Green Carbon Black Recycled from Scrap Tires for Water Purification. Coatings, 14(4), 389. https://doi.org/10.3390/coatings14040389






