Investigating the Impact of Physical Vapour Deposition (PVD)-Coated Cutting Tools on Stress Corrosion Cracking Susceptibility in Turning Super Duplex Stainless Steel
Abstract
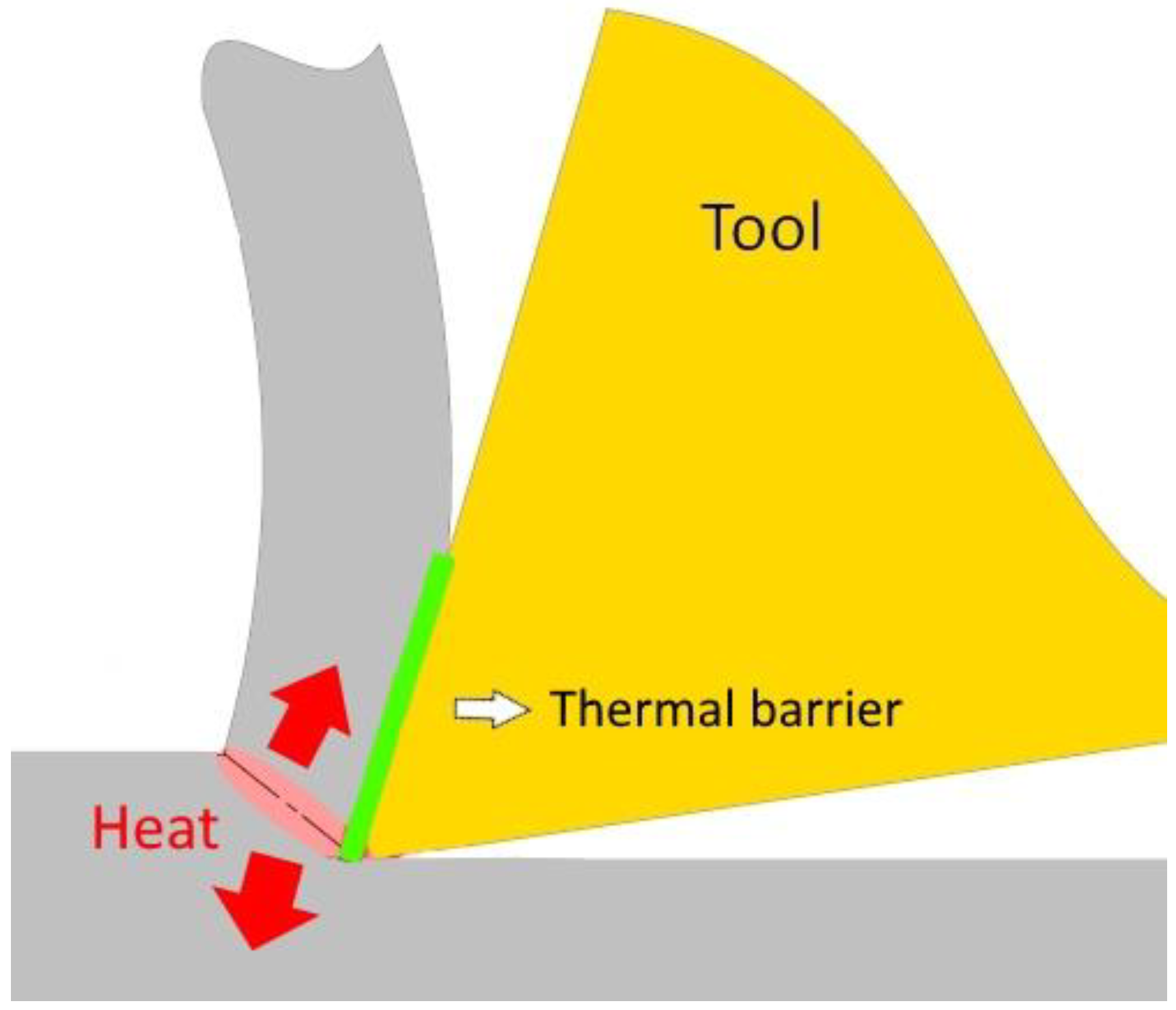
1. Introduction
2. Materials and Methods
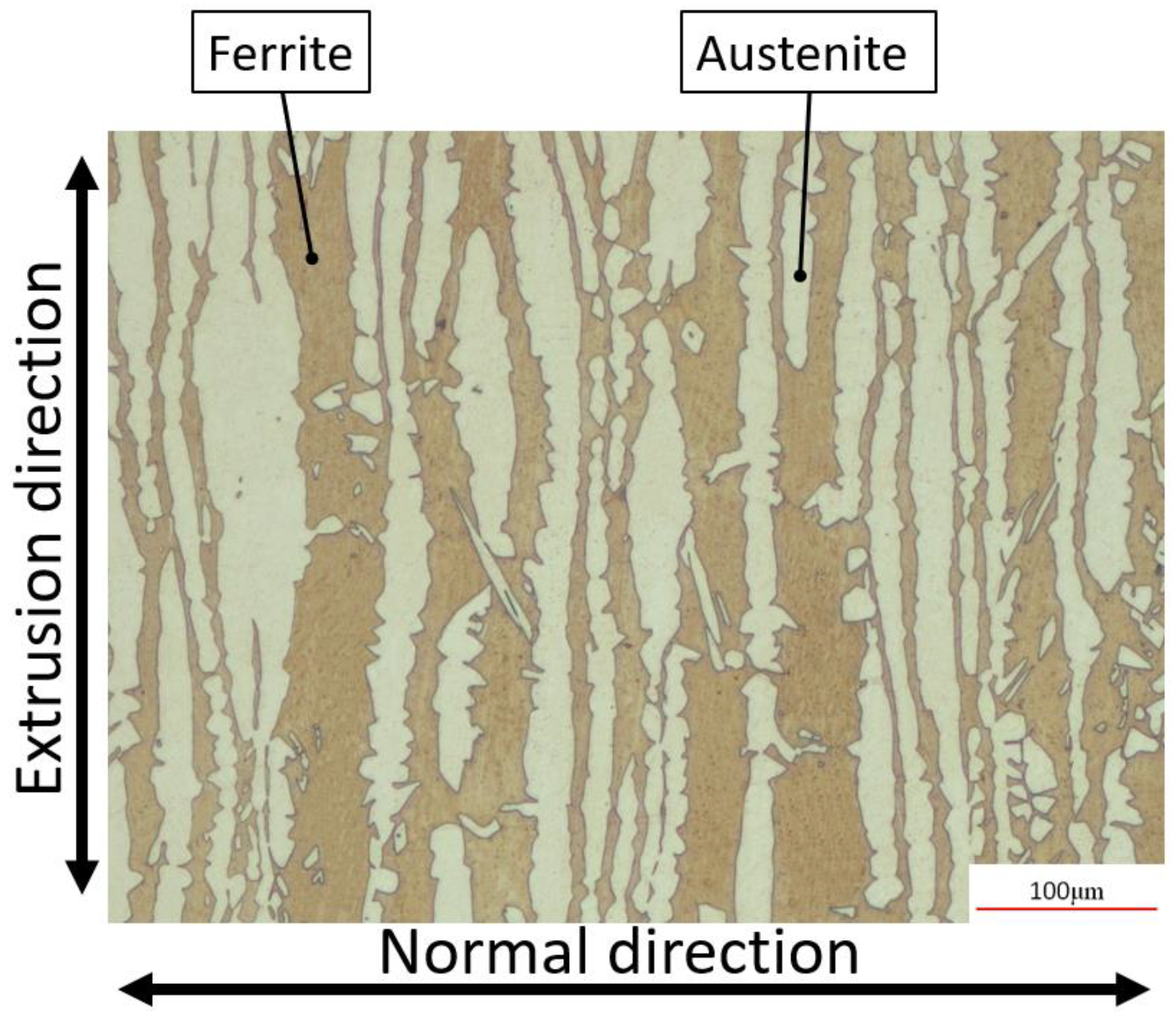
2.1. Materials
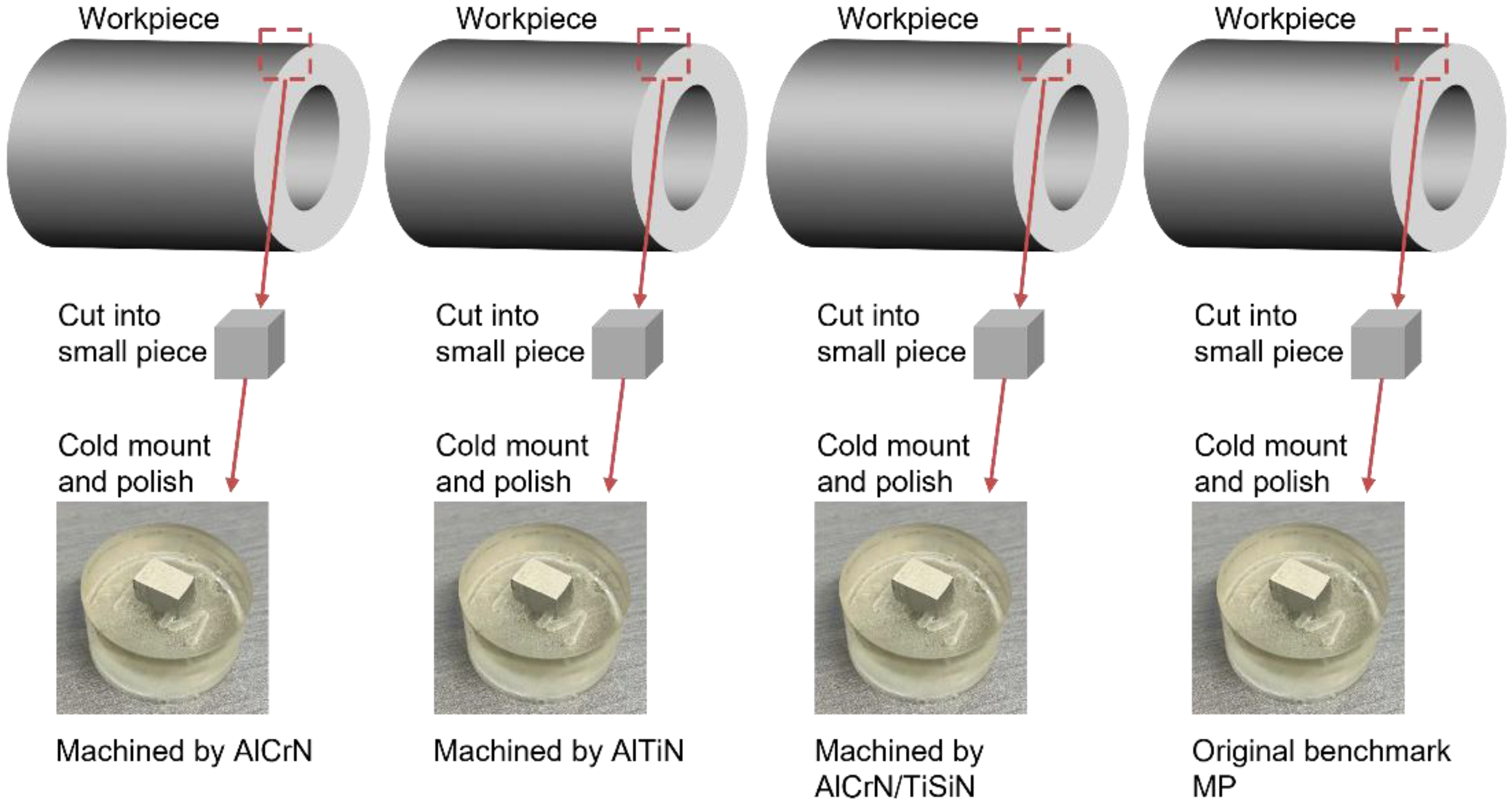
2.2. Machined Surface Characterization
2.3. Electrochemical Measurements
2.4. SCC Susceptibility (ASTM G36) Test
3. Results and Discussion
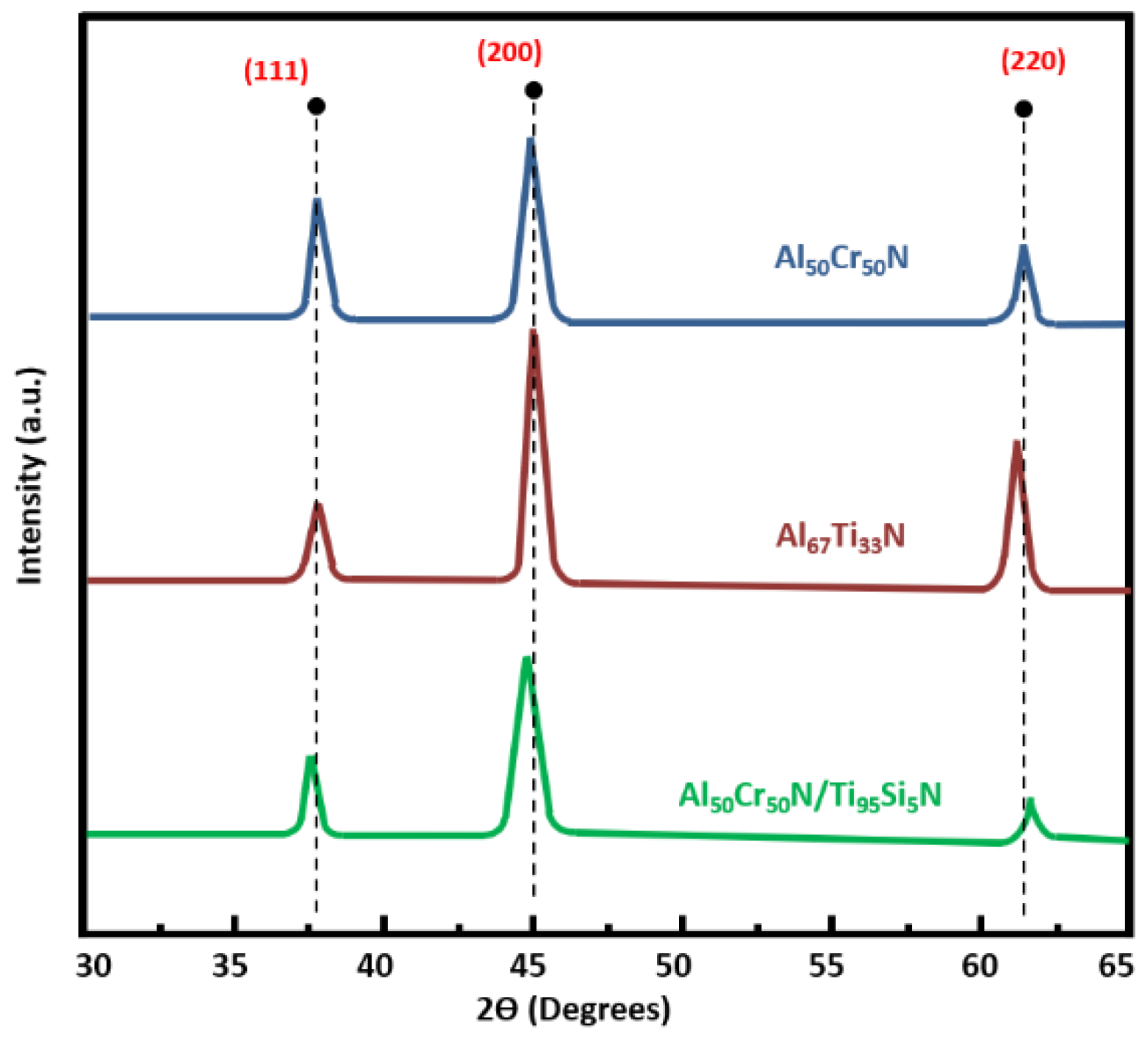
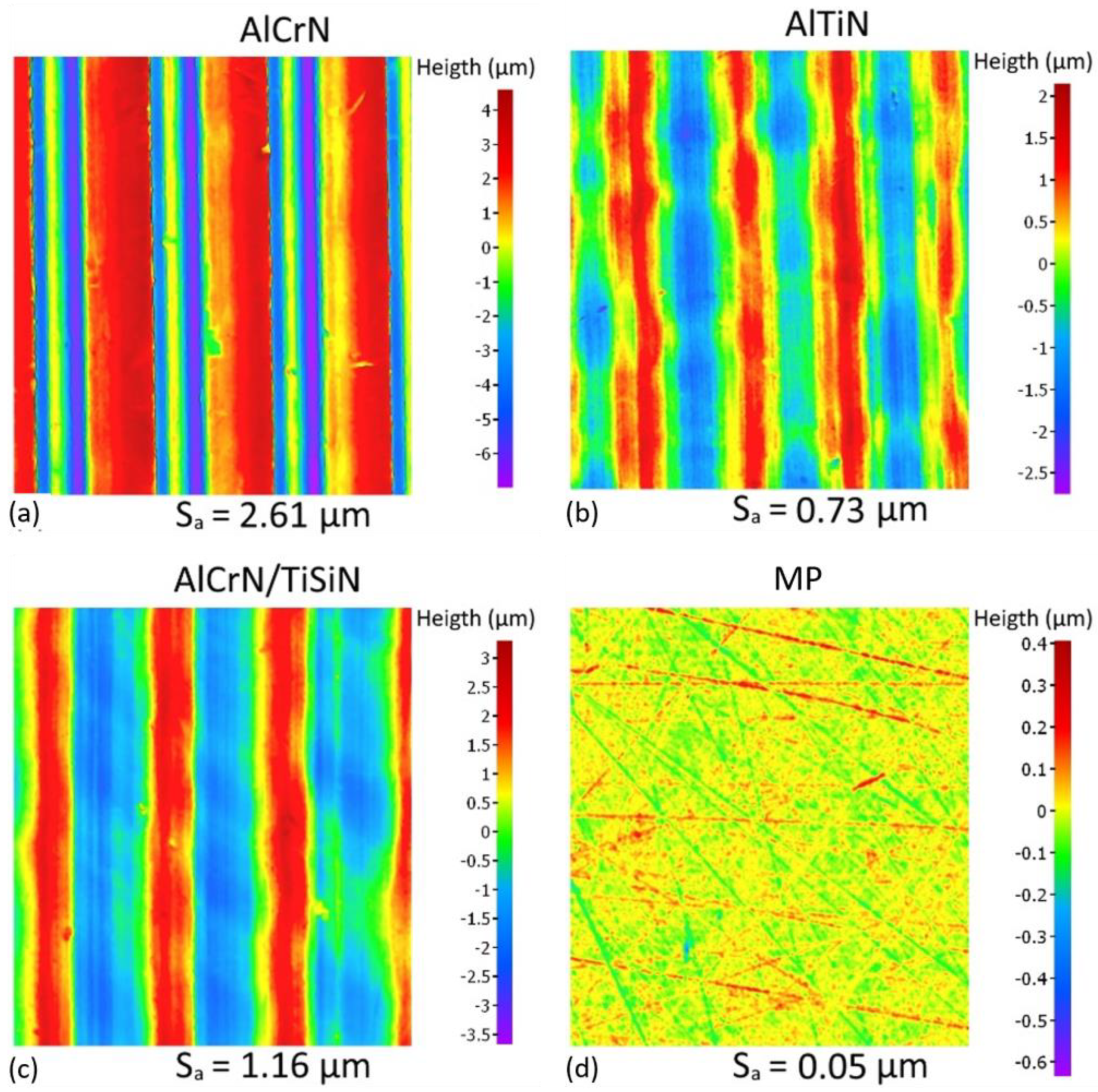
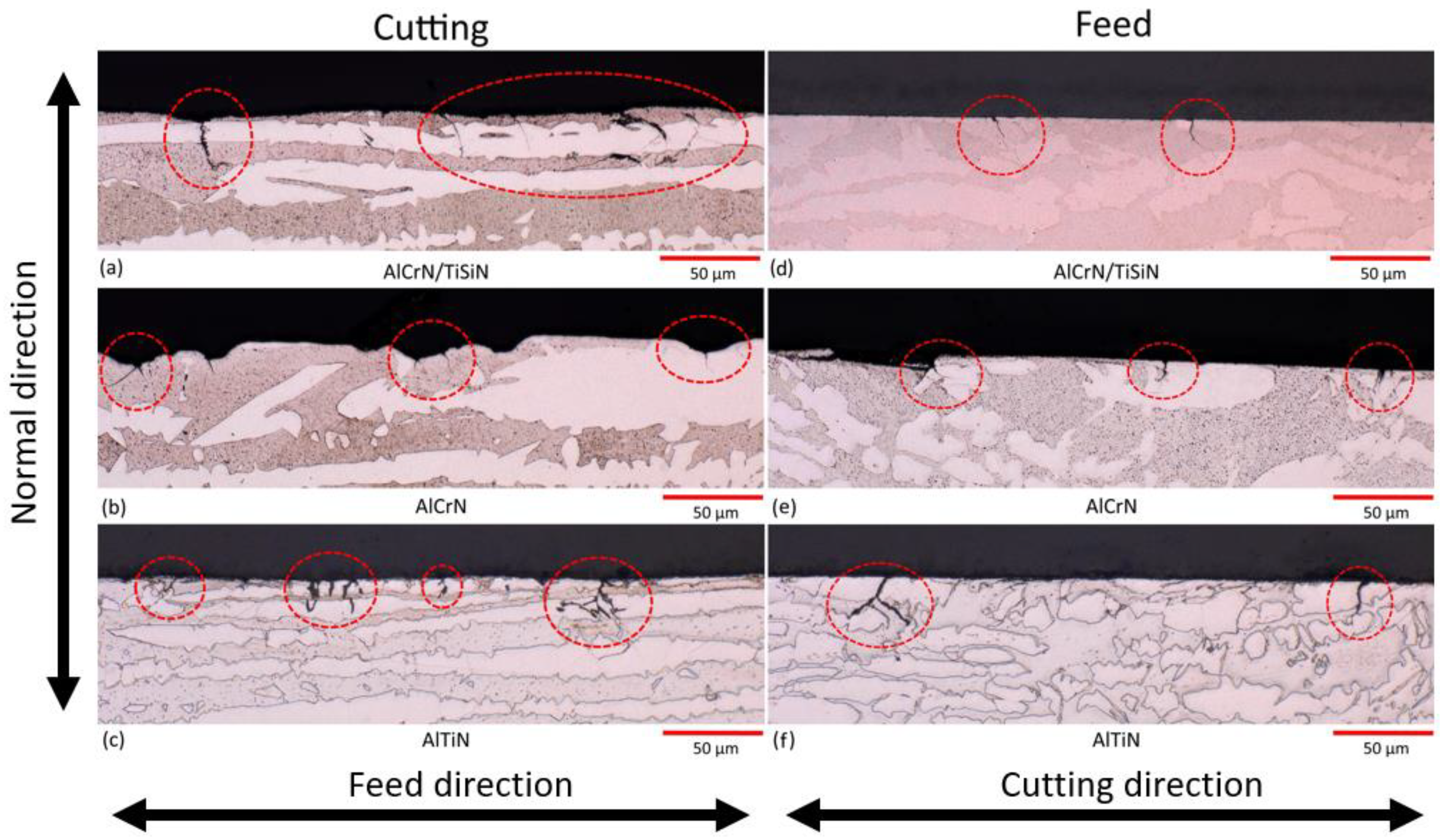
3.1. Machined Surface Characterization
3.2. Electrochemical Measurements
3.3. SCC Susceptibility Testing
4. Conclusions
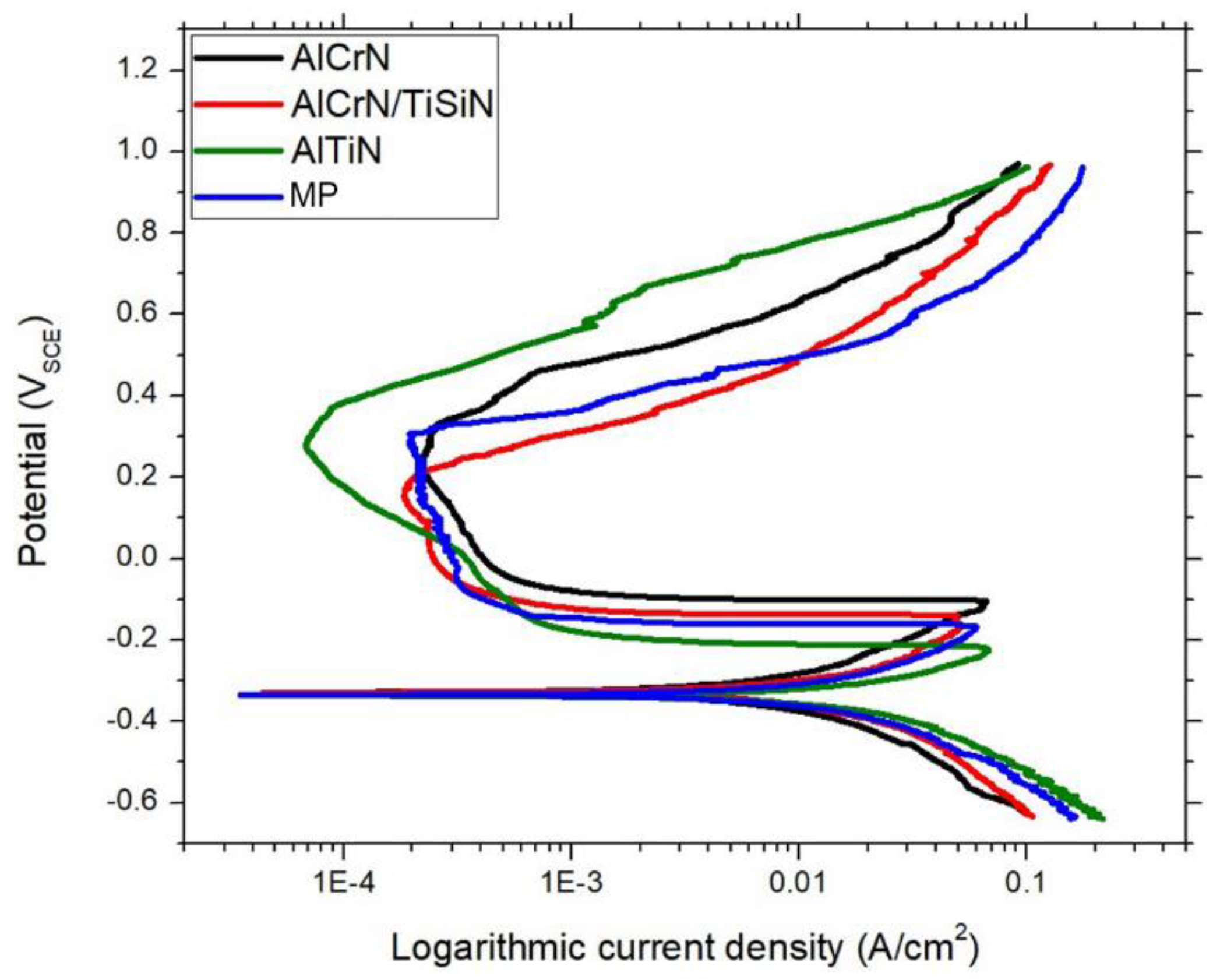
- Compared to the other evaluated samples, the surface machined with an AlTiN-coated insert has an exceptional corrosion resistance. The AlTiN-coated tool’s capability to prevent surface corrosion is enhanced by early passivation and a broader passive zone, whose behaviour is comparable to that of the polished benchmark sample.
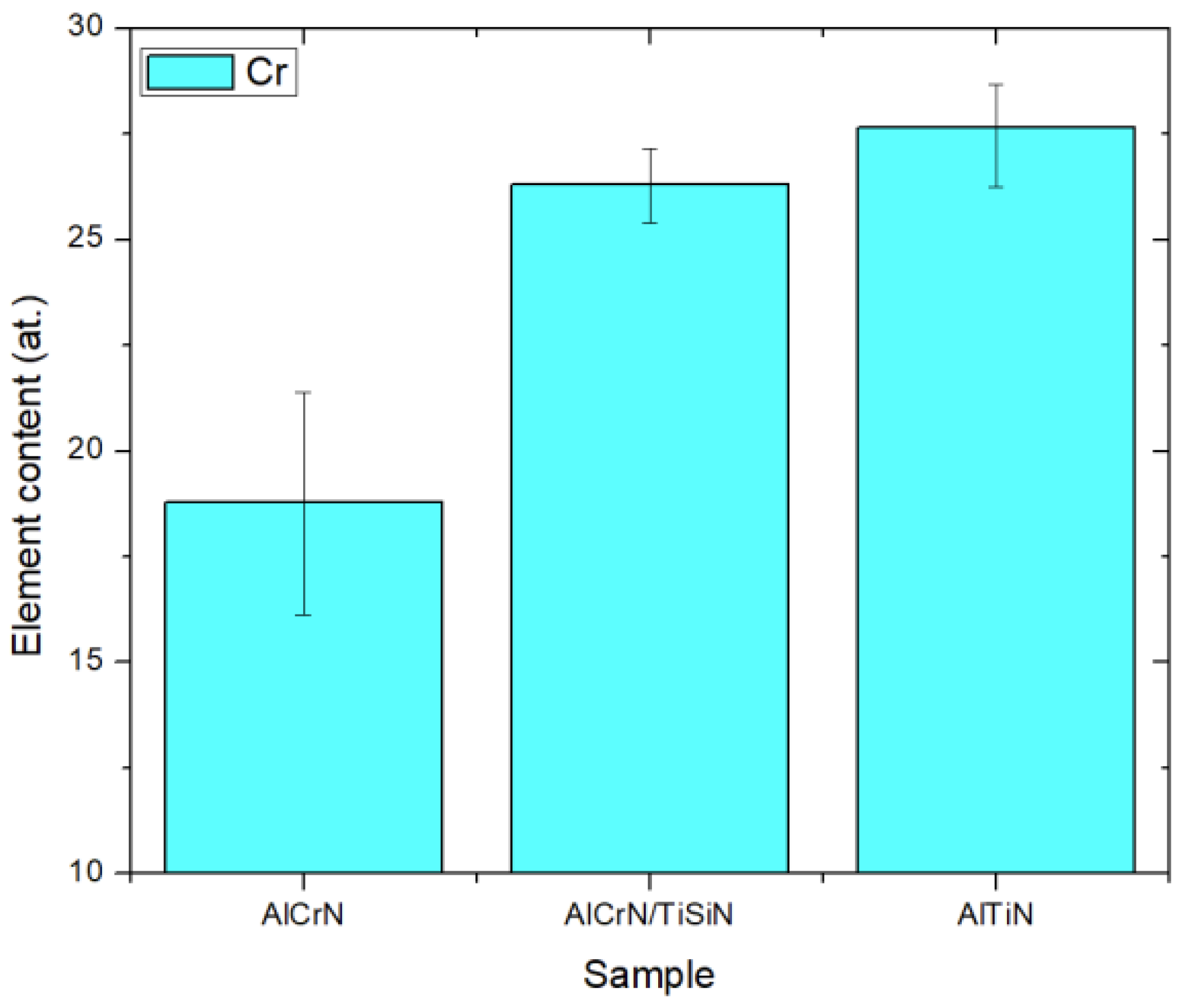
- Major differences in a surface’s electrochemistry can be attributed to the different Cr contents that each machined surface has. The low Cr content on the surface machined by the AlCrN-coated insert resulted in more corrosion susceptibility when compared to other samples.
- The oxide thickness present on each machined surface contributes to different potential stabilization times during OCP. The longer transition time is consistent with the presence of a thicker oxide film on the surface machined with the AlCrN-coated insert.
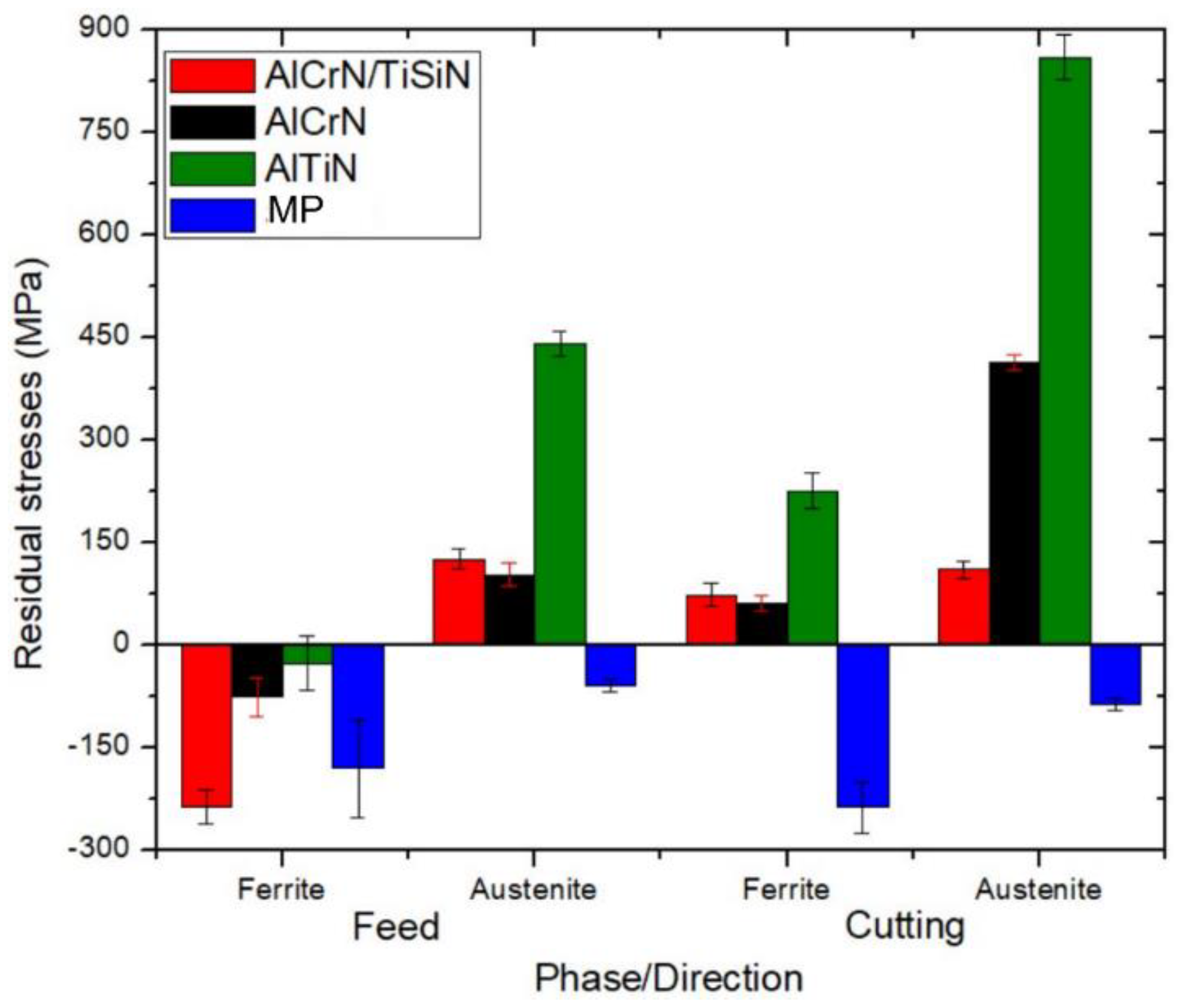
- SCC response is directly related to the residual stresses generated during the cutting process, where each individual phase can have a different stress state. The SCC susceptibility did not present any relation to the electrochemical behaviour of each sample.
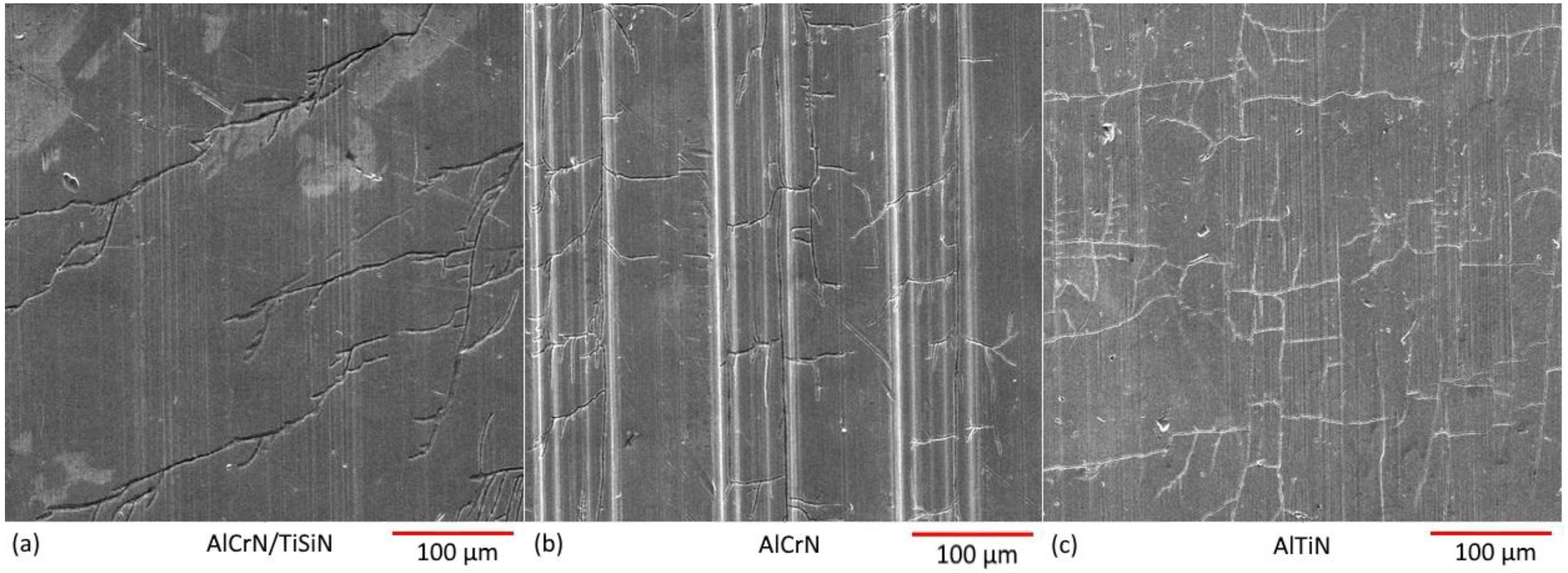
- In addition to presenting less corrosion susceptibility given by the polarization measurement results, the high tensile nature of AlTiN residual stresses resulted in a high number of cracks in its surface.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tan, H.; Jiang, Y.; Deng, B.; Sun, T.; Xu, J.; Li, J. Effect of annealing temperature on the pitting corrosion resistance of super duplex stainless steel UNS S32750. Mater. Charact. 2009, 60, 1049–1054. [Google Scholar] [CrossRef]
- Pardal, J.M.; Tavares, S.S.M.; Fonseca, M.C.; de Souza, J.A.; Côrte, R.R.A.; de Abreu, H.F.G. Influence of the grain size on deleterious phase precipitation in superduplex stainless steel UNS S32750. Mater. Charact. 2009, 60, 165–172. [Google Scholar] [CrossRef]
- Cvijović, Z.; Radenković, G. Microstructure and pitting corrosion resistance of annealed duplex stainless steel. Corros. Sci. 2006, 48, 3887–3906. [Google Scholar] [CrossRef]
- Pohl, M.; Storz, O.; Glogowski, T. Effect of intermetallic precipitations on the properties of duplex stainless steel. Mater. Charact. 2007, 58, 65–71. [Google Scholar] [CrossRef]
- Smiderle, J.; Pardal, J.M.; Tavares, S.S.M.; Vidal, A.C.N. Premature failure of superduplex stainless steel pipe by pitting in sea water environment. Eng. Fail. Anal. 2014, 46, 134–139. [Google Scholar] [CrossRef]
- de Paiva, J.M.F.; Torres, R.D.; Amorim, F.L.; Covelli, D.; Tauhiduzzaman, M.; Veldhuis, S.; Dosbaeva, G.; Fox-Rabinovich, G. Frictional and wear performance of hard coatings during machining of superduplex stainless steel. Int. J. Adv. Manuf. Technol. 2017, 92, 423–432. [Google Scholar] [CrossRef]
- Fernández-Abia, A.I.; Barreiro, J.; Fernández-Larrinoa, J.; de Lacalle, L.N.L.; Fernández-Valdivielso, A.; Pereira, O.M. Behaviour of PVD Coatings in the Turning of Austenitic Stainless Steels. Procedia Eng. 2013, 63, 133–141. [Google Scholar] [CrossRef]
- Paiva, J.M.F.; Amorim, F.L.; Soares, P.C.; Veldhuis, S.C.; Mendes, L.A.; Torres, R.D. Tribological behavior of superduplex stainless steel against PVD hard coatings on cemented carbide. Int. J. Adv. Manuf. Technol. 2017, 90, 1649–1658. [Google Scholar] [CrossRef]
- Navas, V.G.; Gonzalo, O.; Bengoetxea, I. Effect of cutting parameters in the surface residual stresses generated by turning in AISI 4340 steel. Int. J. Mach. Tools Manuf. 2012, 61, 48–57. [Google Scholar] [CrossRef]
- Nilsson, J.-O. Super duplex stainless steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Rajaguru, J.; Arunachalam, N. Investigation on machining induced surface and subsurface modifications on the stress corrosion crack growth behaviour of super duplex stainless steel. Corros. Sci. 2018, 141, 230–242. [Google Scholar] [CrossRef]
- Topolska, S.; Łabanowski, J. Effect of microstructure on impact toughness of duplex and superduplex stainless steels. J. Achiev. Mater. Manuf. Eng. 2009, 36, 142–149. [Google Scholar]
- Cui, J.; Park, I.-S.; Kang, C.-Y.; Miyahara, K. Degradation of Impact Toughness due to Formation of R Phase in High Nitrogen 25Cr-7Ni-Mo Duplex Stainless Steels. ISIJ Int. 2001, 41, 192–195. [Google Scholar] [CrossRef]
- Bastos, I.N.; Tavares, S.S.M.; Dalard, F.; Nogueira, R.P. Effect of microstructure on corrosion behavior of superduplex stainless steel at critical environment conditions. Scr. Mater. 2007, 57, 913–916. [Google Scholar] [CrossRef]
- Zhang, Z.; Jing, H.; Xu, L.; Han, Y.; Zhao, L.; Zhang, J. Influence of microstructure and elemental partitioning on pitting corrosion resistance of duplex stainless steel welding joints. Appl. Surf. Sci. 2017, 394, 297–314. [Google Scholar] [CrossRef]
- Tavares, S.S.M.; Pardal, J.M.; Lima, L.D.; Bastos, I.N.; Nascimento, A.M.; de Souza, J.A. Characterization of microstructure, chemical composition, corrosion resistance and toughness of a multipass weld joint of superduplex stainless steel UNS S32750. Mater. Charact. 2007, 58, 610–616. [Google Scholar] [CrossRef]
- Nowacki, J.; Łukojc, A. Microstructural transformations of heat affected zones in duplex steel welded joints. Mater. Charact. 2006, 56, 436–441. [Google Scholar] [CrossRef]
- Tavares, S.S.M.; Silva, V.G.; Pardal, J.M.; Corte, J.S. Investigation of stress corrosion cracks in a UNS S32750 superduplex stainless steel. Eng. Fail. Anal. 2013, 35, 88–94. [Google Scholar] [CrossRef]
- Krolczyk, G.; Gajek, M.; Legutko, S. Effect of the cutting parameters impact on tool life in duplex stainless steel turning process. Teh. Vjesn. 2013, 20, 587–592. [Google Scholar]
- Bordinassi, E.; Filho, M.; Batalha, G.; Delijaicov, S.; Lima, N. Superficial integrity analysis in a super duplex stainless steel after turning. J. Achiev. Mater. Manuf. Eng. 2006, 18, 335–338. [Google Scholar]
- de Oliveira Junior, C.A.; Diniz, A.E.; Bertazzoli, R. Correlating tool wear, surface roughness and corrosion resistance in the turning process of super duplex stainless steel. J. Braz. Soc. Mech. Sci. Eng. 2014, 36, 775–785. [Google Scholar] [CrossRef]
- Shaw, M. Metal Cutting Principles, 2nd ed.; Oxford Unversity Press: New York, NY, USA, 2005. [Google Scholar]
- Chen, W. Cutting forces and surface finish when machining medium hardness steel using CBN tools. Int. J. Mach. Tools Manuf. 2000, 40, 455–466. [Google Scholar] [CrossRef]
- Stephenson, D.A.; Agapiou, J.S. Metal Cutting Theory and Practice; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Vetter, J.; Eriksson, A.O.; Reiter, A.; Derflinger, V.; Kalss, W. Quo Vadis: AlCr-Based Coatings in Industrial Applications. Coatings 2021, 11, 344. [Google Scholar] [CrossRef]
- Pereira, C.B.G.; He, Q.; Soares, P.; Meruvia, M.S.; de Souza, G.B.; Amorim, F.L.; De Paiva, J.M.; Veldhuis, S.C.; Torres, R.D. Tribological characterization of PVD TiSiN/AlCrN coating: A comprehensive study on thermal effect. Mater. Charact. 2023, 203, 113135. [Google Scholar] [CrossRef]
- He, Q.; Paiva, J.M.; Kohlscheen, J.; Beake, B.D.; Veldhuis, S.C. Study of wear performance and tribological characterization of AlTiN PVD coatings with different Al/Ti ratios during ultra-high speed turning of stainless steel 304. Int. J. Refract. Metals Hard Mater. 2021, 96, 105488. [Google Scholar] [CrossRef]
- Chen, W.; Yan, A.; Wang, C.; Deng, Y.; Chen, D.C.; Xiao, H.; Zhang, D.; Meng, X. Microstructures and mechanical properties of AlCrN/TiSiN nanomultilayer coatings consisting of fcc single-phase solid solution. Appl. Surf. Sci. 2020, 509, 145303. [Google Scholar] [CrossRef]
- ASTM G36; Standard Practice for Evaluating Stress-Corrosion-Cracking Resistance of Metals and Alloys in a Boiling Magnesium Chloride Solution. ASTM: West Conshohocken, PA, USA, 2018.
- Grzesik, W.; Ruszaj, A. Vibration-Assisted Machining Processes BT—Hybrid Manufacturing Processes: Physical Fundamentals, Modelling and Rational Applications; Grzesik, W., Ruszaj, A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 81–104. [Google Scholar] [CrossRef]
- Filho, T.K.; He, Q.; Paiva, J.M.; Veldhuis, S.C. An analysis of different cutting strategies to improve tool life when machining Ti-5Al-5V-5Mo-3Cr alloy. J. Manuf. Process. 2023, 102, 50–66. [Google Scholar] [CrossRef]
- Qi, R.; Mao, X.; Zhang, K.; Xia, R. Accurate Clamping Method of Multipoint Flexible Fixture for Large Complex Surface. Math. Probl. Eng. 2021, 2021, 5568801. [Google Scholar] [CrossRef]
- Sun, W.; Luo, M.; Zhang, D. Machining vibration monitoring based on dynamic clamping force measuring in thin-walled components milling. Int. J. Adv. Manuf. Technol. 2020, 107, 2211–2226. [Google Scholar] [CrossRef]
- Thamizhmanii, S.; Hasan, S. Investigation of Surface Roughness and Flank Wear by CBN and PCBN Tools on Hard Cr-Mo Steel. J. Achiev. Mater. Manuf. Eng. 2008, 31, 415–421. [Google Scholar]
- Denape, J. Wear Mechanisms. In Tribology of Ceramics and Composites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar] [CrossRef]
- Mazid, A.M.; Imam, T.; Ahsan, K.B.; Khandoker, N. Characterising surface roughness of Ti-6Al-4V alloy machined using coated and uncoated carbide tools with variable nose radius by machine learning. Eng. Appl. Artif. Intell. 2023, 124, 106546. [Google Scholar] [CrossRef]
- Khoei, A.A.; He, Q.; DePaiva, J.M.; Bose, B.; Veldhuis, S.C. Assessment of the tribological and wear performance of new duplex AlTiN-based coating systems at high temperatures when threading super duplex stainless steel. J. Manuf. Process. 2023, 98, 285–301. [Google Scholar] [CrossRef]
- He, Q.; DePaiva, J.M.; Kohlscheen, J.; Veldhuis, S.C. Analysis of the performance of PVD AlTiN coating with five different Al/Ti ratios during the high-speed turning of stainless steel 304 under dry and wet cooling conditions. Wear 2022, 492–493, 204213. [Google Scholar] [CrossRef]
- Deshpande, N.C.; Vasudevan, H. A Model for Residual Stress in the Dry Turning of Duplex Stainless Steels. Key Eng. Mater. 2022, 933, 25–31. [Google Scholar] [CrossRef]
- Simon, N.; Erdle, H.; Walzer, S.; Gibmeier, J.; Böhlke, T.; Liewald, M. Residual stresses in deep-drawn cups made of duplex stainless steel X2CrNiN23-4. Forsch. Im Ingenieurwesen 2021, 85, 795–806. [Google Scholar] [CrossRef]
- Payares-Asprino, C.; de Escalona, P.M.; Sanchez, A. Residual stress in machined duplex stainless steel welds developed through automatic process. In Proceedings of the IV International Conference on Welding and Joining of Materials—ICONWELD 2018, San Miguel, Peru, 6–8 August 2018; Pontificia Universidad Católica del Perú: San Miguel, Peru, 2019; pp. 222–230. [Google Scholar]
- Santos, C.E.D.; Carneiro, J.R.G.; da Silva, G.C.; Brito, P.P.; Santos, Í.B.D.; Campos, T.R. Residual stress and surface microhardness post-milling in 2205 duplex steel. Int. J. Adv. Manuf. Technol. 2021, 113, 3445–3455. [Google Scholar] [CrossRef]
- Wan, M.; Ye, X.-Y.; Wen, D.-Y.; Zhang, W.H. Modeling of machining-induced residual stresses. J. Mater. Sci. 2019, 54, 1–35. [Google Scholar] [CrossRef]
- Wang, F.; Mao, K.; Ying, H.; Zhu, Y.; Yin, L. Equivalent parameters-based residual thermal stress fields modeling and design for square coated indexable cutting inserts. Int. J. Adv. Manuf. Technol. 2019, 102, 567–581. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, M.; Guan, R.; Liu, L.; Qian, J. Generation mechanism of insert residual stress while cutting 508III steel. Int. J. Adv. Manuf. Technol. 2017, 91, 247–255. [Google Scholar] [CrossRef]
- Fox-Rabinovich, G.; Totten, G.E. Self-Organization During Friction—Advanced Surface-Engineered Materials and Systems Design, 1st ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Ajo, H.; Blankenship, D.; Clark, E. Analysis of Passivated A-286 Stainless Steel Surfaces for Mass Spectrometer Inlet Systems by Auger Electron and X-ray Photoelectron Spectroscopy and Scanning Electron Microscopy. Metallogr. Microstruct. Anal. 2014, 3, 263–271. [Google Scholar] [CrossRef]
- Rar, A.; Hofmann, S.; Yoshihara, K.; Kajiwara, K. Optimization of depth resolution parameters in AES sputter profiling of GaAs/AlAs multilayer structures. Appl. Surf. Sci. 1999, 144–145, 310–314. [Google Scholar] [CrossRef]
- Kerber, S.J.; Tverberg, J. Stainless steel surface analysis: AES and XPS analysis of the passivation layer on stainless steel can help determine how well it will resist corrosion. Adv. Mater. Process. 2000, 158, 33–36. [Google Scholar]
- Dikusar, A.I.; Silkin, S.A. Formation and Breakdown of Oxide Films in High-Rate Anodic Dissolution of Chromium–Nickel Steels in Electrolytes for Electrochemical Machining. Surf. Eng. Appl. Electrochem. 2022, 58, 313–322. [Google Scholar] [CrossRef]
- Yuan, J.; Fox-Rabinovich, G.S.; Veldhuis, S.C. Control of tribofilm formation in dry machining of hardened AISI D2 steel by tuning the cutting speed. Wear 2018, 402–403, 30–37. [Google Scholar] [CrossRef]
- Chen, W.; Wu, W.; Huang, Y.; Wu, D.; Li, B.; Wang, C.; Wang, Y.; Li, S. Influence of annealing on microstructures and mechanical properties of arc-deposited AlCrTiSiN coating. Surf. Coat. Technol. 2021, 421, 127470. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Z.; Wang, B.; Wang, C.; Cheung, C.F. Friction behaviors in the metal cutting process: State of the art and future perspectives. Int. J. Extrem. Manuf. 2022, 5, 12002. [Google Scholar] [CrossRef]
- Wang, J.; Liu, H.; Kijkla, P.; Kumseranee, S.; Punpruk, S.; Mohamed, M.E.-S.; Saleh, M.A.; Gu, T. Comparison of 304 SS, 2205 SS, and 410 SS Corrosion by Sulfate-Reducing Desulfovibrio ferrophilus. J. Chem. 2021, 2021, 3268404. [Google Scholar] [CrossRef]
- Philip, A.M.; Chakraborty, K. Some studies on the machining behaviour of 316L austenitic stainless steel. Mater. Today Proc. 2022, 56, 681–685. [Google Scholar] [CrossRef]
- Becker, H.; Dickinson, E.J.F.; Lu, X.; Bexell, U.; Proch, S.; Moffatt, C.; Stenström, M.; Smith, G.; Hinds, G. Assessing potential profiles in water electrolysers to minimise titanium use. Energy Environ. Sci. 2022, 15, 2508–2518. [Google Scholar] [CrossRef]
- Hassan, H.M.; Attia, A.A.; Zordok, W.A.; Eldesoky, A.M. Potentiodynamic polarization, Electrochemical Impedance Spectroscopy (EIS) and Density functional theory studies of Sulfa Guanidine Azomethine as Efficient Corrosion Inhibitors for Nickel Surface in hydrochloeic acid solution. Int. J. Sci. Eng. Res. 2014, 5, 369–380. [Google Scholar]
- Zhou, L.; Zhao, Q.; Lv, G.; Dou, Z.; Mu, W.; Zhang, T. Effect of NH4Cl on the rusting leaching behavior of metallic phase of iron in reduced ilmenite during TiO2 purification: An electrochemical and density-functional theory study. Appl. Surf. Sci. 2023, 638, 158019. [Google Scholar] [CrossRef]
- Hassel, T.; Maier, H.J.; Alkhimenko, A.; Davydov, A.; Shaposhnikov, N.; Turichin, G.; Klimova, O. Investigation of the mechanical properties and corrosion behaviour of hybrid L 80 Type 1 and duplex steel joints produced by magnetically impelled arc butt welding. J. Adv. Join. Process. 2022, 5, 100109. [Google Scholar] [CrossRef]
- Wang, L.; Ding, Y.; Lu, Q.; Guo, Z.; Liu, Y.; Cui, Z. Microstructure and corrosion behavior of welded joints between 2507 super duplex stainless steel and E690 low alloy steel. Corros. Commun. 2023, 11, 1–11. [Google Scholar] [CrossRef]
- Sung, C.; Shin, B.-H.; Chung, W. Effect of Solution Annealing on Austenite Morphology and Pitting Corrosion of Super Duplex Stainless Steel UNS S 32750. Int. J. Electrochem. Sci. 2021, 16, 210813. [Google Scholar] [CrossRef]
- Kim, J.-H.; Choi, S.-W.; Park, D.-H.; Lee, J.-M. Charpy impact properties of stainless steel weldment in liquefied natural gas pipelines: Effect of low temperatures. Mater. Des. (1980–2015) 2015, 65, 914–922. [Google Scholar] [CrossRef]
- Rajaguru, J.; Arunachalam, N. A comprehensive investigation on the effect of flood and MQL coolant on the machinability and stress corrosion cracking of super duplex stainless steel. J. Mater. Process. Technol. 2020, 276, 116417. [Google Scholar] [CrossRef]
- Sharma, L.; Sharma, K. Dissimilar welding of super duplex stainless steel (SDSS) and pipeline steel—A brief overview. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- George, P.; Wins, K.L.D.; Dhas, D.S.E.J.; George, P.; Beatrice, B.A. Machinability, weldability and surface treatment studies of SDSS 2507 material—A review. Mater. Today Proc. 2021, 46, 7682–7687. [Google Scholar] [CrossRef]
- Wickström, L.; Mingard, K.; Hinds, G.; Turnbull, A. Microcrack clustering in stress corrosion cracking of 22Cr and 25Cr duplex stainless steels. Corros. Sci. 2016, 109, 86–93. [Google Scholar] [CrossRef]
- Pereira, H.B.; Azevedo, C.R.F. Can the drop evaporation test evaluate the stress corrosion cracking susceptibility of the welded joints of duplex and super duplex stainless steels? Eng. Fail. Anal. 2019, 99, 235–247. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]






| Cr | Ni | Mo | Mn | Cu | Si | W | N | C | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 25.0 | 7.1 | 4.0 | 1.1 | 0.8 | 0.7 | 0.6 | 0.3 | 0.03 | Bal. |
| Machined Surface | Ecorr (mVSCE) | Epp (mVSCE) | Eb (mVSCE) | Passivation Range |
|---|---|---|---|---|
| AlCrN | −334 ± 16 | −70 ± 3 | +300 ± 15 | 370 mV |
| AlCrN/TiSiN | −324 ± 16 | −139 ± 7 | +137 ± 7 | 276 mV |
| AlTiN | −331 ± 16 | −214 ± 10 | +256 ± 13 | 470 mV |
| MP (Baseline) | −329 ± 16 | −161 ± 8 | +247 ± 12 | 408 mV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Locks, E.; He, Q.; DePaiva, J.M.; Guimaraes, M.; Arif, A.F.; Veldhuis, S.C.; Kish, J.R. Investigating the Impact of Physical Vapour Deposition (PVD)-Coated Cutting Tools on Stress Corrosion Cracking Susceptibility in Turning Super Duplex Stainless Steel. Coatings 2024, 14, 290. https://doi.org/10.3390/coatings14030290
Locks E, He Q, DePaiva JM, Guimaraes M, Arif AF, Veldhuis SC, Kish JR. Investigating the Impact of Physical Vapour Deposition (PVD)-Coated Cutting Tools on Stress Corrosion Cracking Susceptibility in Turning Super Duplex Stainless Steel. Coatings. 2024; 14(3):290. https://doi.org/10.3390/coatings14030290
Chicago/Turabian StyleLocks, Edinei, Qianxi He, Jose M. DePaiva, Monica Guimaraes, Abul Fazal Arif, Stephen C. Veldhuis, and Joey R. Kish. 2024. "Investigating the Impact of Physical Vapour Deposition (PVD)-Coated Cutting Tools on Stress Corrosion Cracking Susceptibility in Turning Super Duplex Stainless Steel" Coatings 14, no. 3: 290. https://doi.org/10.3390/coatings14030290
APA StyleLocks, E., He, Q., DePaiva, J. M., Guimaraes, M., Arif, A. F., Veldhuis, S. C., & Kish, J. R. (2024). Investigating the Impact of Physical Vapour Deposition (PVD)-Coated Cutting Tools on Stress Corrosion Cracking Susceptibility in Turning Super Duplex Stainless Steel. Coatings, 14(3), 290. https://doi.org/10.3390/coatings14030290







