Improved High-Temperature Stability and Hydrogen Penetration through a Pd/Ta Composite Membrane with a TaTiNbZr Intermediate Layer
Abstract
1. Introduction
2. Experimental Details
2.1. Specimen Preparation
2.2. Annealing and Hydrogen Permeation Test
2.3. Micro-Structure Characterization
3. Results and Discussions
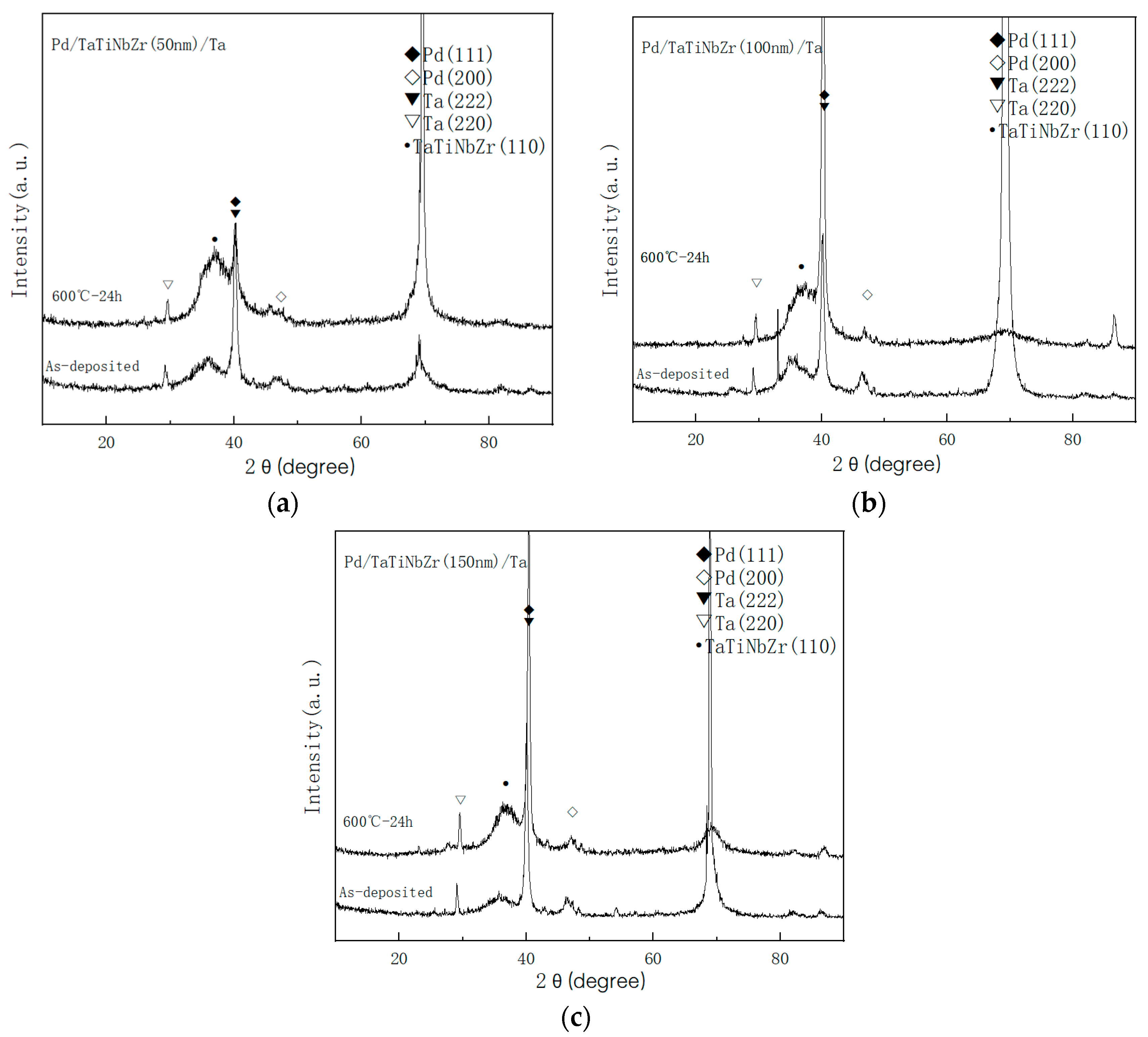
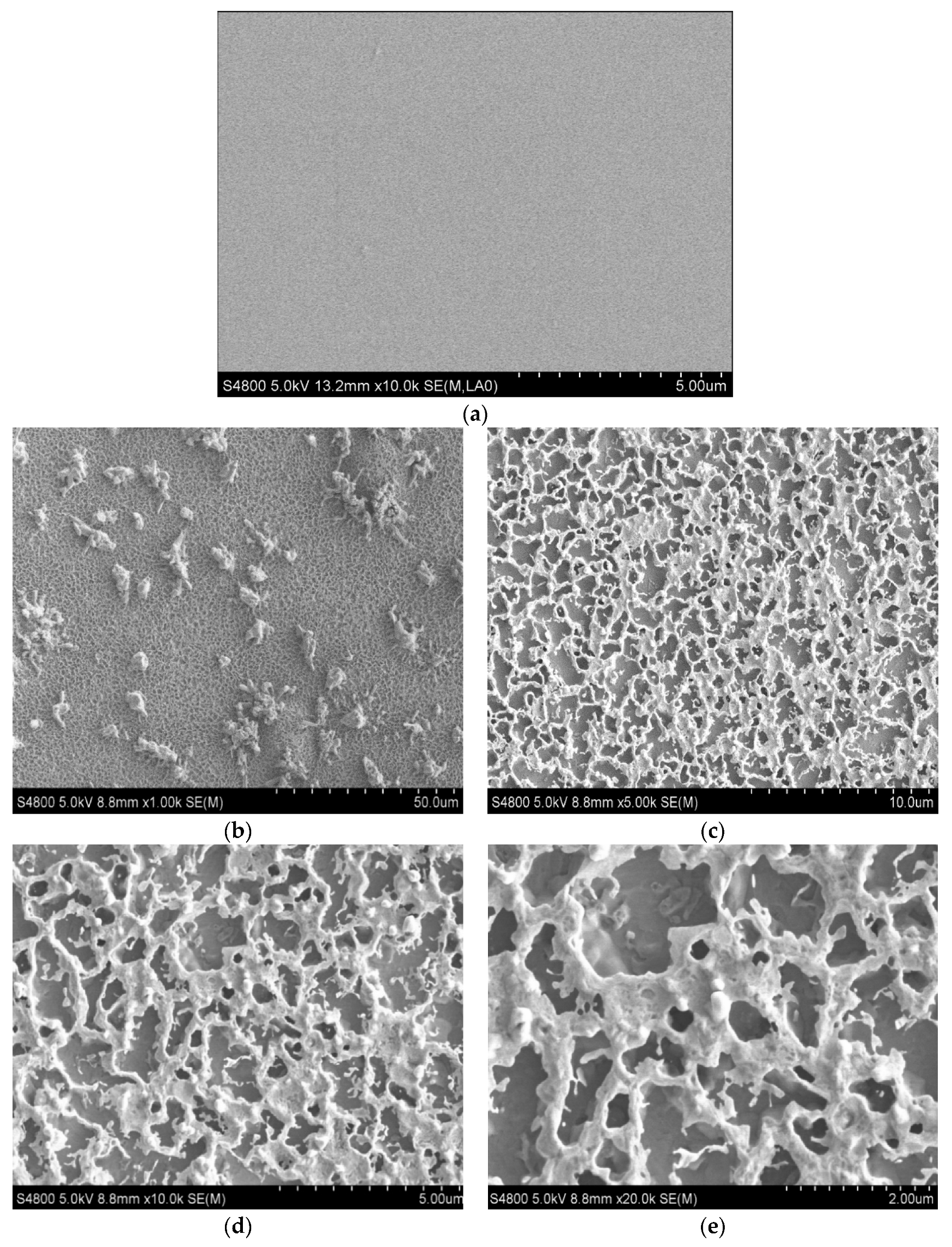
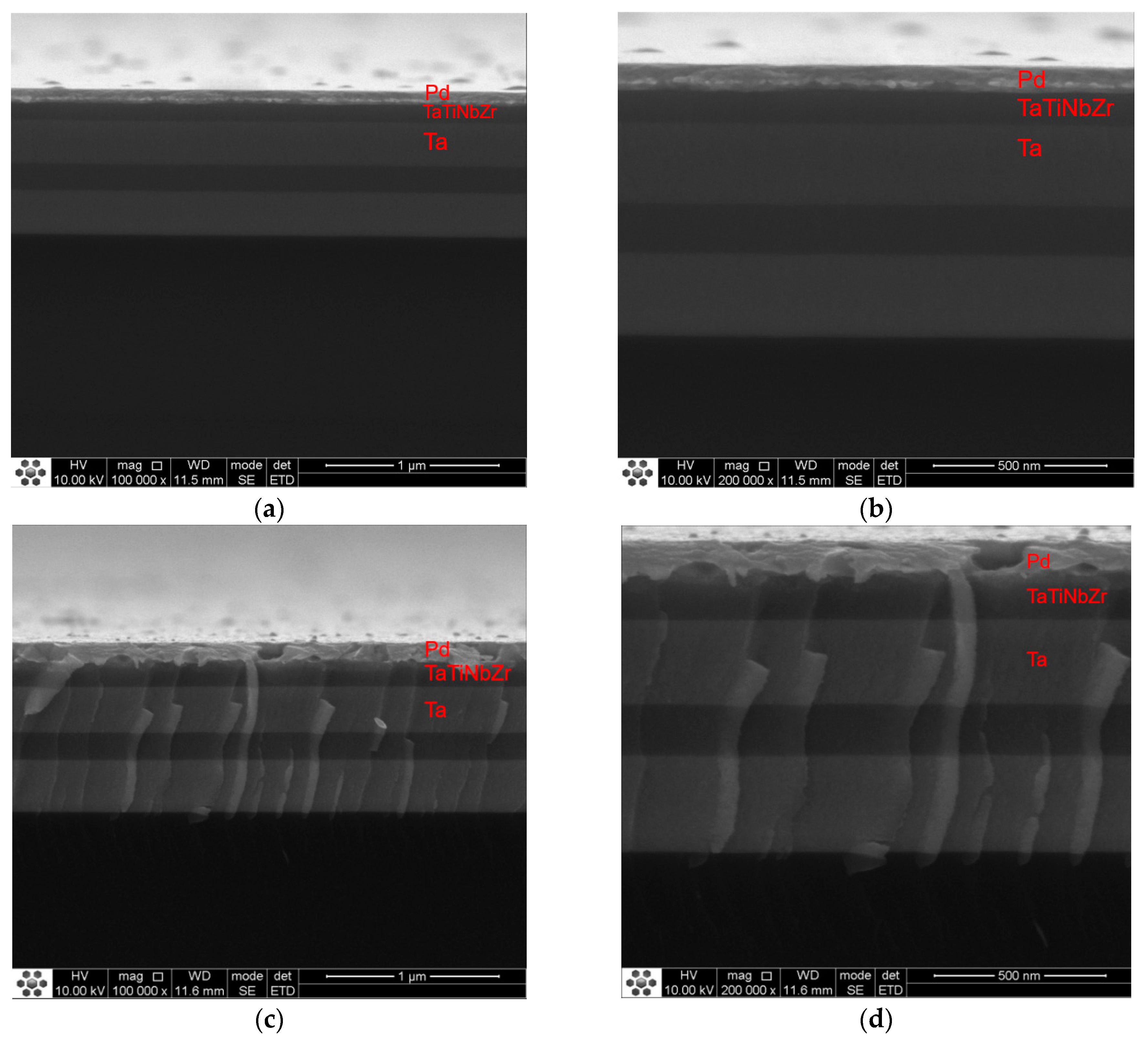
3.1. Influence of Annealing Temperature on Microstructure of Multilayer Films
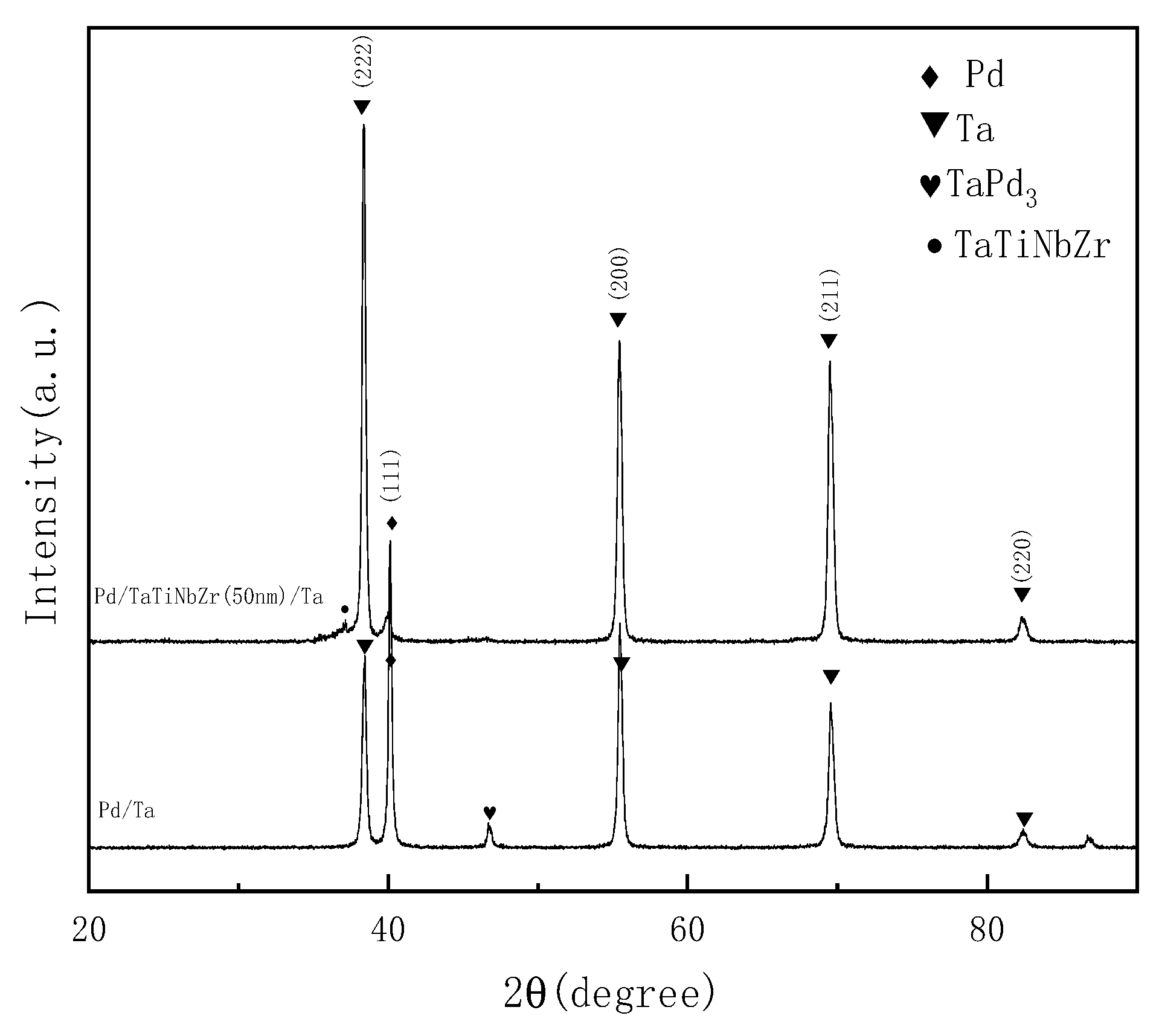
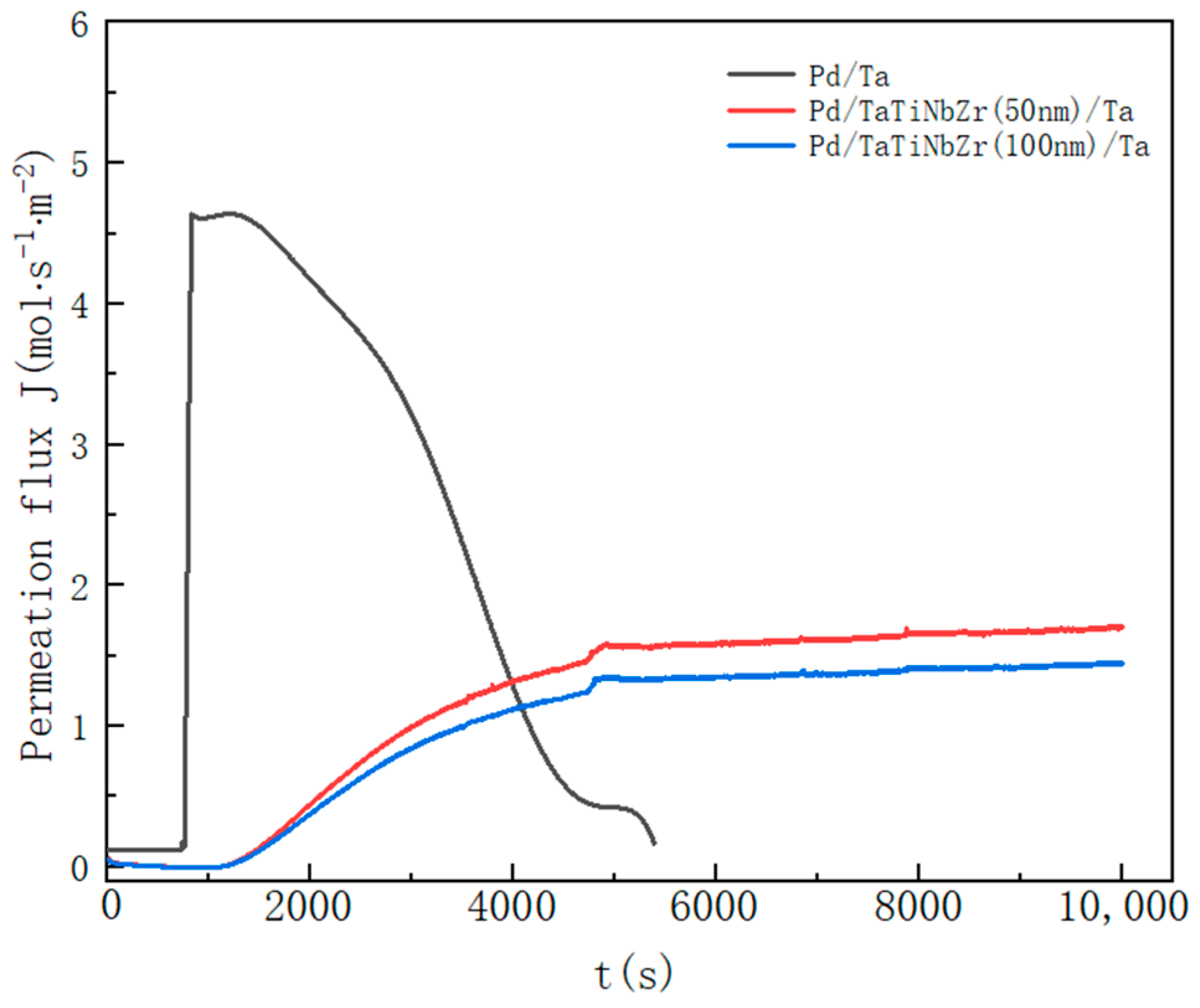
3.2. Hydrogen Permeation through Pd/TaTiNbZr/Ta and Pd/Ta
4. Conclusions
- After the addition of an amorphous TaTiNbZr barrier layer, Pd/TaTiNbZr/Ta can maintain structural and morphological stability before and after annealing.
- The high-temperature hydrogen permeation experiment shows that the barrier layer can effectively increase the working time of the multilayer film. Pd/TaTiNbZr(50 nm)/Ta has better hydrogen permeation performance than Pd/TaTiNbZr(100 nm)/Ta, indicating that the thickness of the barrier layer is a key factor affecting the hydrogen permeation ability.
- A layer of TaTiNbZr was inserted between a Ta substrate and a Pd film as a barrier layer. No TaPd3 was produced after the hydrogen infiltration experiment at 600 °C, indicating that TaTiNbZr can effectively block the mutual diffusion of Ta and Pd.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hassanpouryouzband, A.; Wilkinson, M.; Haszeldine, R.S. Hydrogen energy futures–foraging or farming? Chem. Soc. Rev. 2024, 53, 2258–2263. [Google Scholar] [CrossRef] [PubMed]
- Hassanpouryouzband, A.; Joonaki, E.; Edlmann, K.; Haszeldine, R.S. Offshore geological storage of hydrogen: Is this our best option to achieve net-zero? ACS Energy Lett. 2021, 6, 2181–2186. [Google Scholar] [CrossRef]
- Alimov, V.N.; Hatano, Y.; Busnyuk, A.O.; Livshits, D.A.; Notkin, M.E.; Livshits, A.I. Hydrogen permeation through the Pd-Nb-Pd composite membrane: Surface effects and thermal degradation. Int. J. Hydrogen Energy 2011, 36, 7737–7746. [Google Scholar] [CrossRef]
- Cooney, D.A.; Way, J.D.; Wolden, C.A. A comparison of the performance and stability of Pd/BCC metal composite membranes for hydrogen purification. Int. J. Hydrogen Energy 2014, 39, 19009–19017. [Google Scholar] [CrossRef]
- Jo, Y.S.; Lee, C.H.; Kong, S.Y.; Lee, K.Y.; Yoon, C.W.; Nam, S.W.; Han, J. Characterization of a Pd/Ta composite membrane and its application to a large scale high-purity hydrogen separation from mixed gas. Sep. Purif. Technol. 2018, 200, 221–229. [Google Scholar] [CrossRef]
- Park, Y.; Kwak, Y.; Yu, S.; Badakhsh, A.; Lee, Y.-J.; Jeong, H.; Kim, Y.; Sohn, H.; Nam, S.W.; Yoon, C.W.; et al. Degradation mechanism of a Pd/Ta composite membrane: Catalytic surface fouling with inter-diffusion. J. Alloys Compd. 2021, 854, 157196. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, J.; Ikehara, T.; Maeda, R.; Nishimura, C. Characterization and permeation of microfabricated palladium membrane. Mater. Trans. 2006, 47, 255–258. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Maeda, R.; Komaki, M.; Nishimura, C. Hydrogen permeation and diffusion of metallic composite membranes. J. Membr. Sci. 2006, 269, 60–65. [Google Scholar] [CrossRef]
- Zhang, Y.; Gwak, J.; Murakoshi, Y.; Ikehara, T.; Maeda, R.; Nishimura, C. Hydrogen permeation characteristics of thin Pd membrane prepared by microfabrication technology. J. Membr. Sci. 2006, 277, 203–209. [Google Scholar] [CrossRef]
- Demange, D.; Glugla, M.; Günther, K.; Le, T.L.; Simon, K.H.; Wagner, R.; Welte, S. Tritium processing tests for the validation of upgraded PERMCAT mechanical design. Fusion Sci. Technol. 2008, 54, 14–17. [Google Scholar] [CrossRef]
- Liang, X.; Li, X.; Nagaumi, H.; Guo, J.; Gallucci, F.; van Sint Annaland, M.; Liu, D. Degradation of Pd/Nb30Ti35Co35/Pd hydrogen permeable membrane: A numerical description. J. Membr. Sci. 2020, 601, 117922. [Google Scholar] [CrossRef]
- Wong, T.; Yu, Z.; Suzuki, K.; Gibson, M.; Ishikawa, K.; Aoki, K. Effect of Annealing on the Hydrogen Permeation and Mechanical Behaviour of Nb-Ni-Zr Alloy Membranes. Mater. Sci. Forum 2010, 654–656, 2839–2842. [Google Scholar] [CrossRef]
- Chin, H.-S.; Suh, J.-Y.; Park, K.-W.; Lee, W.; Fleury, E. Hydrogen permeability of glass-forming Ni-Nb-Zr-Ta crystalline membranes. Met. Mater. Int. 2011, 17, 541–545. [Google Scholar] [CrossRef]
- Nozaki, T.; Hatano, Y. Hydrogen permeation through a Pd/Ta composite membrane with a HfN intermediate layer. Int. J. Hydrogen Energy 2013, 38, 11983–11987. [Google Scholar] [CrossRef]
- Jayalakshmi, S.; Choi, Y.; Kim, Y.; Fleury, E. Hydrogenation properties of Ni-Nb-Zr-Ta amorphous ribbons. Intermetallics 2010, 18, 1988–1993. [Google Scholar] [CrossRef]
- Nozaki, T.; Hatano, Y.; Yamakawa, E.; Hachikawa, A.; Ichinose, K. Improvement of high temperature stability of Pd coating on Ta by HfN intermediate layer. Int. J. Hydrogen Energy 2010, 35, 12454–12460. [Google Scholar] [CrossRef]
- Liang, S.C.; Tsai, D.-C.; Chang, Z.-C.; Lin, T.-N.; Shiao, M.-H.; Shieu, F.-S. Thermally Stable TiVCrZrHf Nitride Films as Diffusion Barriers in Copper Metallization. Electrochem. Solid State Lett. 2012, 15, H5–H8. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W.; Gan, J.Y. Diffusion barrier properties of AlMoNbSiTaTiVZr high-entropy alloy layer between copper and silicon. Thin Solid Film. 2008, 516, 5527–5530. [Google Scholar] [CrossRef]
- Chang, S.Y.; Li, C.-E.; Huang, Y.-C.; Hsu, H.-F.; Yeh, J.-W.; Lin, S.-J. Structural and thermodynamic factors of suppressed interdiffusion kinetics in multi-component high-entropy materials. Sci. Rep. 2014, 4, 4162. [Google Scholar] [CrossRef]
- Steward, S. Review of Hydrogen Isotope Permeability through Materials; Lawrence Livermore National Lab. (LLNL): Livermore, CA, USA, 1983. [Google Scholar]
- Lee, D.-Y. Hydrogen Permeation Properties of Pd-Coated Ni-Nb-Ti-Zr Amorphous Alloys. Met. Mater. Int. 2008, 14, 545–548. [Google Scholar] [CrossRef]
- Li, J.; Pu, G.; Sun, H.; Du, X.; Lin, L.; Ren, D.; Zhang, K.; Liu, B. Effect of He-ions irradiation on the microstructure and mechanical properties of TiTaNbZr refractory medium-entropy alloy film. Vacuum 2023, 217, 112545. [Google Scholar] [CrossRef]
- Paglieri, S.N.; Wermer, J.R.; Buxbaum, R.E.; Ciocco, M.V.; Howard, B.H.; Morreale, B.D. Development of membranes for hydrogen separation: Pd coated V-10Pd. Energy Mater. 2008, 3, 169–176. [Google Scholar] [CrossRef]
- Nambu, T.; Shimizu, K.; Matsumoto, Y.; Rong, R.; Watanabe, N.; Yukawa, H.; Morinaga, M.; Yasuda, I. Enhanced hydrogen embrittlement of Pd-coated niobium metal membrane detected by in situ small punch test under hydrogen permeation. J. Alloys Compd. 2007, 446, 588–592. [Google Scholar] [CrossRef]
- Peachey, N.M.; Snow, R.C.; Dye, R.C. Composite Pd/Ta metal membranes for hydrogen separation. J. Membr. Sci. 1996, 111, 123–133. [Google Scholar] [CrossRef]
- Edlund, D.J.; McCarthy, J. The relationship between intermetallic diffusion and flux decline in composite-metal membranes-implications for achieving long membrane lifetime. J. Membr. Sci. 1995, 107, 147–153. [Google Scholar] [CrossRef]
- Konishi, S.; Glugla, M.; Hayashi, T. Fuel cycle design for ITER and its extrapolation to DEMO. Fusion Eng. Des. 2008, 83, 954–958. [Google Scholar] [CrossRef]
- Glugla, M.; Dörr, L.; Lässer, R.; Murdoch, D.; Yoshida, H. Recovery of tritium from different sources by the ITER Tokamak exhaust processing system. Fusion Eng. Des. 2002, 61–62, 569–574. [Google Scholar] [CrossRef]
- Liang, X.; Li, X.; Chen, R.; Nagaumi, H.; Guo, J.; Liu, D. Enhancement of hydrogen permeation stability at high temperatures for Pd/Nb30Ti35Co35/Pd composite membranes by HfN intermediate layer. J. Membr. Sci. 2022, 643, 120062. [Google Scholar] [CrossRef]
- Li, P.F.; Ma, Y.; Ma, H.; Ta, S.; Yang, Z.; Han, X.; Kai, M.; Chen, J.; Cao, Z. Enhanced diffusion barrier property of nanolayered NbMoTaW/TiVCr high entropy alloy for copper metallization. J. Alloys Compd. 2022, 895, 162574. [Google Scholar] [CrossRef]
- Fang, J.-S.; Yang, L.-C.; Lee, Y.-C. Low resistivity Fe-Co-B-Ti-Nb amorphous thin film as a copper barrier. J. Alloys Compd. 2014, 586, S348–S352. [Google Scholar] [CrossRef]
- Liu, Q. Experimental Study on Hydrogen Permeation Behavior in Niobium Driven by Gas and Plasma. Master’s Thesis, Xihua University, Chengdu, China, March 2023. [Google Scholar]
- Jiang, C.; Li, R.; Wang, X.; Shang, H.; Zhang, Y.; Liaw, P.K. Diffusion Barrier Performance of AlCrTaTiZr/AlCrTaTiZr-N High-Entropy Alloy Films for Cu/Si Connect System. Entropy 2020, 22, 234. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Wang, H.; Li, Q.; Wang, L.; Zhang, W.; Zhang, L.; Guo, S.; Liu, Y.; Liu, S.; Ma, Q. Effect of Mo on interdifussion behaviors and interfacial characteristics in multicomponent diffusion couple of FeCoCrNi high entropy alloys and diamond. Mater. Des. 2022, 215, 110522. [Google Scholar] [CrossRef]
- Jing, W.-N.; Liu, J.; Guo, H.; Wang, S.; Bi, H.; Chen, B.; Chen, J.; Wang, H.; Wei, J.; Ye, Z.; et al. Gas- and plasma-driven hydrogen permeation behavior of stagnant eutectic-solid GaInSn/Fe double-layer structure. Chin. Phys. B 2023, 32, 045201. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Liu, B.; Pu, G. Improved High-Temperature Stability and Hydrogen Penetration through a Pd/Ta Composite Membrane with a TaTiNbZr Intermediate Layer. Coatings 2024, 14, 370. https://doi.org/10.3390/coatings14030370
Sun H, Liu B, Pu G. Improved High-Temperature Stability and Hydrogen Penetration through a Pd/Ta Composite Membrane with a TaTiNbZr Intermediate Layer. Coatings. 2024; 14(3):370. https://doi.org/10.3390/coatings14030370
Chicago/Turabian StyleSun, Haoxin, Bo Liu, and Guo Pu. 2024. "Improved High-Temperature Stability and Hydrogen Penetration through a Pd/Ta Composite Membrane with a TaTiNbZr Intermediate Layer" Coatings 14, no. 3: 370. https://doi.org/10.3390/coatings14030370
APA StyleSun, H., Liu, B., & Pu, G. (2024). Improved High-Temperature Stability and Hydrogen Penetration through a Pd/Ta Composite Membrane with a TaTiNbZr Intermediate Layer. Coatings, 14(3), 370. https://doi.org/10.3390/coatings14030370






