Albumin–Rutin Nanoparticles: Design, Characterization, and Biophysical Evaluation
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Preparation of Albumin and Albumin–Rutin-Loaded Nanoparticles
2.2.2. Preparation of Dipalmitoylphosphatidylcholine (DPPC) Liposomes and Dipalmitoylphosphatidylcholine (DPPC)/Bovine Serum Albumin (BSA)–Rutin Biohybrids
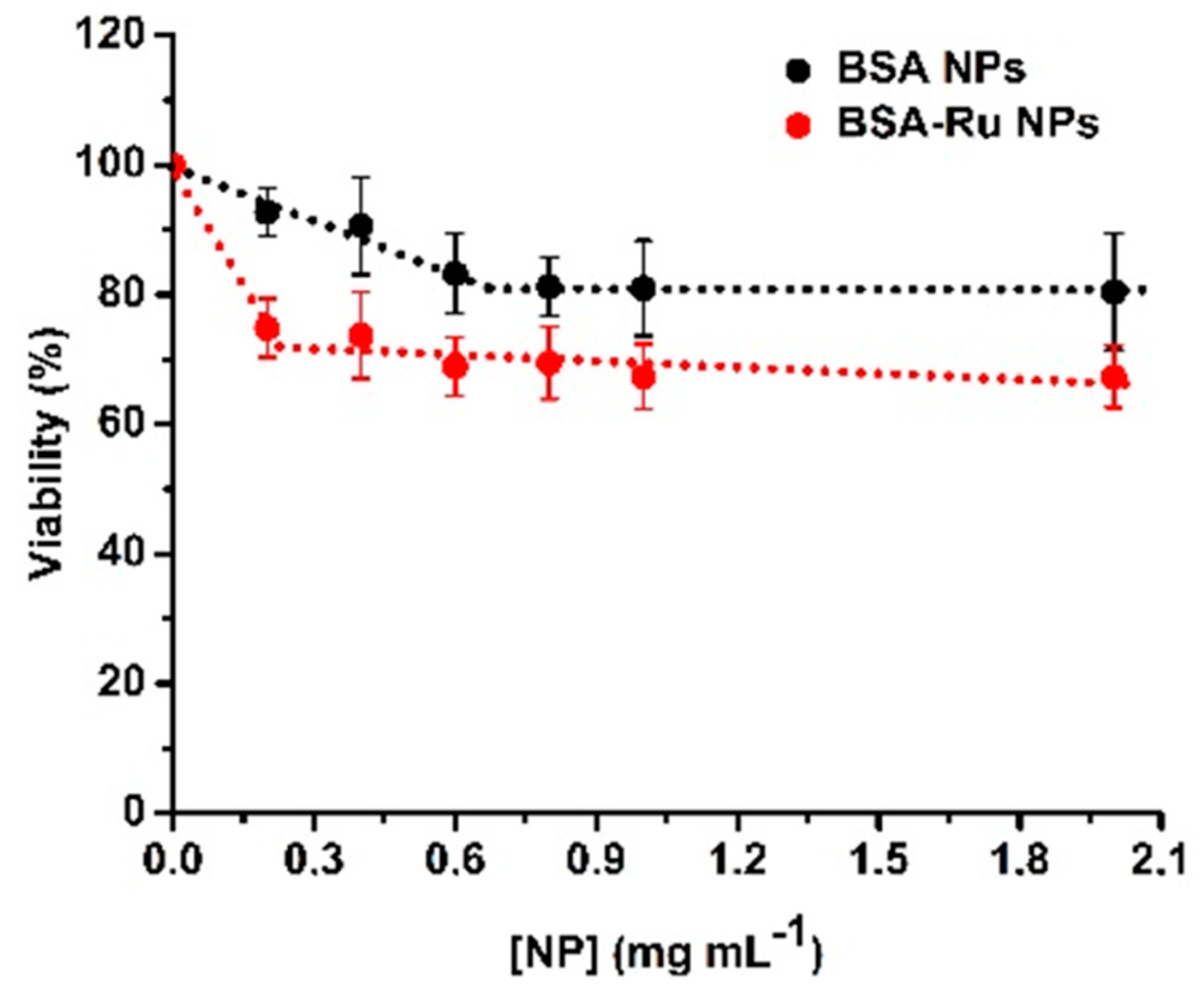
2.2.3. Viability Assay
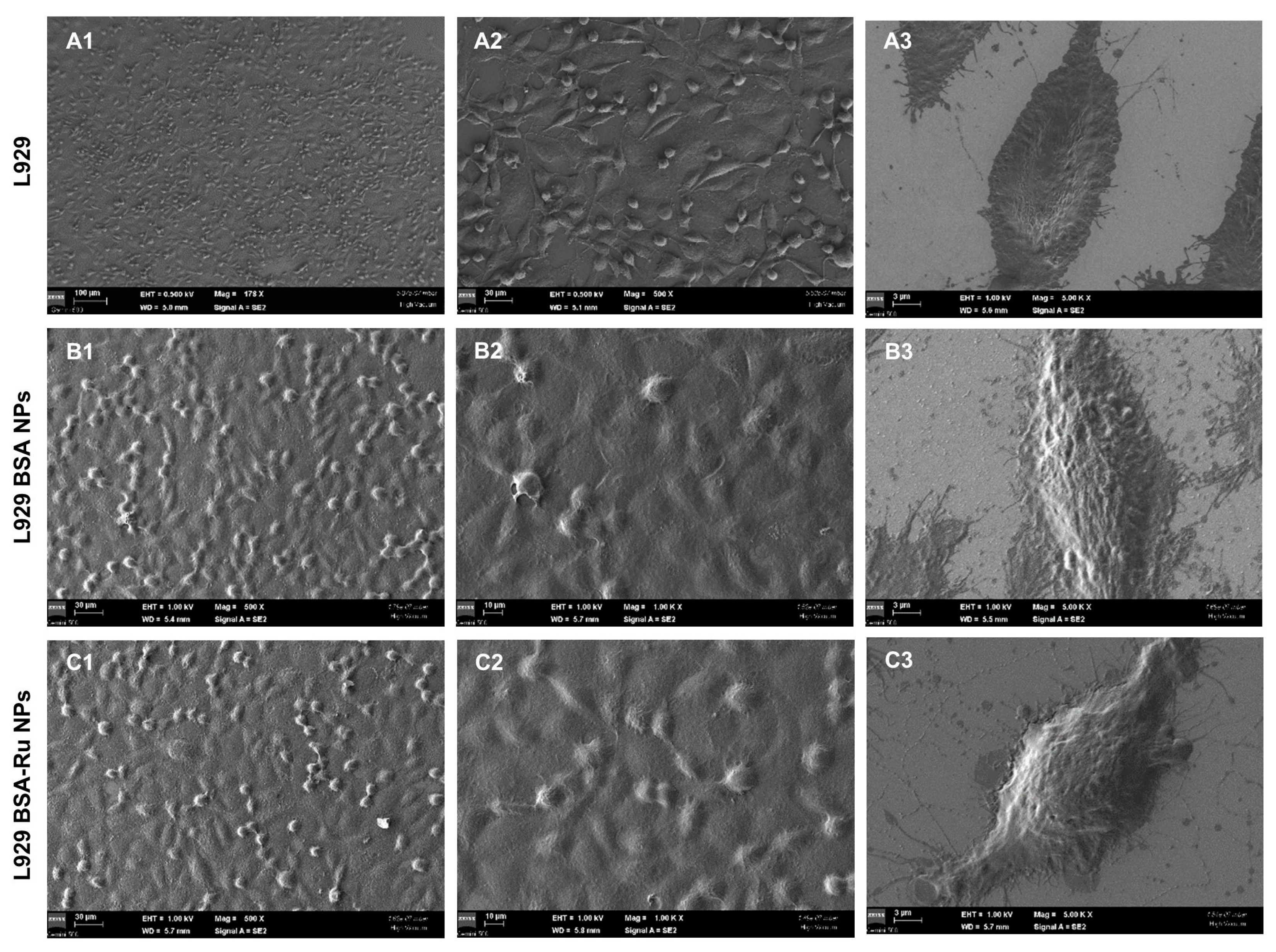
2.2.4. Cell Fixation
2.3. Instrumentation
2.3.1. Atomic Force Microscopy (AFM)
2.3.2. Scanning Electron Microscopy (SEM)
2.3.3. Ultraviolet–Visible Light Absorption Spectroscopy
2.3.4. Surface Plasmon Resonance
2.3.5. Electrochemistry
3. Results and Discussion
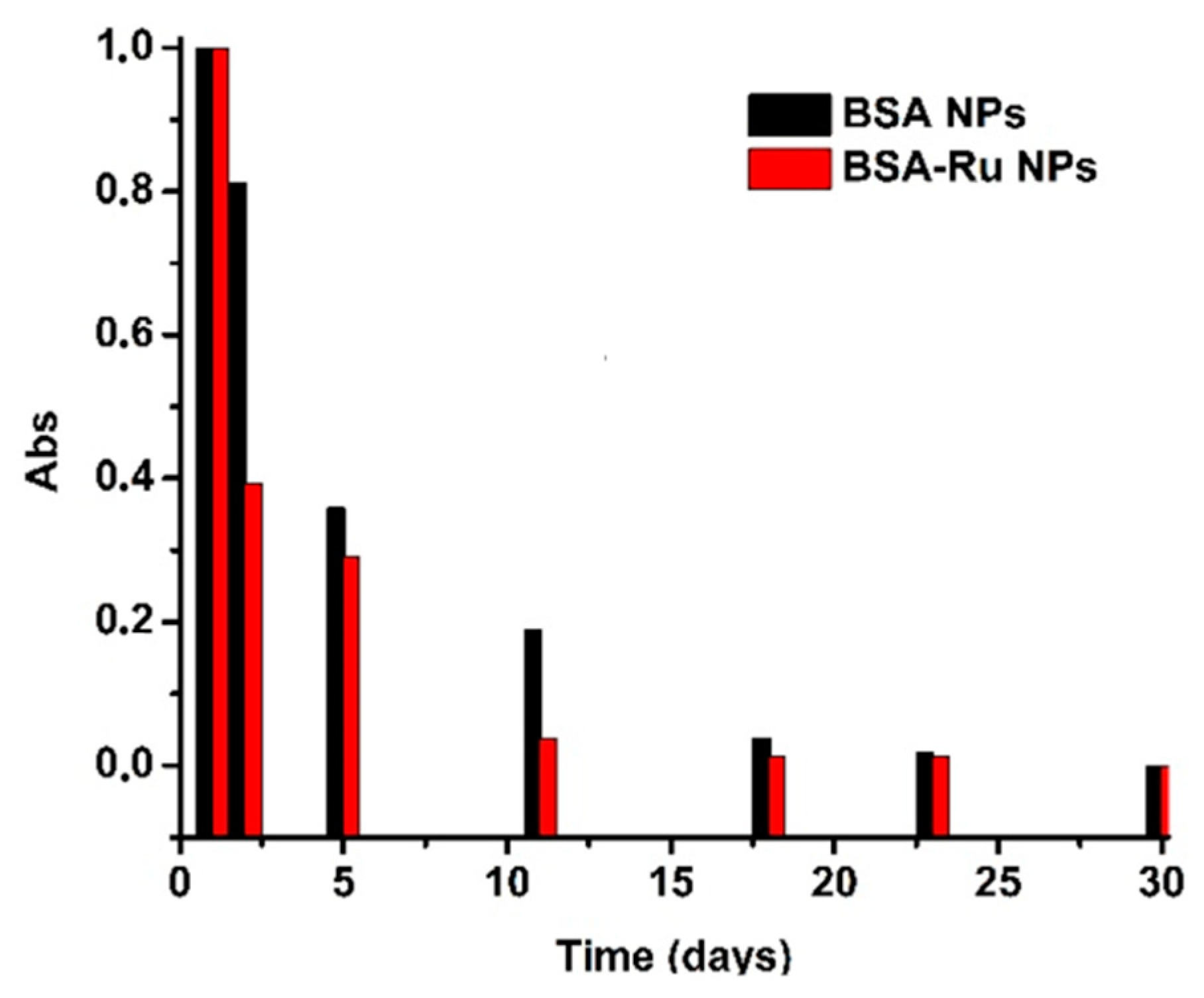
3.1. Morphological Characterization and Stability of Bovine Serum Albumin–Rutin Nanoparticles
3.2. Surface Plasmon Resonance Characterization of Dipalmitoylphosphatidylcholine (DPPC) Liposomes and Dipalmitoylphosphatidylcholine (DPPC)/Bovine Serum Albumin (BSA)–Rutin Biohybrids
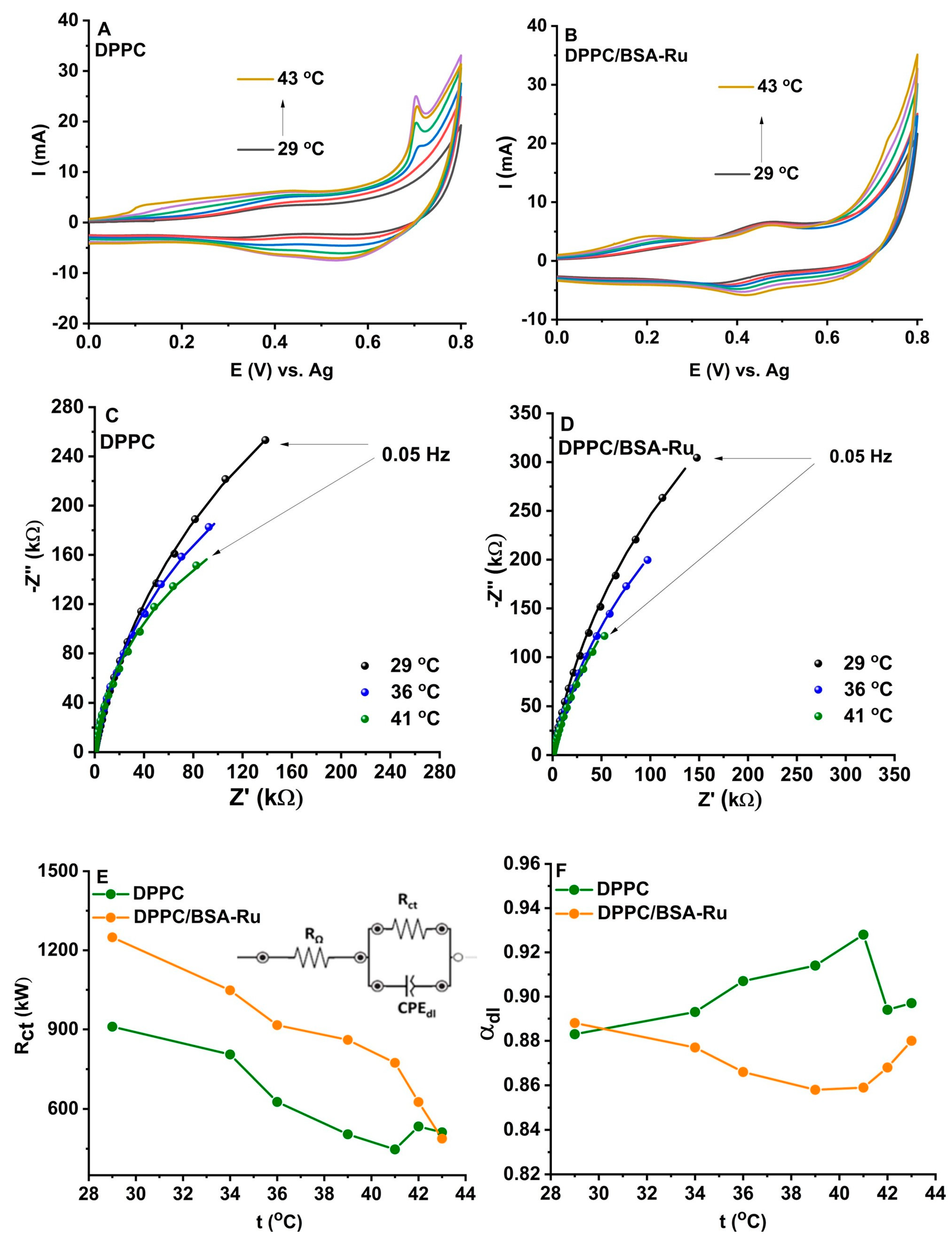
3.3. Electrochemical Characterization of Dipalmitoylphosphatidylcholine (DPPC) Liposomes and Dipalmitoylphosphatidylcholine (DPPC)/Bovine Serum Albumin (BSA)–Rutin Biohybrids
3.3.1. Cyclic Voltammetry
3.3.2. Electrochemical Impedance
3.4. The Effect of Bovine Serum Albumin–Rutin Nanoparticles at a Cellular Level
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hassanin, I.; Elzoghby, A. Albumin-based nanoparticles: A promising strategy to overcome cancer drug resistance. Cancer Drug Resist. 2020, 3, 930–946. [Google Scholar] [CrossRef]
- Verma, D.; Gulati, N.; Kaul, S.; Mukherjee, S.; Nagaich, U. Protein based nanostructures for drug delivery. J. Pharm. 2018, 2018, 9285854. [Google Scholar] [CrossRef]
- Rempel, S.A.; Ge, S.; Gutiérrez, J.A. SPARC: A potential diagnostic marker of invasive meningiomas. Clin. Cancer Res. 1999, 5, 237–241. [Google Scholar] [PubMed]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2020, 65, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Bronze-Uhle, E.S.; Costa, B.C.; Ximenes, V.F.; Lisboa-Filho, P.N. Synthetic nanoparticles of bovine serum albumin with entrapped salicylic acid. Nanotechnol. Sci. Appl. 2017, 10, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Jahanban-Esfahlan, A.; Dastmalchi, S.; Davaran, S. A simple improved desolvation method for the rapid preparation of albumin nanoparticles. Int. J. Biol. Macromol. 2016, 91, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Barbinta-Patrascu, M.-E.; Iftimie, S.; Cazacu, N.; Stan, D.L.; Costas, A.; Balan, A.E.; Chilom, C.G. Bio-Entities Based on Albumin Nanoparticles and Biomimetic Cell Membranes: Design, Characterization and Biophysical Evaluation. Coatings 2023, 13, 671. [Google Scholar] [CrossRef]
- Amighi, F.; Emam-Djomeh, Z.; Labbafi-Mazraeh-Shahi, M. Effect of different cross-linking agents on the preparation of bovine serum albumin nanoparticles. J. Iran. Chem. Soc. 2020, 17, 1223–1235. [Google Scholar] [CrossRef]
- Imamura, K.; Ogawa, T.; Sakiyama, T.; Nakanishi, K. Effects of Types of Sugar on the Stabilization of Protein in the Dried State. J. Pharm. Sci. 2003, 92, 266–274. [Google Scholar] [CrossRef] [PubMed]
- An, F.F.; Zhang, X.H. Strategies for preparing albumin-based nanoparticles for multifunctional bioimaging and drug delivery. Theranostics 2017, 7, 3667–3689. [Google Scholar] [CrossRef]
- Zielińska, D.; Szawara-Nowak, D.; Zieliński, H. Determination of the antioxidant activity of rutin and its contribution to the antioxidant capacity of diversifed buckwheat origin material by updated analytical strategies. Pol. J. Food Nutr. Sci. 2010, 60, 315–321. [Google Scholar]
- Pandey, P.; Khan, F.; Qari, H.A.; Oves, M. Rutin (Bioflavonoid) as Cell Signaling Pathway Modulator: Prospects in Treatment and Chemoprevention. Pharmaceuticals 2021, 14, 1069. [Google Scholar] [CrossRef] [PubMed]
- Bonechi, C.; Donati, A.; Tamasi, G.; Leone, G.; Consumi, M.; Rossi, C.; Lamponi, S.; Magnani, A. Protective effect of quercetin and rutin encapsulated liposomes on induced oxidative stress. Biophys. Chem. 2018, 233, 55–63. [Google Scholar] [CrossRef]
- Sengupta, P.; Sardar, P.S.; Roy, P.; Dasgupta, S.; Bose, A. Investigation on the interaction of Rutin with serum albumins: Insights from spectroscopic and molecular docking techniques. J. Photochem. Photobiol. B 2018, 183, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Sandu, N.; Chilom, C.G.; Popescu, A.I. Spectroscopic Insights on the Binding of Rutin to Bovine Serum Albumin. Rom. J. Phys. 2020, 65, 703. [Google Scholar]
- Guruvayoorappan, C.; Kuttan, G. Rutin inhibits nitric oxide and tumor necrosis factor-alpha production in lipopolysaccharide and concanavalin-a stimulated macrophages. Drug Metab. Drug Interact. 2007, 22, 263–278. [Google Scholar] [CrossRef]
- Wang, H.; Wang, L.; Guo, S.; Liu, Z.; Zhao, L.; Qiao, R.; Li, C. Rutin-Loaded Stimuli-Responsive Hydrogel for Anti-Inflammation. ACS Appl. Mater. Interfaces 2022, 14, 26327–26337. [Google Scholar] [CrossRef]
- Matsuo, M.; Sasaki, N.; Saga, K.; Kaneko, T. Cytotoxicity of Flavonoids toward Cultured Normal Human Cells. Biol. Pharm. Bull. 2005, 28, 253–259. [Google Scholar] [CrossRef]
- Abreu, A.C.; Serra, S.C.; Borges, A.; Saavedra, M.J.; Mcbain, A.J.; Salgado, A.J.; Simões, M. Combinatorial Activity of Flavonoids with Antibiotics Against Drug-Resistant Staphylococcus aureus. Microb. Drug Resist. 2015, 21, 600–609. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Bahorun, T.; Aruoma, O.I. Chemopreventive actions of polyphenolic compounds in cancer. BioFactors 2006, 27, 19–35. [Google Scholar] [CrossRef]
- Gong, G.; Qin, Y.; Huang, W.; Zhou, S.; Yang, X.; Li, D. Rutin inhibits hydrogen peroxide-induced apoptosis through regulating reactive oxygen species mediated mitochondrial dysfunction pathway in human umbilical vein endothelial cells. Eur. J. Pharmacol. 2010, 628, 27–35. [Google Scholar] [CrossRef]
- Negahdari, R.; Bohlouli, S.; Sharifi, S.; Maleki Dizaj, S.; Rahbar Saadat, Y.; Khezri, K.; Jafari, S.; Ahmadian, E.; Gorbani Jahandizi, N.; Raeesi, S. Therapeutic benefits of rutin and its nanoformulations. Phytother. Res. 2021, 35, 1719–1738. [Google Scholar] [CrossRef]
- Budiman, A.; Rusdin, A.; Aulifa, D.L. Current Techniques of Water Solubility Improvement for Antioxidant Compounds and Their Correlation with Its Activity: Molecular Pharmaceutics. Antioxidants 2023, 12, 378. [Google Scholar] [CrossRef]
- Hornok, V. Serum Albumin Nanoparticles: Problems and Prospects. Polymers 2021, 13, 3759. [Google Scholar] [CrossRef]
- Giri, T.K. Alginate Containing Nanoarchitectonics for Improved Cancer Therapy. In Nanoarchitectonics for Smart Delivery and Drug Targeting; William Andrew Publishing; Elsevier: Amsterdam, The Netherlands, 2016; pp. 565–588. [Google Scholar]
- David, M.; Budziak-Wieczorek, I.; Karcz, D.; Florescu, M.; Matwijczuk, A. Insight into dual fluorescence effects induced by molecular aggregation occurring in membrane model systems containing 1,3,4-thiadiazole derivatives. Eur. Biophys. J. 2021, 50, 1083–1101. [Google Scholar] [CrossRef]
- Pedrozo, R.C.; Antônio, E.; Khalil, N.M.; Mainardes, R.M. Bovine serum albumin–based nanoparticles containing the flavonoid rutin produced by nano spray drying. Braz. J. Pharm. Sci. 2020, 56, e17692. [Google Scholar] [CrossRef]
- Kızılbey, K. Optimization of Rutin-Loaded PLGA Nanoparticles Synthesized by Single-Emulsion Solvent Evaporation Method. ACS Omega 2019, 4, 555–562. [Google Scholar] [CrossRef]
- Rohiwal, S.S.; Tiwari, A.P.; Verma, G.; Pawar, S.H. Preparation and evaluation of bovine serum albumin nanoparticles for ex vivo colloidal stability in biological media. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 28–37. [Google Scholar] [CrossRef]
- Enache, T.A.; Oliveira-Brett, A.M. Phenol and para-substituted phenols electrochemical oxidation pathways. J. Electroanal. Chem. 2011, 655, 9–16. [Google Scholar] [CrossRef]
- Sun, W.; Yang, M.; Li, Y.; Jiang, Q.; Liu, S.; Jiao, K. Electrochemical behavior and determination of rutin on a pyridinium-based ionic liquid modified carbon paste electrode. J. Pharm. Biomed. Anal. 2008, 48, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Naumowicz, M.; Figaszewski, Z.A. Pore formation in lipid bilayer membranes made of phosphatidylcholine and cholesterol followed by means of constant current. Cell Biochem. Biophys. 2013, 66, 109–119. [Google Scholar] [CrossRef] [PubMed][Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chilom, C.G.; Balan, A.E.; Enache, T.A.; Oprea, D.; Enculescu, M.; Florescu, M.; David, M. Albumin–Rutin Nanoparticles: Design, Characterization, and Biophysical Evaluation. Coatings 2024, 14, 220. https://doi.org/10.3390/coatings14020220
Chilom CG, Balan AE, Enache TA, Oprea D, Enculescu M, Florescu M, David M. Albumin–Rutin Nanoparticles: Design, Characterization, and Biophysical Evaluation. Coatings. 2024; 14(2):220. https://doi.org/10.3390/coatings14020220
Chicago/Turabian StyleChilom, Claudia G., Adriana Elena Balan, Teodor Adrian Enache, Daniela Oprea, Monica Enculescu, Monica Florescu, and Melinda David. 2024. "Albumin–Rutin Nanoparticles: Design, Characterization, and Biophysical Evaluation" Coatings 14, no. 2: 220. https://doi.org/10.3390/coatings14020220
APA StyleChilom, C. G., Balan, A. E., Enache, T. A., Oprea, D., Enculescu, M., Florescu, M., & David, M. (2024). Albumin–Rutin Nanoparticles: Design, Characterization, and Biophysical Evaluation. Coatings, 14(2), 220. https://doi.org/10.3390/coatings14020220









