Abstract
Central venous catheters (CVCs) are largely used to administer chemotherapy, hemodialysis, and other treatments. Mostly made of polydimethylsiloxane (PDMS), these medical devices present an intrinsic risk of infection due to the possible formation of biofilm, thus increasing the risk of complications. Drug-releasing polymer coatings are a well-recognized strategy for combating biofilm formation. However, adhesion of the coating to the substrate over time is a major challenge. Therefore, this work aimed to design a chitosan-based coating designed to have maximum adhesion and stability to guarantee sustained drug release and antibacterial properties for at least 14 days. A coating composed of chitosan (CS) as a drug carrier, caffeic acid (CA) and copper sulphate (Cu) as crosslinkers, and moxifloxacin (Mox) as an antibiotic, was deposited through a controlled casting process onto functionalized PDMS surface. PDMS surface modification was investigated by X-ray photoelectron spectroscopy (XPS), and Fourier-transfer infrared (FTIR). Antibiotic release over time was measured in pseudo-physiological conditions (pH 7.4 and at 37 °C). Indirect cytotoxicity assays were performed on human dermal fibroblasts (HDF). The adhesion of the as-designed coating was evaluated by a specially designed pull-off test, before and after aging for 14 days in PBS. XPS and FTIR analyses confirmed the successful PDMS surface modification. The CS-CA-Cu-Mox coating resulted in being non-cytotoxic towards HDF and exhibited sustained moxifloxacin release for up to 49 days. Furthermore, the CS-CA and CS-CA-Cu coatings presented antibacterial activity for 21 days against E. coli, and for 14 days against S. aureus. Importantly, the coating maintained stable adhesion after 14 days in pseudo-physiological conditions. This study provides new insights into the adhesion behavior of polymeric coatings for medical devices, which is rarely reported in the literature.
1. Introduction
The term healthcare-associated infections (HAIs) define infections that are usually acquired after hospitalization, generally within 30 days after receiving healthcare [1]. The Public Health Agency of Canada (2014) reported that more than 200,000 patients develop HAIs each year, and their associated complications affect approximately 8000 patients yearly [2]. Central line-associated bloodstream infections (CLABSI), which account for 42% of HAI cases, are bacterial infections that occur with a central venous catheter (CVC), due to biofilm formation. In the United States of America, CLABSI is estimated to be the eighth leading cause of death, as well as associated with increased hospital care costs, hospitalization length, morbidity, and mortality rates from 14 to 40% [3,4]. To tackle CLASBI, physicians make use of antimicrobial lock therapy [5], which involves instilling an antibiotic solution into the hub of the CVC when not in use, and antibiotics administration, to treat the infection [6]. However, those treatments are not sufficient to effectively control CLABSI [7]. A promising approach to limit nosocomial infections could be the use of antibacterial coatings on catheter surfaces. In the literature, the main strategies for achieving antibacterial effects rely on (i) contact-killing (e.g., chitosan coating); (ii) anti-adhesion (e.g., PEG); and (iii) antibacterial agent release (e.g., silver, copper, and antibiotic delivery system) [8]. For implants, drug release systems are the most investigated due to the high versatility in terms of antibacterial agent and coating composition, and the fact that antibacterial agents are delivered directly on-site. Indeed, if administered locally, the antibiotics concentration at the site of infection can be higher than systemic administration. However, controlling the release of the antibacterial over time remains a challenge. To this end, the development of a controlled drug release within the limits of the therapeutic window Is crucial [9].
An antibacterial coating is mainly composed of two components: (1) a drug carrier (polymer), and (2) an antibacterial agent. Chitosan (CS) is a natural polymer [10,11] of particular interest. In fact, over the years, several groups have demonstrated the potential of chitosan-based delivery systems when loaded with anti-inflammatory drugs [12], as well as growth factors and vaccines [13]. Nevertheless, to achieve controlled release, a chitosan-based system needs to be optimized. It has been shown that pure CS does not provide sustained release of the drug [14,15]. Thus, a crosslinker capable of modulating CS drug release properties needs to be added. Studies have shown that natural chemical compounds such as polyphenols (e.g., caffeic acid, tannic acid, dopamine) are extremely versatile crosslinkers [16]. Interestingly, caffeic acid (CA), a polyphenol extracted from coffee beans, fruits, and propolis [17], features also antimicrobial [17], antiviral [18], and anti-inflammatory activities [18]. The crosslinking efficiency of CA is associated with its catechol moiety, which upon oxidation, is converted to ortho-quinone, an electrophilic compound capable of reacting with a variety of nucleophilic functions, such as alcohol and amines found in the CS structure, leading to Michael addition or/and Schiff Base products [19], as shown in Figure 1. Furthermore, it has been demonstrated that the oxidation of catechol in quinones could be induced by metal ions, such as iron, copper, etc. [20,21]. It is worth noting that copper shows antibacterial properties. Therefore, by adding copper to the coating formulation, catechol oxidation and degree of crosslinking can be expected to be improved, as can the antibacterial activity of the system, if released.
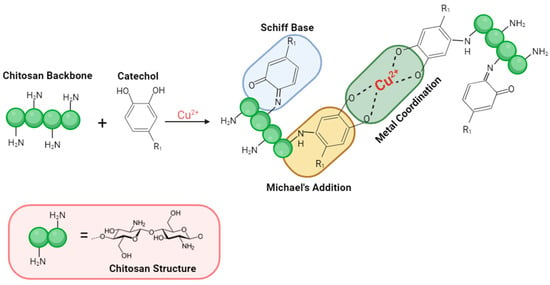
Figure 1.
General reaction scheme of oxidized catechol (ortho-quinone) with chitosan.
As previously mentioned, an antibacterial coating is mainly composed of a drug carrier, herein CS, and an antibacterial agent. The latter must be effective against CLABSI-associated bacteria. Antibiotics with broad-spectrum efficacy and with low minimum inhibition concentration (MIC) values, remain the safest and most common approach for such applications. For that reason, moxifloxacin (Mox), a third-generation fluoroquinolone, was selected. Mox’s main mechanism is based on inhibition of DNA replication [22] in Gram-positive and Gram-negative strains.
Once the formulation of the antibacterial coating is established, the adhesion between the coating and the surface of the medical device needs to be guaranteed. The adhesion on a medical device is of utmost importance to withstand the stresses caused by the implantation environment, e.g., pH, ions concentration, proteins, etc., and to avoid degradation, delamination, and detachment of the coating from the surface [7]. CVCs are usually made of polydimethylsiloxane (PDMS), an organosilicon polymer with an inorganic backbone that contributes to its biocompatibility, inertness, and hydrophobicity [23]. Thus, to ensure coating adhesion, it is necessary to activate the PDMS surface. For that purpose, techniques such as plasma and wet chemical processes have been reported [24]. Common approaches to modifying PDMS surfaces are piranha solution [25], and other strong chemicals. Even with the effectiveness for surface modification, the coating’s adhesion remains weak due to low interactions between the coating and the substrate [26]. Functionalization constitutes another approach and has the potential to improve covalent bonds and/or strong intermolecular interactions between the coating and the substrate, thus enhancing coating adhesion. To this end, dopamine (DA), a natural compound known to self-polymerize at alkaline pH [27], has been reported to strongly adhere to various substrates [28], including PDMS [29,30]. Therefore, PDMS functionalization with dopamine prior to coating appears to be a promising strategy. However, assessing the coating’s adhesion on soft polymers, such as PDMS, still constitutes an issue. Indeed, in the literature, the adhesion of chitosan on metallic substrates was largely reported. In opposition, on soft substrates such as PDMS, it is still rare. For instance, the adhesion strength of chitosan-based coating on Ti or Ti alloys implants for orthopedic, bone, or dental applications assessed by a pull-off test, ranged from 2.5 MPa to 5 MPa [31] In contrast, the adhesion strength of chitosan-based coating for soft polymer was around 3.9 N (0.039 MPa·cm2) [32], meaning that adhesion strength is not only dependent on the coating composition or substrate activation/functionalization, but on the nature of the substrate itself. To our best knowledge, chitosan-based coating adhesion strength on PDMS has never been reported, nor have the problems associated with obtaining a stable, adherent coating with sustained drug release and antibacterial properties.
Therefore, this study aimed to develop an adherent chitosan-based coating for CVCs for short-term use, which should present sustained drug release and antibacterial properties for at least 14 days. Moxifloxacin was used as the loaded antibiotic, with caffeic acid and copper as the crosslinker. To promote the adhesion of the coatings, PDMS samples were functionalized with dopamine. Then, the coatings were characterized, and their performances were assessed in terms of the moxifloxacin kinetic release profile, antibacterial efficacy, cytotoxicity, and adhesion strength stability in a simulated physiological environment.
2. Materials and Methods
2.1. Materials
Chitosan medium molecular weight, caffeic acid, copper sulphate, dopamine, moxifloxacin, sodium hydroxide, phosphate buffer saline (PBS), and ethanol were purchased at Sigma-Aldrich (Oakville, ON, Canada), and used without further purification, as well as medical-grade PDMS (70 duro @ .062”, CS Hyde Co., Lake Villa, IL, USA) and super glue (Krazy Glue, Newell Office Brands, Atlanta, GA, USA).
2.2. Methodology
2.2.1. Chitosan Formulations
Chitosan formulations used herein were based on a previous article from our group, in which tannic acid and iron (II) were used as crosslinkers to modulate the controlled release of gentamicin. The results indicated that release modulation occurs when the catechol/metal ion ratio was around 1:0.2 [33]. Consequently, the proportion of caffeic acid and copper presented herein follows this ratio.
A 1.5% (w/w) chitosan solution (CS) was prepared by dissolving chitosan in 1% acetic acid water solution. Then, 10 mg/mL of caffeic acid (CA) dissolved in 0.1M NaOH was added, followed by the addition of an aqueous solution of CuSO4 at 20 mg/mL. Finally, an aqueous solution of moxifloxacin (Mox) (10 mg/mL) was added to the formulations. Table 1 shows the amount and the addition order of each compound.

Table 1.
Chitosan formulations.
2.2.2. Surface Modification Surface Activation and Chitosan Coating
PDMS samples were cut into two different sizes: 1 cm2, for samples used for the surface characterization and drug release tests, and 2 cm2, for those in the pull-off test. Before use, PDMS samples were cleaned in an ultrasonic (US) bath for 5 min, first in ultrapure water, then in ethanol, and then dried using an air gun. Prior to PDMS functionalization with dopamine (DOPA), the surface was activated by sodium hydroxide (NaOH). This activation step was carried out by immersing the samples in a 2.5 M NaOH solution for 20 min in a US bath, followed by washing them twice in a US bath, in water, for 10 min and then air-dried. The PDMS-NaOH samples were then placed in a multi-well plate, covered with a dopamine solution 2 mg/mL pH 8.5 in PBS, allowed to react at room temperature for 24 h in the dark, washed with water, and air-dried. Finally, the functionalized PDMS-Dopa samples were coated with the different chitosan solutions (formulation given in Table 1), in which 0.7 mL of CS solution was placed on the 1 cm2 samples and 1.4 mL of those of 2 cm2 the samples were incubated (37 °C) overnight for drying.
2.2.3. Surface Characterization
X-ray Photoelectron Spectroscopy (XPS) analyses were carried out using Physical Electronics PHI 5600-ci equipment (Chanhassen, MN, USA). A standard aluminum X-ray source (1486.6 eV) was used to record survey spectra with charge compensation, while high-resolution spectra were acquired using a standard magnesium X-ray source without neutralization. The detection was set at an angle of 45° concerning the surface normal, and the analyzed area was 0.5 mm2. Three measurements for each sample were performed for each sample to assess the homogeneity of the chemical composition of three different samples. The curve fitting procedures for C1s were performed employing a least-square Gaussian-Lorentzian peak fitting procedure, after Shirley background subtraction. The C1s peaks were set at 285 eV (C-C and C-H) as reference.
Static contact angle measurements were obtained using a VCA 2500 XE system (AST®, Billerica, MA, USA). Analyses were performed at room temperature, with 1 µL droplets of ultrapure water deposited on three different areas per sample, and on three different samples.
The surface roughness of PDMS samples was assessed by profilometry measurements on a 1 mm2 scan area. Images were acquired using a Bruker Dektak XT Profilometer (Billerica, MA, USA) with a tip radius of 12.5 μm and a stylus force of 3 mg. The thickness value was estimated by profilometry measurements. Before the coating was added, an adhesive tape was placed on the PDMS-Dopa substrate, covering an area of around 0.5 cm2. After coating, the tape was carefully removed.
2.2.4. Bacterial Survival Test
The bacteria of interest, Escherichia coli (ATCC 8937) and Staphylococcus aureus (ATCC 6538) were seeded on fresh sterile Mueller–Hinton agar in Petri dishes and incubated overnight at 37 °C in an inverted position. Then, a single colony was picked and incubated in 20 mL of fresh sterile Mueller–Hinton broth overnight at 37 °C under shaking at 150 rpm. After the bacterial growth, sterile glycerol at 15% v/v was added for cryoprotection and the bacteria suspension aliquots were frozen at −20 °C. The CFU/mL of the stocks after thawing was determined by utilizing the log dilution method.
To assess the antibacterial activity of the proposed coating, indirect bacterial susceptibility tests were performed. Briefly, the samples were immersed in 2 mL of sterile Mueller–Hinton broth and incubated. Eluates were collected at different time points, i.e., 6 h, 1 day, 3 days, 7 days, and then every 7 days, where the solutions (eluates) were collected and replaced by an equivalent volume of fresh sterile Mueller–Hinton broth. All the eluates were cryopreserved at −20 °C until analysis. The bacterial stock suspension was thawed and diluted to a final concentration of approximately 1 × 106 CFU/mL in sterile Mueller–Hinton broth in a 96-well culture plate. Subsequently, 100 µL of antibiotic solution eluates from the films was added to the wells, resulting in a final volume of 200 µL. Negative controls (blanks) were prepared by adding the inoculum to 100 µL of sterile antibiotic-free Mueller–Hinton broth. The plates were incubated at 35 °C under shaking at 200 rpm until OD600 reached 0.6–0.8. Positive controls were prepared by adding antibiotics at a concentration of 10 µg/mL (gentamicin). The OD600 values of the samples were measured and compared to the negative controls. The bacterial survival was then calculated using Equation (1). Experiments were carried out at least in triplicate.
2.2.5. Moxifloxacin Releases Kinetic Characterization
Drug release tests were carried out in PBS pH 7.4 at 37 °C. First, the samples were weighed (approximately 1 mg of coating, in triplicate) and deposited in an Eppendorf, and then 2 mL of PBS was added; with this volume, it is expected that the medium will not be saturated with the drug and/or without incidence of negative effects on the drug release from the coating, as it is described by Siepmann J. et al. (2020) [34]. Then, every 7 days, the solutions were collected and replaced by an equivalent volume of fresh PBS. At each time point, the total volume of the medium was removed, and fresh PBS solution was added. The moxifloxacin quantification was performed by fluorescence spectrometry (λexc. 287 nm and λem. 480 nm), and the Mox concentration was determined from a previous calibration curve, as described by Ocaña et al. [35]. The calibration curve used to quantify Moxifloxacin is shown in Figure 2. The results were analyzed and reported as a percentage of cumulative release. The limit of detection was 0.124 µg/mL and the limit of quantification was 0.412 µg/mL.
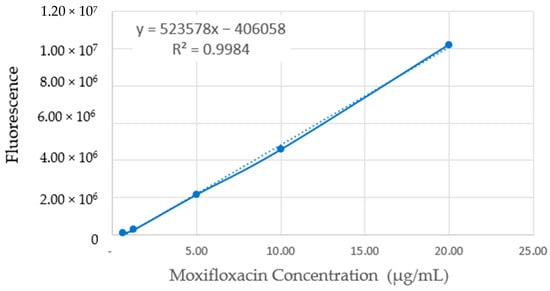
Figure 2.
Moxifloxacin calibration curve using fluorescence spectrometry.
2.2.6. Adhesion Strength (Pull-Off Test)
Coated PDMS samples were aged in PBS solution for 7 days, 14 days, 21 days, and 28 days. After these periods, the samples were gently dried, and a pull-off test was performed. The pull-off test was carried out following the norm ISO 4624:2016—Pull-off test for adhesion [36]. Using the MACH-1 V500CSST Mechanical Tester (Laval, QC, Canada), t indenter, the 2 cm2 samples were positioned between two metallic plates, which were fixed in the holder, and then a thin layer of super glue was added to the flat surface of the indenter. Next, the indenter was placed in contact with the sample and a force of approximately 2 N was applied. To ensure that the entire surface would be in contact with the glue, the glue was left to dry for ~10 min. The test was carried out at a speed of 0.07 mm/s, and at the latest approximately 1.5 min.
The adhesion force was calculated using Equation (2):
where σ is the adhesion strength [kPa], F is the adhesion force [N], and A is the area of the indenter (0.0064 m2). The test was performed with five replicates of each condition.
2.2.7. Indirect Viability Test
Before starting the tests, all samples were sterilized by UV irradiation. Shortly, each side of the samples underwent two 15 min cycles of UV irradiation. After that, the samples were stored in a sterile 24 multi-well plate until use.
Human dermal fibroblasts (HDFs) were used in the following experiments. Briefly, HDFs were cultured in Dulbecco’s modified Eagle’s medium (D-MEM) with 10% fetal bovine serum (FBS), penicillin (100 U/mL), and streptomycin (100 U/mL). The cells were maintained at 37 °C in a saturated atmosphere at 5% CO2. Media was changed every two days until a 90%–95% confluence was reached. At this point, the cells were detached from the plate using trypsin and then re-plated at a ratio of 1:5. The cells were used in passage 7 for the present experiments.
To evaluate the effects of the proposed films on cell viability, an indirect cytotoxicity assay was performed based on the ISO 10993-5:2009 [37] procedure. Sterile films were immersed in 660 µL of DMEM supplemented with 1% penicillin and streptomycin and incubated at 37 °C in a saturated atmosphere at 5% CO2 for 1, 3, and 7 days. After the incubation, the different media were collected from samples and subsequently used for the cytotoxicity test. In the meantime, HDFs were seeded in the wells of 96 multi-well plates at a density of 20,000 cells/cm2 and incubated at 37 °C, 5% CO2 for 24 h in a complete medium. The day after, the medium was removed, and 100 µL of the extracts, supplemented with 10% of FBS, were added to the well containing the cells. Normal HDF complete medium was used as a control. The cells were then incubated for 24 h. Afterwards, the extracts were removed and 100 µL of 1X solution of resazurin sodium salt in the complete medium was added to the cells and incubated for 4 h at 37 °C and 5% CO2. After incubation, the solutions containing the now reduced resorufin product were collected and fluorescence intensity at a 545 nmex/590 nmem wavelength was measured with a SpectraMax i3x Multi-Mode Plate Reader (Molecular Devices, San Jose, CA, USA). Fluorescence intensity is proportional to cell viability.
2.2.8. Statistical Analysis
Statistical significance was determined using a one-way ANOVA parametric approach followed by Tukey’s post hoc test, conducted with InStat™ software (GraphPad Prism 10, Boston, MA, USA). Results with p-values less than 0.05 were regarded as statistically significant. The results are expressed as mean ± standard deviation.
3. Results
3.1. Physico-Chemical Surface Characterization
The changes in the chemical composition of the sample’s surface were assessed by XPS survey analyses, and the results given as relative atomic percentages are shown in Table 2. For the untreated PDMS (control), the main components detected were carbon, oxygen, and silicon, with 54.1 ± 0.9%, 26.5 ± 0.4%, and 19.4 ± 0.5%, respectively. These results are consistent with the expected composition of PDMS, according to its chemical composition (O-Si(CH3)2-), and the literature [38]. Surprisingly, the NaOH (PDMS-NaOH) surface treatment did not significantly alter the chemical composition of the PDMS surface. In addition, it was observed that the roughness of the PDMS-NaOH sample increased slightly compared with the crude PDMS (Table 2), from 0.15 ± 0.01 µm to 0.22 ± 0.04 µm. This suggests that the NaOH treatment resulted in surface etching, rather than surface activation.

Table 2.
Physico-chemical surface characterization.
The PDMS functionalization by dopamine (PDMS-Dopa) was confirmed by the increase in nitrogen from 0 to 2.8 ± 0.6% and carbon from 54.1 ± 0.9% to 59.5 ± 1.3%, respectively (Table 2). Moreover, the amount of silicon content decreased from 19.4 ± 0.3% to 13.8 ± 0.3%, indicating the deposition of a thin dopamine layer on the PDMS surface. While the surface roughness increased (Figure 3), the wettability of PDMS-Dopa significantly decreased, with a WCA value of 56 ± 3° (Table 2), similar to what was reported by Yang and Zhao (2011) [29]. This hydrophilic behavior could be explained by the presence of nucleophilic groups such as hydroxyl and amine groups after the polymerization process of dopamine [39].
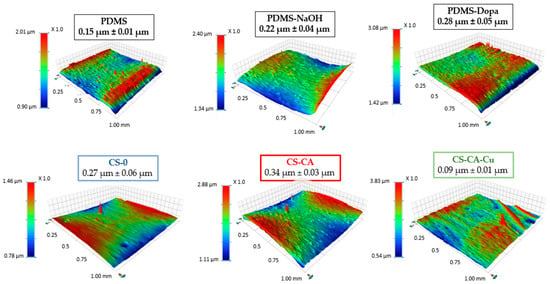
Figure 3.
Mapping profilometry and roughness values.
Once coated with the different chitosan formulations, the samples displayed different wettability (Table 2) and roughness values (Figure 3), while the thickness of the coatings was similar, between 11–13 µm (Table 2). For instance, the coating CS-0 featured a hydrophobic surface, with the WCA value around 112.8 ± 0.5°, close to the chitosan films reported in the literature [33], while roughness (Figure 3) remained unchanged compared to the PDMS-Dopa sample. Nonetheless, the formulations with caffeic acid showed a significant decrease in the WCA value, CS-CA 73.7 ± 0.2° and CS-CA-Cu 53 ± 1°, compared to CS-0 coating. It is worth noting that the CS-CA-Cu sample exhibited the smoothest coating overall, 0.09 ± 0.01 µm, with the highest hydrophilic character. These changes may be associated with interactions or bonds between CA and CS molecules, which appeared to be enhanced in the presence of Cu. In fact, it is well reported in the literature that some coordination bonds could be formed between the polyphenol and the metallic ion [40] and between polyphenol and CS [41]. Moreover, Siripatrawan et al. noted that the strong intramolecular interactions between polyphenol and CS led to an increase in water affinity [42], which agrees with the lower WCA angle value obtained with the CS-CA-Cu coating.
Regarding the chemical composition, the XPS survey analyses revealed a clear difference in surface composition compared to PDMS-Dopa (Table 2). After coating, the silicon content decreased significantly, from 13.8 ± 0.3% to less than 5%, whereas the other components’ contents increased, as expected due to CS and CA chemical composition. Interestingly, PDMS-CS-CA-Cu exhibited the lowest C amount overall while the highest O percentage, meaning that adding Cu in the formulation changed the interactions/bonds between the molecules, as previously mentioned. To further investigate the chemical bonds/interactions involved, high-resolution C1s were performed, and the resulting spectra are shown in Figure 4A. All spectra were fitted with three bands associated with C–C/C–H, C–O/C–N and C=O/O-C-O (glycosidic group from CS) at 285.0 eV, 286.7 eV and 288.3–288.7 eV, respectively [43,44]. In the CS-CA sample, the increase in the C-C/C-H band when compared to CS-0, from 48% to 59%, was correlated with the aromatic ring from CA. By adding Cu to the formulation, the C-O/C-N peak contribution increased compared to the CS-CA sample. This, combined with the fact that in the XPS survey data, an increase in O content (Table 2) was observed, could be associated with the fact that Cu induced CA oxidation, and hence CS crosslinking. It should also be pointed out that in the XPS survey analyses, no Cu was detected on the CS-CA-Cu surface (Table 2), whereas Chevallier et al. detected iron on XPS with Fe and TA used as chitosan crosslinkers. The absence of Cu on the surface suggests the total incorporation of copper into the coating, and it can be assumed that CA-Cu improved crosslinking efficiency resulting in a smooth surface, as shown by profilometry analysis. That said, XPS is a surface technique capable of an analyzing depth of approximately 5 nm, thus a complementary more in-depth analysis was performed by FTIR, which detects approximately 1 µm.
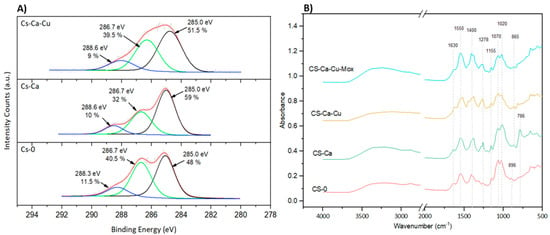
Figure 4.
Surface characterization. (A) High-resolution XPS for carbon (C1s); (B) FTIR spectrum.
The FTIR results are shown in Figure 4B. The characteristic spectra of CS were observed for the CS-0 coating. The bands at 1630 cm−1 and 1550 cm−1 were attributed to Amide I (C=O) and Amide II (NH(CO)) bonds, respectively [45]. The peak at around 1400 cm−1 is frequently attributed to the deformation of C-H [46], the sugar unit at 1155–1020 cm−1 from C–O–C and C–N stretching and the band at 896 cm−1 from the glycosidic bond [45] were also detected. Adding CA (CS-CA and CS-CA-Mox) had no impact on the peaks corresponding to the chitosan sugar unit, and the CS structure remained unaffected by the process and the components used. The addition of CA (CS-CA) induced chemical interactions on the atoms present at the glycosidic bond, as indicated by a peak shift to 865 cm−1. In addition, a new peak at 786 cm−1 appeared corresponding to the out-of-plane C-H bending [47], associated with the aromatic ring from CA. Interestingly, the addition of Cu significantly decreased this peak as the catechol groups were converted into ortho-quinones due to oxidation, also observed in the XPS experiments. The presence of Mox led to an increase in complexity when attempting to discern the individual effects of each compound. The FTIR and XPS results demonstrated the formation of different chemical interactions in the coating, which will have a significant impact on drug release.
3.2. Drug Release Kinects
Drug release tests conducted in PBS 37 °C pH 7.4 were performed to characterize the release kinetic of moxifloxacin (Figure 5). The formulations were able to present a sustained drug release for nearly 50 days. The CS-0-Mox released 90% of the contained Mox within 49 days, while in the same time range CS-CA-Mox released almost 50% and CS-CA-Cu-Mox reached close to 65%.
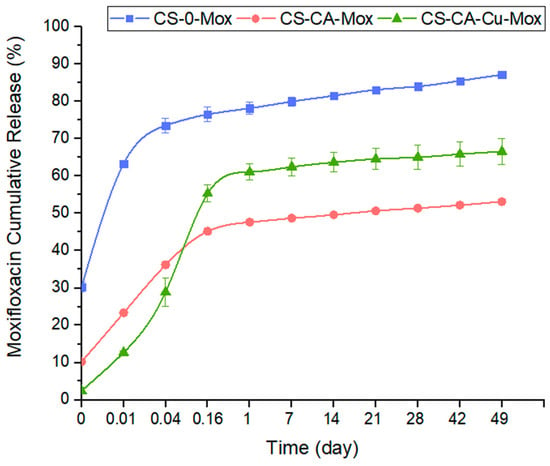
Figure 5.
Cumulative moxifloxacin release from PDMS-Dopa, where 0.01 d (20 min), 0.04 d (1 h), and 0.16 d (4 h).
As can be noted in Figure 5, all formulations presented a burst release. This type of burst release is usually associated with different factors such as drug molecular weight, drug–polymer interactions [48], Mox solubility in water, and crosslinking degree [49]. The absence of crosslinkers in the CS-0-Mox coating may explain why this formulation exhibited the highest burst release. With the addition of crosslinkers, CA and CA-Cu, a lower burst release was observed due to stronger interactions or crosslinking degree involved. Surprisingly, Cu addition did not delay the burst release as expected. This may be explained by the fact that Cu interacts also with CA [50], reducing the CA–Mox complex formation, and hence the faster release of Mox.
This being said, the coating proposed here, whatever its composition, exhibited a longer sustained release, up to 49 days, compared with other chitosan-based coatings described in the literature. For example, Chen et al. electrodeposited a cefazolin-loaded chitosan-based coating onto a titanium substrate and, after 21 days, almost 100% of the drug content was released [51]. Soares et al. studied the release of drugs from a chitosan and polycaprolactone-based coating dip-coated onto sandblasted stainless steel for the release of microspheres loaded with daptomycin or vancomycin, and only after 100 h (≈4 days) all the drug-loaded was released [52].
From the cumulative release graphs (Figure 5), it is possible to make a rough estimation of the amount of drug released relative to the total amount loaded, enabling us to estimate the longevity of the coating in terms of drug release capacity. Nevertheless, to know whether the amount of drug released is sufficient to inhibit bacterial growth and meet the therapeutic window, it is necessary to consider drug release from a different angle. To that end, the concentration of Mox release per time point was calculated, and the results are presented in Figure 6. From 1 day to 49 days, whatever the composition of the coating, the amount of Mox released was constant and sustained, with a release speed of around 0.006 µg Mox/h. Moreover, this sustained Mox released concentration over time was higher than the minimum inhibitory concentration (MIC) for both S. aureus and E. coli.
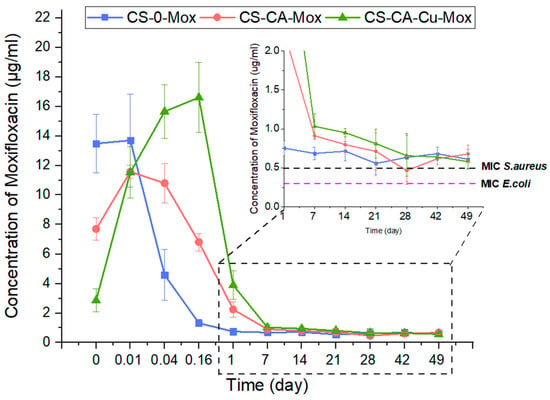
Figure 6.
Moxifloxacin release. Concentration of Mox released per time point. Green and purple dashed lines are correlated to S. aureus and E. coli MIC, respectively, where 0.01 d (20 min), 0.04 d (1 h), and 0.16 d (4 h).
3.3. Antibacterial Assay µ
The bacterial survival assay over time (Figure 7) was derived from the MIC90 assessment, wherein the activity of the coating was considered effective if the bacterial survival was below 10%. For the bacteria used in this test, the moxifloxacin MIC found was 0.5 µg/mL for S. aureus and 0.03 µg/mL for E. coli, in accordance with Eucast Database (bacteria chosen as representative Gram-negative and Gram-positive pathogens, respectively, and E. coli among the most common strains in the case of bloodstream infection [53]). Since the formulations without Mox did not present antimicrobial activity as expected, the focus was made only on the formulations with the antibiotic. The formulation CS-0-Mox was contaminated after 14 days and 3 days for E. coli and S. aureus, respectively, whereas CS-CA-Mox and CS-CA-Cu-Mox coatings were able to prevent bacterial growth up to 21 days for E. coli and 14 days for S. aureus. It is noteworthy that the copper used in the coating formulation did not add additional antibacterial activity. In addition, the antibacterial results did not completely fit with the release kinetic data shown in Figure 5, where Mox amounts were higher than MIC values, regardless of the coating composition and bacteria strains. Since the release kinetics were carried out in phosphate-buffered saline (PBS) and the antibacterial tests were performed in Mueller–Hinton broth, the antibacterial activity of Mox can be expected to be effective over the longer term in a natural physiological environment as found in blood vessels (proteins, acids, carbohydrates, blood flow, etc.).
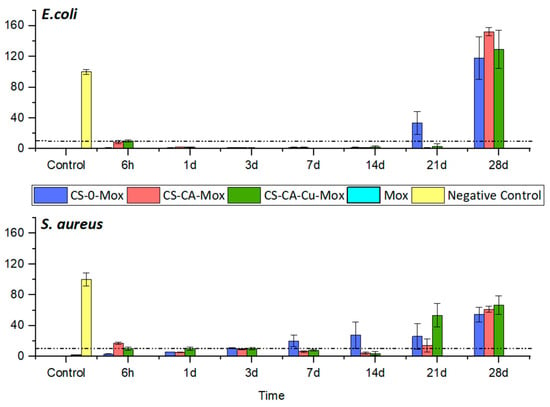
Figure 7.
Bacterial survival test for S. aureus and E. coli. The chart presents the results of different chitosan coatings compared to positive control (CTRL Pos) and negative control (CTRL Neg) conditions. The graphic shows the mean percentage of bacterial survival ± SD.
These results appear noteworthy compared to those obtained for other chitosan-based coatings reported in the literature. For example, in the study by Farrokhi-Rad et al. [31], the number of S. aureus colonies on the vancomycin-loaded coating was about 27% less than on the one without drug loading after 20 h, with no longer-term evaluation. For Wang et al. [54], the silver-loaded chitosan coating, S. aureus anti-adhesion rates on Ti substrate were around 90% after 3 days compared to the uncoated sample, but no longer-term studies were carried out. For the E. coli strain, it is also challenging to make comparisons, as most studies of chitosan-based coatings assess the reduction in bacterial colonization on the modified surface in the short-term, e.g., 80% after 15 h [53] and 37% after 48 h [55]. Moreover, the chitosan-based coatings were developed more as repellent coatings, e.g., chitosan-fatty acid derivatives, and not as a drug release system, as developed in this work.
3.4. Indirect Cytotoxicity
To properly assess the possible toxic effects of using this formulation as an antimicrobial coating for medical devices, cytotoxicity for vein cells (human dermal fibroblasts, HDF) was performed. Indirect viability tests were performed on HDFs that were put in contact with the extract obtained by the different samples to evaluate their effects on cell viability (Figure 8). In the extracts obtained after 1 day of incubation, the PDMS condition did not alter cell viability over time compared to the CTRL (yellow bar) one, as expected [23]. The chitosan coatings, both with and without moxifloxacin, also did not change the cell viability in a significant way compared to the control condition; although, in the conditions containing caffeic acid, CS-CA, and CS-CA-Mox, both showed a significantly decreased viability compared to all the other tested conditions. As for the CS-CA-Cu formulation, the coating with no moxifloxacin still presented a good viability level, close to the control condition. Still, the CS-CA-Cu-Mox coating significantly reduced the HDF viability compared to the other tested conditions.
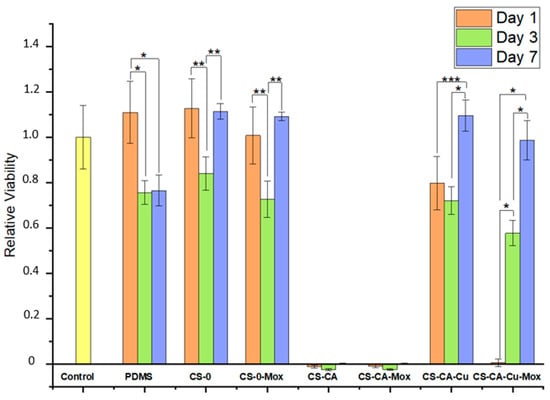
Figure 8.
Indirect viability of HDF. The results were normalized based on the control (CTRL). The graph presents the cell viability ratio ± SD. Tukey’s test was performed individually for each collection of time points. The graphic shows the mean relative viability ± SD recorded from HDFs treated with the different experimental conditions. The results have been normalized against the CTRL condition. Averages followed by the same symbol are statistically equal (95% significance), where * p ˂ 0.0001; ** p < 0.005; *** p < 0.05.
Regarding the extracts from day 3, the extracts obtained from PDMS, CS-0, CS-0-Mox and CS-CA-Cu, CS-CA-Cu-Mox did not exert any effects on cell viability. In fact, cells showed a viability comparable to the CTRL condition. However, once again the extracts obtained from the CS-CA (without and with Mox) conditions still induced significant cytotoxic effects on the treated cells. Finally, with the extract collected on day 7, the same tendency was observed. The extracts from the CS-CA (without and with Mox) films still induced a significant decrease in cell viability, whereas all the other tested conditions did not alter the viability of the treated HDFs compared to the CTRL condition.
3.5. Adhesion Strength of Chitosan-Based Coating
As described above, in the development of an antibacterial coating for medical devices, adhesion strength plays a crucial role in the longevity and effectiveness of the coating. Thus, the adhesion strength of the developed coatings was assessed. First, the adhesion strength of the PDMS-Dopa surface was determined to be 2.67 kPa ± 0.20 kPa (Figure 9), a value subsequently used as a control. Then, once coated with the different chitosan-based formulations, the adhesion strengths were measured before ageing (Coated-PDMS) and after ageing in the pseudo-physiological medium for 7 and 14 days (PBS 7d, PBS 14 d), and the results are given in Figure 9.
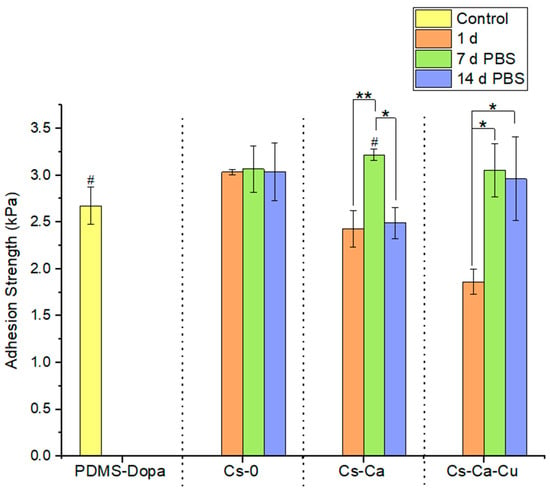
Figure 9.
Adhesion strength PDMS-Dopa chitosan-based coating. Tukey’s test was performed individually for each collection of time points. Averages followed by the same symbol are statistically equal (95% significance), where * p ˂ 0.0001; ** p < 0.005; # p < 0.005.
The non-crosslinked CS coating CS-0 displayed the highest adhesion strength of 3.03 ± 0.03 kPa compared to crosslinked coatings: CS-CA and CS-CA-Cu with 2.43 ± 0.20 kPa and 1.86 ± 0.13 kPa, respectively (Figure 9). Furthermore, it is surprising to note that the crosslinked coatings appear to have a lower adhesion strength than the control (PDMS-Dopa), which was no longer the case after aging in PBS. Indeed, the adhesion strength of all crosslinked coatings on PDMS-dopa increased significantly after aging in PBS, whereas for CS-0 it remained unchanged. For example, the CS-CA-Cu coating increased from 1.86 ± 0.13 kPa to 3.05 ± 0.29 kPa and 2.96 ± 0.45 kPa for 7 d PBS and 14 d PBS, respectively, i.e., a 1.6-fold increase. These behaviors can be explained by the fact that the crosslinking reaction takes time and was not yet fully settled after the coating was added to the surface (i.e., samples 1d). To corroborate this hypothesis, the adhesion strengths of coatings aged on the bench for 14 days were also evaluated: CS-0 showed an adhesion strength of 3.45 ± 0.62 kPa, CS-CA of 3.63 ± 0.43 kPa, and CS-CA-Cu of 3.51 ± 0.38 kPa. These results demonstrate that not only was the crosslinking reaction in the coating incomplete, but also that of the chitosan-based coating with the functionalized PDMS surface (PDMS-Dopa). Indeed, the CS-0 coating also exhibited an increase in adhesion strength after 14 days of aging. Of note, the highest adhesion strength increase was observed for CA-CA-Cu, i.e., a 1.8-fold increase. In the literature, Park et al. demonstrated that the adhesion strength of chitosan matrices relied on the functional groups available in the chitosan coating formulation, such as OH and NH2 [56]. In their study, they showed that the adhesion strength of chitosan/oxidized caffeic acid decreased when the amount of oxidized CA was higher due to the decrease in free functional groups on chitosan, which are already involved in the reaction with oxidized CA. That said, the adhesion strength of chitosan-based coatings appears promising and demonstrated a certain stability of the coatings even in a pseudo-physiological environment. Whether the adhesion strength presented herein is sufficient to guarantee coating stability during use in a hospital environment requires further study.
4. Conclusions
In this study, the effects of chitosan-based coating compositions on their adhesion strength to PDMS, as well as its efficacy as a short-term drug delivery system (up to 2 weeks) were investigated. Surface chemical analyses demonstrated that both PDMS surface functionalization and coating grafting were effective. When CA and CA-Cu, which were shown to act as CS crosslinkers, were added, the CS-based coatings became more hydrophilic and smoother than CS-0. It was also observed that increasing the degree of crosslinking impacted the release kinetics of the antibiotic. All formulations showed sustained release for up to 49 days. However, the initial burst release appeared to be lower in the CS-CA and CS-CA-Cu coatings than in CS-0. In addition, the amount of Mox concentration released over time proved to be constant after the first day and was higher than the MIC values for S. aureus and E. coli. While the formulation CS-0-Mox was contaminated after 14 days and 21 days for E. coli and S. aureus, respectively, the CS-CA-Mox and CS-CA-Cu-Mox coatings were able to prevent bacterial growth for up to 21 and 14 days against E. coli and S. aureus, respectively. The CS-CA-Cu formulation mitigated the cytotoxic effects observed on HDF when compared to coatings containing CA alone. Furthermore, the adhesion strength of the coating on PDMS, assessed by a pull-off test, indicated that the coating process presented herein was highly adherent over time even in a pseudo-physiological medium. Among all the formulations tested, CS-CA-Cu demonstrated the most promising performance, maintaining antibacterial activity for up to 21 days against S. aureus and E. coli, while exhibiting no cytotoxic effect.
Author Contributions
Conceptualization, F.d.S.V., P.C. and B.D.; Methodology, F.d.S.V., P.C., H.J.W., F.C. and B.D.; Validation, P.C., H.J.W., B.D. and D.M.; Formal analysis, F.d.S.V., P.C. and F.C.; Investigation, F.d.S.V. and H.J.W.; Resources, D.M.; Writing—original draft, F.d.S.V.; Writing—review & editing, P.C., H.J.W., F.C. and D.M.; Visualization, F.d.S.V.; Supervision, P.C. and D.M.; Project administration, D.M.; Funding acquisition, D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially supported by the Parque Científico e Tecnológico de Biociências BIOPARK, Prati–Donaduzzi Farmacêutica Co. in Toledo, Brazil, the Natural Science and Engineering Research Council of Canada (Discovery program and Alliance project), and the Quebec Ministry of Economy and Innovation (under the hub of PRIMA, Quebec).
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki and approved by the Ethics Committee of CR-CHU de Québec—Université Laval (CER#SCH11-09-091, date of approval 2011-11-25).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This work was supported in a joint effort between Associação de Pesquisa, Ensino e Extensão BIOPARK, Prati–Donaduzzi Pharmaceutical Industry, Parque Científico e Tecnológico de Biociências BIOPARK, and the Natural Science and Engineering Research Council of Canada, under the Alliance Program. The authors thank Fernanda Heloisa Simch, and Nathália Fernanda Sczesny, for technical support in the execution of experiments, as well as Cecilia Zorzi Bueno, for help and guidance all over the research. Special thanks to Carmen Prati-Donaduzzi, and Luiz Donaduzzi, for providing support and guidance throughout the project.
Conflicts of Interest
This study received funding from BIOPARK and Prati–Donaduzzi Farmacêutica Co. The funder was not involved in the study design, collection, analysis, and interpretation of data, the writing of this article or the decision to submit it for publication. All authors declare no other competing interests.
References
- Revelas, A. Healthcare—Associated infections: A public health problem. Niger. Med. J. 2012, 53, 59. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Canada. Report on the State of Public Health in Canada 2013; Public Health Agency of Canada: Ottawa, ON, Canada, 2013. [Google Scholar]
- Umscheid, C.A.; Mitchell, M.D.; Doshi, J.A.; Agarwal, R.; Williams, K.; Brennan, P.J. Estimating the Proportion of Healthcare-Associated Infections that Are Reasonably Preventable and the Related Mortality and Costs. Infect. Control Hosp. Epidemiol. 2011, 32, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Siempos, I.I.; Kopterides, P.; Tsangaris, I.; Dimopoulou, I.; Armaganidis, A.E. Impact of catheter-related bloodstream infections on the mortality of critically ill patients: A meta-analysis. Crit. Care Med. 2009, 37, 2283–2289. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, M.; Dunais, B.; Roger, P.M. Antimicrobial lock therapy in central-line associated bloodstream infections: A systematic review. Infection 2015, 43, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Marschall, J. Current strategies for the prevention and management of central line-associated bloodstream infections. Infect. Drug Resist. 2010, 3, 147. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Hu, B.; Rosenthal, V.D.; Gao, X.; He, L. Device-associated infection rates in 398 intensive care units in Shanghai, China: International Nosocomial Infection Control Consortium (INICC) findings. Int. J. Infect. Dis. 2011, 15, e774–e780. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef]
- Alasvand, N.; Urbanska, A.M.; Rahmati, M.; Saeidifar, M.; Gungor-Ozkerim, P.S.; Sefat, F.; Rajadas, J.; Mozafari, M. Therapeutic Nanoparticles for Targeted Delivery of Anticancer Drugs; No. 2011; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Tolesa, L.D.; Gupta, B.S.; Lee, M.J. Chitin and chitosan production from shrimp shells using ammonium-based ionic liquids. Int. J. Biol. Macromol. 2019, 130, 818–826. [Google Scholar] [CrossRef]
- Chelu, M.; Musuc, A.M. Advanced Biomedical Applications of Multifunctional Natural and Synthetic Biomaterials. Processes 2023, 11, 2696. [Google Scholar] [CrossRef]
- Kausar, R.; Khan, A.U.; Jamil, B.; Shahzad, Y.; ul-Haq, I. Development and pharmacological evaluation of vancomycin loaded chitosan films. Carbohydr. Polym. 2021, 256, 117565. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M.S. Chitosan as a Vehicle for Growth Factor Delivery: Various Preparations and Their Applications in Bone Tissue Regeneration; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 104. [Google Scholar] [CrossRef]
- Younis, M.K.; Tareq, A.Z.; Kamal, I.M. Optimization of Swelling, Drug Loading and Release from Natural Polymer Hydrogels. IOP Conf. Ser. Mater. Sci. Eng. 2018, 454, 012017. [Google Scholar] [CrossRef]
- Wiggers, H.J.; Chevallier, P.; Copes, F.; Simch, F.H.; da Silva Veloso, F.; Genevro, G.M.; Mantovani, D. Quercetin-Crosslinked Chitosan Films for Controlled Release of Antimicrobial Drugs. Front. Bioeng. Biotechnol. 2022, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xu, Z.; Liu, G.; Wu, J. Polyphenols as a versatile component in tissue engineering. Acta Biomater. 2021, 119, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.P.; Hansch, C. An approach towards the quantitative structure-activity relationships of caffeic acid and its derivatives. ChemBioChem 2004, 5, 1188–1195. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, Y.; Yan, Y. Caffeic acid production enhancement by engineering a phenylalanine over-producing Escherichia coli strain. Biotechnol. Bioeng. 2013, 110, 3188–3196. [Google Scholar] [CrossRef]
- Liu, F.; Ma, C.; Gao, Y.; McClements, D.J. Food-Grade Covalent Complexes and Their Application as Nutraceutical Delivery Systems: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 76–95. [Google Scholar] [CrossRef]
- Yan, G.; Chen, G.; Peng, Z.; Shen, Z.; Tang, X.; Sun, Y.; Zeng, X.; Lin, L. The Cross-Linking Mechanism and Applications of Catechol–Metal Polymer Materials. Adv. Mater. Interfaces 2021, 8, 2100239. [Google Scholar] [CrossRef]
- Mumtaz, A.; Mahmud, T.; Elsegood, M.R.; Weaver, G.W. Synthesis and Characterization of New Schiff Base Transition Metal Complexes Derived from Drug Together with Biological Potential Study. J. Nucl. Med. Radiat. Ther. 2016, 07, 2. [Google Scholar] [CrossRef]
- Hooper, D.C. Mechanisms of Action of Antimicrobials: Focus on Fluoroquinolones Inhibition of Cell Wall Synthesis. 2001. Available online: https://academic.oup.com/cid/article/32/Supplement_1/S9/298541 (accessed on 15 October 2023).
- Kaliyathan, A.V.; Mathew, A.; Rane, A.V.; Kanny, K.; Thomas, S. Natural Rubber and Silicone Rubber-Based Biomaterials; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Tu, Q.; Wang, J.C.; Zhang, Y.; Liu, R.; Liu, W.; Ren, L.; Shen, S.; Xu, J.; Zhao, L.; Wang, J. Surface modification of poly(dimethylsiloxane) and its applications in microfluidics-based biological analysis. Rev. Anal. Chem. 2012, 31, 177–192. [Google Scholar] [CrossRef]
- Koh, K.S.; Chin, J.; Chia, J.; Chiang, C.L. Quantitative studies on PDMS-PDMS interface bonding with piranha solution and its swelling effect. Micromachines 2012, 3, 427–441. [Google Scholar] [CrossRef]
- Bhatt, P.; Kumar, V.; Subramaniyan, V.; Nagarajan, K.; Sekar, M.; Chinni, S.V.; Ramachawolran, G. Plasma Modification Techniques for Natural Polymer-Based Drug Delivery Systems. Pharmaceutics 2023, 15, 2066. [Google Scholar] [CrossRef]
- Marras-Marquez, T.; Peña, J.; Veiga-Ochoa, M.D. Robust and versatile pectin-based drug delivery systems. Int. J. Pharm. 2015, 479, 265–276. [Google Scholar] [CrossRef]
- Ding, Y.H.; Floren, M.; Tan, W. Mussel-inspired polydopamine for bio-surface functionalization. Biosurf. Biotribol. 2016, 2, 121–136. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, B. Adhesion Properties of Self-Polymerized Dopamine Thin Film. Open Surf. Sci. J. 2011, 3, 115–122. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Farrokhi-Rad, M.; Fateh, A.; Shahrabi, T. Electrophoretic deposition of vancomycin loaded halloysite nanotubes-chitosan nanocomposite coatings. Surf. Coat. Technol. 2018, 349, 144–156. [Google Scholar] [CrossRef]
- Li, Y.; Ren, J.; Wang, B.; Lu, W.; Wang, H.; Hou, W. Development of biobased multilayer films with improved compatibility between polylactic acid-chitosan as a function of transition coating of SiOx. Int. J. Biol. Macromol. 2020, 165, 1258–1263. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, P.; Wiggers, H.J.; Copes, F.; Bueno, C.Z.; Mantovani, D. Prolonged Antibacterial Activity in Tannic Acid–Iron Complexed Chitosan Films for Medical Device Applications. Nanomaterials 2023, 13, 484. [Google Scholar] [CrossRef] [PubMed]
- Siepmann, J.; Siepmann, F. Sink conditions do not guarantee the absence of saturation effects. Int. J. Pharm. 2020, 577, 119009. [Google Scholar] [CrossRef] [PubMed]
- Ocaña, J.A.; Barragán, F.J.; Callejón, M. Spectrofluorimetric determination of moxifloxacin in tablets, human urine and serum. Analyst 2000, 125, 2322–2325. [Google Scholar] [CrossRef] [PubMed]
- ISO 4624:2016; Paints and Varnishes Pull-Off Test for Adhesion. ISO: Geneve, Switzerland, 2016.
- ISO 10993-5:2009; Biological Evaluation of Medical Devices Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneve, Switzerland, 2009.
- Nourmohammadi, J.; Hajibabaei, T.; Amoabediny, G.; Jafari, S.H. Aminosilane Layer Formation Inside the PDMS Tubes Improves Wettability and Cytocompatibility of Human Endothelial Cells the Preparation and Characterization of Nanofibrous Scaffold for Endothelial Progenitor Cell Expansion View Project. Available online: https://www.researchgate.net/publication/278753721 (accessed on 15 October 2023).
- Lu, M.; Ding, L.; Zhong, T.; Dai, Z. Improving Hydrophilicity and Adhesion of PDMS through Dopamine Modification Assisted by Carbon Dioxide Plasma. Coatings 2023, 13, 126. [Google Scholar] [CrossRef]
- Razaviamri, S.; Wang, K.; Liu, B.; Lee, B.P. Catechol-based antimicrobial polymers. Molecules 2021, 26, 559. [Google Scholar] [CrossRef]
- Silvestre, J.; Delattre, C.; Michaud, P.; de Baynast, H. Optimization of chitosan properties with the aim of a water resistant adhesive development. Polymers 2021, 13, 4031. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Saleviter, S.; Chanlek, N.; Nakajima, H.; Abdullah, J.; Yusof, N.A. X-ray photoelectron spectroscopy analysis of chitosan–graphene oxide-based composite thin films for potential optical sensing applications. Polymers 2021, 13, 478. [Google Scholar] [CrossRef]
- Li, P.C.; Liao, G.M.; Kumar, S.R.; Shih, C.M.; Yang, C.C.; Wang, D.M.; Lue, S.J. Fabrication and Characterization of Chitosan Nanoparticle-Incorporated Quaternized Poly(Vinyl Alcohol) Composite Membranes as Solid Electrolytes for Direct Methanol Alkaline Fuel Cells. Electrochim. Acta 2016, 187, 616–628. [Google Scholar] [CrossRef]
- Branca, C.; D’Angelo, G.; Crupi, C.; Khouzami, K.; Rifici, S.; Ruello, G.; Wanderlingh, U. Role of the OH and NH vibrational groups in polysaccharide-nanocomposite interactions: A FTIR-ATR study on chitosan and chitosan/clay films. Polymer 2016, 99, 614–622. [Google Scholar] [CrossRef]
- Cárdenas, G.; Miranda, S.P. FTIR and TGA studies of chitosan composite films. J. Chil. Chem. Soc. 2004, 49, 291–295. [Google Scholar] [CrossRef]
- Yamada, T.; Mizuno, M. Infrared Spectroscopy in the Middle Frequency Range for Various Imidazolium Ionic Liquids—Common Spectroscopic Characteristics of Vibrational Modes with In-Plane +C(2)-H and +C(4,5)-H Bending Motions and Peak Splitting Behavior Due to Local Symmetry Breaking of Vibrational Modes of the Tetrafluoroborate Anion. ACS Omega 2021, 6, 1709–1717. [Google Scholar] [CrossRef]
- Yoo, J.; Won, Y.Y. Phenomenology of the Initial Burst Release of Drugs from PLGA Microparticles. ACS Biomater. Sci. Eng. 2020, 6, 6053–6062. [Google Scholar] [CrossRef]
- Martinez, A.W.; Caves, J.M.; Ravi, S.; Li, W.; Chaikof, E.L. Effects of crosslinking on the mechanical properties, drug release and cytocompatibility of protein polymers. Acta Biomater. 2014, 10, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Herrero, A.C.; Sánchez-Sánchez, C.; Chérioux, F.; Martínez, J.I.; Abad, J.; Floreano, L.; Verdini, A.; Cossaro, A.; Mazaleyrat, E.; Guisset, V.; et al. Copper-assisted oxidation of catechols into quinone derivatives. Chem. Sci. 2021, 12, 2257–2267. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.T.; Chien, H.W.; Cheng, C.Y.; Huang, H.M.; Song, T.Y.; Chen, Y.C.; Wu, C.H.; Hsueh, Y.H.; Wang, Y.H.; Ou, S.F. Drug-release dynamics and antibacterial activities of chitosan/cefazolin coatings on Ti implants. Prog. Org. Coat. 2021, 159, 106385. [Google Scholar] [CrossRef]
- Soares, I.; Faria, J.; Marques, A.; Ribeiro, I.A.; Baleizão, C.; Bettencourt, A.; Ferreira, I.M.; Baptista, A.C. Drug Delivery from PCL/Chitosan Multilayer Coatings for Metallic Implants. ACS Omega 2022, 7, 23096–23106. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, A.; Goszczyńska, A.; Gołda-Cępa, M.; Kotarba, A.; Sobolewski, P.; El Fray, M. Biofunctional catheter coatings based on chitosan-fatty acids derivatives. Carbohydr. Polym. 2019, 225, 115263. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.B.; Quan, Y.H.; Xu, Z.M.; Zhao, Q. Preparation of highly effective antibacterial coating with polydopamine/chitosan/silver nanoparticles via simple immersion. Prog. Org. Coat. 2020, 149, 105967. [Google Scholar] [CrossRef]
- Vakili, N.; Asefnejad, A. Titanium coating: Introducing an antibacterial and bioactive chitosan-alginate film on titanium by spin coating. Biomed. Tech. 2020, 65, 621–630. [Google Scholar] [CrossRef]
- Park, M.K.; Li, M.X.; Yeo, I.; Jung, J.; Yoon, B.I.L.; Joung, Y.K. Balanced adhesion and cohesion of chitosan matrices by conjugation and oxidation of catechol for high-performance surgical adhesives. Carbohydr. Polym. 2020, 248, 116760. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).