Effect of Pulse Electrodeposition Mode on Microstructures and Properties of Ni-TiN Composite Coatings
Abstract
1. Introduction
2. Experiment
2.1. Sample Preparation
2.2. Test Methods
3. Results and Discussion
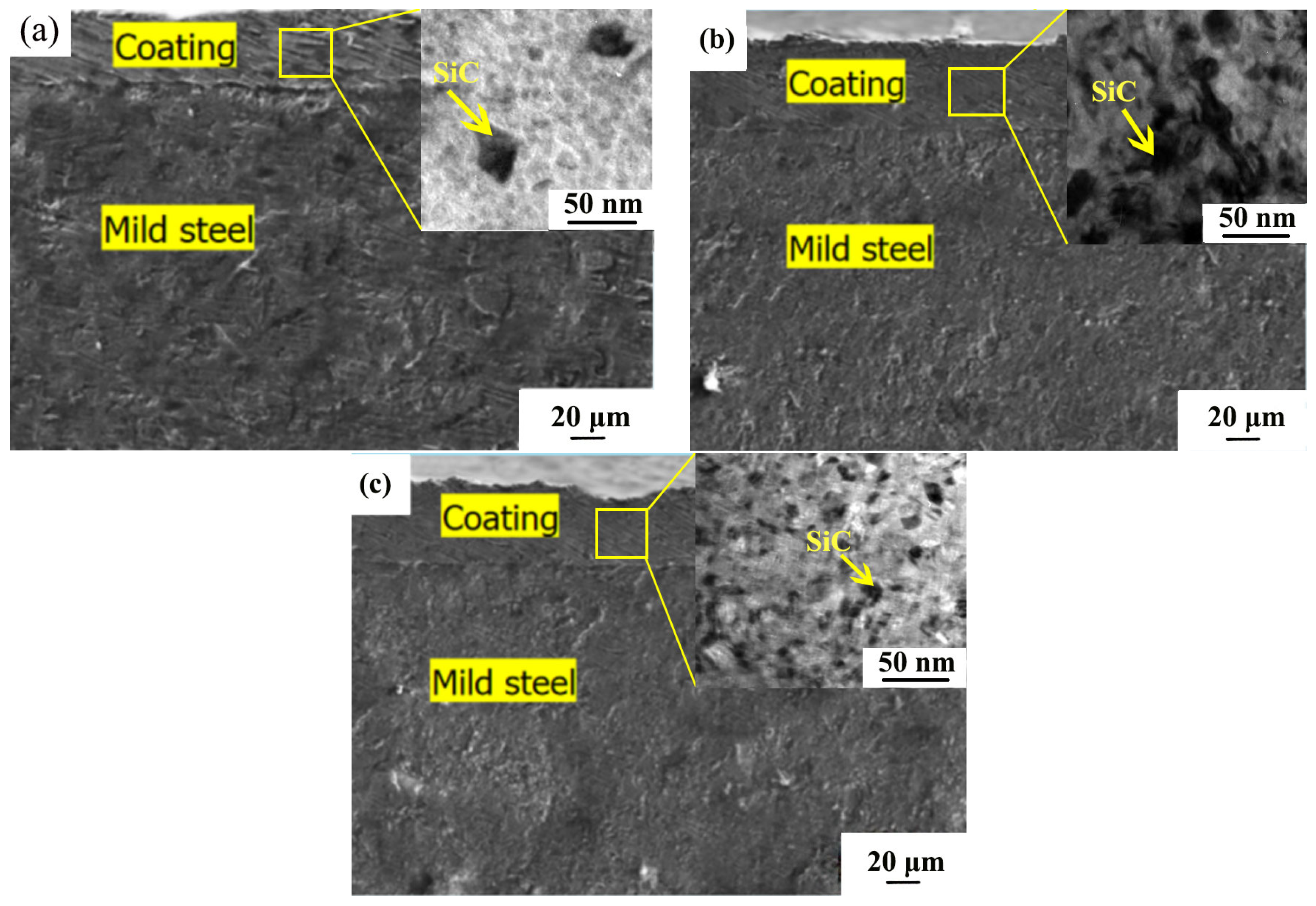
3.1. SEM Morphology
3.2. Interface Bonding Force
3.3. Microhardness
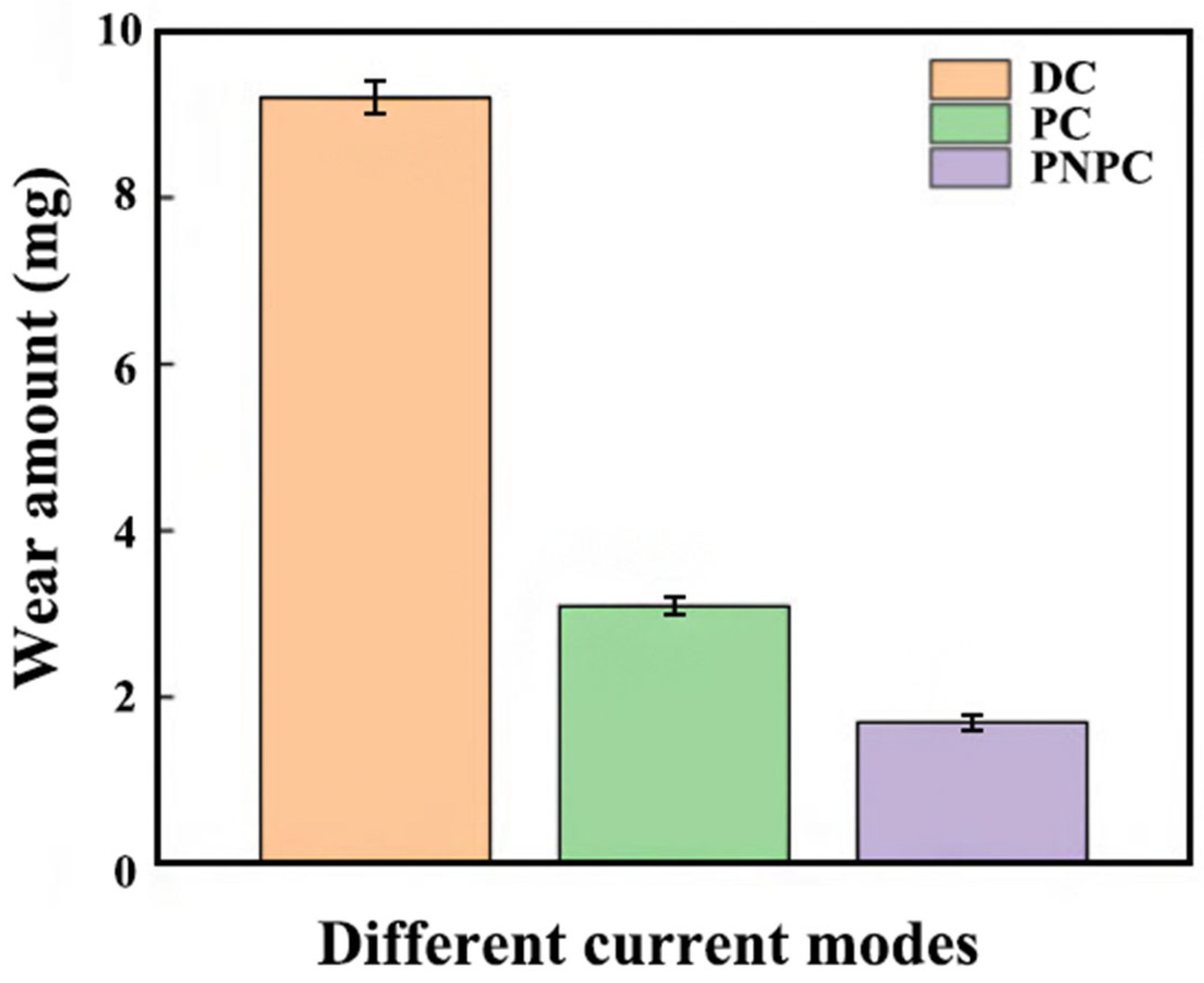
3.4. Friction and Wear Property
3.5. Corrosion Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Podoynitsyn, S.N.; Tsyganova, T.V.; Mchedlishvili, B.V. Current-voltage characteristics and electric breakdown of metal-coated track-etched membranes. Pet. Chem. 2015, 55, 866–870. [Google Scholar] [CrossRef]
- Zhao, K.L.; Shen, L.; Qiu, M.B.; Tian, Z.J.; Jiang, W. Preparation and properties of nanocomposite coatings by pulsed current-jet electrodeposition. Int. J. Electrochem. Sci. 2017, 12, 8578–8590. [Google Scholar] [CrossRef]
- Zeng, Y.B.; Qu, N.S.; Hu, X.Y. Preparation and Characterization of Electrodeposited Ni-CeO2 Nanocomposite Coatings with High Current Density. Int. J. Electrochem. Sci. 2014, 9, 8145–8154. [Google Scholar] [CrossRef]
- Novikov, S.V.; Peretyagin, P.Y.; Dolzhikova, E.Y.; Torrecillas, R. Formation of structure in hard-alloy coatings from powders under passage of a powerful pulse of electric current. Met. Sci. Heat Treat. 2016, 57, 596–602. [Google Scholar] [CrossRef]
- Ma, C.; He, H.; Xia, F.; Xiao, Z.; Liu, Y. Performance of Ni-SiC composites deposited using magnetic-field-assisted electrodeposition under different magnetic-field directions. Ceram. Int. 2023, 49, 35907–35916. [Google Scholar] [CrossRef]
- Zhai, L.L.; Wang, Q.; Zhang, J.W.; Ban, C.Y. Effect of alternating current electric field on microstructure and properties of laser cladding Ni-Cr-B-Si coating. Ceram. Int. 2019, 45, 16873–16879. [Google Scholar] [CrossRef]
- Xia, F.; Yan, P.; Ma, C.; Wang, B.; Liu, Y. Effect of different heat-treated temperatures upon structural and abrasive performance of Ni-TiN composite nanocoatings. J. Mater. Res. Technol. 2023, 27, 2874–2881. [Google Scholar] [CrossRef]
- Guo, S.J.; Yang, Z.G.; Deng, S.H.; Wang, S.; Wang, X. Effect of pulse electrical parameters on the microstructure and performance of Ni-TiN nanocoatings prepared by pulse electrodeposition technique. Trans. Indian Inst. Met. 2022, 3, 75. [Google Scholar] [CrossRef]
- Li, Q.; Xia, F.; Liu, G.; Yao, L. Microstructure and properties of jet pulse electrodeposited Ni-TiN nanocoatings. Ceram. Int. 2022, 31, 8823–8829. [Google Scholar] [CrossRef]
- Nrgin, E.; Yoruk, G.; Ozdemir, O. Characterization of Ni3Al and Ti3Al coatings produced by electric current activated sintering method. Acta Phys. Pol. A 2013, 123, 245–247. [Google Scholar]
- Pokhmurs’ka, H.V.; Holovchuk, M.Y.; Dz’oba, Y.V.; Hvozdets’kyi, V.M.; Dzyubyk, L.V. Influence of the composition of charge of powder wires on the structure and properties of electric-arc coating. Mater. Sci. 2018, 53, 868–874. [Google Scholar] [CrossRef]
- Zhanga, R.F.; Shan, D.Y.; Chen, R.S.; Han, E.H. Effects of electric parameters on properties of anodic coatings formed on magnesium alloys. Mater. Chem. Phys. 2008, 107, 356–363. [Google Scholar] [CrossRef]
- Wang, Z.W.; Yu, Y.T. Thickness and conductivity measurement of multilayered electricity-conducting coating by pulsed eddy current technique: Experimental investigation. IEEE Trans. Instrum. Meas. 2019, 68, 3166–3172. [Google Scholar] [CrossRef]
- Xia, F.F.; Ma, C.Y.; Wang, J.D. Prediction of nano TiN particles content in the Ni-TiN composite coating based on AR model. Appl. Mech. Mater. 2011, 130–134, 998–1000. [Google Scholar] [CrossRef]
- Xia, Q.X.; Wang, J.K.; Liu, G.J.; Wei, H.; Li, D.Q.; Yao, Z.P.; Jiang, Z.H. Effects of electric parameters on structure and thermal control property of PEO ceramic coatings on Ti alloys. Surf. Coat. Technol. 2016, 307, 1284–1290. [Google Scholar] [CrossRef]
- Nagai, A.; Ma, C.F.; Kishi, S.; Inuzuka, M.; Nakamura, M.; Horiuchi, N.; Nishio, K.; Yamashita, K. Surface properties of Al2O3-YSZ ceramic composites modified by a combination of biomimetic coatings and electric. Appl. Surf. Sci. 2012, 262, 45–50. [Google Scholar] [CrossRef]
- Xue, J.Y.; Zhang, Z.; Li, B.B.; Zhao, Y.S.; Ding, L.J. Temperature-dependent adaptive conductivity coating for surface charge release and electric field control under electro-thermal coupling field. High Volt. 2023, 8, 1082–1092. [Google Scholar] [CrossRef]
- Srivastava, M.; William, G.V.; Jain, A.; Rajam, K.S. Influence of SiC particle size on the structure and tribological properties of Ni-Co composites. Surf. Coat. Technol. 2007, 202, 310–318. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, K.; Li, Q.; Ma, C.; Xia, F. Pulse electrodeposited Ni-SiC thin coatings by using experimental system designed for potential industrial application. Int. J. Electrochem. Sci. 2021, 16, 21044. [Google Scholar] [CrossRef]
- Li, C.; Xia, F.; Ma, C.; Li, Q. Research on the corrosion behavior of Ni-SiC nanocoating prepared using a jet electrodeposition technique. J. Mater. Eng. Perform. 2021, 30, 6336–6344. [Google Scholar] [CrossRef]
- Wasekar, N.; Bathini, L.; Sundararajan, G. Tribological behavior of pulsed electrodeposited Ni-W/SiC nanocomposites. Mater. Eng. Perform. 2018, 27, 5236–5245. [Google Scholar] [CrossRef]
- Xia, F.; Zhao, X.; Jiang, M.; Ma, C. Influence of nozzle-fluid velocity on morphology and wear resistance of jet flow electrodeposited Ni-doped SiC composites. AIP Adv. 2019, 9, 065310. [Google Scholar] [CrossRef]
- Wang, F.; Fu, X.; Shen, M.; Xu, Y.; Duan, S.; Wang, Q.; Wang, H.; Cao, H.; Lin, J. Preparation of Ni-P-SiC composite coatings by magnetic field-enhanced jet electrodeposition. Int. J. Elecrochem. Sci. 2020, 15, 10432–10452. [Google Scholar] [CrossRef]
- Fu, X.; Wang, F.; Chen, X.; Lin, J.; Cao, H. Corrosion resistance of Ni-P/SiC and Ni-P composite coatings prepared by magnetic field-enhanced jet electrodeposition. RSC Adv. 2021, 10, 34167–34176. [Google Scholar] [CrossRef]
- Şahin, İ.; Faik, Y.L.; Levent, U. Optimisation on machining parametres by EDM of TiN coated Ti6Al4V alloys. Adv. Mater. Process. Technol. 2023, 10, 960–970. [Google Scholar] [CrossRef]
- Urtekin, L.; Aydin, E.; Sevim, A.; Gk, K.; Uslan, B. Experimental determination of biofilm and mechanical properties of surfaces obtained by CO2 laser gas-assisted nitriding of Ti-6Al-4V alloy. Surf. Rev. Lett. 2022, 29, 2250154. [Google Scholar] [CrossRef]








| Current Mode | Test Microhardness (HV) | Mean Microhardness (HV) | ||||
|---|---|---|---|---|---|---|
| DC | 313.5 | 276.9 | 318.3 | 324.1 | 354.2 | 317.4 |
| PC | 377.0 | 342.5 | 402.6 | 377.4 | 405.9 | 381.1 |
| PNPC | 479.3 | 533.9 | 486.4 | 470.0 | 482.6 | 490.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; He, H.; Xia, F.; Cao, M. Effect of Pulse Electrodeposition Mode on Microstructures and Properties of Ni-TiN Composite Coatings. Coatings 2024, 14, 1384. https://doi.org/10.3390/coatings14111384
Ma C, He H, Xia F, Cao M. Effect of Pulse Electrodeposition Mode on Microstructures and Properties of Ni-TiN Composite Coatings. Coatings. 2024; 14(11):1384. https://doi.org/10.3390/coatings14111384
Chicago/Turabian StyleMa, Chunyang, Hongxin He, Fafeng Xia, and Mengyu Cao. 2024. "Effect of Pulse Electrodeposition Mode on Microstructures and Properties of Ni-TiN Composite Coatings" Coatings 14, no. 11: 1384. https://doi.org/10.3390/coatings14111384
APA StyleMa, C., He, H., Xia, F., & Cao, M. (2024). Effect of Pulse Electrodeposition Mode on Microstructures and Properties of Ni-TiN Composite Coatings. Coatings, 14(11), 1384. https://doi.org/10.3390/coatings14111384




