CoMoO4 Nanoflowers Doped with La Element for Advanced Electrode Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of CoMoO4 Material
2.2. Preparation of La-CoMoO4 Material
2.3. Preparation of CNTs Electrode Materials
2.4. Assembly of Components
2.5. Material Characterization
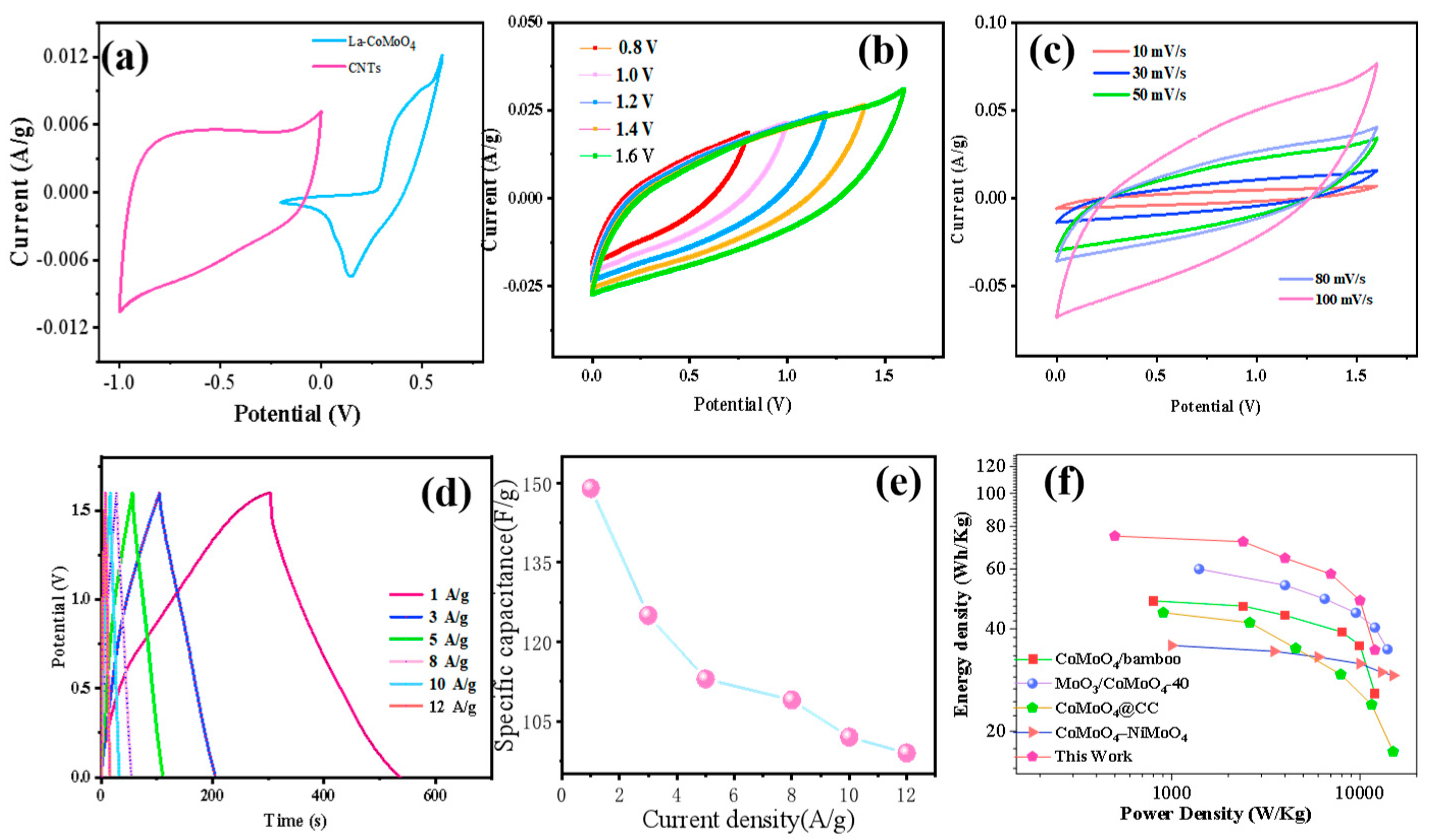
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahman, M.M.; Oni, A.O.; Gemechu, E.; Kumar, A. Assessment of energy storage technologies: A review. Energy Convers. Manag. 2020, 223, 11329. [Google Scholar] [CrossRef]
- Shen, X.; Zhang, X.-Q.; Ding, F.; Huang, J.-Q.; Xu, R.; Chen, X.; Yan, C.; Su, F.-Y.; Chen, C.-M.; Liu, X.; et al. Advanced electrode materials in lithium batteries: Retrospect and prospect. Energy Mater. Adv. 2021, 2021, 1205324. [Google Scholar] [CrossRef]
- Rajkumar, S.; Gowri, S.; Dhineshkumar, S.; Merlin, J.P.; Sathiyan, A. Investigation on NiWO4/PANI composite as an electrode material for energy storage devices. New J. Chem. 2021, 45, 20612–20623. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Q.; Gao, S.; Tang, G.; Liu, K.; He, S.; Huang, C. Biomass derived carbon as binder-free electrode materials for supercapacitors. Carbon 2019, 155, 706–726. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, Y.; Cheng, T.; Lai, W.Y.; Pang, H.; Huang, W. Flexible supercapacitors based on paper substrates: A new paradigm for low-cost energy storage. Chem. Soc. Rev. 2015, 44, 5181–5199. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Zhao, Z.; Hu, Z.; Liu, X.; Yu, G. Flexible sodium-ion based energy storage devices: Recent progress and challenges. Energy Storage Mater. 2020, 26, 83–104. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.; Zhang, X.; Li, X.; Zhang, G.; Cao, P. 3D printed micro-electrochemical energy storage devices: From design to integration. Adv. Funct. Mater. 2021, 31, 2104909. [Google Scholar] [CrossRef]
- Xu, T.; Du, H.; Liu, H.; Liu, W.; Zhang, X.; Si, C.; Liu, P.; Zhang, K. Advanced nanocellulose-based composites for flexible functional energy storage devices. Adv. Mater. 2021, 33, 2101368. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zang, W.; Li, J.; Jiang, Y.; Zou, H.; Ning, N.; Tian, M.; Zhang, L. CB/PDMS electrodes for dielectric elastomer generator with low energy loss, high energy density and long life. Compos. Commun. 2022, 31, 101132. [Google Scholar] [CrossRef]
- Song, L.; Zheng, T.; Zheng, L.; Lu, B.; Chen, H.; He, Q.; Zheng, W.; Hou, Y.; Lian, J.; Wu, Y.; et al. Cobalt-doped basic iron phosphate as bifunctional electrocatalyst for long-life and high-power-density rechargeable zinc-air batteries. Appl. Catal. B Environ. 2022, 300, 120712. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Najib, S.; Erdem, E. Current progress achieved in novel materials for supercapacitor electrodes: Mini review. Nanoscale Adv. 2019, 1, 2817–2827. [Google Scholar] [CrossRef]
- Chen, G.Z. Supercapacitor and supercapattery as emerging electrochemical energy stores. Int. Mater. Rev. 2017, 62, 173–202. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Han, Y.; Li, T. Flexible supercapacitor: Overview and outlooks. J. Energy Storage 2021, 42, 103053. [Google Scholar] [CrossRef]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Mevada, C.; Mukhopadhyay, M. Limitations and recent advances in high mass loading asymmetric supercapacitors based on pseudocapacitive materials. Ind. Eng. Chem. Res. 2021, 60, 1096–1111. [Google Scholar] [CrossRef]
- Wu, M.; Yu, S.; Wang, X.; Li, L. Ultra-high energy storage density and ultra-wide operating temperature range in Bi2Zn2/3Nb4/3O7 thin film as a novel lead-free capacitor. J. Power Sources 2021, 497, 229879. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Liu, B.; Su, Z. A critical review on the application and recent developments of post-modified biochar in supercapacitors. J. Clean. Prod. 2021, 310, 127428. [Google Scholar] [CrossRef]
- Liu, S.; Xu, Y.; Wu, J.; Huang, J. Huang Celery-derived porous carbon materials for superior performance supercapacitors. Nanoscale Adv. 2021, 3, 5363–5372. [Google Scholar] [CrossRef]
- Liu, X.; Liu, L.; Yan, W.; Wang, Y.; Huang, C.; Wang, Z. Hierarchical Fe3O4@FeS2 nanocomposite as high-specific-capacitance electrode material for supercapacitors. Energy Technol. 2020, 8, 2000544. [Google Scholar] [CrossRef]
- Forouzandeh, P.; Kumaravel, V.; Pillai, S.C. Electrode materials for supercapacitors: A review of recent advances. Catalysts 2020, 10, 969. [Google Scholar] [CrossRef]
- Abazari, R.; Sanati, S.; Morsali, A.; Dubal, D.P. High specific capacitance of a 3D-metal–organic framework-confined growth in CoMn2O4 nanostars as advanced supercapacitor electrode materials. J. Mater. Chem. A 2021, 9, 11001–11012. [Google Scholar] [CrossRef]
- Xiong, S.; He, Y.; Zhang, X.; Wu, B.; Chu, J.; Wang, X.; Zhang, R.; Gong, M.; Li, Z.; Chen, Z. Hydrothermal synthesis of high specific capacitance electrode material using porous bagasse biomass carbon hosting MnO2 nanospheres. Biomass Convers. Biorefinery 2021, 11, 1325–1334. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Moore, J.; Nagaiah, N.; Zhai, L.; Jung, Y.; Thomas, J. Asymmetric supercapacitor electrodes and devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhou, D.; Pang, L.; Sun, S.; Zhou, T.; Su, J. Perspectives on working voltage of aqueous supercapacitors. Small 2022, 18, 2106360. [Google Scholar] [CrossRef]
- Song, F.; Ao, X.; Chen, Q. Effect of heteroatom doping on the charge storage and operating voltage window of nickel-based sulfide composite electrodes in alkaline electrolytes. Chem. Eng. J. 2022, 427, 130885. [Google Scholar] [CrossRef]
- Gong, K.; Lee, H.; Choi, Y.; Jung, G.; Keum, K.; Kim, J.W.; Ha, J.S. A flexible supercapacitor with high energy density and wide range of temperature tolerance using a high-concentration aqueous gel electrolyte. Electrochim. Acta 2024, 475, 143585. [Google Scholar] [CrossRef]
- nil Kumar, Y.; Yadav, A.A.; Al-Asbahi, B.A.; Kang, S.W.; Moniruzzaman, M. Sulfur nanoparticle-decorated nickel cobalt sulfide hetero-nanostructures with enhanced energy storage for high-performance supercapacitors. Molecules 2022, 27, 7458. [Google Scholar] [CrossRef] [PubMed]
- Kadam, V.S.; Jagtap, C.V.; Lokhande, P.E.; Bulakhe, R.N.; Kang, S.W.; Yadav, A.A.; Pathan, H.M. One-step deposition of nanostructured Ni(OH)2/rGO for supercapacitor applications. J. Mater. Sci. Mater. Electron. 2023, 34, 1083. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Ko, S.; Kang, S.W. Chemically synthesized iron-oxide-based pure negative electrode for solid-state asymmetric supercapacitor devices. Materials 2022, 15, 6133. [Google Scholar] [CrossRef]
- Wang, J.; Chang, J.; Wang, L.; Hao, J. One-step and low-temperature synthesis of CoMoO4 nanowire arrays on Ni foam for asymmetric supercapacitors. Ionics 2018, 24, 3967–3973. [Google Scholar] [CrossRef]
- Ilayas, T.; Anjum, S.; Raja, M.Y.A.; Khurram, R.; Sattar, M.; Mansoor, A. Rietveld refinement, 3D view and electrochemical properties of rare earth lanthanum doped nickel ferrite to fabricate high performance electrodes for supercapacitor applications. Ceram. Int. 2023, 49, 28864–28877. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Durai, G.; Tatarchuk, T.; Sumathi, M.; Kuppusami, P.; Qin, J.; Choi, M.Y. Synthesis of hierarchical structured rare earth metal–doped Co3O4 by polymer combustion method for high performance electrochemical supercapacitor electrode materials. Ionics 2020, 26, 2051–2061. [Google Scholar] [CrossRef]
- He, W.X.; Jiang, S.; Pang, M.G.; Li, J.W.; Pang, M.; Mao, M.M.; Wang, R.W.; Yang, H.; Pan, Q.L.; Zhao, J. A free-standing NiMoO4@Mg-Co(OH)F core-shell nanocomposites supported on Ni foam for asymmetric supercapacitor applications. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130883. [Google Scholar] [CrossRef]
- Wang, G.G.; Chen, Q.Y.; Zhang, J.; An, X.G.; Liu, Q.; Xie, L.S.; Yao, W.T.; Sun, X.P.; Kong, Q.Q. NiMoO4 nanorods with oxygen vacancies self-supported on Ni foam towards high-efficiency electrocatalytic conversion of nitrite to ammonia. J. Colloid Interface Sci. 2023, 647, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Wang, X.E.; Zhang, X.; Wang, B.; Li, Y.; Zeng, X.; Zhang, X.F.; Fan, M.Q.; Yang, X.D. Designed formation of hierarchical core-shell NiCo2S4@NiMoO4 arrays on cornstalk biochar as battery-type electrodes for hybrid supercapacitors. J. Alloys Compd. 2023, 937, 168403. [Google Scholar] [CrossRef]
- Sun, Y.S.; Liu, Z.Q.; Zheng, X.Z.; Wang, C.X.; Wang, J.H.; Jiang, M.Y.; Jiang, D.G.; Liu, J.Q. Construction of KCu7S4@NiMoO4 three-dimensional core-shell hollow structure with high hole mobility and fast ion transport for high-performance hybrid supercapacitors. Compos. Part B Eng. 2023, 249, 110409. [Google Scholar] [CrossRef]
- Chen, H.; Hu, H.; Han, F.; Liu, J.; Zhang, Y.; Zheng, Y. CoMoO4/bamboo charcoal hybrid material for high-energy-density and high cycling stability supercapacitors. Dalton Trans. 2020, 49, 10799–10807. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Zhou, H.; Kang, M. POM-derived MoO3/CoMoO4 mixed oxides directed by glucose for high-performance supercapacitors. New J. Chem. 2022, 46, 16914–16921. [Google Scholar] [CrossRef]
- Chen, C.; Deng, H.; Wang, C.; Luo, W.; Huang, D.; Jin, T. Petal-like CoMoO4 clusters grown on carbon cloth as a binder-free electrode for supercapacitor application. ACS Omega 2021, 6, 19616–19622. [Google Scholar] [CrossRef]
- Wang, J.; Hong, W.; Fu, Y. Urea-assisted synthesis of CoMoO4–NiMoO4 microspheres for the electrochemical energy storage. Ionics 2023, 29, 3077–3086. [Google Scholar] [CrossRef]




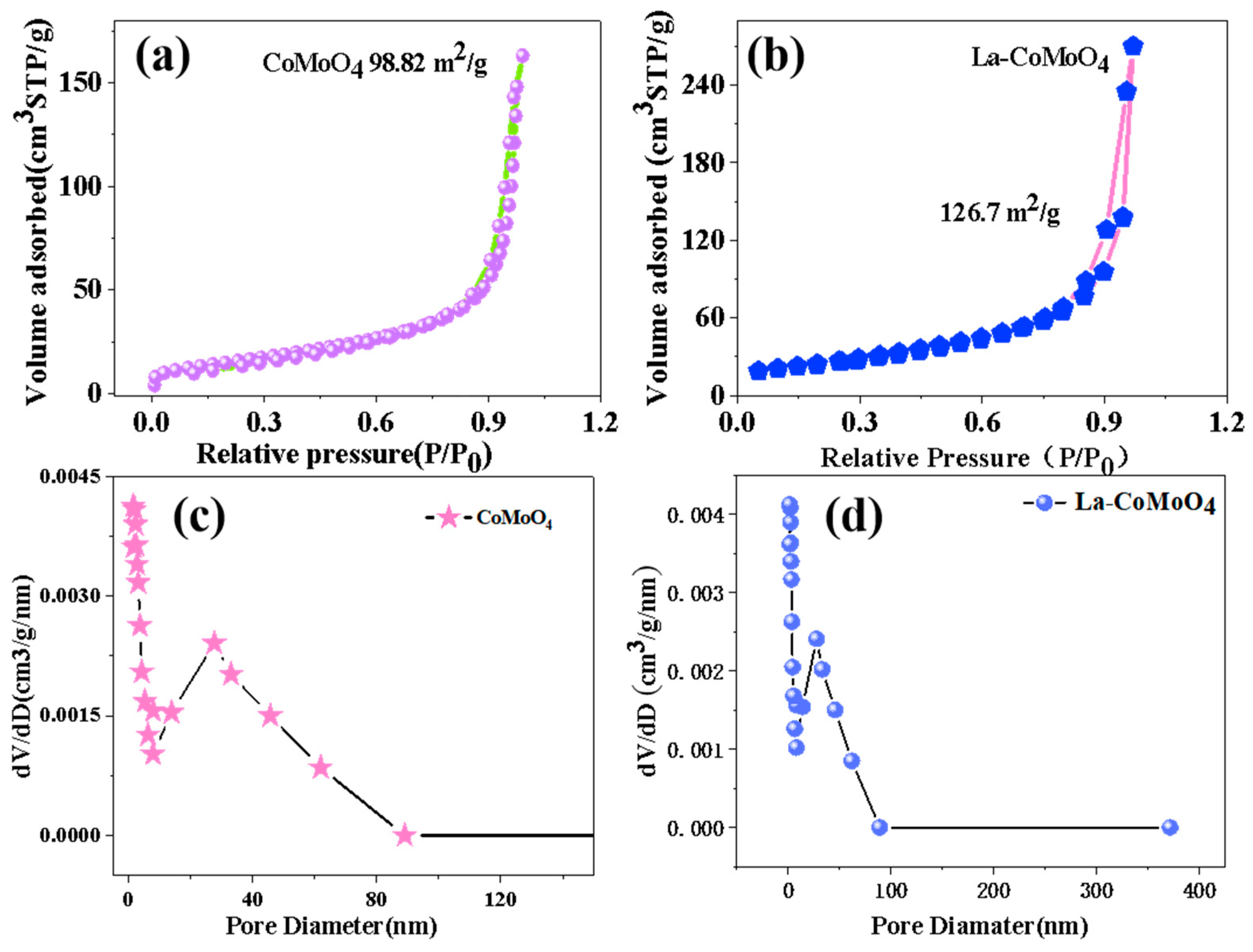
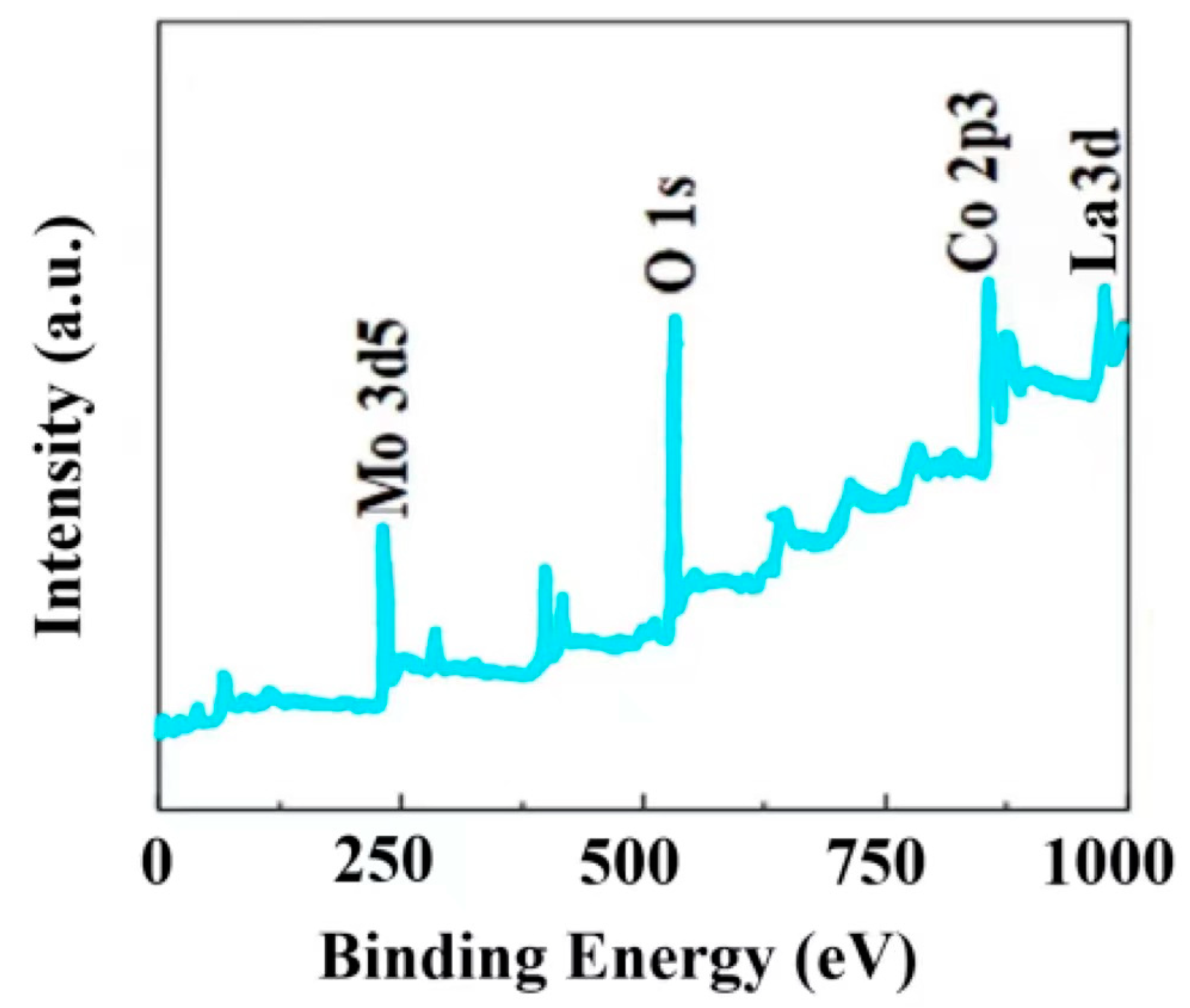
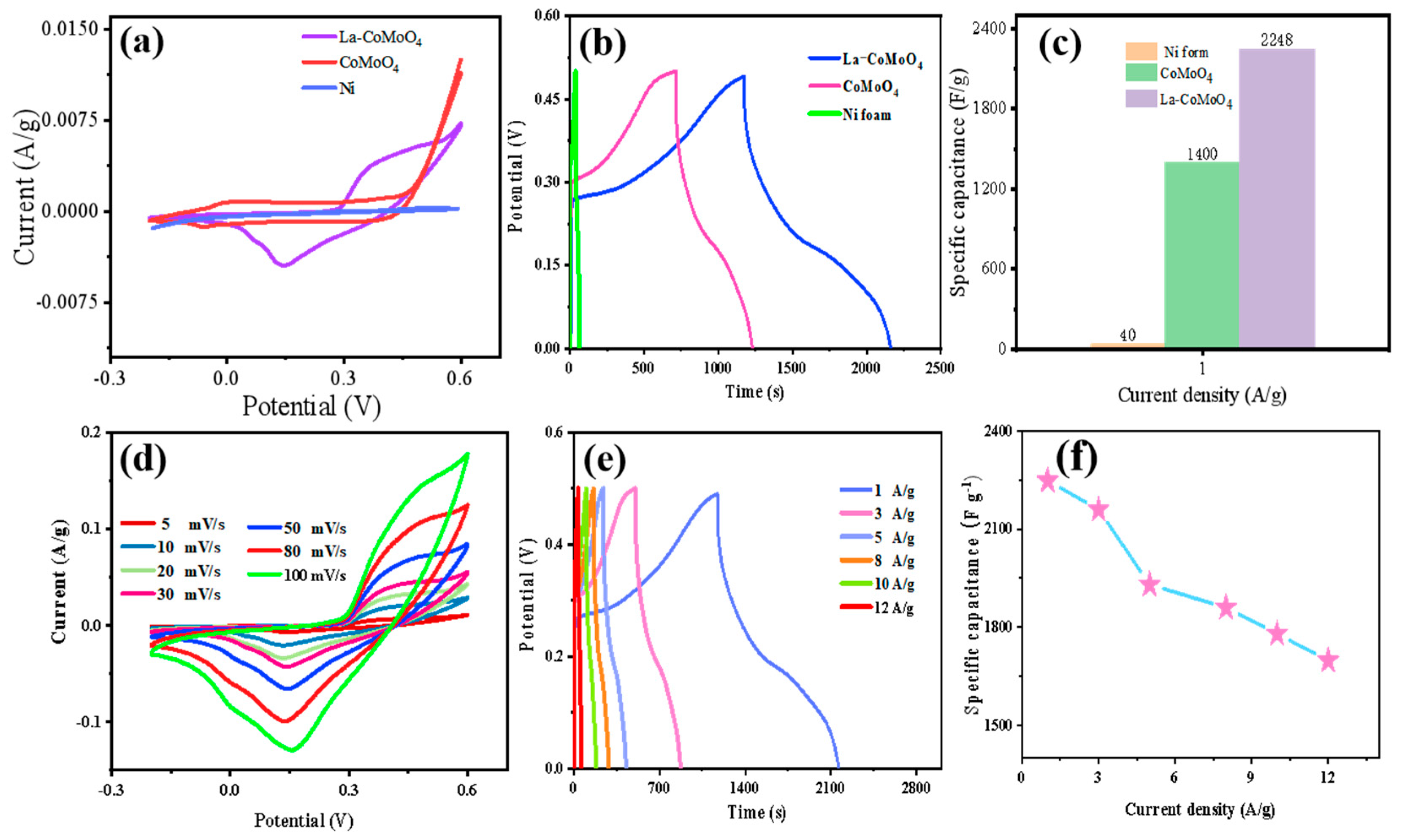
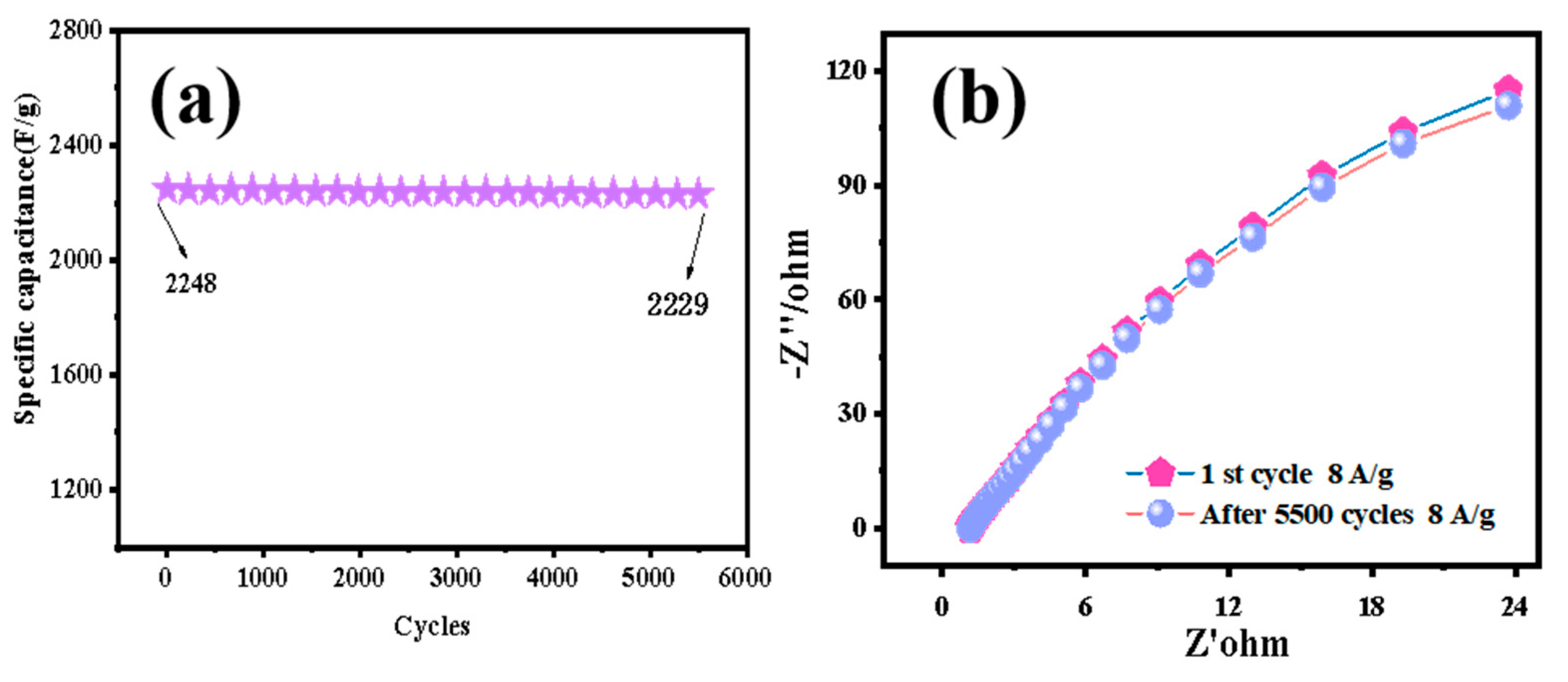

| Electrode Material | Current Density [1 A/g] | Specific Capacitance [F/g] | Number of Cycles | Capacity Retention Rate (%) | Ref |
|---|---|---|---|---|---|
| KCu7S4@NiMoO4 | 1 | 1194.6 | 10,000 | 92.3% | [34] |
| NiMoO4@Mg-Co(OH)F/NF | 1 | 1630 | 6000 | 86% | [35] |
| NiCo2S4@NiMoO4 | 5 | 1447 | 10,000 | 88% | [36] |
| CNTs/NiMoO4 | 20 | 727.2 | 2000 | 86.8% | [37] |
| La-CoMoO4 | 1 | 2248 | 5500 | 99.2% | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Liu, Y.; Li, D.; Ma, T.; Wang, J. CoMoO4 Nanoflowers Doped with La Element for Advanced Electrode Materials. Coatings 2024, 14, 388. https://doi.org/10.3390/coatings14040388
Chen D, Liu Y, Li D, Ma T, Wang J. CoMoO4 Nanoflowers Doped with La Element for Advanced Electrode Materials. Coatings. 2024; 14(4):388. https://doi.org/10.3390/coatings14040388
Chicago/Turabian StyleChen, Donghua, Yang Liu, Danting Li, Tenghao Ma, and Jing Wang. 2024. "CoMoO4 Nanoflowers Doped with La Element for Advanced Electrode Materials" Coatings 14, no. 4: 388. https://doi.org/10.3390/coatings14040388
APA StyleChen, D., Liu, Y., Li, D., Ma, T., & Wang, J. (2024). CoMoO4 Nanoflowers Doped with La Element for Advanced Electrode Materials. Coatings, 14(4), 388. https://doi.org/10.3390/coatings14040388






