Abstract
Deposited by a reactive atmospheric pressure non-thermal TiCl4/O2/Ar plasma, anatase TiO2 single crystal sheet-connected film exhibits two large exposed {001} facets and a high concentration of oxygen defects. Strong white photoluminescence centered at 542 nm has been observed with naked eyes, whose internal quantum efficiency is 0.62, and whose intensity is comparable to that of commercial fluorescent lamp interior coatings. Based on the simulation results of a hybrid global–analytical model developed on this atmospheric pressure non-equilibrium plasma system, the mechanism of a self-confined growth of single crystal sheets was proposed. A high concentration of oxygen defects is in situ incorporated into the anatase crystal lattice without damaging its crystallographic orientation. This method opens a new way to construct 3D porous metal-oxide single crystal sheet-connected films with two exposing high energy surfaces and a large concentration of oxygen defects.
1. Introduction
Appropriately designed anatase TiO2 single crystal sheet-connected films with two exposed {001} reactive facets and highly reduced characteristics are highly desirable for scientific research and practical applications [1,2,3]. Desirable characteristics include directly connected boundaries, high carrier mobility, low incident light reflection loss, large specific surface area, and large reactive surfaces. Constructing such architecture films is expected to have interesting photo, electromagnetic, cytokine, chemical, and catalyst effects and applications to photocatalysts, photonics crystals, spintronic devices, anticancer or gene therapy modalities, photo/electrochromic devices, gas sensors, chemical degradation, water splitting, solar cells, etc. [1,2,4,5,6,7]. Recently, anatase micro/nano powders composed of single crystal sheets or flowerlike clusters with a large percentage of exposed {001} faces have been synthesized through a high concentration of hydrofluoric acid (HF) as a crystal shape controller [3]. Anatase TiO2 architecture films were constructed from its powders through post-binding and annealing processes. This could have problems of nanoparticle coagulation, weak boundary connection, or crystal phase transformation. Post-vacuum annealing, ion implantation, plasma treatment, or electron bombardment are often applied to reduce TiO2 surface via the introduction of oxygen defects, and to improve its surface reactivity [8,9,10]. Plasma sputtering and plasma-enhanced chemical vapor deposition (PECVD) have been applied to fabricate anatase crystalline films, but with no obvious exposed {001} facets [11]. The growth of anatase single crystal sheet-connected architecture film with two exposed {001} facets and a high surface reactivity is an area that has had little success until now, to the best of our knowledge. Two challenges have to be taken to obtain such structured anatase films in one deposition step. One is that the highly reactive surface diminishes rapidly during crystal growth; this is due to the minimization of surface energy [12]. The other is that the regular crystal lattice growth is usually interrupted by incorporating oxygen defects into the crystal facets and interconnecting omnidirectional crystal sheets into the film architecture. This suggests that a well-designed crystal growth process has to balance the opposite aspects of high surface reactivity, crystal lattice regularity, defect introduction, and crystal sheet interconnection.
Hydrothermal [13] and sol-gel [14] methods are widely employed in the preparation of {001} facets TiO2 films. The processes are usually complicated and can last for several hours. Fluorine ions (F−) are always used as crystal shape controllers in the process [15]. Atmospheric pressure plasma deposition has been well studied in recent decades due to its low-cost equipment, simple steps, and its capability for continuous process [16,17]. Meanwhile, the plasma provides a unique (non-thermal equilibrium) deposition environment when compared to traditional chemical process.
In this paper, we provide a one-step, crystal-shape, controller-free method to deposit single crystal sheet-connected {001} facets TiO2 film. The TiO2 film exhibits unusually strong white photoluminescence due to the oxygen defects generated during the plasma deposition.
2. Experimental
The apparatus was a homemade atmospheric pressure plasma reactor, composed of two coaxial quartz glass tubes or parallel quartz plates with a gap of 1.5 mm (Figure 1a). A 13.56 MHz radio-frequency power supply (RF-10S/PWT, Advanced Energy Co., Ltd., Denver, CO, USA) was applied to ignite the plasma in the 5.4 × 2.0 × 0.15 cm3 space between the two electrodes. The power was set at 100 W. Argon (Ar, 99.99%, Shanghai Central Gases Co., Ltd., Shanghai, China) was used as the discharge gas, oxygen (O2, 99.99%, Shanghai Central Gases Co., Ltd., China) was used as the oxidant, and titanium tetrachloride (TiCl4, 98.0%, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) was selected as the titanium precursor. A bubbler containing TiCl4 was immersed in a water bath which was heated and maintained at 40 °C. The connecting tube from the bubbler to the discharge chamber was kept at 70 °C using a heating band in ordwer to prevent the TiCl4 vapor from condensing. TiCl4/O2/Ar (0.17/30/203 sccm) was fed into the reactor through mass flow controllers. The deposition time was 1 h.

Figure 1.
(a) Scheme of the plasma deposition equipment. The SEM images of the films, (b) in-glow, (d) afterglow. (c,e) are the relative magnified images corresponding to (b,d).
The morphology and crystal structure of the deposited films were examined using a field emission scanning electron microscope (FESEM, Hitachi S-4800, Tokyo, Japan) and a transmission electron microscope (TEM, JEM-2100, JEOL Ltd., Tokyo, Japan). The chemical states of the samples were investigated using X-ray photoelectron spectroscopy (XPS, ThermoFisher Scientific ESCALAB 250Xi, Waltham, MA, USA), equipped with Al-Kα X-ray radiation (1486 eV). For XPS analysis, Avantage software (version 5.934, from Thermo Fisher) was employed to deconvolve and analyze the detailed binding energy and the ratios of species. The photoluminescence (PL) of all the samples was measured using a 325 nm He-Cd laser (Renishaw) with a pump power of 30 mW.
3. Results and Discussion
The FE-SEM images of the deposited films in the in-glow zone and in the after-glow zone are shown in Figure 1b,d, respectively. They show that both films are composed of single crystal sheets which connect with each other. According to the symmetries of anatase TiO2, the two square surfaces and the eight isosceles trapezoidal surfaces in Figure 1c are, respectively, recognized to be {001} and {101} facets. Importantly, almost all the connected crystal sheets stand vertically on the substrate, especially in the after-glow zone, which are exposed high {001} facets, as shown in Figure 1e. Cl− plays an important role in promoting the {001} facets of TiO2 [18]. The plasma sheath will repel negatively charged ions or particles, causing them to be unable to reach the surface of the substrate and participate in chemical reactions [19]. In the “in-glow” region, the sheath is strong, but in the after-glow region, the sheath is weak. A weak plasma sheath leads to more Cl ions reaching the surface, which promotes the growth of the film 001 facets.
Figure 2a shows the XRD spectra of the films deposited in the in-glow and after-glow conditions. Both films exhibit the typical pattern for anatase TiO2. Peaks are identified at 2θ values of 25.4°, 38.1°, 48.2°, 53.9°, 55.2°, 62.9°, 68.9°, 70.5°, and 75.5°, corresponding to the crystal planes of (101), (004), (200), (105) (211), (204), (116), (200), and (215), respectively, agreeing with the standard XRD pattern of anatase TiO2 (JCPDS files No. 21-1272). Figure 2b shows the Raman spectra of the TiO2 films. These are in agreement with the XRD results. The samples show the five Raman-active modes of Eg, Eg, A1g, A1g, and Eg, corresponding to 144, 197, 399, 515, and 639 cm−1 [20].
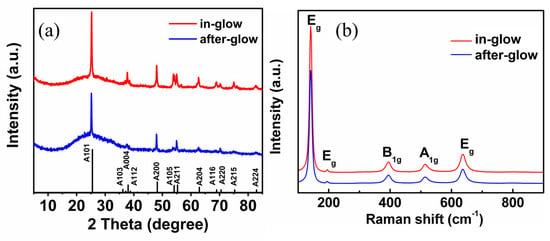
Figure 2.
(a) XRD, and (b) Raman spectra of TiO2 thin films deposited in in-glow and after-glow.
Figure 3a–d show the single crystal growth process in the 120 min deposition in the after-glow condition. After 1 min (Figure 3a), the TiO2 seeds are observed on the substrate, and, after 10 min (Figure 3b), the film shows then step-growth of the crystals. The crystals keep growing and then presented both {001} and {101} facets (Figure 3c) after 30 min. Figure 3d shows the crystals after deposition for 2 h. Huge TiO2 crystals with exposed high {101} facets are presented. High resolution transmission electron microscopy (HR-TEM) images and selected-area electron diffraction (SAED) patterns are given in Figure 3e,f, which clearly show the single crystal characteristics. As shown in Figure 3e and in its inset, the (200) and (020) atomic planes have a lattice spacing of 0.189 nm, and the SAED spot pattern is well-indexed into the zone, ascribing the two flat square surfaces to the {001} facets of the anatase single crystal [21]. The rotational Moiré fringes and the corresponding SAED spot pattern in Figure 3f and in its inset confirm the existence of the dislocation structure of the crystal sheets [22], which corresponds to the dislocation steps in Figure 1. The results indicate that grain sizes and shapes grow with the deposited time, showing the 001 facet TiO2 crystalline growth processes. The density of these films, alongside the presence of visible voids in the composition, has not yet been studied.

Figure 3.
The SEM images of the TiO2 film deposited in after-glow conditions for (a) 1 min, (b) 10 min, (c) 30 min, and (d) 120 min. HRTEM images and SAED (insets) of (e) the {001} facet, and (f) rotational Moiré fringes. The insets in (e,f) are the selected-area electron diffraction pattern, and arrows indicate the corresponding lattice parameters.
According to the Wulff construction, the lower reactive {101} facet of anatase is more thermodynamically stable (surface energy = 0.44 J m−2) than the higher reactive {001} facet (surface energy = 0.90 J m−2), and dominates more than 90% of the single-crystalline particles of anatase under equilibrium conditions [15]. In this atmospheric pressure non-equilibrium reactive plasma system, high densities of reactive species, such as Ar+, Ar2+, e, TiOCl2, O, Cl−, Cl2, ClO, etc., can be generated in the plasma gas phase [23]. It is demonstrated that a self-confined growth and the connections of the anatase TiO2 single crystal sheets with the two exposed {001} reactive crystal facets, as well as Cl ions all play a role in the control of surface morphology in TiO2 crystal growth [18]. Because the binding energy of Cl-Cl (D0Cl-Cl = 242.6 kJ mol−1) is much lower than that of Cl-Ti (D0Cl-Ti = 494 kJ mol−1), the bonding of Cl to the TiO2 crystal surface will lower the surface energy of the (001) surface to almost the same extent as that of the (101) surface, and will also form anatase TiO2 crystals with large {001} facets. Therefore, under the circumstances of a high density of Cl− containing precursor species, the newly formed nanosized anatase TiO2 nuclei come with exposed {001} facets. Subsequently, these loosely and randomly packed anatase TiO2 nuclei redevelop into branched or cross-linked crystal sheets with large {001} facets. When the branched or cross-linked crystal sheets grow large enough via a self-assembly extension recrystallization process (a self-confined Ostwald process in the reactive plasma) and combine together, a porous 3D film architecture, composed of micro/nano anatase TiO2 single crystal sheets with two exposed {001} facets, can be constructed directly on the substrate surface without any catalysts or templates after a certain time of deposition.
A high concentration of oxygen defects is in situ introduced into the anatase TiO2 single crystal sheets. The O 1s band is obviously different from that of pure unreduced anatase TiO2, and has a shoulder peak at about 531.5 eV, as shown in Figure 4a,b. The O 1s band can be resolved and assigned to three valence states at 530.1 ± 0.1 eV, 531.5 ± 0.1 eV, and 533.0 ± 0.1 eV. The peak with a binding energy at 531.6 ± 0.1 eV, which is 1.6 eV higher than the common Ti-O bond, is caused by the lower electron density of oxygen ions when compared to that of the O2−. This can be attributed to the oxygen ion O− with specific coordination, particularly integrated into the bulk structure near the surface and associated with oxygen defects in the subsurface [24]. The resolved band at 531.5 ± 0.1 eV covers, respectively, 41.0% and 25.2% of the O 1s spectrum for the TiO2 film deposited in the in-glow zone and in the after-glow zone. The peak of 533.1 ± 0.1 eV is related to the Ti-OH bond [25]. According to the references, O 532 eV is often related to defects or oxygen vacancy [26]. And the oxygen vacancy in TiO2 has a great influence on photoluminescence, as they will induce a higher number of photoexcited carriers to recombine cause more photoexcited carriers to be recombined [27]. The thermal effect of plasma treatment can accelerate the formation of the above-mentioned defects in TiO2. And, because of the destruction of O-Ti-O and Ti-O-Ti bonds via the plasma bombard, the residual electrons can migrate from the lattice Ti and O atom, and then create the defective lattice structure of TiO2 [28]. The thin film in the “in-glow” period will be subjected to more plasma bombarding and heat than the “after-glow” film. The high-resolution XPS spectra of Ti 2p are shown in Figure 4c,d. The peaks with a binding energy at 458 eV and 464 eV are Ti4+. The thin film deposited in the in-glow period shows Ti3+ at the binding energy of 457.2 eV [29]. However, the thin film deposited in the after-glow period does not show any Ti3+ peak.
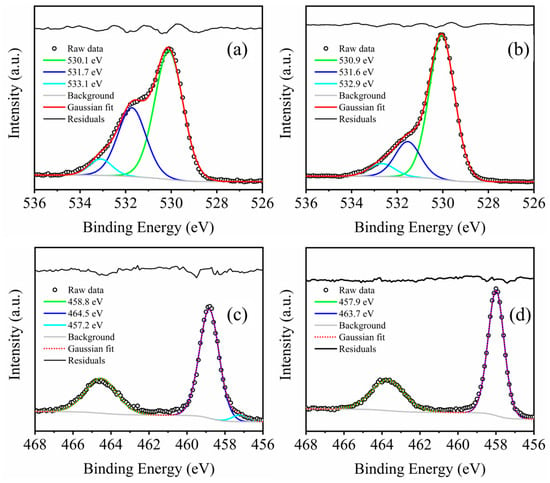
Figure 4.
(a,b) represent the resolved peaks of O 1s spectrum, and (c,d) represent the Ti 2p spectrum of the films obtained in the in-glow zone and in the afterglow zone, respectively.
The large percentage of oxygen defects in the film is confirmed by the PL spectrum of the film. As seen from Figure 5, the deposited films exhibit light emissions from 390 nm to 700 nm, centered at 542 nm, which is strong enough to be viewed easily with the naked eye. The strong PL, centered at 542 nm, are attributed to oxygen vacancies (OVs) [30,31]. Furthermore, the PL intensity increases with the percentage of the oxygen defects, and is higher for the film deposited in the in-glow zone than in the after-glow zone. TiO2 powder (Degussa, P25) and commercial fluorescent coating (get from Opple T8 fluorescent lamp) were also tested at the same condition for use as a contrast.
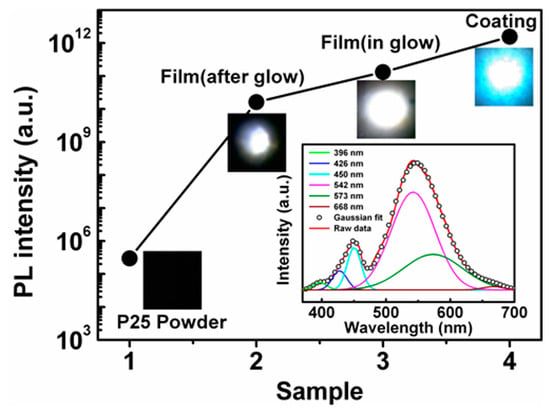
Figure 5.
The PL intensity of (1) the P25 powder, (2) the after-glow film, (3) the in-glow film, and (4) the commercial fluorescent coating, as well as the PL spectrum (inset), of the in-glow film.
4. Conclusions
In summary, 3D porous anatase TiO2 single crystal sheet-connected films with two large exposed {001} reactive facets and a high percentage of oxygen defects has been successfully deposited onto the substrate in one step, without any catalysts or templates. This provides a feasible way for the fast synthesis of 3D porous metal-oxide films that are composed of single crystal sheets connected with each other, and vertically aligned on the substrate. A self-confined crystal sheet growth mechanism has been proposed for the architecture formation, based on the hybrid analytical global model simulation and the experimental results. Unusually, strong white photoluminescence centered at 542 nm has been observed for the first time, and is mainly caused by the high percentage of oxygen defects introduced into the crystal in situ.
Author Contributions
Conceptualization, T.H.; methodology, T.H.; validation, T.H. and D.W.; investigation, Y.X.; writing—original draft preparation, T.H.; writing—review and editing, Y.X. and J.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The National Natural Science Foundation of China NSFC (Nos. 12205040, 12075054).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Diebold, U.; Ruzycki, N.; Herman, G.S.; Selloni, A. One step towards bridging the materials gap: Surface studies of TiO2 anatase. Catal. Today 2003, 85, 93–100. [Google Scholar] [CrossRef]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638–641. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xue, J.; Shen, Q.; Jia, S.; Gao, J.; Liu, X.; Jia, H. Black single-crystal TiO2 nanosheet array films with oxygen vacancy on {001} facets for boosting photocatalytic CO2 reduction. J. Alloys Compd. 2021, 870, 159400. [Google Scholar] [CrossRef]
- Tian, X.; Cui, X.; Lai, T.; Ren, J.; Yang, Z.; Xiao, M.; Wang, B.; Xiao, X.; Wang, Y. Gas sensors based on TiO2 nanostructured materials for the detection of hazardous gases: A review. Nano Mater. Sci. 2021, 3, 390–403. [Google Scholar] [CrossRef]
- Zhang, J.; Lei, Y.; Cao, S.; Hu, W.; Piao, L.; Chen, X. Photocatalytic hydrogen production from seawater under full solar spectrum without sacrificial reagents using TiO2 nanoparticles. Nano Res. 2022, 15, 2013–2022. [Google Scholar] [CrossRef]
- Aschauer, U.; He, Y.; Cheng, H.; Li, S.-C.; Diebold, U.; Selloni, A. Influence of Subsurface Defects on the Surface Reactivity of TiO2: Water on Anatase (101). J. Phys. Chem. C 2010, 114, 1278–1284. [Google Scholar] [CrossRef]
- Grätzel, M. Photoelectrochemical cells. Nature 2001, 414, 338–344. [Google Scholar] [CrossRef]
- Cai, R.; Kubota, Y.; Shuin, T.; Sakai, H.; Hashimoto, K.; Fujishima, A. Induction of Cytotoxicity by Photoexcited TiO2 Particles1. Cancer Res. 1992, 52, 2346–2348. [Google Scholar]
- Lou, B.-S.; Chen, W.-T.; Diyatmika, W.; Lu, J.-H.; Chang, C.-T.; Chen, P.-W.; Lee, J.-W. High power impulse magnetron sputtering (HiPIMS) for the fabrication of antimicrobial and transparent TiO2 thin films. Curr. Opin. Chem. Eng. 2022, 36, 100782. [Google Scholar] [CrossRef]
- Han, X.; Kuang, Q.; Jin, M.; Xie, Z.; Zheng, L. Synthesis of Titania Nanosheets with a High Percentage of Exposed (001) Facets and Related Photocatalytic Properties. J. Am. Chem. Soc. 2009, 131, 3152–3153. [Google Scholar] [CrossRef]
- Shahvardanfard, F.; Cha, G.; Denisov, N.; Osuagwu, B.; Schmuki, P. Photoelectrochemical performance of facet-controlled TiO2 nanosheets grown hydrothermally on FTO. Nanoscale Adv. 2021, 3, 747–754. [Google Scholar] [CrossRef]
- Yan, Y.; Keller, V.; Keller, N. On the role of BmimPF6 and P/F- containing additives in the sol-gel synthesis of TiO2 photocatalysts with enhanced activity in the gas phase degradation of methyl ethyl ketone. Appl. Catal. B Environ. 2018, 234, 56–69. [Google Scholar] [CrossRef]
- Butburee, T.; Kotchasarn, P.; Hirunsit, P.; Sun, Z.; Tang, Q.; Khemthong, P.; Sangkhun, W.; Thongsuwan, W.; Kumnorkaew, P.; Wang, H.; et al. New understanding of crystal control and facet selectivity of titanium dioxide ruling photocatalytic performance. J. Mater. Chem. A 2019, 7, 8156–8166. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; He, T.; Ding, K.; Huang, X.; Li, H.; Shi, J.; Guo, Y.; Zhang, J. The Effects of Thermal and Atmospheric Pressure Radio Frequency Plasma Annealing in the Crystallization of TiO2 Thin Films. Coatings 2019, 9, 357. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Li, L.; Ding, K.; Guo, Y.; Shi, J.; Huang, X.; Zhang, J. Synergistic Effect of Plasma Discharge and Substrate Temperature in Improving the Crystallization of TiO2 Film by Atmospheric Pressure Plasma Enhanced Chemical Vapor Deposition. Plasma Chem. Plasma Process. 2019, 39, 937–947. [Google Scholar] [CrossRef]
- Liu, G.; Yang, H.G.; Pan, J.; Yang, Y.Q.; Lu, G.Q.; Cheng, H.-M. Titanium Dioxide Crystals with Tailored Facets. Chem. Rev. 2014, 114, 9559–9612. [Google Scholar] [CrossRef]
- Xu, Y.; He, T.; Zhang, Y.; Wang, H.; Guo, Y.; Shi, J.; Du, C.; Zhang, J. Insights into the low-temperature deposition of a dense anatase TiO2 film via an atmospheric pressure pulse-modulated plasma. Plasma Process. Polym. 2021, 18, 2100050. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; He, T.; Li, Y.; Guo, Y.; Shi, J.; Xu, Y.; Zhang, J. The effects of radio frequency atmospheric pressure plasma and thermal treatment on the hydrogenation of TiO2 thin film. Plasma Sci. Technol. 2023, 25, 065504. [Google Scholar] [CrossRef]
- Ni, J.; Fu, S.; Wu, C.; Maier, J.; Yu, Y.; Li, L. Self-Supported Nanotube Arrays of Sulfur-Doped TiO2 Enabling Ultrastable and Robust Sodium Storage. Adv. Mater. 2016, 28, 2259–2265. [Google Scholar] [CrossRef]
- Zhu, M.; Chikyow, T.; Ahmet, P.; Naruke, T.; Murakami, M.; Matsumoto, Y.; Koinuma, H. A high-resolution transmission electron microscopy investigation of the microstructure of TiO2 anatase film deposited on LaAlO3 and SrTiO3 substrates by laser ablation. Thin Solid Film. 2003, 441, 140–144. [Google Scholar] [CrossRef]
- Leblanc, A.; Ding, K.; Lieberman, M.A.; Wang, D.X.; Zhang, J.; Jun Shi, J. Hybrid model of atmospheric pressure Ar/O2/TiCl4 radio-frequency capacitive discharge for TiO2 deposition. J. Appl. Phys. 2014, 115, 183302. [Google Scholar] [CrossRef]
- Sanjinés, R.; Tang, H.; Berger, H.; Gozzo, F.; Margaritondo, G.; Lévy, F. Electronic structure of anatase TiO2 oxide. J. Appl. Phys. 1994, 75, 2945–2951. [Google Scholar] [CrossRef]
- He, Z.; Que, W.; Chen, J.; He, Y.; Wang, G. Surface chemical analysis on the carbon-doped mesoporous TiO2 photocatalysts after post-thermal treatment: XPS and FTIR characterization. J. Phys. Chem. Solids 2013, 74, 924–928. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.-X.; He, L.; Wang, L.-Y.; Liu, X.-L.; Liu, J.-W.; Li, Y.-Z.; Tian, G.; Zhao, H.; Yang, X.-H.; et al. Interfacial co-existence of oxygen and titanium vacancies in nanostructured TiO2 for enhancement of carrier transport. Nanoscale 2020, 12, 8364–8370. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, J.; Feng, Z.; Chen, T.; Lian, Y.; Wang, X.; Li, C. Photoluminescence Characteristics of TiO2 and Their Relationship to the Photoassisted Reaction of Water/Methanol Mixture. J. Phys. Chem. C 2007, 111, 693–699. [Google Scholar] [CrossRef]
- Kong, X.; Xu, Y.; Cui, Z.; Li, Z.; Liang, Y.; Gao, Z.; Zhu, S.; Yang, X. Defect enhances photocatalytic activity of ultrathin TiO2 (B) nanosheets for hydrogen production by plasma engraving method. Appl. Catal. B Environ. 2018, 230, 11–17. [Google Scholar] [CrossRef]
- Bharti, B.; Kumar, S.; Lee, H.-N.; Kumar, R. Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci. Rep. 2016, 6, 32355. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Zhang, L.D.; Meng, G.W.; Li, G.H.; Zhang, X.Y.; Liang, C.H.; Chen, W.; Wang, S.X. Preparation and photoluminescence of highly ordered TiO2 nanowire arrays. Appl. Phys. Lett. 2001, 78, 1125–1127. [Google Scholar] [CrossRef]
- Serpone, N.; Lawless, D.; Khairutdinov, R. Size Effects on the Photophysical Properties of Colloidal Anatase TiO2 Particles: Size Quantization versus Direct Transitions in This Indirect Semiconductor? J. Phys. Chem. 1995, 99, 16646–16654. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).