The Addition of MoO3 or SiO2 Nano-/Microfillers Thermally Stabilized and Mechanically Reinforce the PVDF-HFP/PVP Polymer Composite Thin Films
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
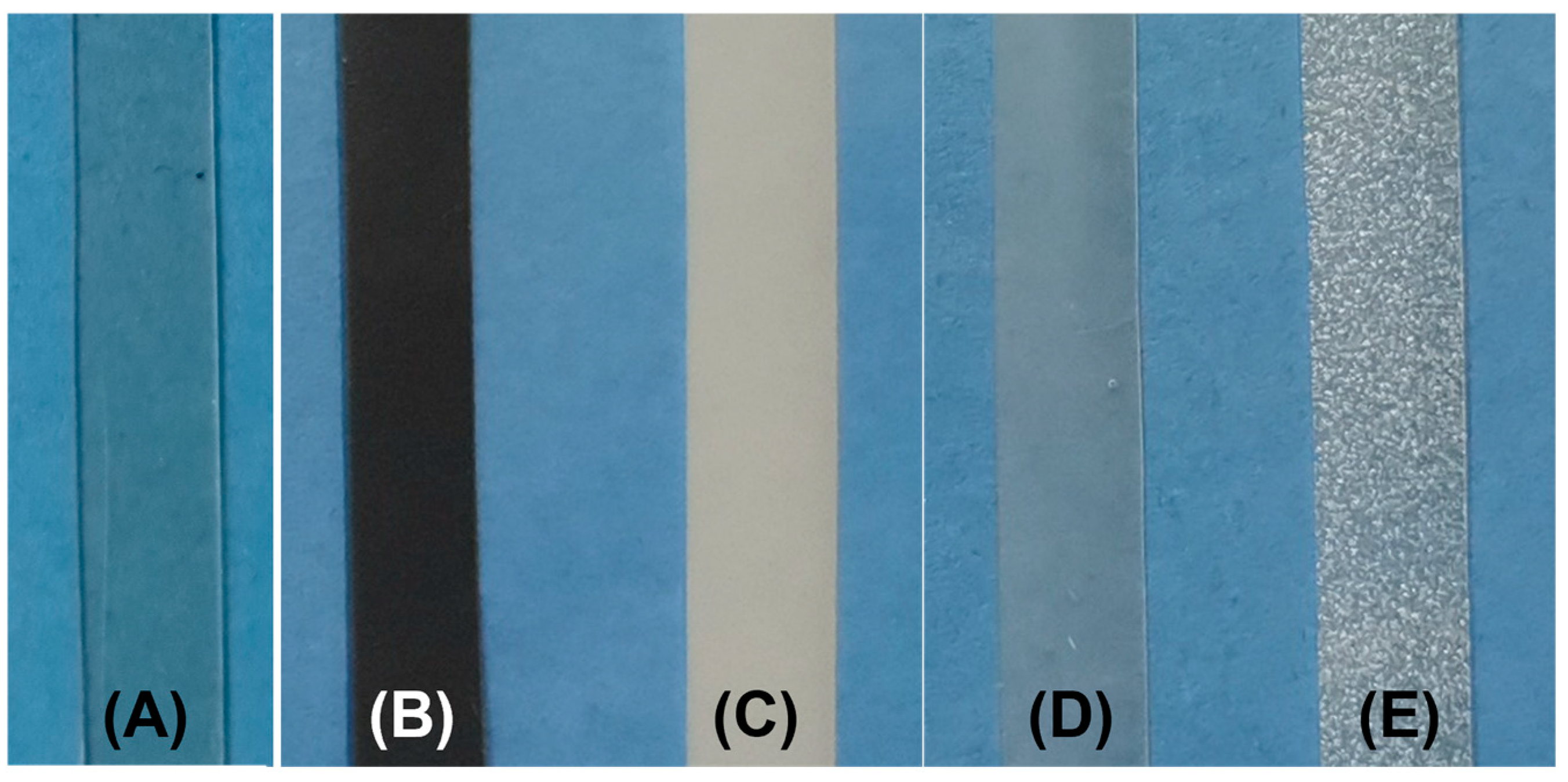
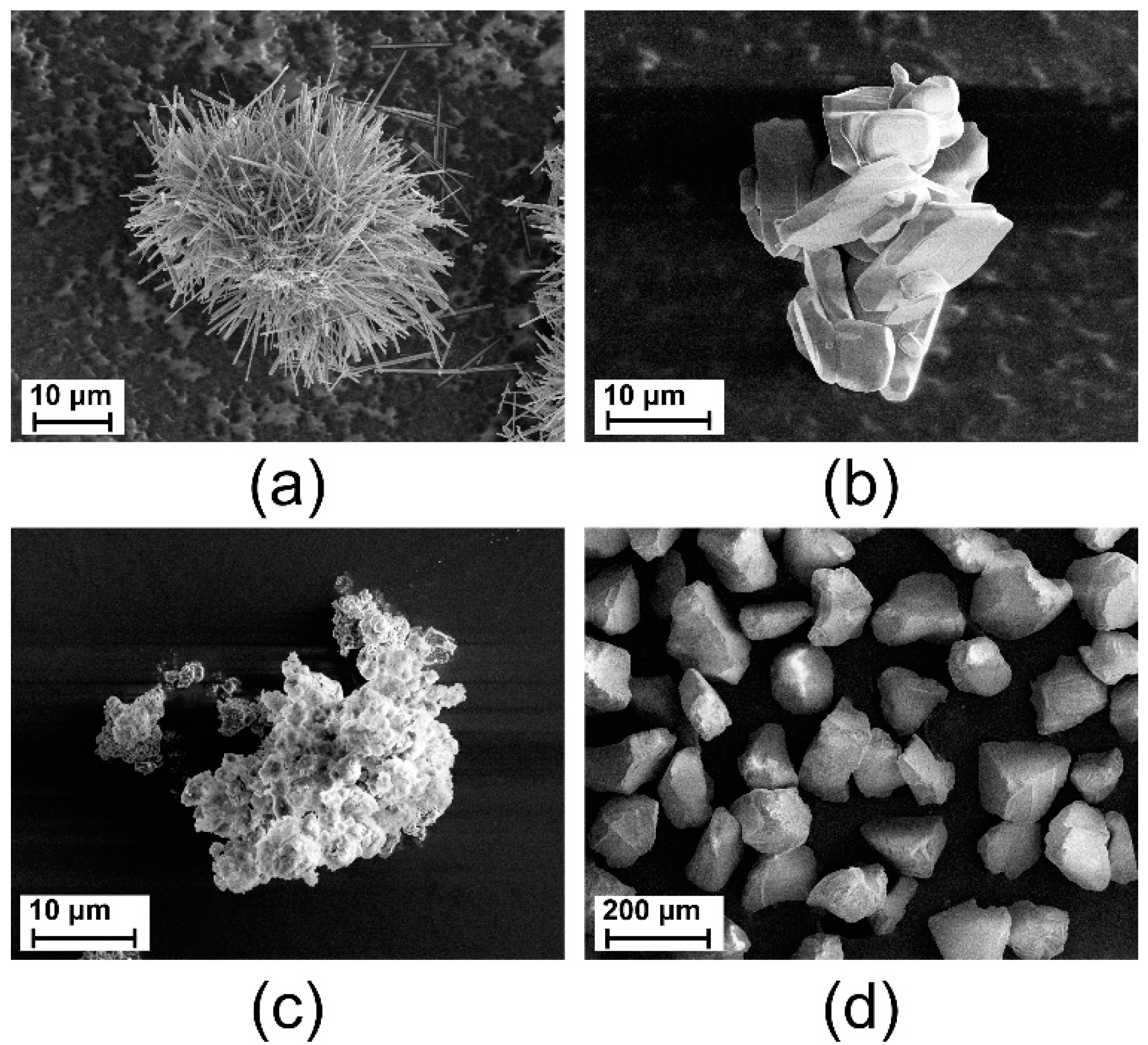
3.1. Morphology
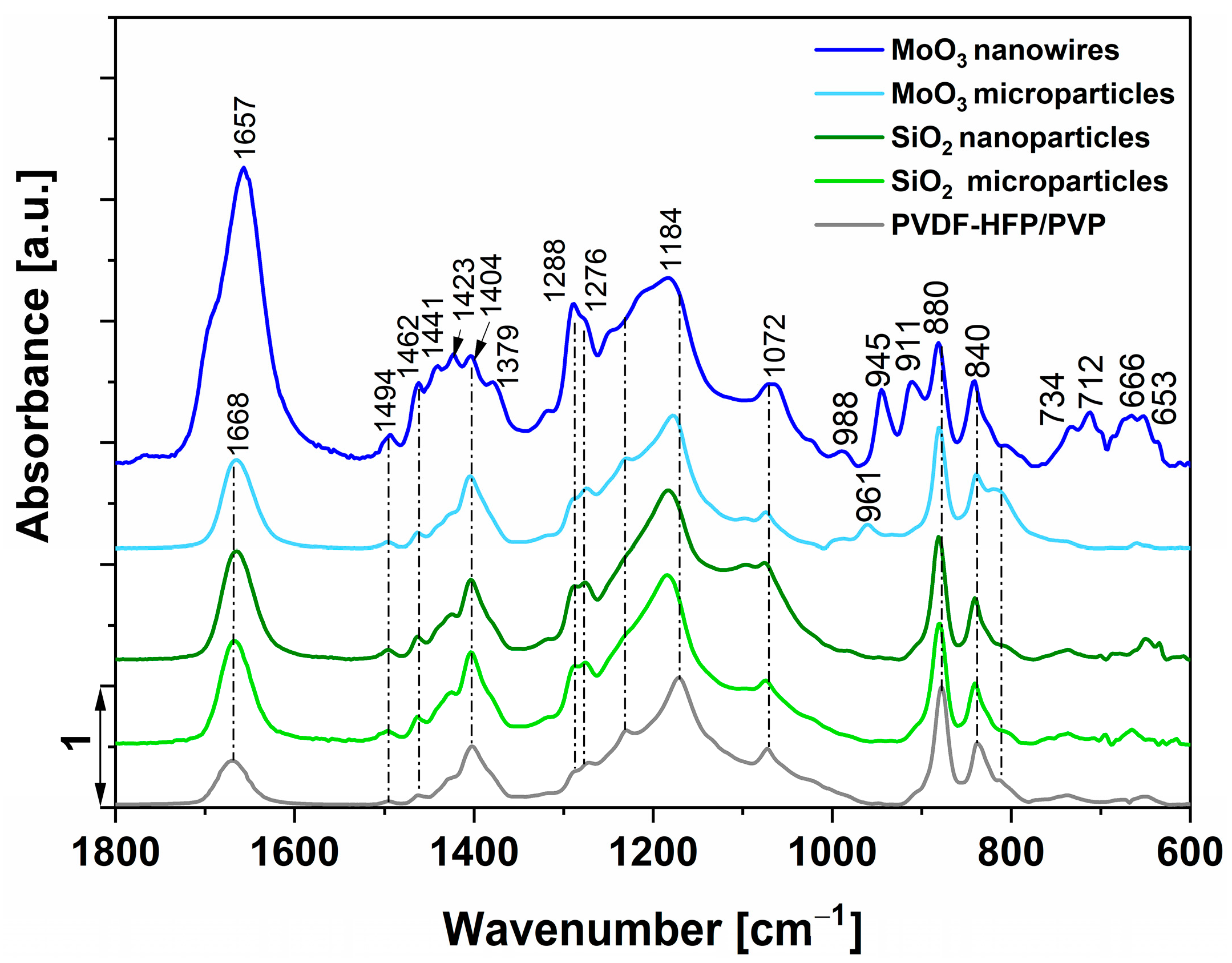
3.2. Structural Properties
3.3. Thermal Properties
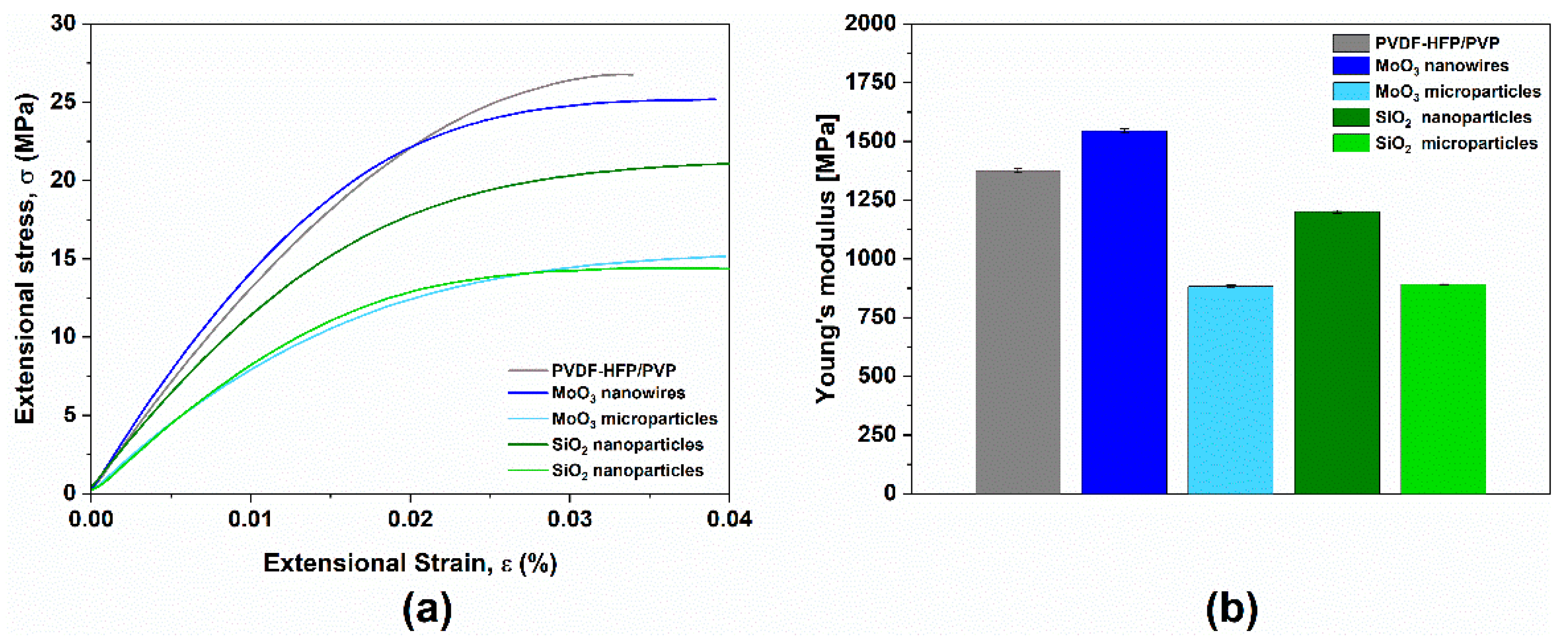
3.4. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.; Masud, A.K.M. Evaluation of Effective Thermal Conductivity of Multiwalled Carbon Nanotube Reinforced Polymer Composites Using Finite Element Method and Continuum Model. Procedia Eng. 2014, 90, 129–135. [Google Scholar] [CrossRef][Green Version]
- Morampudi, P.; Namala, K.K.; Gajjela, Y.K.; Barath, M.; Prudhvi, G. Review on Glass Fiber Reinforced Polymer Composites. Mater. Today Proc. 2021, 43, 314–319. [Google Scholar] [CrossRef]
- Miao, C.; Hamad, W.Y. Cellulose Reinforced Polymer Composites and Nanocomposites: A Critical Review. Cellulose 2013, 20, 2221–2262. [Google Scholar] [CrossRef]
- Jotiram, G.A.; Palai, B.K.; Bhattacharya, S.; Aravinth, S.; Gnanakumar, G.; Subbiah, R.; Chandrakasu, M. Investigating Mechanical Strength of a Natural Fibre Polymer Composite Using SiO2 Nano-Filler. Mater. Today Proc. 2022, 56, 1522–1526. [Google Scholar] [CrossRef]
- Vivekchand, S.R.C.; Ramamurty, U.; Rao, C.N.R. Mechanical Properties of Inorganic Nanowire Reinforced Polymer–Matrix Composites. Nanotechnology 2006, 17, S344. [Google Scholar] [CrossRef]
- Jordan, J.; Jacob, K.I.; Tannenbaum, R.; Sharaf, M.A.; Jasiuk, I. Experimental Trends in Polymer Nanocomposites—A Review. Mater. Sci. Eng. A 2005, 393, 1–11. [Google Scholar] [CrossRef]
- Cho, J.; Joshi, M.S.; Sun, C.T. Effect of Inclusion Size on Mechanical Properties of Polymeric Composites with Micro and Nano Particles. Compos. Sci. Technol. 2006, 66, 1941–1952. [Google Scholar] [CrossRef]
- Mayeen, A.; Santhosh, A.; Joseph, N.; Jose, J.; Manoj, A.; Joseph, S.; Bhat, S.; John, H. Flexible, Electrospun Boron Nitride Nanosheets (BNNS)/Hydroxyapatite –PVDF Nanofibers with Superior Piezoelectric/Ferroelectric, Biocompatible Features for Effective Bone Tissue Regeneration. J. Alloys Compd. 2024, 1002, 175111. [Google Scholar] [CrossRef]
- Kar, E.; Ghosh, P.; Pratihar, S.; Tavakoli, M.; Sen, S. SiO2 Nanoparticles Incorporated Poly(Vinylidene) Fluoride Composite for Efficient Piezoelectric Energy Harvesting and Dual-Mode Sensing. Energy Technol. 2023, 11, 2201143. [Google Scholar] [CrossRef]
- Alam, M.M.; Crispin, X. The Past, Present, and Future of Piezoelectric Fluoropolymers: Towards Efficient and Robust Wearable Nanogenerators. Nano Res. Energy 2023, 2, e9120076. [Google Scholar] [CrossRef]
- Janićijević, A.; Filipović, S.; Sknepnek, A.; Salević-Jelić, A.; Jančić-Heinemann, R.; Petrović, M.; Petronijević, I.; Stamenović, M.; Živković, P.; Potkonjak, N.; et al. Structural, Mechanical, and Barrier Properties of the Polyvinylidene Fluoride-Bacterial Nanocellulose-Based Hybrid Composite. Polymers 2024, 16, 1033. [Google Scholar] [CrossRef]
- Al Rashid, A.; Al-Maslamani, N.A.; Abutaha, A.; Hossain, M.; Koç, M. Cobalt Iron Oxide (CoFe2O4) Reinforced Polyvinyl Alcohol (PVA) Based Magnetoactive Polymer Nanocomposites for Remote Actuation. Mater. Sci. Eng. B 2025, 311, 117838. [Google Scholar] [CrossRef]
- Jiang, Z.; Feng, W.; Lin, Z.; Cai, Y.; Liu, M.; Hu, X.; Zeng, X.; Liu, X.; Wu, Y.; Zhang, Y.; et al. One-Pot Construction of All-in-One Flexible Asymmetrical Supercapacitors for Extreme-Condition Applications. Chem. Eng. J. 2024, 501, 157553. [Google Scholar] [CrossRef]
- Behera, R.; Elanseralathan, K. Modeling of Polyvinylidene Fluoride Nanocomposite Utilizing BaTiO3@SiO2 for Energy Storage. Mater. Today Proc. 2022, 62, 6554–6560. [Google Scholar] [CrossRef]
- Lee, H.; Yamaguchi, K.; Nagaishi, T.; Murai, M.; Kim, M.; Wei, K.; Zhang, K.-Q.; Kim, I.S. Enhancement of Mechanical Properties of Polymeric Nanofibers by Controlling Crystallization Behavior Using a Simple Freezing/Thawing Process. RSC Adv. 2017, 7, 43994–44000. [Google Scholar] [CrossRef]
- Chakhchaoui, N.; Farhan, R.; Eddiai, A.; Meddad, M.; Cherkaoui, O.; Mazroui, M.; Boughaleb, Y.; Van Langenhove, L. Improvement of the Electroactive β-Phase Nucleation and Piezoelectric Properties of PVDF-HFP Thin Films Influenced by TiO2 Nanoparticles. Mater. Today Proc. 2021, 39, 1148–1152. [Google Scholar] [CrossRef]
- Zhang, S.; Tong, W.; Wang, J.; Wang, W.; Wang, Z.; Zhang, Y. Modified Sepiolite/PVDF-HFP Composite Film with Enhanced Piezoelectric and Dielectric Properties. J. Appl. Polym. Sci. 2020, 137, 48412. [Google Scholar] [CrossRef]
- Gradišar Centa, U.; Mihelčič, M.; Bobnar, V.; Remškar, M.; Slemenik Perše, L. The Effect of PVP on Thermal, Mechanical, and Dielectric Properties in PVDF-HFP/PVP Thin Film. Coatings 2022, 12, 1241. [Google Scholar] [CrossRef]
- Centa, U.G.; Sterniša, M.; Višić, B.; Federl, Ž.; Možina, S.S.; Remškar, M. Novel Nanostructured And Antimicrobial PVDF-HFP/PVP/MoO 3 Composite. Surf. Innov. 2020, 9, 256–266. [Google Scholar] [CrossRef]
- Jiang, Y.; Tohgo, K.; Yang, H. Study of the Effect of Particle Size on the Effective Modulus of Polymeric Composites on the Basis of the Molecular Chain Network Microstructure. Comput. Mater. Sci. 2010, 49, 439–443. [Google Scholar] [CrossRef]
- Tcherdyntsev, V.V. Reinforced Polymer Composites. Polymers 2021, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Riggleman, R.A.; Toepperwein, G.; Papakonstantopoulos, G.J.; Barrat, J.-L.; de Pablo, J.J. Entanglement Network in Nanoparticle Reinforced Polymers. J. Chem. Phys. 2009, 130, 244903. [Google Scholar] [CrossRef]
- Woigk, W.; Fuentes, C.A.; Rion, J.; Hegemann, D.; van Vuure, A.W.; Dransfeld, C.; Masania, K. Interface Properties and Their Effect on the Mechanical Performance of Flax Fibre Thermoplastic Composites. Compos. Part A Appl. Sci. Manuf. 2019, 122, 8–17. [Google Scholar] [CrossRef]
- Žigon, J.; Centa, U.G.; Remškar, M.; Humar, M. Application and Characterization of a Novel PVDF-HFP/PVP Polymer Composite with MoO3 Nanowires as a Protective Coating for Wood. Sci. Rep. 2023, 13, 3429. [Google Scholar] [CrossRef] [PubMed]
- Sterniša, M.; Gradišar Centa, U.; Drnovšek, A.; Remškar, M.; Smole Možina, S. Pseudomonas Fragi Biofilm on Stainless Steel (at Low Temperatures) Affects the Survival of Campylobacter Jejuni and Listeria Monocytogenes and Their Control by a Polymer Molybdenum Oxide Nanocomposite Coating. Int. J. Food Microbiol. 2023, 394, 110159. [Google Scholar] [CrossRef] [PubMed]
- Centa, U.G.; Sterniša, M.; Slemenik, L. Perše Surface and Mechanical Properties of Polymer Nanocomposite PVDF-HFP/PVP/SiO2 with Anti-Fouling Properties; SECTOR: Seville, Spain, 2024; pp. 11–14. [Google Scholar]
- Cao, J.H.; Zhu, B.K.; Xu, Y.Y. Structure and Ionic Conductivity of Porous Polymer Electrolytes Based on PVDF-HFP Copolymer Membranes. J. Memb. Sci. 2006, 281, 446–453. [Google Scholar] [CrossRef]
- Shir Mohammadi, M.; Hammerquist, C.; Simonsen, J.; Nairn, J.A. The Fracture Toughness of Polymer Cellulose Nanocomposites Using the Essential Work of Fracture Method. J. Mater. Sci. 2016, 51, 8916–8927. [Google Scholar] [CrossRef]
- Gradišar Centa, U.; Kocbek, P.; Belcarz, A.; Škapin, S.D.; Remškar, M. Polymer Blend Containing MoO3 Nanowires with Antibacterial Activity against Staphylococcus Epidermidis ATCC 12228. J. Nanomater. 2020, 2020, 9754024. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Zdarta, J.; Szatkowski, T.; Wysokowski, M.; Nowacka, M.; Szwarc-Rzepka, K.; Bartczak, P.; Siwińska-Stefańska, K.; Ehrlich, H.; Jesionowski, T. Silica/Lignosulfonate Hybrid Materials: Preparation and Characterization. Open Chem. 2014, 12, 719–735. [Google Scholar] [CrossRef]
- Li, Z.; Jiang, X.; Liu, H.; Yao, Z.; Liu, A.; Ming, L. Evaluation of Hydrophilic and Hydrophobic Silica Particles on the Release Kinetics of Essential Oil Pickering Emulsions. ACS Omega 2022, 7, 8651–8664. [Google Scholar] [CrossRef]
- Dash, S.; Mohanty, H.S.; Ravikant; Kumar, A.; Thomas, R.; Pradhan, D.K. Ferroelectric Ceramic Dispersion to Enhance the β Phase of Polymer for Improving Dielectric and Ferroelectric Properties of the Composites. Polym. Bull. 2021, 78, 5317–5336. [Google Scholar] [CrossRef]
- Jangra, M.; Gupta, M.; Hussain, S. Probing the Local Interactions and Their Effects on the Dielectric Properties of PVDF/PVP Composite Films. J. Mol. Struct. 2024, 1307, 137913. [Google Scholar] [CrossRef]
- Kobayashi, M.; Tashiro, K.; Tadokoro, H. Molecular Vibrations of Three Crystal Forms of Poly(Vinylidene Fluoride). Macromolecules 1975, 8, 158–171. [Google Scholar] [CrossRef]
- Bai, H.; Wang, X.; Zhou, Y.; Zhang, L. Preparation and Characterization of Poly(Vinylidene Fluoride) Composite Membranes Blended with Nano-Crystalline Cellulose. Prog. Nat. Sci. Mater. Int. 2012, 22, 250–257. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A Critical Analysis of the α, β and γ Phases in Poly(Vinylidene Fluoride) Using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef]
- Borodko, Y.; Habas, S.E.; Koebel, M.; Yang, P.; Frei, H.; Somorjai, G.A. Probing the Interaction of Poly(Vinylpyrrolidone) with Platinum Nanocrystals by UV−Raman and FTIR. J. Phys. Chem. B 2006, 110, 23052–23059. [Google Scholar] [CrossRef] [PubMed]
- Al-Oweini, R.; El-Rassy, H. Synthesis and Characterization by FTIR Spectroscopy of Silica Aerogels Prepared Using Several Si(OR)4 and R′′Si(OR′)3 Precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar] [CrossRef]
- Mishra, K.; Hashmi, S.A.; Rai, D.K. Protic Ionic Liquid-Based Gel Polymer Electrolyte: Structural and Ion Transport Studies and Its Application in Proton Battery. J. Solid State Electrochem. 2014, 18, 2255–2266. [Google Scholar] [CrossRef]
- Paramee, S.; Guo, R.; Bhalla, A.S.; Manuspiya, H. A Comparison of Shear-mixing and Solvent-induced on Phase Behavior, Thermal and Dielectric Properties of PVDF-HFP/MOF Composites. J. Appl. Polym. Sci. 2022, 139, e52741. [Google Scholar] [CrossRef]
- Lopes, A.C.; Costa, C.M.; Tavares, C.J.; Neves, I.C.; Lanceros-Mendez, S. Nucleation of the Electroactive γ Phase and Enhancement of the Optical Transparency in Low Filler Content Poly(Vinylidene)/Clay Nanocomposites. J. Phys. Chem. C 2011, 115, 18076–18082. [Google Scholar] [CrossRef]
- Lizundia, E.; Reizabal, A.; Costa, C.M.; Maceiras, A.; Lanceros-Méndez, S. Electroactive γ-Phase, Enhanced Thermal and Mechanical Properties and High Ionic Conductivity Response of Poly (Vinylidene Fluoride)/Cellulose Nanocrystal Hybrid Nanocomposites. Materials 2020, 13, 743. [Google Scholar] [CrossRef]
- Yuan, D.; Li, Z.; Thitsartarn, W.; Fan, X.; Sun, J.; Li, H.; He, C. β Phase PVDF-Hfp Induced by Mesoporous SiO2 Nanorods: Synthesis and Formation Mechanism. J. Mater. Chem. C Mater. 2015, 3, 3708–3713. [Google Scholar] [CrossRef]
- Klapiszewski, Ł.; Królak, M.; Jesionowski, T. Silica Synthesis by the Sol-Gel Method and Its Use in the Preparation of Multifunctional Biocomposites. Open Chem. 2014, 12, 173–184. [Google Scholar] [CrossRef]
- Yuennan, J.; Muensit, N. Preparation and Characterization of Flexible PVDF-HFP Film for Piezoelectric Applications. IOP Conf. Ser. Mater. Sci. Eng. 2020, 715, 012107. [Google Scholar] [CrossRef]
- Bahloul, C.; Ez-Zahraoui, S.; Eddiai, A.; Cherkaoui, O.; Mazraoui, M.; Semlali, F.-Z.; El Achaby, M. Ferrite-Doped Rare-Earth Nanoparticles for Enhanced β-Phase Formation in Electroactive PVDF Nanocomposites. RSC Adv. 2024, 14, 38872–38887. [Google Scholar] [CrossRef]

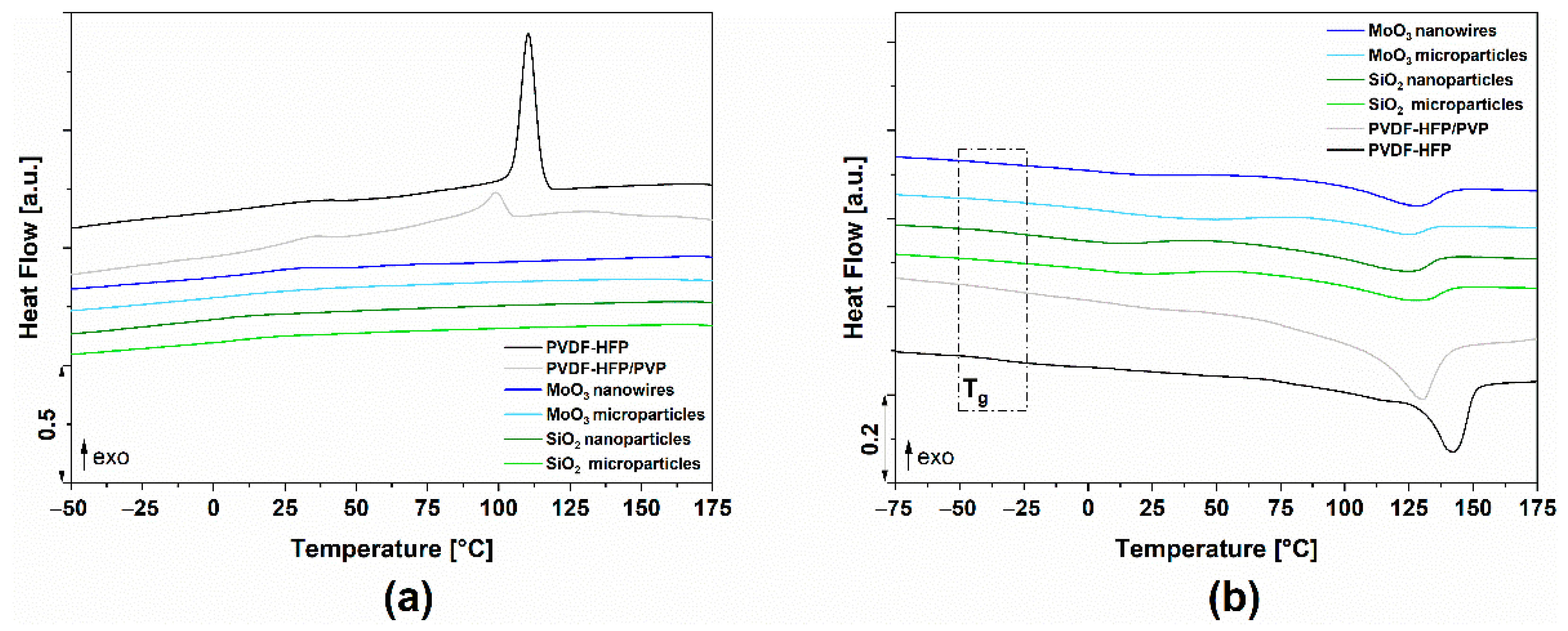

| Polymer Films with | Tg [°C] | Tm [°C] | ΔHm [J/g] | Tc [°C] | ΔHc [J/g] | XC [%] |
|---|---|---|---|---|---|---|
| PVDF-HFP | −35.66 | 142.00 | 36.42 | 110.39 | 32.60 | 34.79 |
| PVDF-HFP/PVP | −40.33 | 130.19 | 30.78 | 98.49 | 10.09 | 40.01 |
| MoO3 nanowires | −38.42 | 127.50 | 8.87 | 29.60 | 2.31 | 12.28 |
| MoO3 microparticles | −30.96 | 124.41 | 4.31 | 22.53 | 0.42 | 5.96 |
| SiO2 nanoparticles | −32.82 | 124.36 | 9.19 | 15.46 | 0.93 | 12.72 |
| SiO2 microparticles | −32.95 | 128.07 | 7.94 | 20.96 | 0.73 | 11.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradišar Centa, U.; Pogačnik Krajnc, A.; Slemenik Perše, L.; Šobak, M.; Mihelčič, M. The Addition of MoO3 or SiO2 Nano-/Microfillers Thermally Stabilized and Mechanically Reinforce the PVDF-HFP/PVP Polymer Composite Thin Films. Coatings 2024, 14, 1603. https://doi.org/10.3390/coatings14121603
Gradišar Centa U, Pogačnik Krajnc A, Slemenik Perše L, Šobak M, Mihelčič M. The Addition of MoO3 or SiO2 Nano-/Microfillers Thermally Stabilized and Mechanically Reinforce the PVDF-HFP/PVP Polymer Composite Thin Films. Coatings. 2024; 14(12):1603. https://doi.org/10.3390/coatings14121603
Chicago/Turabian StyleGradišar Centa, Urška, Anja Pogačnik Krajnc, Lidija Slemenik Perše, Matic Šobak, and Mohor Mihelčič. 2024. "The Addition of MoO3 or SiO2 Nano-/Microfillers Thermally Stabilized and Mechanically Reinforce the PVDF-HFP/PVP Polymer Composite Thin Films" Coatings 14, no. 12: 1603. https://doi.org/10.3390/coatings14121603
APA StyleGradišar Centa, U., Pogačnik Krajnc, A., Slemenik Perše, L., Šobak, M., & Mihelčič, M. (2024). The Addition of MoO3 or SiO2 Nano-/Microfillers Thermally Stabilized and Mechanically Reinforce the PVDF-HFP/PVP Polymer Composite Thin Films. Coatings, 14(12), 1603. https://doi.org/10.3390/coatings14121603







