Hydrophobic Modification of Bi2O3-Doped Si-Ti Composite Film on a Wood Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Hydrophobic Modification of Bi2O3-Doped Si-Ti Composite Film on Wood Surface
2.3. Characterisation of Structure and Properties
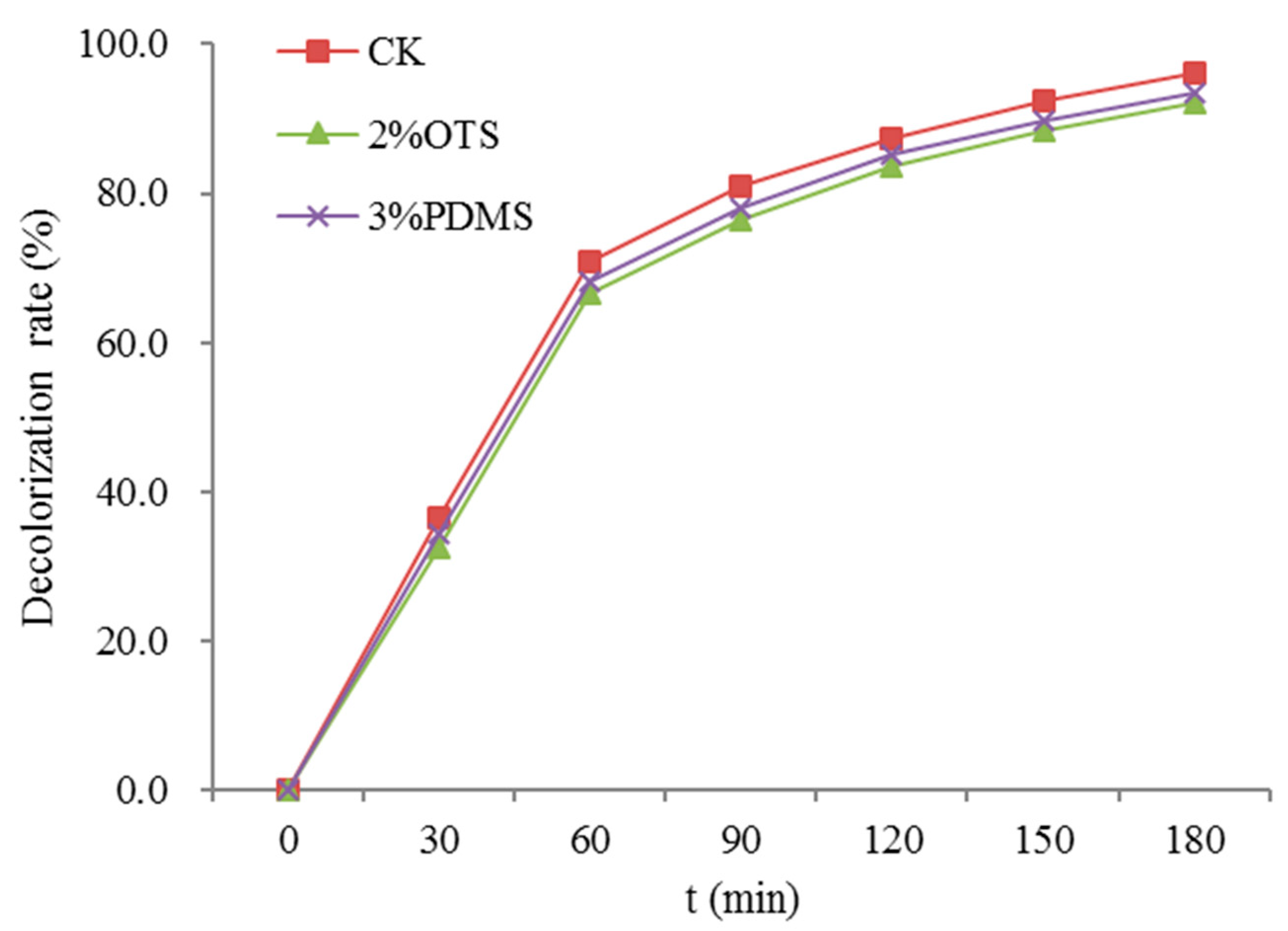
3. Results and Discussion
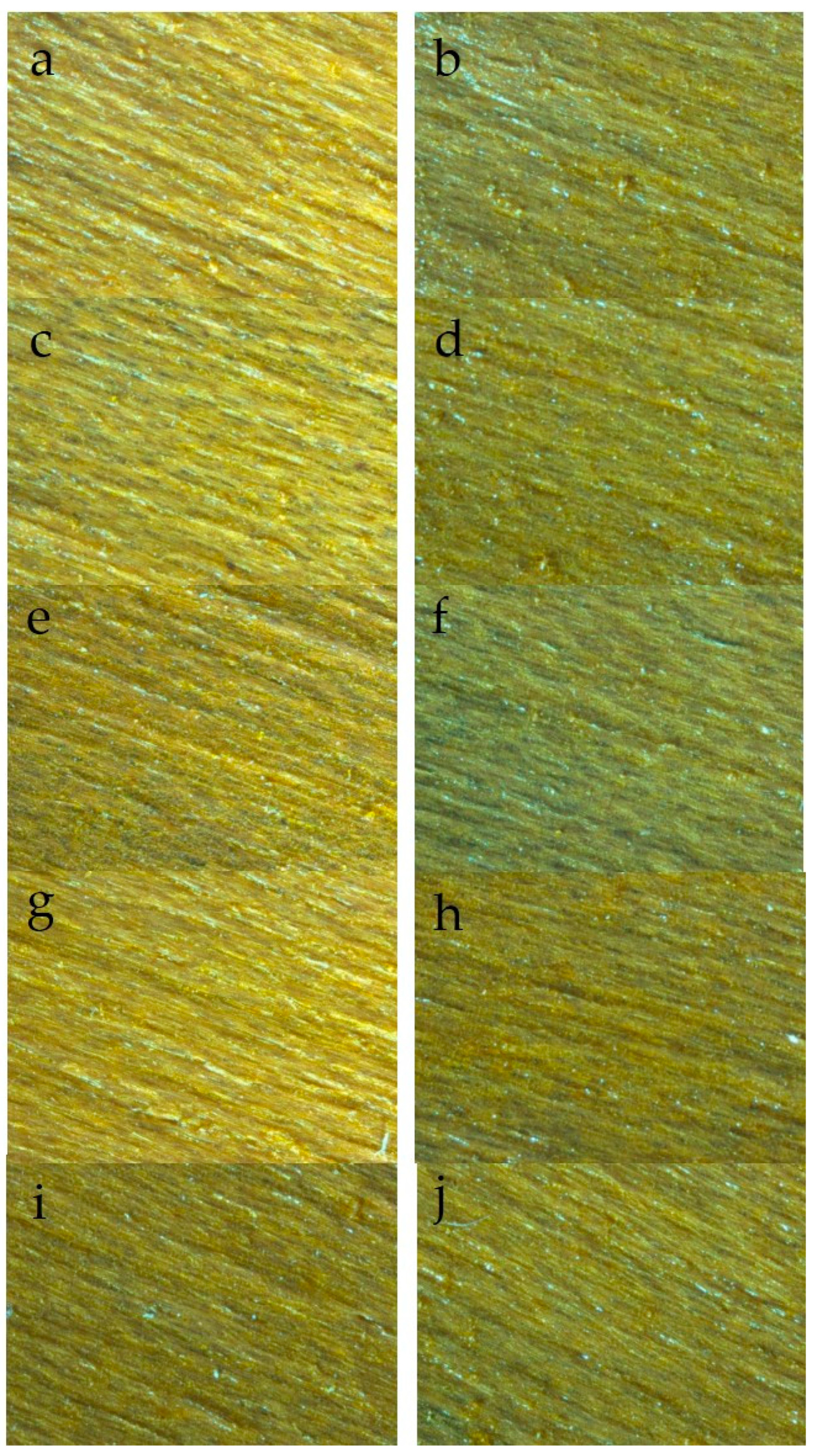
3.1. Surface Morphology Analysis
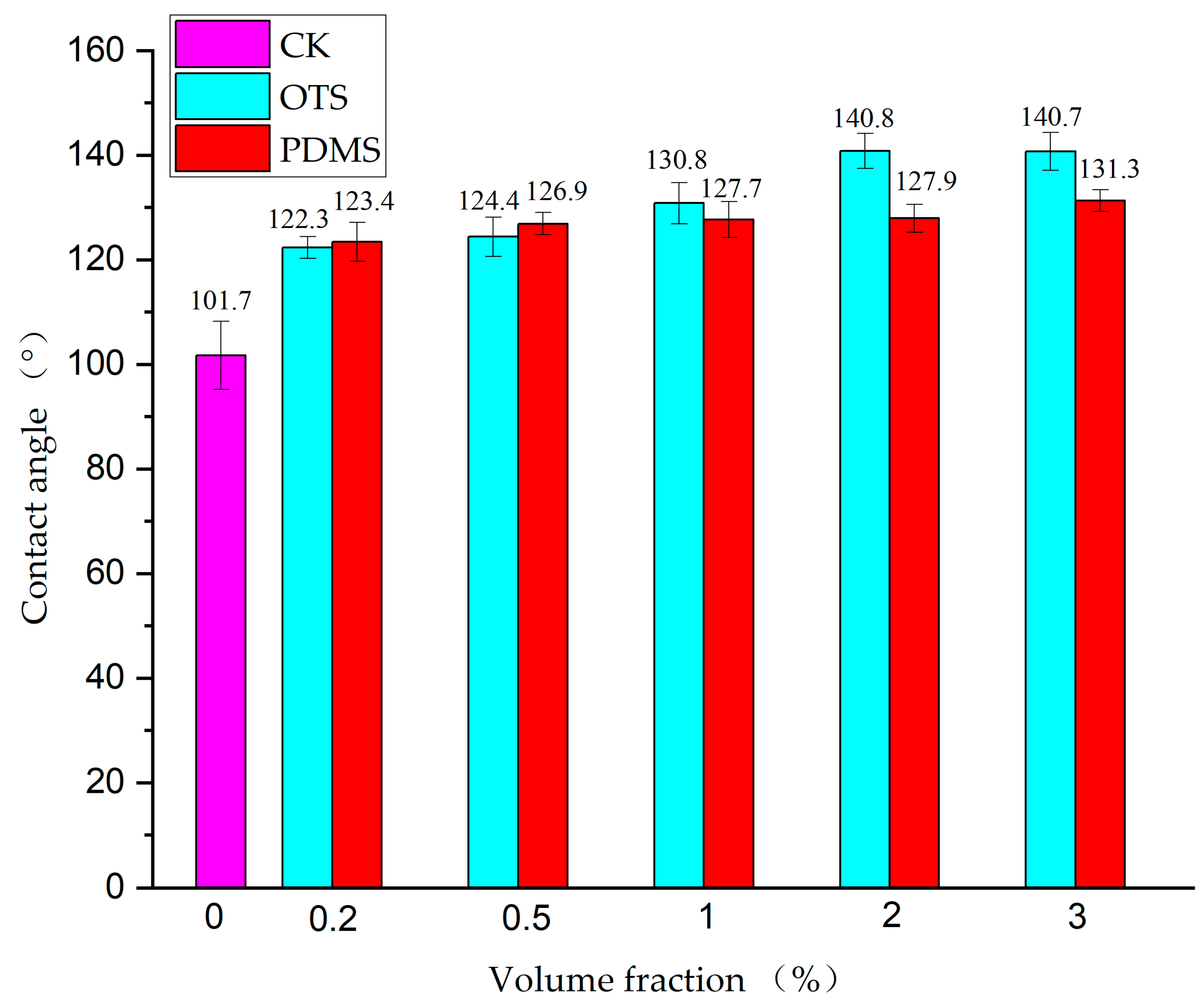
3.2. Surface Wettability Analysis
3.3. FTIR Analysis
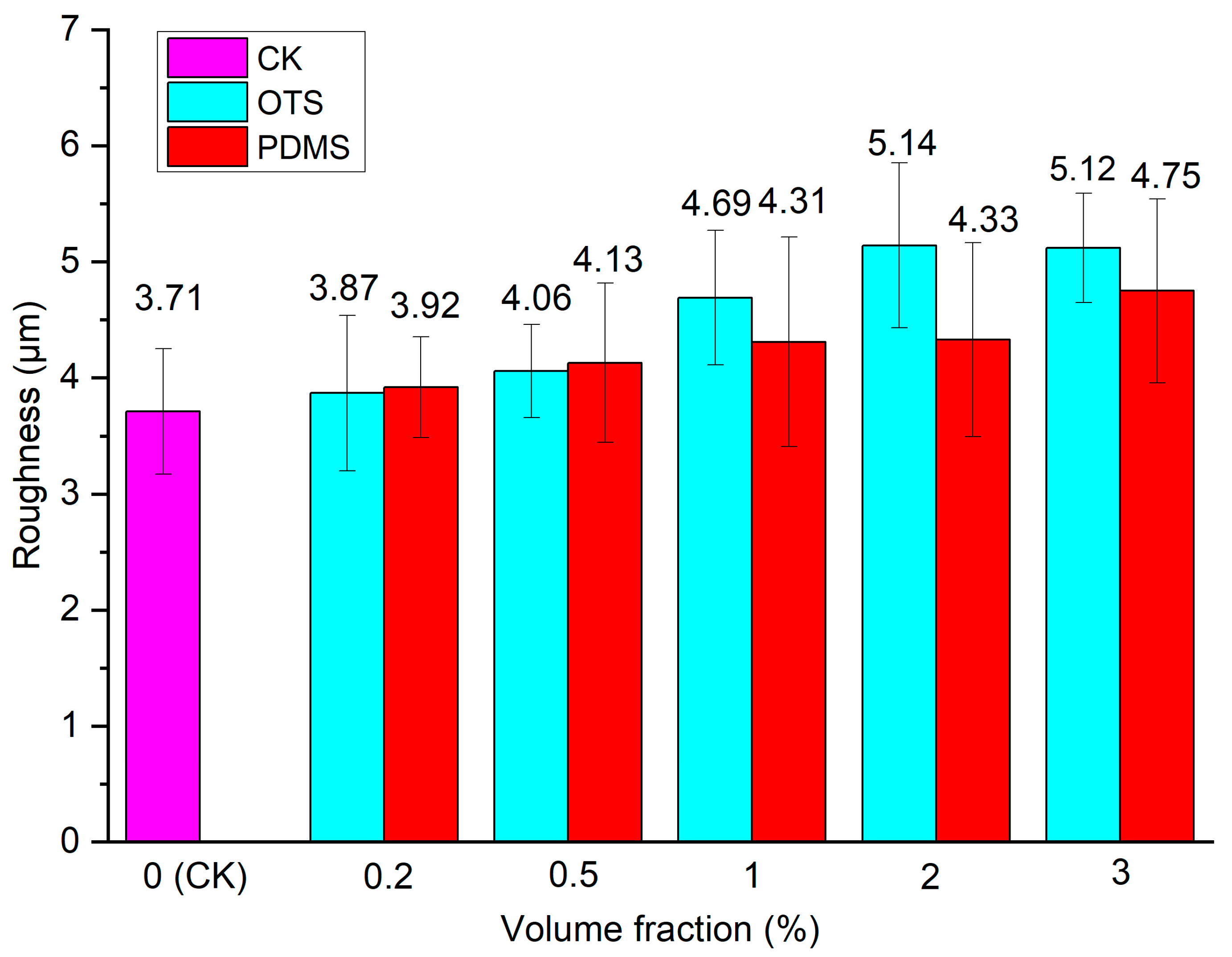
3.4. Surface Roughness Analysis
3.5. Surface Free Energy Analysis
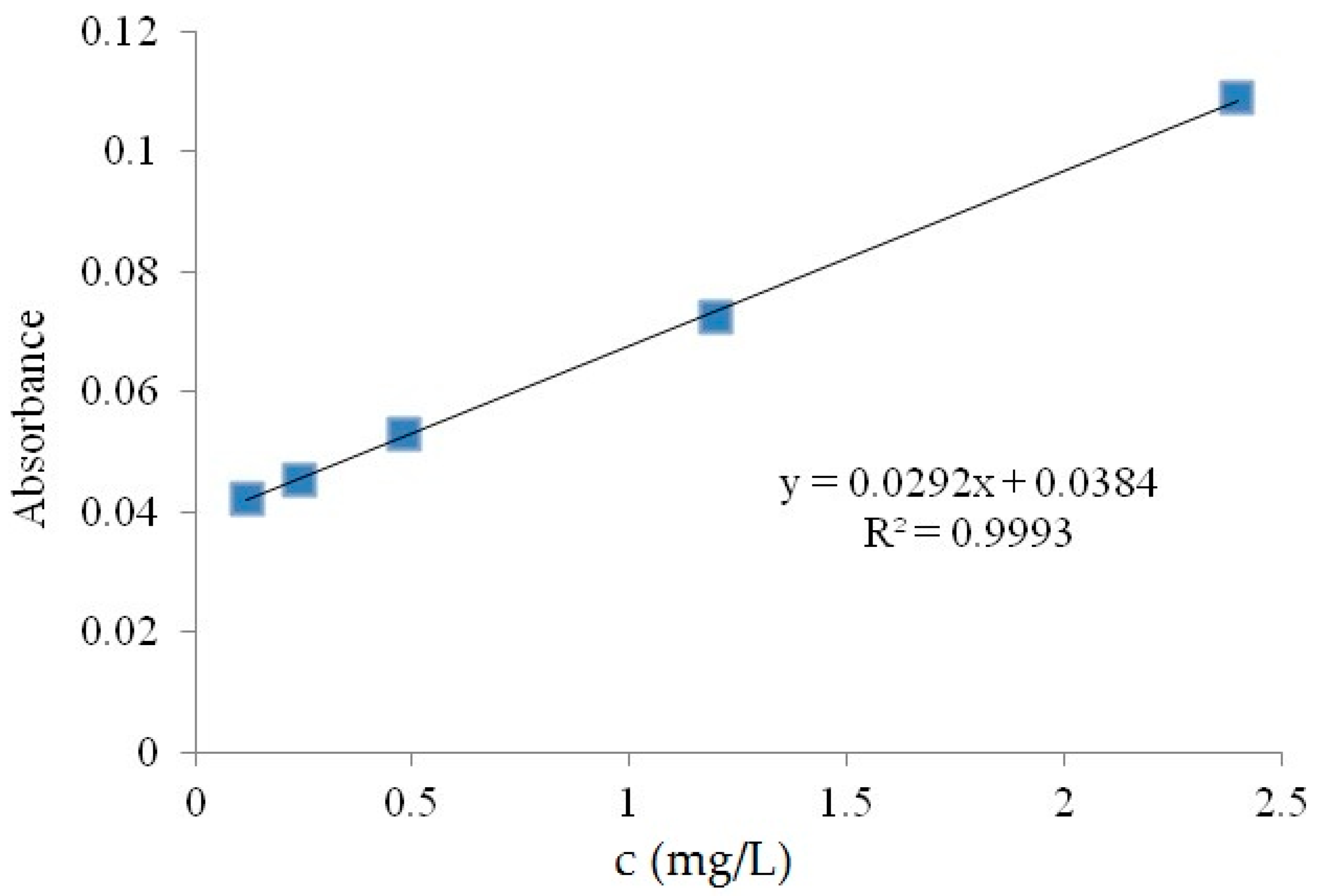
3.6. Photocatalytic Activity Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsieh, C.; Chang, B.; Lin, J. Improvement of water and oil repellency on wood substrates by using fluorinated silica nanocoating. Appl. Surf. Sci. 2011, 257, 7997–8002. [Google Scholar] [CrossRef]
- Wang, J.; Lu, Y.; Chu, Q.; Ma, C.; Cai, L.; Shen, Z.; Chen, H. Facile construction of superhydrophobic surfaces by coating fluoroalkylsilane/silica composite on a modified hierarchical structure of wood. Polymers 2020, 12, 813. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, K.; Dong, Y.; Zhang, W.; Zhang, S.; Li, J. Bulk superhydrophobility of wood via in-situ deposition of ZnO rods in wood structure. Surf. Coat. Technol. 2020, 383, 125240. [Google Scholar] [CrossRef]
- Wang, K. Preparation and Mechanism of Superhydrophobic Wood Based on Surface Micro/Nano Structure Design. Master’s Thesis, Beijing Forestry University, Beijing, China, 2019; pp. 65–76. [Google Scholar]
- Yang, T.; Xia, M.; Chen, S.; Mu, M.; Yuan, G. Enhancing the thermal stability of silica-mineralized wood via layer-by-layer self-assembly. J. Therm. Anal. Calorim. 2021, 145, 309–318. [Google Scholar] [CrossRef]
- Liu, M.; Qing, Y.; Wu, Y.; Liang, J.; Luo, S. Facile fabrication of superhydrophobic surfaces on wood substrates via a one-step hydrothermal process. Appl. Surf. Sci. 2015, 330, 332–338. [Google Scholar] [CrossRef]
- Yang, R.; Zuo, S.; Song, B.; Mao, H.; Huang, Z.; Wu, Y.; Cai, L.; Ge, S.; Lian, H.; Xia, C. Hollow mesoporous microspheres coating for super-hydrophobicity wood with high thermostability and abrasion performance. Polymers 2020, 12, 2856. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, M.; Xu, Y.; Wang, S.; Liu, F.; Ma, M.; Zang, D.; Gao, Z. One-step synthesis of unique silica particles for the fabrication of bionic and stably superhydrophobic coatings on wood surface. Adv. Powder Technol. 2014, 25, 530–535. [Google Scholar] [CrossRef]
- Wang, X.; Liu, S.; Chang, H.; Liu, J. Sol-gel deposition of TiO2 nanocoatings on wood surfaces with enhanced hydrophobicity and photostability. Wood Fiber Sci. 2014, 46, 109–117. [Google Scholar]
- Zheng, R.; Tshabalala, M.; Li, Q.; Wang, H. Construction of hydrophobic wood surfaces by room temperature deposition of rutile (TiO2) nanostructures. Appl. Surf. Sci. 2015, 328, 453–458. [Google Scholar] [CrossRef]
- Gao, L.; Gan, W.; Xiao, S.; Zhan, X.; Li, J. A robust superhydrophobic antibacterial Ag-TiO2 composite film. Ceram. Int. 2016, 42, 2170–2179. [Google Scholar] [CrossRef]
- Gao, Z.; Ma, M.; Zhai, X.; Zhang, M.; Zang, D.; Wang, C. Improvement of chemical stability and durability of superhydrophobic wood surface via filming TiO2 coated CaCO3 micro-nano-composite particles. RSC Adv. 2015, 5, 63978–63984. [Google Scholar] [CrossRef]
- Gao, L.; Lu, Y.; Cao, J.; Li, J.; Sun, Q. Reversible Photocontrol of Wood-Surface Wettability Between Superhydrophilicity and Superhydrophobicity Based on a TiO2 Film. J. Wood Chem. Technol. 2015, 35, 365–373. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, W.; Chen, Y. Structure and photocatalytic properties of Mn-doped TiO2 loaded on wood-based activated carbon fiber composites. Materials 2017, 10, 631. [Google Scholar] [CrossRef] [PubMed]
- Hung, K.; Wu, J. Characteristics and thermal decomposition kinetics of wood-SiO2 composites derived by the sol-gel process. Holzforschung 2017, 71, 233–240. [Google Scholar] [CrossRef]
- Xu, E.; Zhang, Y.; Lin, L. Improvement of mechanical, hydrophobicity and thermal properties of Chinese fir wood by impregnation of nano silica sol. Polymers 2020, 12, 1632. [Google Scholar] [CrossRef]
- Yu, L.; Cai, J.; Wang, Y.; Tang, Z.; Zhu, J. Improved dimensional stability of nano-SiO2/wax modified ACQ-treated southern pine. Bioresources 2017, 12, 7515–7524. [Google Scholar] [CrossRef]
- Dirna, F.; Rahayu, I.; Zaini, L.; Darmawan, W.; Prihatini, E. Improvement of fast-growing wood species characteristics by MEG and nano SiO2 impregnation. J. Korean Wood Sci. Technol. 2020, 48, 41–49. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Cheng, S.; Qin, Z.; Fu, Y. Photocatalytic performance and kinetic studies of a wood surface loaded with Bi2O3-doped silicon–titanium composite film. Polymers 2023, 15, 25. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y. Control and preparation to wettability of material surfaces. Mat. Eng. 2008, 4, 74–80. [Google Scholar]
- Wenzel, R. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Cassie, A.-B.-D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Liu, B.; Feng, Q.; Kuang, C. Survey of measurement methods for surface roughness. Opt. Instrum. 2004, 26, 54–58. [Google Scholar]
- Fu, Y.; Li, G.; Yu, H.; Liu, Y. Hydrophobic modification of wood via surface-initiated ARGET ATRP of MMA. Appl. Surf. Sci. 2012, 258, 2529–2533. [Google Scholar] [CrossRef]
- Sun, X.; Li, L.; Xu, X.; Song, G.; Tu, J.; Yan, P.; Zhang, W.; Hu, K. Preparation of hydrophobic SiO2/PTFE sol and antireflective coatings for solar glass cover. Optik 2020, 212, 164704. [Google Scholar] [CrossRef]
- He, F.; Wang, J.; Deng, D. Effect of Bi2O3 on structure and wetting studies of Bi2O3-ZnO-B2O3 glasses. J. Alloy Compd. 2011, 509, 6332–6336. [Google Scholar] [CrossRef]
- Queeney, K.T.; Weldon, M.K.; Chang, J.P.; Chabal, Y.J.; Gurevich, A.B.; Sapjeta, J.; Opila, R.L. Infrared spectroscopic analysis of the Si/SiO2 interface structure of thermally oxidized silicon. J. Appl. Phys. 2000, 87, 1322–1330. [Google Scholar] [CrossRef]
- Zhou, L.; Yan, S.; Tian, B.; Zhang, J.; Anpo, M. Preparation of TiO2–SiO2 film with high photocatalytic activity on PET substrate. Mater. Lett. 2006, 60, 396–399. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, S. Surface Roughness. Plat. Finish. 2009, 31, 32–34. [Google Scholar]
- Wang, M.; Wang, J.; Li, L. Analysis of wood surface roughness. J. Beijing For. Univ. 2005, 27, 14–18. [Google Scholar]


| Liquid | Surface Tension (mJ/m2) | ||||||
|---|---|---|---|---|---|---|---|
| γL | |||||||
| Distilled water | 72.8 | 21.8 | 51.0 | 21.8 | 51.0 | 25.5 | 25.5 |
| Diiodomethane | 50.8 | 50.8 | 0 | 50.8 | 0 | 0 | 0 |
| Test Solution | Modifiers | Contact Angle/° | |||||
|---|---|---|---|---|---|---|---|
| 0% (CK) | 0.2% | 0.5% | 1% | 2% | 3% | ||
| Distilled water | OTS | 101.7 | 122.3 | 124.4 | 130.8 | 140.8 | 140.7 |
| PDMS | 123.4 | 126.9 | 127.7 | 127.9 | 131.3 | ||
| Diiodomethane | OTS | 94.5 | 113 | 114.9 | 121.1 | 128.5 | 128.5 |
| PDMS | 115.7 | 118.6 | 119.5 | 120.2 | 121.3 | ||
| Surface Free Energy mJ/m2 | OTS | PDMS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CK | 0.2% | 0.5% | 1% | 2% | 3% | 0.2% | 0.5% | 1% | 2% | 3% | |
| γs | 5.21 | 3.13 | 2.94 | 2.37 | 1.62 | 1.62 | 3.00 | 2.69 | 2.61 | 2.58 | 2.33 |
| 3.29 | 2.17 | 2.07 | 1.73 | 1.35 | 1.35 | 2.02 | 1.86 | 1.81 | 1.77 | 1.72 | |
| 1.92 | 0.96 | 0.87 | 0.64 | 0.27 | 0.28 | 0.97 | 0.82 | 0.80 | 0.81 | 0.62 | |
| /γs | 0.63 | 0.69 | 0.70 | 0.73 | 0.83 | 0.83 | 0.67 | 0.69 | 0.69 | 0.69 | 0.74 |
| /γs | 0.37 | 0.31 | 0.30 | 0.27 | 0.17 | 0.17 | 0.33 | 0.31 | 0.31 | 0.31 | 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Gan, L.; Cheng, S.; Fu, Y.; Wei, P. Hydrophobic Modification of Bi2O3-Doped Si-Ti Composite Film on a Wood Surface. Coatings 2024, 14, 371. https://doi.org/10.3390/coatings14030371
Liu Z, Gan L, Cheng S, Fu Y, Wei P. Hydrophobic Modification of Bi2O3-Doped Si-Ti Composite Film on a Wood Surface. Coatings. 2024; 14(3):371. https://doi.org/10.3390/coatings14030371
Chicago/Turabian StyleLiu, Zhigao, Linshuang Gan, Si Cheng, Yunlin Fu, and Penglian Wei. 2024. "Hydrophobic Modification of Bi2O3-Doped Si-Ti Composite Film on a Wood Surface" Coatings 14, no. 3: 371. https://doi.org/10.3390/coatings14030371
APA StyleLiu, Z., Gan, L., Cheng, S., Fu, Y., & Wei, P. (2024). Hydrophobic Modification of Bi2O3-Doped Si-Ti Composite Film on a Wood Surface. Coatings, 14(3), 371. https://doi.org/10.3390/coatings14030371






