Recent Progress on the Tribology of Pure/Doped Diamond-like Carbon Coatings and Ionic Liquids
Abstract
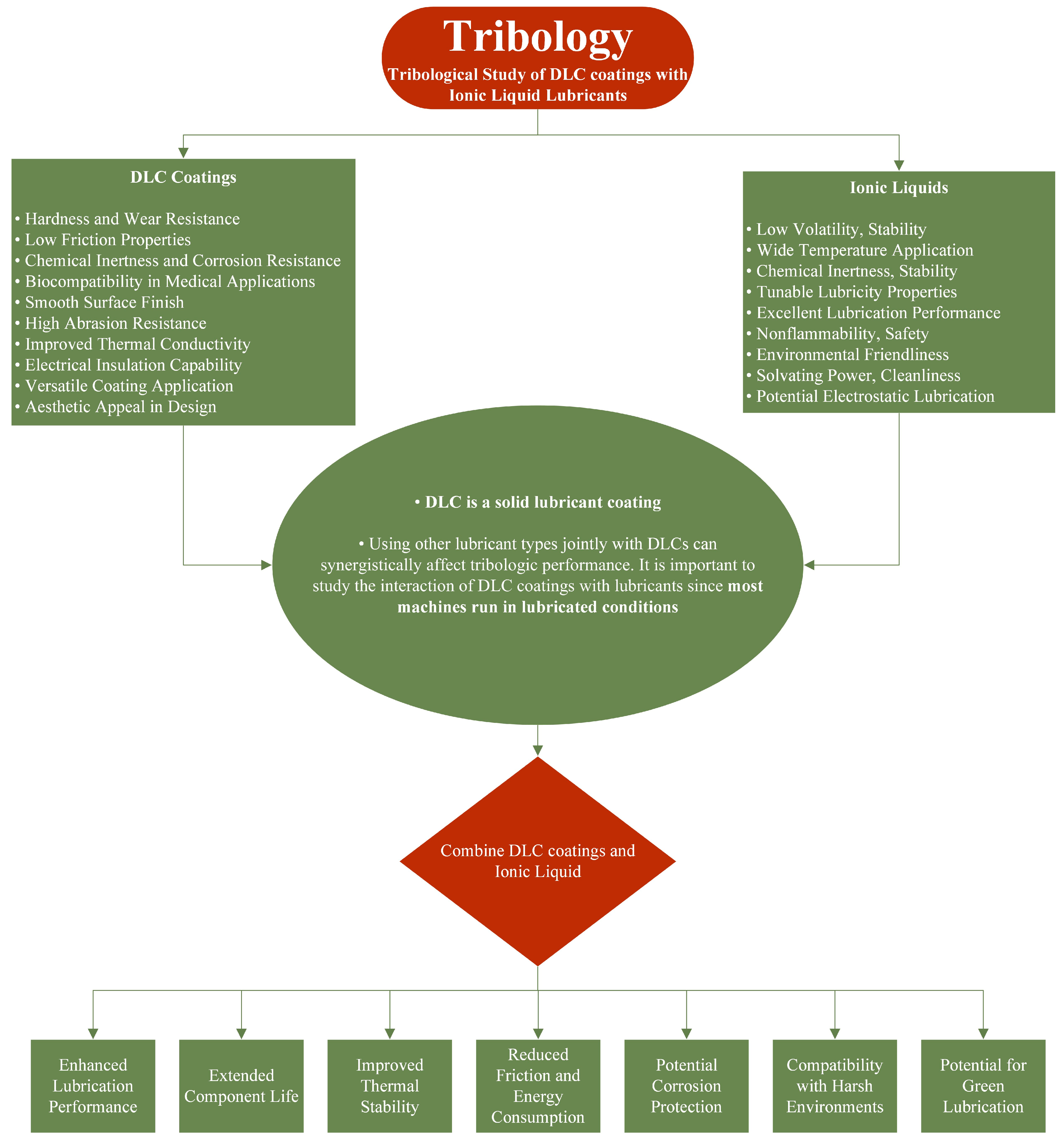
1. Introduction
2. Diamond-like Carbon Coatings
2.1. Why DLC Coatings?
2.2. Major Research Trends in the Production Process of DLCs
2.3. Characteristics of DLC Coatings
2.3.1. Thermal Stability of DLC Coatings
2.3.2. Chemical Resistance of DLC Films
2.3.3. Friction and Wear
3. Ionic Liquids
3.1. Characteristics
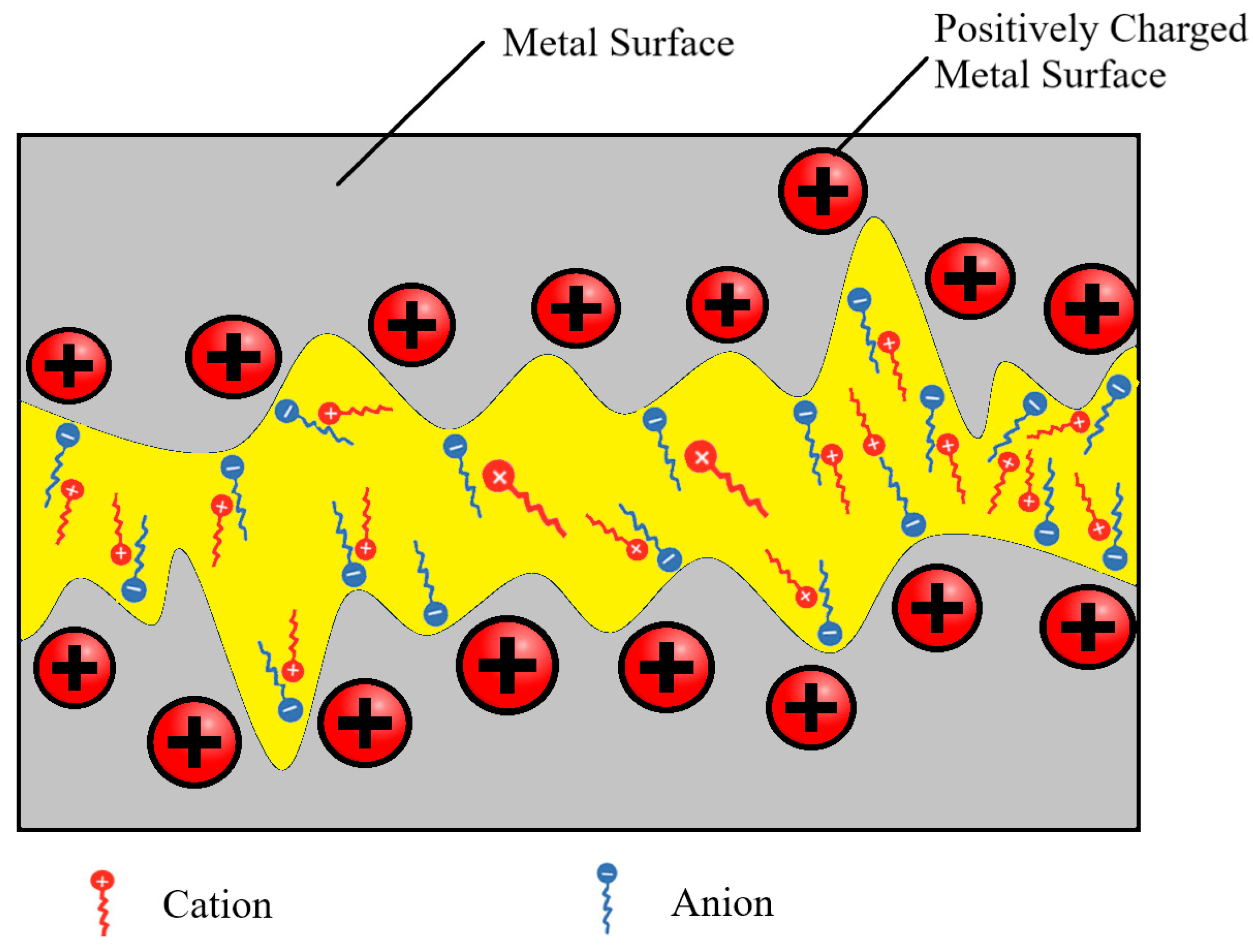
3.1.1. Cationic Structure and Anionic Structure
3.1.2. Thermal Stability
3.1.3. Corrosion Behavior of Ionic Liquids
3.2. Ionic Liquids in Tribology
3.2.1. Tribological Behavior of Ionic Lubricants
3.2.2. Ionic Liquids as Main Lubricant
3.2.3. Ionic Liquids as an Additive
3.2.4. Ionic Liquids as Extreme Temperature Lubricants
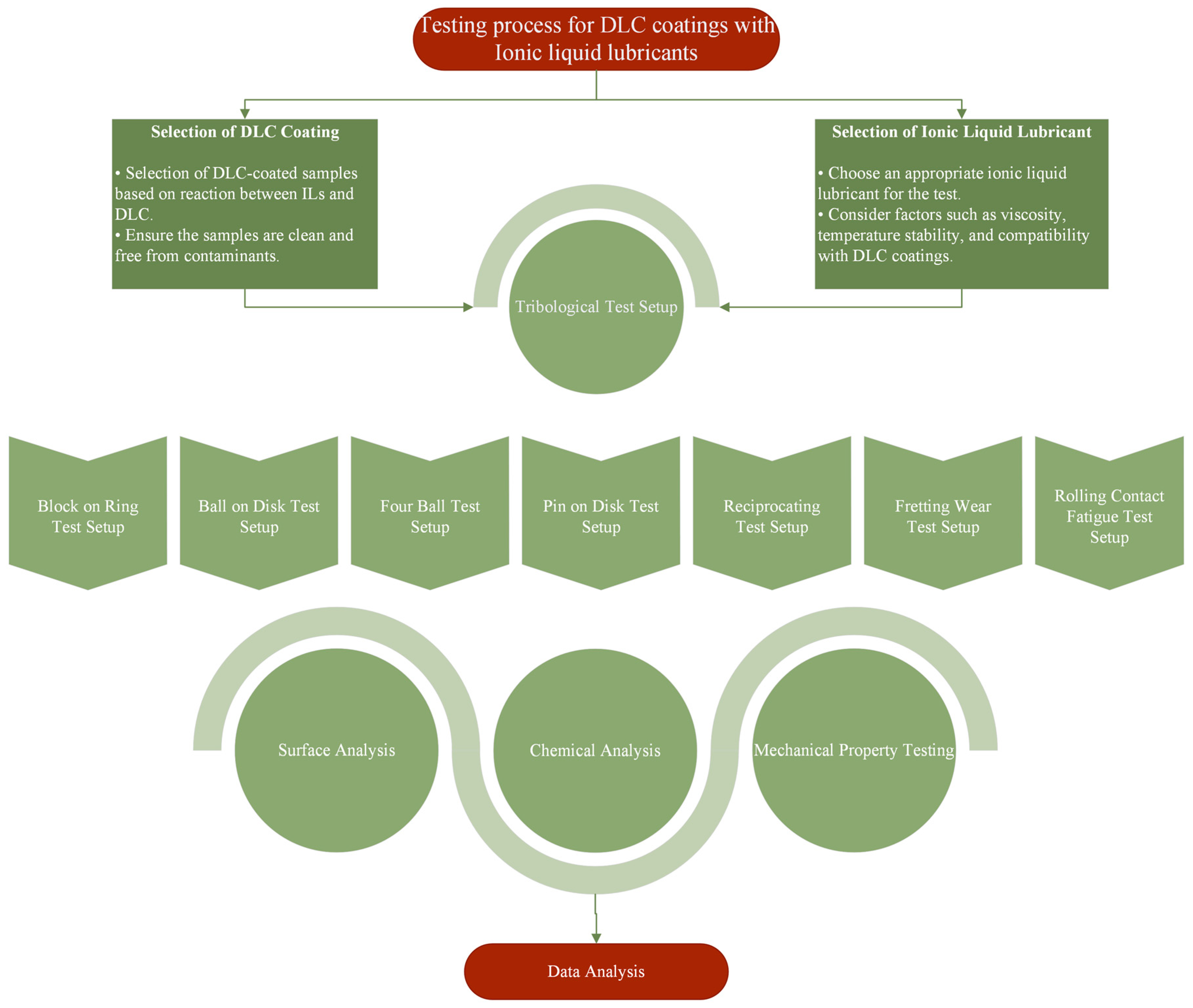
4. Tribological Testing of DLC Coatings with Ionic Liquid Lubricants
4.1. Tribology of Pure Diamond-like Carbon Coatings and Ionic Liquids
4.2. Tribology of Doped Diamond-like Carbon Coatings and Ionic Liquids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holmberg, K.; Erdemir, A. The Impact of Tribology on Energy Use and CO2 Emission Globally and in Combustion Engine and Electric Cars. Tribol. Int. 2019, 135, 389–396. [Google Scholar] [CrossRef]
- Holmberg, K.; Andersson, P.; Erdemir, A. Global Energy Consumption Due to Friction in Passenger Cars. Tribol. Int. 2012, 47, 221–234. [Google Scholar] [CrossRef]
- Pinkus, O.; Wilcock, D.F. Strategy for Energy Conservation through Tribology; American Society of Mechanical Engineers: New York, NY, USA, 1977. [Google Scholar]
- Kimura, Y.; Tanaka, M.; Enomoto, Y. Tribology Research in Japan—A State-of-the-Art Report. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 1996, 210, 159–172. [Google Scholar] [CrossRef]
- National Research Council of Canada. Associate Committee on Tribology. A Strategy for Tribology in Canada: Enhancing Reliability and Efficiency through the Reduction of Wear and Friction; National Research Council of Canada: Ottawa, ON, Canada, 1986.
- What Is the Kyoto Protocol?|UNFCCC. Available online: https://unfccc.int/kyoto_protocol (accessed on 21 March 2023).
- The Paris Agreement|UNFCCC. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement (accessed on 21 March 2023).
- Q&A: Commission Proposal on the New Euro 7 Standards. Available online: https://ec.europa.eu/commission/presscorner/detail/en/qanda_22_6496 (accessed on 21 March 2023).
- Reeves, C.J.; Siddaiah, A.; Menezes, P.L. A Review on the Science and Technology of Natural and Synthetic Biolubricants. J. Bio-Tribo-Corros. 2017, 3, 11. [Google Scholar] [CrossRef]
- Chan, C.-H.; Tang, S.W.; Mohd, N.K.; Lim, W.H.; Yeong, S.K.; Idris, Z. Tribological Behavior of Biolubricant Base Stocks and Additives. Renew. Sustain. Energy Rev. 2018, 93, 145–157. [Google Scholar] [CrossRef]
- Nowak, P.; Kucharska, K.; Kamiński, M. Ecological and Health Effects of Lubricant Oils Emitted into the Environment. Int. J. Environ. Res. Public. Health 2019, 16, 3002. [Google Scholar] [CrossRef]
- Spikes, H. The History and Mechanisms of ZDDP. Tribol. Lett. 2004, 17, 469–489. [Google Scholar] [CrossRef]
- Ferdeghini, C.; Guazzelli, L.; Pomelli, C.S.; Ciccioli, A.; Brunetti, B.; Mezzetta, A.; Vecchio Ciprioti, S. Synthesis, Thermal Behavior and Kinetic Study of N-Morpholinium Dicationic Ionic Liquids by Thermogravimetry. J. Mol. Liq. 2021, 332, 115662. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chen, C.-Y.; Wu, J.-H.; Kang, X. Fire and Explosion Hazards of Ionic Liquid 1-Methyl-1-Propylpyrrolidinium Bis(Trifluoromethanesulfonyl)Imide. J. Loss Prev. Process Ind. 2019, 60, 233–240. [Google Scholar] [CrossRef]
- Barulli, L.; Mezzetta, A.; Brunetti, B.; Guazzelli, L.; Vecchio Ciprioti, S.; Ciccioli, A. Evaporation Thermodynamics of the Tetraoctylphosphonium Bis(Trifluoromethansulfonyl)Imide([P8888]NTf2) and Tetraoctylphosphonium Nonafluorobutane-1-Sulfonate ([P8888]NFBS) Ionic Liquids. J. Mol. Liq. 2021, 333, 115892. [Google Scholar] [CrossRef]
- Nancarrow, P.; Mohammed, H. Ionic Liquids in Space Technology–Current and Future Trends. ChemBioEng Rev. 2017, 4, 106–119. [Google Scholar] [CrossRef]
- Street, K.W.; Morales, W.; Koch, V.R.; Valco, D.J.; Richard, R.M.; Hanks, N. Evaluation of Vapor Pressure and Ultra-High Vacuum Tribological Properties of Ionic Liquids. Tribol. Trans. 2011, 54, 911–919. [Google Scholar] [CrossRef]
- Palacio, M.; Bhushan, B. A Review of Ionic Liquids for Green Molecular Lubrication in Nanotechnology. Tribol. Lett. 2010, 40, 247–268. [Google Scholar] [CrossRef]
- Reeves, C.J.; Siddaiah, A.; Menezes, P.L. Ionic Liquids: A Plausible Future of Bio-Lubricants. J. Bio Tribo-Corros. 2017, 3, 18. [Google Scholar] [CrossRef]
- Cai, M.; Yu, Q.; Liu, W.; Zhou, F. Ionic Liquid Lubricants: When Chemistry Meets Tribology. Chem. Soc. Rev. 2020, 49, 7753–7818. [Google Scholar] [CrossRef] [PubMed]
- Predel, T.; Pohrer, B.; Schlücker, E. Ionic Liquids as Alternative Lubricants for Special Applications. Chem. Eng. Technol. 2010, 33, 132–136. [Google Scholar] [CrossRef]
- Zhou, Y.; Weber, J.; Viola, M.B.; Qu, J. Is More Always Better? Tribofilm Evolution and Tribological Behavior Impacted by the Concentration of ZDDP, Ionic Liquid, and ZDDP-Ionic Liquid Combination. Wear 2019, 432–433, 202951. [Google Scholar] [CrossRef]
- Khanmohammadi, H.; Wijanarko, W.; Cruz, S.; Evaristo, M.; Espallargas, N. Triboelectrochemical Friction Control of W- and Ag-Doped DLC Coatings in Water-Glycol with Ionic Liquids as Lubricant Additives. RSC Adv. 2022, 12, 3573–3583. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Xue, Q. DLC-Based Solid–Liquid Synergetic Lubricating Coatings for Improving Tribological Behavior of Boundary Lubricated Surfaces under High Vacuum Condition. Wear 2011, 271, 889–898. [Google Scholar] [CrossRef]
- González, R.; Battez, A.H.; Viesca, J.L.; Higuera-Garrido, A.; Fernández-González, A. Lubrication of DLC Coatings with Two Tris(Pentafluoroethyl)Trifluorophosphate Anion-Based Ionic Liquids. Tribol. Trans. 2013, 56, 887–895. [Google Scholar] [CrossRef]
- Yan, M.; Wang, X.; Zhang, S.; Zhang, S.; Sui, X.; Li, W.; Hao, J.; Liu, W. Friction and Wear Properties of GLC and DLC Coatings under Ionic Liquid Lubrication. Tribol. Int. 2020, 143, 106067. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, M.; Tailor, S. Self-Lubricating Composite Coatings: A Review of Deposition Techniques and Material Advancement. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Tasdemir, H.A.; Tokoroyama, T.; Kousaka, H.; Umehara, N.; Mabuchi, Y. Friction and Wear Performance of Boundary-Lubricated DLC/DLC Contacts in Synthetic Base Oil. Procedia Eng. 2013, 68, 518–524. [Google Scholar] [CrossRef][Green Version]
- Santhosh, N.; Shankar, G.; Nunthavarawong, P. Mechanical and Tribological Performance of Diamond-Like Carbon Coatings: An Overview. In Diamond-Like Carbon Coatings; CRC Press: Boca Raton, FL, USA, 2023; pp. 255–274. [Google Scholar]
- Yue, Z.; Fan, X.; Wang, Y.; Li, H.; Zhang, J.; Zhu, M. Fretting Behaviors of Self-Mated Diamond-like Carbon Films: The Evolution of Fretting Regime and Transfer Film. Carbon 2023, 203, 695–705. [Google Scholar] [CrossRef]
- Zia, A.W.; Zhou, Z.; Li, L.K.-Y. Chapter 7—Structural, Mechanical, and Tribological Characteristics of Diamond-like Carbon Coatings. In Nanomaterials-Based Coatings; Nguyen Tri, P., Rtimi, S., Ouellet Plamondon, C.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 171–194. ISBN 978-0-12-815884-5. [Google Scholar]
- Liu, J.; Yang, T.; Cao, H.; Deng, Q.; Pan, C.; Wen, F. Diamond-like Carbon Films for Tribological Modification of Rubber. Nanotechnol. Rev. 2022, 11, 2839–2856. [Google Scholar] [CrossRef]
- Vieira, V.F.; Shigaki, Y.; Martins, P.S.; Ba, E.C.T.; Dias, C.A.R. Nanoindentation Test of a DLC Coated High-Speed Steel Substrate Using a Two-Dimensional Axisymmetric Finite Element Method. Diam. Relat. Mater. 2023, 134, 109792. [Google Scholar] [CrossRef]
- Roy, R.K.; Lee, K.R. Biomedical Applications of Diamond-like Carbon Coatings: A Review. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 83, 72–84. [Google Scholar] [CrossRef]
- Beake, B.D.; Mcmaster, S.J.; Liskiewicz, T.W.; Neville, A. Influence of Si-and W-Doping on Micro-Scale Reciprocating Wear and Impact Performance of DLC Coatings on Hardened Steel. Tribol. Int. 2021, 160, 107063. [Google Scholar] [CrossRef]
- Diamond-like Carbon (DLC) Market Size, Share, Scope, Trends & Forecast. Available online: https://www.verifiedmarketresearch.com/product/diamond-like-carbon-dlc-market/ (accessed on 12 March 2023).
- Ferreira, F.; Serra, R.; Cavaleiro, A.; Oliveira, J. Diamond-like Carbon Coatings Deposited by Deep Oscillation Magnetron Sputtering in Ar-Ne Discharges. Diam. Relat. Mater. 2019, 98, 107521. [Google Scholar] [CrossRef]
- Vetter, J. 60years of DLC Coatings: Historical Highlights and Technical Review of Cathodic Arc Processes to Synthesize Various DLC Types, and Their Evolution for Industrial Applications. Surf. Coat. Technol. 2014, 257, 213–240. [Google Scholar] [CrossRef]
- Deng, X.; Kousaka, H.; Tokoroyama, T.; Umehara, N. Thermal Stability and High-Temperature Tribological Properties of AC: H and Si-DLC Deposited by Microwave Sheath Voltage Combination Plasma. Tribol. Online 2013, 8, 257–264. [Google Scholar] [CrossRef]
- Ali, A.; Hirakuri, K.K.; Friedbacher, G. Roughness and Deposition Mechanism of DLC Films Prepared by r.f. Plasma Glow Discharge. Vacuum 1998, 51, 363–368. [Google Scholar] [CrossRef]
- Karuzskii, A.L.; Melnik, N.N.; Murzin, V.N.; Nozdrin, V.S.; Perestoronin, A.V.; Volchkov, N.A.; Zhurkin, B.G. Pulsed-Laser Deposition of “Diamond-like” Carbon Coating on YBa2Cu3O7 High-Tc Superconductor Films. Appl. Surf. Sci. 1996, 92, 457–460. [Google Scholar] [CrossRef]
- Wang, A.-Y.; Lee, K.-R.; Ahn, J.-P.; Han, J.H. Structure and Mechanical Properties of W Incorporated Diamond-like Carbon Films Prepared by a Hybrid Ion Beam Deposition Technique. Carbon 2006, 44, 1826–1832. [Google Scholar] [CrossRef]
- de Fátima Magalhaes Mariano, S.; de Dios Mitma Pillaca, E.J.; Ueda, M.; de Moraes Oliveira, R. Influence of the Magnetic Field on DLC Coatings Grown by Plasma Immersion Ion Implantation and Deposition in Crossed Fields. Surf. Coat. Technol. 2014, 256, 47–51. [Google Scholar] [CrossRef]
- Bulíř, J.; Novotný, M.; Jelínek, M.; Kocourek, T.; Studnička, V. Plasma Study and Deposition of DLC/TiC/Ti Multilayer Structures Using Technique Combining Pulsed Laser Deposition and Magnetron Sputtering. Surf. Coat. Technol. 2005, 200, 708–711. [Google Scholar] [CrossRef]
- Lin, Z.; Lv, S.-B.; Yu, Z.-J.; Li, M.; Lin, T.-Y.; Ba, D.-C.; Choi, C.-K.; Lee, I.-S. Effect of Bias Voltage on Diamond-like Carbon Film Deposited on PMMA Substrate. Surf. Coat. Technol. 2008, 202, 5386–5389. [Google Scholar] [CrossRef]
- Zia, A.W.; Birkett, M. Deposition of Diamond-like Carbon Coatings: Conventional to Non-Conventional Approaches for Emerging Markets. Ceram. Int. 2021, 47, 28075–28085. [Google Scholar] [CrossRef]
- Chowdhury, S.; Laugier, M.T.; Rahman, I.Z. Effect of Target Self-Bias Voltage on the Mechanical Properties of Diamond-like Carbon Films Deposited by RF Magnetron Sputtering. Thin Solid. Film. 2004, 468, 149–154. [Google Scholar] [CrossRef]
- Sheeja, D.; Tay, B.K.; Lau, S.P.; Shi, X. Tribological Properties and Adhesive Strength of DLC Coatings Prepared under Different Substrate Bias Voltages. Wear 2001, 249, 433–439. [Google Scholar] [CrossRef]
- Wu, K.Y.; Zhao, G.R.; Li, Z.; Gong, Z.B. Effects of Electrode Distance on Mechanical and Tribological Properties of Hydrogenated Dlc Films Deposited by Dc-Pulse PECVD. Surf. Rev. Lett. 2020, 28, 2050045. [Google Scholar] [CrossRef]
- Lux, H.; Edling, M.; Lucci, M.; Kitzmann, J.; Villringer, C.; Siemroth, P.; De Matteis, F.; Schrader, S. The Role of Substrate Temperature and Magnetic Filtering for DLC by Cathodic Arc Evaporation. Coatings 2019, 9, 345. [Google Scholar] [CrossRef]
- Zavaleyev, V.; Walkowicz, J.; Greczynski, G.; Hultman, L. Effect of Substrate Temperature on Properties of Diamond-like Films Deposited by Combined DC Impulse Vacuum-Arc Method. Surf. Coat. Technol. 2013, 236, 444–449. [Google Scholar] [CrossRef]
- Kang, Y.; Li, B.; Zhao, J.; Ge, B.; Weng, M.; Shi, Z.; Zhao, Y. Effect of Structure on the Secondary Electron Emission of Tetrahedral Amorphous Carbon Films. Vacuum 2020, 172, 109043. [Google Scholar] [CrossRef]
- Yadav, V.S.; Sahu, D.K.; Singh, M.; Kumar, K.; Dhubkarya, D.C.; Singh, Y. Characterization of Nano-Crystalline Diamond like Carbon (DLC) Films with Substrate Temperature Using Dense Plasma Focusing Method. In Proceedings of the AIP Conference Proceedings, San Francisco, CA, USA, 20–22 October 2009; American Institute of Physics: College Park, MD, USA, 2010; Volume 1247, pp. 363–373. [Google Scholar]
- Lei, Y.; Jiang, J.; Wang, Y.; Bi, T.; Zhang, L. Structure Evolution and Stress Transition in Diamond-like Carbon Films by Glancing Angle Deposition. Appl. Surf. Sci. 2019, 479, 12–19. [Google Scholar] [CrossRef]
- Kinoshita, H.; Ippei, I.; Sakai, H.; Ohmae, N. Synthesis and Mechanical Properties of Carbon Nanotube/Diamond-like Carbon Composite Films. Diam. Relat. Mater. 2007, 16, 1940–1944. [Google Scholar] [CrossRef]
- Guo, C.Q.; Pei, Z.L.; Fan, D.; Gong, J.; Sun, C. Microstructure and Tribomechanical Properties of (Cr, N)-DLC/DLC Multilayer Films Deposited by a Combination of Filtered and Direct Cathodic Vacuum Arcs. Diam. Relat. Mater. 2015, 60, 66–74. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, K.W. Effects of Sliding Velocity and Ambient Temperature on the Friction and Wear of a Boundary-Lubricated, Multi-Layered DLC Coating. Wear 2014, 315, 95–102. [Google Scholar] [CrossRef]
- Zia, A.W.; Hussain, S.A.; Baig, M.M.F.A. Optimizing Diamond-like Carbon Coatings—From Experimental Era to Artificial Intelligence. Ceram. Int. 2022, 48, 36000–36011. [Google Scholar] [CrossRef]
- Jean, M.D.; Liu, C.D.; Wang, S.F.; Li, C.H.; Kao, K.H. Design and Optimization of Surface Properties for Diamond-Like Carbon Films by Sputtering Depositions. Appl. Mech. Mater. 2013, 401–403, 762–766. [Google Scholar] [CrossRef]
- Fang, J.; Sheen, M.T.; Jean, M. Der Analysis of Surface Properties of Diamond-Like Carbon Films by a Sputtering Deposition. Adv. Mater. Res. 2013, 662, 505–510. [Google Scholar] [CrossRef]
- Kalita, K.; Ghadai, R.K. Optimization of Plasma Enhanced Chemical Vapor Deposition Process Parameters for Hardness Improvement of Diamond-like Carbon Coatings. Sci. Iranica. Trans. B Mech. Eng. 2022, 29, 1795–1805. [Google Scholar] [CrossRef]
- Solis-Romero, J.; Rodríguez-Molina, A.; Solis-Cordova, J.J.; Neville, A. Parametric Optimisation of Friction and Wear of a Multi-Layered a-C:H Coating on AISI 52100 Steel. Mater Lett 2022, 318, 132166. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Wang, D.-Y. Structural and Electrical Properties of Cr Doped A-C:H Films Synthesized by a Cathodic-Arc Activated Deposition Process. Surf. Coat. Technol. 2006, 200, 3170–3174. [Google Scholar] [CrossRef]
- Tang, X.S.; Wang, H.J.; Feng, L.; Shao, L.X.; Zou, C.W. Mo Doped DLC Nanocomposite Coatings with Improved Mechanical and Blood Compatibility Properties. Appl. Surf. Sci. 2014, 311, 758–762. [Google Scholar] [CrossRef]
- Khun, N.W.; Lee, P.M.; Toh, W.Q.; Liu, E. Tribological Behavior of Nickel-Doped Diamond-Like Carbon Thin Films Prepared on Silicon Substrates via Magnetron Sputtering Deposition. Tribol. Trans. 2016, 59, 845–855. [Google Scholar] [CrossRef]
- Majeed, S.; Siraj, K.; Naseem, S.; Khan, M.F.; Irshad, M.; Faiz, H.; Mahmood, A. Structural and Optical Properties of Gold-Incorporated Diamond-like Carbon Thin Films Deposited by RF Magnetron Sputtering. Mater. Res. Express 2017, 4, 076403. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, H.; Wei, Q.; Long, H.; Deng, Z.; Yu, Z.; Xie, Y.; Wang, J.; Ma, L.; Zhou, K. A Niobium and Nitrogen Co-Doped DLC Film Electrode and Its Electrochemical Properties. J. Electrochem. Soc. 2017, 164, H1091–H1098. [Google Scholar] [CrossRef]
- Wang, L.; Jin, J.; Zhu, C.; Li, G.; Kuang, X.; Huang, K. Effects of HiPIMS Pulse-Length on Plasma Discharge and on the Properties of WC-DLC Coatings. Appl. Surf. Sci. 2019, 487, 526–538. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, L. Synergistic Improving of Tribological Properties of Amorphous Carbon Film Enhanced by F–Si-Doped Multilayer Structure under Corrosive Environment. Surf. Coat. Technol. 2015, 276, 626–635. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, M.; Yang, Y.; Zhang, Y.; Yan, F.; Li, H. Excellent Mechanical, Tribological and Anti-Corrosive Performance of Novel Ti-DLC Nanocomposite Thin Films Prepared via Magnetron Sputtering Method. Carbon 2019, 151, 136–147. [Google Scholar] [CrossRef]
- Jo, Y.J.; Zhang, T.F.; Son, M.J.; Kim, K.H. Synthesis and Electrochemical Properties of Ti-Doped DLC Films by a Hybrid PVD/PECVD Process. Appl. Surf. Sci. 2018, 433, 1184–1191. [Google Scholar] [CrossRef]
- Rana, R.; Walia, R.S.; Murtaza, Q. Characterization and Parametric Optimization of Performance Parameters of DLC-Coated Tungsten Carbide (WC) Tool Using TOPSIS. Coatings 2021, 11, 760. [Google Scholar] [CrossRef]
- Liu, L.; Huang, J.; He, X.; Wang, T.; He, Z.; Du, K.; Diao, X. Preparation and Characterization of High Quality Diamond like Carbon Films on Si Microspheres. Mater. Lett. 2018, 220, 309–312. [Google Scholar] [CrossRef]
- Sharifahmadian, O.; Mahboubi, F.; Yazdani, S. Comparison between Corrosion Behaviour of DLC and N-DLC Coatings Deposited by DC-Pulsed PACVD Technique. Diam. Relat. Mater. 2019, 95, 60–70. [Google Scholar] [CrossRef]
- Carvalho, I.; Dias, N.; Henriques, M.; Calderon, V.S.; Ferreira, P.; Cavaleiro, A.; Carvalho, S. Antibacterial Effects of Bimetallic Clusters Incorporated in Amorphous Carbon for Stent Application. ACS Appl. Mater. Interfaces 2020, 12, 24555–24563. [Google Scholar] [CrossRef]
- Antunes, J.; Matos, K.; Carvalho, I.; Carvalho, S.; Ferreira, F.; Cruz, S.M.A. Physical Vapor Deposition Technology in Personal Protective Equipment Production: Improved Antibacterial and Hydrophobic Character of Textiles. Coatings 2022, 12, 1399. [Google Scholar] [CrossRef]
- Bociaga, D.; Komorowski, P.; Batory, D.; Szymanski, W.; Olejnik, A.; Jastrzebski, K.; Jakubowski, W. Silver-Doped Nanocomposite Carbon Coatings (Ag-DLC) for Biomedical Applications—Physiochemical and Biological Evaluation. Appl. Surf. Sci. 2015, 355, 388–397. [Google Scholar] [CrossRef]
- Tallant, D.R.; Parmeter, J.E.; Siegal, M.P.; Simpson, R.L. The Thermal Stability of Diamond-like Carbon. Diam. Relat. Mater. 1995, 4, 191–199. [Google Scholar] [CrossRef]
- Zeng, Q.; Ning, Z. High-Temperature Tribological Properties of Diamond-like Carbon Films: A Review. Rev. Adv. Mater. Sci. 2021, 60, 276–292. [Google Scholar] [CrossRef]
- Salah, N.; Alshahrie, A.; Iqbal, J.; Hasan, P.M.Z.; Abdel-Wahab, M.S. Tribological Behavior of Diamond-like Carbon Thin Films Deposited by the Pulse Laser Technique at Different Substrate Temperatures. Tribol. Int. 2016, 103, 274–280. [Google Scholar] [CrossRef]
- Zhang, T.F.; Wan, Z.X.; Ding, J.C.; Zhang, S.; Wang, Q.M.; Kim, K.H. Microstructure and High-Temperature Tribological Properties of Si-Doped Hydrogenated Diamond-like Carbon Films. Appl. Surf. Sci. 2018, 435, 963–973. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Zhang, J.; Chen, Q.; Ootani, Y.; Higuchi, Y.; Ozawa, N.; Martin, J.M.; Adachi, K.; Kubo, M. Tribochemical Reactions and Graphitization of Diamond-like Carbon against Alumina Give Volcano-Type Temperature Dependence of Friction Coefficients: A Tight-Binding Quantum Chemical Molecular Dynamics Simulation. Carbon 2018, 133, 350–357. [Google Scholar] [CrossRef]
- Yu, W.; Wang, J.; Huang, W.; Cui, L.; Wang, L. Improving High Temperature Tribological Performances of Si Doped Diamond-like Carbon by Using W Interlayer. Tribol. Int. 2020, 146, 106241. [Google Scholar] [CrossRef]
- Field, J.E. The Properties of Diamond; Academic Press: Cambridge, MA, USA, 1979; ISBN 0122553500. [Google Scholar]
- Rajak, D.K.; Kumar, A.; Behera, A.; Menezes, P.L. Diamond-Like Carbon (DLC) Coatings: Classification, Properties, and Applications. Appl. Sci. 2021, 11, 4445. [Google Scholar] [CrossRef]
- Outka, D.A.; Hsu, W.L.; Boehme, D.R.; Yang, N.Y.C.; Johnsen, H.A.; Clift, W.M.; Headley, T.J. Compilation of Diamond-Like Carbon Properties for Barriers and Hard Coatings; Sandia National Labs.: Livermore, CA, USA, 1994. [Google Scholar]
- Ohtake, N.; Hiratsuka, M.; Kanda, K.; Akasaka, H.; Tsujioka, M.; Hirakuri, K.; Hirata, A.; Ohana, T.; Inaba, H.; Kano, M.; et al. Properties and Classification of Diamond-Like Carbon Films. Materials 2021, 14, 315. [Google Scholar] [CrossRef]
- Wongpanya, P.; Pintitraratibodee, N.; Thumanu, K.; Euaruksakul, C. Improvement of Corrosion Resistance and Biocompatibility of 316L Stainless Steel for Joint Replacement Application by Ti-Doped and Ti-Interlayered DLC Films. Surf. Coat. Technol. 2021, 425, 127734. [Google Scholar] [CrossRef]
- Fayed, S.M.; Wu, H.; Chen, D.; Li, S.; Zhou, Y.; Wang, H.; Sadawy, M.M. Influence of Positive Pulse Voltages on Structure, Mechanical, and Corrosion Inhibition Characteristics of Si/DLC Coatings. Surf. Coat. Technol. 2022, 445, 128749. [Google Scholar] [CrossRef]
- Talha Hanif, M.; Zahid, R.; Mufti, R.; Waqas, M.; Naveed, T. A Review on Tribological Study of DLC Coatings in Combination with Bio Based Lubricants. Int. J. Mater. Sci. Appl. 2021, 10, 61. [Google Scholar] [CrossRef]
- Donnet, C.; Grill, A. Friction Control of Diamond-like Carbon Coatings. Surf. Coat. Technol. 1997, 94–95, 456–462. [Google Scholar] [CrossRef]
- Enke, K.; Dimigen, H.; Hübsch, H. Frictional Properties of Diamondlike Carbon Layers. Appl. Phys. Lett. 1980, 36, 291–292. [Google Scholar] [CrossRef]
- Liu, Y.; Erdemir, A.; Meletis, E.I. An Investigation of the Relationship between Graphitization and Frictional Behavior of DLC Coatings. Surf. Coat. Technol. 1996, 86–87, 564–568. [Google Scholar] [CrossRef]
- Miyake, S.; Takahashi, S.; Watanabe, I.; Yoshihara, H. Friction and Wear Behavior of Hard Carbon Films. A S L E Trans. 2008, 30, 121–127. [Google Scholar] [CrossRef]
- Zhang, T.F.; Xie, D.; Huang, N.; Leng, Y. The Effect of Hydrogen on the Tribological Behavior of Diamond like Carbon (DLC) Coatings Sliding against Al2O3 in Water Environment. Surf. Coat. Technol. 2017, 320, 619–623. [Google Scholar] [CrossRef]
- Kokaku, Y.; Kitoh, M. Influence of Exposure to an Atmosphere of High Relative Humidity on Tribological Properties of Diamondlike Carbon Films. J. Vac. Sci. Technol. A Vac. Surf. Film. 1989, 7, 2311–2314. [Google Scholar] [CrossRef]
- Yoon, E.-S.; Kong, H.; Lee, K.-R. Tribological Behavior of Sliding Diamond-like Carbon Films under Various Environments. Wear 1998, 217, 262–270. [Google Scholar] [CrossRef]
- Kim, D.-W.; Kim, K.-W. Effects of Sliding Velocity and Normal Load on Friction and Wear Characteristics of Multi-Layered Diamond-like Carbon (DLC) Coating Prepared by Reactive Sputtering. Wear 2013, 297, 722–730. [Google Scholar] [CrossRef]
- Al Mahmud, K.A.H.; Kalam, M.A.; Masjuki, H.H.; Mobarak, H.M.; Zulkifli, N.W.M. An Updated Overview of Diamond-like Carbon Coating in Tribology. Crit. Rev. Solid State Mater. Sci. 2014, 40, 90–118. [Google Scholar] [CrossRef]
- Verma, D.K.; Dewangan, Y.; Singh, A.K.; Mishra, R.; Susan, M.A.B.H.; Salim, R.; Taleb, M.; El Hajjaji, F.; Berdimurodov, E. Ionic Liquids as Green and Smart Lubricant Application: An Overview. Ionics 2022, 28, 4923–4932. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Li, B.; Sarman, S.; Mocci, F.; Lu, Z.-Y.; Yuan, J.; Laaksonen, A.; Fayer, M.D. Microstructural and Dynamical Heterogeneities in Ionic Liquids. Chem. Rev. 2020, 120, 5798–5877. [Google Scholar] [CrossRef]
- Groover, M.P. Fundamentals of Modern Manufacturing: Materials, Processes, and Systems; John Wiley & Sons: Hoboken, NJ, USA, 2020; ISBN 1119722012. [Google Scholar]
- Tu, W.; Chat, K.; Szklarz, G.; Laskowski, L.; Grzybowska, K.; Paluch, M.; Richert, R.; Adrjanowicz, K. Dynamics of Pyrrolidinium-Based Ionic Liquids under Confinement. II. The Effects of Pore Size, Inner Surface, and Cationic Alkyl Chain Length. J. Phys. Chem. C 2019, 124, 5395–5408. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L. Uncovering the Underlying Mechanisms Governing the Solidlike Layering of Ionic Liquids (ILs) on Mica. Langmuir 2020, 36, 2743–2756. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis. Chem. Rev. 1999, 99, 2071–2084. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Zheng, S.S.; Liu, T.Q.; Nie, Y.; Song, K.D. Evaluation of Ionic Liquid “Greenness”-Cytotoxicity of Ionic Liquids. IOP Conf. Ser. Mater. Sci. Eng. 2019, 592, 012031. [Google Scholar] [CrossRef]
- Berthod, A.; Ruiz-Angel, M.J.; Carda-Broch, S. Recent Advances on Ionic Liquid Uses in Separation Techniques. J. Chromatogr. A 2018, 1559, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-Y.; Chiueh, P.-T.; Hsieh, P.-C. A Novel Semi-Analytical Approach for Non-Uniform Vegetated Flows. Adv. Water Resour. 2014, 64, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, F.; Liang, Y.; Liu, W. Tribological Performance of Phosphonium Based Ionic Liquids for an Aluminum-on-Steel System and Opinions on Lubrication Mechanism. Wear 2006, 261, 1174–1179. [Google Scholar] [CrossRef]
- Rahman, M.H.; Khajeh, A.; Panwar, P.; Patel, M.; Martini, A.; Menezes, P.L. Recent Progress on Phosphonium-Based Room Temperature Ionic Liquids: Synthesis, Properties, Tribological Performances and Applications. Tribol. Int. 2022, 167, 107331. [Google Scholar] [CrossRef]
- Sharma, V.; Gabler, C.; Doerr, N.; Aswath, P.B. Mechanism of Tribofilm Formation with P and S Containing Ionic Liquids. Tribol. Int. 2015, 92, 353–364. [Google Scholar] [CrossRef]
- Carrion, F.J.; Sanes, J.; Bermudez, M.D. Effect of Ionic Liquid on the Structure and Tribological Properties of Polycarbonate–Zinc Oxide Nanodispersion. Mater. Lett. 2007, 61, 4531–4535. [Google Scholar] [CrossRef]
- Nooruddin, N.S.; Wahlbeck, P.G.; Carper, W.R. Molecular Modeling of Ionic Liquid Tribology: Semi-Empirical Bonding and Molecular Structure. J. Mol. Struct. Theochem 2007, 822, 1–7. [Google Scholar] [CrossRef]
- Jiménez, A.E.; Bermúdez, M.D.; Iglesias, P.; Carrión, F.J.; Martínez-Nicolás, G. 1-N-Alkyl-3-Methylimidazolium Ionic Liquids as Neat Lubricants and Lubricant Additives in Steel–Aluminium Contacts. Wear 2006, 260, 766–782. [Google Scholar] [CrossRef]
- Xia, Y.; Sasaki, S.; Murakami, T.; Nakano, M.; Shi, L.; Wang, H. Ionic Liquid Lubrication of Electrodeposited Nickel–Si3N4 Composite Coatings. Wear 2007, 262, 765–771. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, S.; Zhou, F.; Wang, H.; Lin, Y.; Xu, T. Tribological Properties of Plasma Nitrided Stainless Steel against SAE52100 Steel under Ionic Liquid Lubrication Condition. Tribol. Int. 2006, 39, 635–640. [Google Scholar] [CrossRef]
- Qu, J.; Truhan, J.J.; Dai, S.; Luo, H.; Blau, P.J. Ionic Liquids with Ammonium Cations as Lubricants or Additives. Tribol. Lett. 2006, 22, 207–214. [Google Scholar] [CrossRef]
- Mu, Z.; Liu, W.; Zhang, S.; Zhou, F. Functional Room-Temperature Ionic Liquids as Lubricants for an Aluminum-on-Steel System. Chem. Lett. 2004, 33, 524–525. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, F.; Liang, Y.; Liu, W. Benzotriazole as the Additive for Ionic Liquid Lubricant: One Pathway towards Actual Application of Ionic Liquids. Tribol. Lett. 2006, 23, 191–196. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, L.; Liu, X.; Qiao, Y. A Comparative Study on the Tribological Behavior of Nanocrystalline Nickel and Coarse-Grained Nickel Coatings under Ionic Liquid Lubrication. Tribol. Lett. 2008, 30, 151–157. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Q.; Ye, C.; Liu, W.; Cui, Z. Friction and Wear Behaviors of Ionic Liquid of Alkylimidazolium Hexafluorophosphates as Lubricants for Steel/Steel Contact. Wear 2004, 256, 44–48. [Google Scholar] [CrossRef]
- Kamimura, H.; Kubo, T.; Minami, I.; Mori, S. Effect and Mechanism of Additives for Ionic Liquids as New Lubricants. Tribol. Int. 2007, 40, 620–625. [Google Scholar] [CrossRef]
- Mu, Z.; Zhou, F.; Zhang, S.; Liang, Y.; Liu, W. Effect of the Functional Groups in Ionic Liquid Molecules on the Friction and Wear Behavior of Aluminum Alloy in Lubricated Aluminum-on-Steel Contact. Tribol. Int. 2005, 38, 725–731. [Google Scholar] [CrossRef]
- Mu, Z.G.; Liang, Y.M.; Zhang, S.X. Phosphonate Functional Group Containing Ionic Liquid Steel/Aluminium Friction Lubrication of the Role of Research. Tribol. J. 2004, 24, 294–298. [Google Scholar]
- Xia, Y.; Wang, L.; Liu, X.; Qiao, Y. Tribological Properties of Phosphor Bronze and Nanocrystalline Nickel Coatings under PAO+ MoDTC and Ionic Liquid Lubricated Condition. Tribol. Lett. 2008, 31, 149–158. [Google Scholar] [CrossRef]
- Feng, R.; Zhao, D.; Guo, Y. Revisiting Characteristics of Ionic Liquids: A Review for Further Application Development. J. Environ. Prot. 2010, 1, 95–104. [Google Scholar] [CrossRef]
- Minami, I.; Hirao, K.; Memita, M.; Mori, S. Investigation of Anti-Wear Additives for Low Viscous Synthetic Esters: Hydroxyalkyl Phosphonates. Tribol. Int. 2007, 40, 626–631. [Google Scholar] [CrossRef]
- Fernandez-Torres, L.C.; Zhao, X.; Chen, Z.; Salmeron, C.; Perry, S.S. Influence of Ethyl Acetate and Alkyl Phosphate Adsorption on the Frictional Properties of VC (100). Tribol. Lett. 2005, 18, 207–213. [Google Scholar] [CrossRef]
- Bertrand, P.A. Effects of Aryl Phosphate Ester Lubricant Additives on Crack Growth in Soda-Lime Glass. Tribol. Lett. 2004, 16, 201–205. [Google Scholar] [CrossRef]
- Minami, I. Development of Novel Lubricity Additives: Hydroxyalkyl Ester of Ortho-Phenylene Phosphate. Tribol. Lett. 1995, 1, 139–146. [Google Scholar] [CrossRef]
- Najman, M.N.; Kasrai, M.; Bancroft, G.M. Chemistry of Antiwear Films from Ashless Thiophosphate Oil Additives. Tribol. Lett. 2004, 17, 217–229. [Google Scholar] [CrossRef]
- Adhvaryu, A.; Erhan, S.Z.; Perez, J.M. Tribological Studies of Thermally and Chemically Modified Vegetable Oils for Use as Environmentally Friendly Lubricants. Wear 2004, 257, 359–367. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, W.; Xue, Q. Tribological Properties of P- and N-containing Organic Compounds as Potential Extreme-pressure and Antiwear Additives. Lubr. Sci. 2003, 15, 173–183. [Google Scholar] [CrossRef]
- Wang, L.; Wood, R.J.K. The Influence of Contact Conditions on Surface Reaction Layers Formed between Steel Surfaces Lubricated by an Aviation Oil. Tribol. Int. 2007, 40, 1655–1666. [Google Scholar] [CrossRef]
- Husnawan, M.; Saifullah, M.G.; Masjuki, H.H. Development of Friction Force Model for Mineral Oil Basestock Containing Palm Olein and Antiwear Additive. Tribol. Int. 2007, 40, 74–81. [Google Scholar] [CrossRef]
- Zhang, L.; Feng, D.; Xu, B. Tribological Characteristics of Alkylimidazolium Diethyl Phosphates Ionic Liquids as Lubricants for Steel–Steel Contact. Tribol. Lett. 2009, 34, 95–101. [Google Scholar] [CrossRef]
- Piatti, E.; Guglielmero, L.; Tofani, G.; Mezzetta, A.; Guazzelli, L.; D’Andrea, F.; Roddaro, S.; Pomelli, C.S. Ionic Liquids for Electrochemical Applications: Correlation between Molecular Structure and Electrochemical Stability Window. J. Mol. Liq. 2022, 364, 120001. [Google Scholar] [CrossRef]
- Karakasidis, T.E.; Sofos, F.; Tsonos, C. The Electrical Conductivity of Ionic Liquids: Numerical and Analytical Machine Learning Approaches. Fluids 2022, 7, 321. [Google Scholar] [CrossRef]
- Nancarrow, P.; Al-Othman, A.; Mital, D.K.; Döpking, S. Comprehensive Analysis and Correlation of Ionic Liquid Conductivity Data for Energy Applications. Energy 2021, 220, 119761. [Google Scholar] [CrossRef]
- Mero, A.; Mezzetta, A.; Nowicki, J.; Łuczak, J.; Guazzelli, L. Betaine and L-Carnitine Ester Bromides: Synthesis and Comparative Study of Their Thermal Behaviour and Surface Activity. J. Mol. Liq. 2021, 334, 115988. [Google Scholar] [CrossRef]
- Amiril, S.A.S.; Rahim, E.A.; Syahrullail, S. A Review on Ionic Liquids as Sustainable Lubricants in Manufacturing and Engineering: Recent Research, Performance, and Applications. J. Clean. Prod. 2017, 168, 1571–1589. [Google Scholar] [CrossRef]
- Banerjee, C.; Mandal, S.; Ghosh, S.; Kuchlyan, J.; Kundu, N.; Sarkar, N. Unique Characteristics of Ionic Liquids Comprised of Long-Chain Cations and Anions: A New Physical Insight. J. Phys. Chem. B 2013, 117, 3927–3934. [Google Scholar] [CrossRef]
- Barnhill, W.C.; Qu, J.; Luo, H.; Meyer, H.M., III; Ma, C.; Chi, M.; Papke, B.L. Phosphonium-Organophosphate Ionic Liquids as Lubricant Additives: Effects of Cation Structure on Physicochemical and Tribological Characteristics. ACS Appl. Mater. Interfaces 2014, 6, 22585–22593. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.M.; Rocha, M.A.A.; Freire, M.G.; Marrucho, I.M.; Coutinho, J.A.P.; Santos, L.M.N.B.F. Evaluation of Cation−Anion Interaction Strength in Ionic Liquids. J. Phys. Chem. B 2011, 115, 4033–4041. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Watanabe, N.; Inada, K.; Ishioka, A.; Hayase, S.; Kawatsura, M.; Minami, I.; Mori, S. Design of Alkyl Sulfate Ionic Liquids for Lubricants. Chem. Lett. 2009, 38, 64–65. [Google Scholar] [CrossRef]
- Yu, B.; Bansal, D.G.; Qu, J.; Sun, X.; Luo, H.; Dai, S.; Blau, P.J.; Bunting, B.G.; Mordukhovich, G.; Smolenski, D.J. Oil-Miscible and Non-Corrosive Phosphonium-Based Ionic Liquids as Candidate Lubricant Additives. Wear 2012, 289, 58–64. [Google Scholar] [CrossRef]
- Liaw, H.-J.; Chen, C.-C.; Chen, Y.-C.; Chen, J.-R.; Huang, S.-K.; Liu, S.-N. Relationship between Flash Point of Ionic Liquids and Their Thermal Decomposition. Green. Chem. 2012, 14, 2001–2008. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Uerdingen, M.; Treber, C.; Balser, M.; Schmitt, G.; Werner, C. Corrosion Behaviour of Ionic Liquids. Green. Chem. 2005, 7, 321–325. [Google Scholar] [CrossRef]
- Zunita, M.; Kevin, Y.J. Ionic Liquids as Corrosion Inhibitor: From Research and Development to Commercialization. Results Eng. 2022, 15, 100562. [Google Scholar] [CrossRef]
- Akintola, S.A.; Oki, M.; Aleem, A.A.; Adediran, A.A.; Akpor, O.B.; Oluba, O.M.; Ogunsemi, B.T.; Ikubanni, P.P. Valorized Chicken Feather as Corrosion Inhibitor for Mild Steel in Drilling Mud. Results Eng. 2019, 4, 100026. [Google Scholar] [CrossRef]
- Ayoola, A.A.; Babalola, R.; Durodola, B.M.; Alagbe, E.E.; Agboola, O.; Adegbile, E.O. Corrosion Inhibition of A36 Mild Steel in 0.5 M Acid Medium Using Waste Citrus Limonum Peels. Results Eng. 2022, 15, 100490. [Google Scholar] [CrossRef]
- Hossain, N.; Chowdhury, M.A.; Rana, M.; Hassan, M.; Islam, S. Terminalia Arjuna Leaves Extract as Green Corrosion Inhibitor for Mild Steel in HCl Solution. Results Eng. 2022, 14, 100438. [Google Scholar] [CrossRef]
- Díaz-Jiménez, V.; Arellanes-Lozada, P.; Likhanova, N.V.; Olivares-Xometl, O.; Chigo-Anota, E.; Lijanova, I.V.; Gómez-Sánchez, G.; Verpoort, F. Investigation of Sulfonium-Iodide-Based Ionic Liquids to Inhibit Corrosion of API 5L X52 Steel in Different Flow Regimes in Acid Medium. ACS Omega 2022, 7, 42975–42993. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.A.; Abdelbasir, S.M.; Ashmawy, A.M.; Ghayad, I.M.; El-Zomrawy, A.A. A Novel Ionic Liquid for Improvement of Lead-Acid Battery Performance and Protection of Its Electrodes against Corrosion. Mater. Chem. Phys. 2022, 292, 126764. [Google Scholar] [CrossRef]
- Naveed, T.; Zahid, R.; Mufti, R.A.; Waqas, M.; Hanif, M.T. A Review on Tribological Performance of Ionic Liquids as Additives to Bio Lubricants. Proc. Inst. Mech. Eng. Part. J J. Eng. Tribol. 2021, 235, 1782–1806. [Google Scholar] [CrossRef]
- Dupont, J.; Suarez, P.A.Z.; Umpierre, A.P.; Souza, R.F. de Pd (II)-Dissolved in Ionic Liquids: A Recyclable Catalytic System for the Selective Biphasic Hydrogenation of Dienes to Monoenes. J. Braz. Chem. Soc. 2000, 11, 293–297. [Google Scholar] [CrossRef]
- García, A.; González, R.; Hernández Battez, A.; Viesca, J.L.; Monge, R.; Fernández-González, A.; Hadfield, M. Ionic Liquids as a Neat Lubricant Applied to Steel–Steel Contacts. Tribol. Int. 2014, 72, 42–50. [Google Scholar] [CrossRef]
- Omotowa, B.A.; Phillips, B.S.; Zabinski, J.S.; Shreeve, J.M. Phosphazene-Based Ionic Liquids: Synthesis, Temperature-Dependent Viscosity, and Effect as Additives in Water Lubrication of Silicon Nitride Ceramics. Inorg. Chem. 2004, 43, 5466–5471. [Google Scholar] [CrossRef]
- Lynden-Bell, R.M.; Atamas, N.A.; Vasilyuk, A.; Hanke, C.G. Chemical Potentials of Water and Organic Solutes in Imidazolium Ionic Liquids: A Simulation Study. Mol. Phys. 2002, 100, 3225–3229. [Google Scholar] [CrossRef]
- Minami, I. Ionic Liquids in Tribology. Molecules 2009, 14, 2286–2305. [Google Scholar] [CrossRef]
- Guo, Y.; Qiao, D.; Han, Y.; Zhang, L.; Feng, D.; Shi, L. Application of Alkylphosphate Ionic Liquids as Lubricants for Ceramic Material. Ind. Eng. Chem. Res. 2015, 54, 12813–12825. [Google Scholar] [CrossRef]
- Zeng, Z.; Phillips, B.S.; Xiao, J.-C.; Shreeve, J.M. Polyfluoroalkyl, Polyethylene Glycol, 1, 4-Bismethylenebenzene, or 1,4-Bismethylene-2,3,5,6-Tetrafluorobenzene Bridged Functionalized Dicationic Ionic Liquids: Synthesis and Properties as High Temperature Lubricants. Chem. Mater. 2008, 20, 2719–2726. [Google Scholar] [CrossRef]
- Qu, J.; Luo, H. Ionic Liquids Containing Symmetric Quaternary Phosphonium Cations and Phosphorus-Containing Anions, and Their Use as Lubricant Additives; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 2018. [Google Scholar]
- Fan, X.; Xue, Q.; Wang, L. Carbon-Based Solid-Liquid Lubricating Coatings for Space Applications-A Review. Friction 2015, 3, 191–207. [Google Scholar] [CrossRef]
- Cai, M.; Guo, R.; Zhou, F.; Liu, W. Lubricating a Bright Future: Lubrication Contribution to Energy Saving and Low Carbon Emission. Sci. China Technol. Sci. 2013, 56, 2888–2913. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Xue, Q. High Vacuum Tribological Performance of DLC-Based Solid–Liquid Lubricating Coatings: Influence of Atomic Oxygen and Ultraviolet Irradiation. Tribol. Int. 2013, 60, 36–44. [Google Scholar] [CrossRef]
- Erdemir, A. Review of Engineered Tribological Interfaces for Improved Boundary Lubrication. Tribol. Int. 2005, 38, 249–256. [Google Scholar] [CrossRef]
- Zhao, W.; Pu, J.; Yu, Q.; Zeng, Z.; Wu, X.; Xue, Q. A Novel Strategy to Enhance Micro/Nano-Tribological Properties of DLC Film by Combining Micro-Pattern and Thin Ionic Liquids Film. Colloids Surf. A Physicochem. Eng. Asp. 2013, 428, 70–78. [Google Scholar] [CrossRef]
- González, R.; Hernández Battez, A.; Blanco, D.; Viesca, J.L.; Fernández-González, A. Lubrication of TiN, CrN and DLC PVD Coatings with 1-Butyl-1-Methylpyrrolidinium Tris(Pentafluoroethyl)Trifluorophosphate. Tribol. Lett. 2010, 40, 269–277. [Google Scholar] [CrossRef]
- Hernández Battez, A.; González, R.; Viesca, J.L.; Fernández-González, A.; Hadfield, M. Lubrication of PVD Coatings with Ethyl-Dimethyl-2-Methoxyethylammonium Tris(Pentafluoroethyl)Trifluorophosphate. Tribol. Int. 2013, 58, 71–78. [Google Scholar] [CrossRef]
- Jia, Z.; Xia, Y.; Li, J.; Pang, X.; Shao, X. Friction and Wear Behavior of Diamond-like Carbon Coating on Plasma Nitrided Mild Steel under Boundary Lubrication. Tribol. Int. 2010, 43, 474–482. [Google Scholar] [CrossRef]
- Milewski, K.; Kudliński, J.; Madej, M.; Ozimina, D. The Interaction between Diamond like Carbon (DLC) Coatings and Ionic Liquids under Boundary Lubrication Conditions. Anal. Zavoda Za Povij. Znan. Hrvat. Akad. Znan. I Umjet. U Dubrovniku 2017, 56, 55–58. [Google Scholar]
- Madej, M. Tribological Properties of Diamond-like Carbon Coatings. Adv. Mater. Res. 2014, 874, 9–15. [Google Scholar] [CrossRef]
- Arshad, M.S.; Čoga, L.; Geue, T.; Kovač, J.; Cruz, S.M.A.; Kalin, M. The W-Cluster Reactive Sites Interaction Model for WDLC Coatings with Ionic Liquids. Tribol. Int. 2023, 185, 108550. [Google Scholar] [CrossRef]
- Arshad, M.S.; Kovač, J.; Cruz, S.; Kalin, M. Physicochemical and Tribological Characterizations of WDLC Coatings and Ionic-Liquid Lubricant Additives: Potential Candidates for Low Friction under Boundary-Lubrication Conditions. Tribol. Int. 2020, 151, 106482. [Google Scholar] [CrossRef]
- Fontes, M.A.; Serra, R.G.H.; Fernandes, F.D.; Cavaleiro Rodrigues de Carvalho, A.A.; Ferreira, F.E.d.S. Comparison of Mechanical and Tribological Properties of Diamond-like Carbon Coatings Doped with Europium and Gadolinium Produced by HiPIMS. Proc. Inst. Mech. Eng. B J. Eng. Manuf. 2022, 09544054221136528. [Google Scholar] [CrossRef]
- Milewski, K.; Madej, M.; Kowalczyk, J.; Ozimina, D. The Influence of Silicon-Doped Diamond-Like Carbon Coating on the Wear of Ionic Liquid Lubricated Friction Pairs. Tribologia 2018, 282, 97–106. [Google Scholar] [CrossRef]
- Feng, X.; Xia, Y. Tribological Properties of Ti-Doped DLC Coatings under Ionic Liquids Lubricated Conditions. Appl. Surf. Sci. 2012, 258, 2433–2438. [Google Scholar] [CrossRef]
- Jelínek, M.; Smetana, K.; Kocourek, T.; Dvořánková, B.; Zemek, J.; Remsa, J.; Luxbacher, T. Biocompatibility and Sp3/Sp2 Ratio of Laser Created DLC Films. Mater. Sci. Eng. B 2010, 169, 89–93. [Google Scholar] [CrossRef]
- Jelínek, M.; Kocourek, T.; Remsa, J.; Mikšovský, J.; Zemek, J.; Smetana, K.; Dvořánková, B.; Luxbacher, T. Diamond/Graphite Content and Biocompatibility of DLC Films Fabricated by PLD. Appl. Phys. A 2010, 101, 579–583. [Google Scholar] [CrossRef]
- Shaikh, S.; Omiya, T.; Cavaleiro, A.; Vilhena, L.; Ramalho, A.; Ferreira, F. Impact of Temperature Variation on Friction Behaviour of Rare Earth-Doped Diamond-like Carbon Coatings with Ionic Liquid Lubricants. Lubricants 2023, 11, 302. [Google Scholar] [CrossRef]
- Sadeghi, M.; Omiya, T.; Fernandes, F.; Vilhena, L.; Ramalho, A.; Ferreira, F. Tribological Behavior of Doped DLC Coatings in the Presence of Ionic Liquid Additive under Different Lubrication Regimes. Coatings 2023, 13, 891. [Google Scholar] [CrossRef]
- Welton, T. Ionic Liquids: A Brief History. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [PubMed]
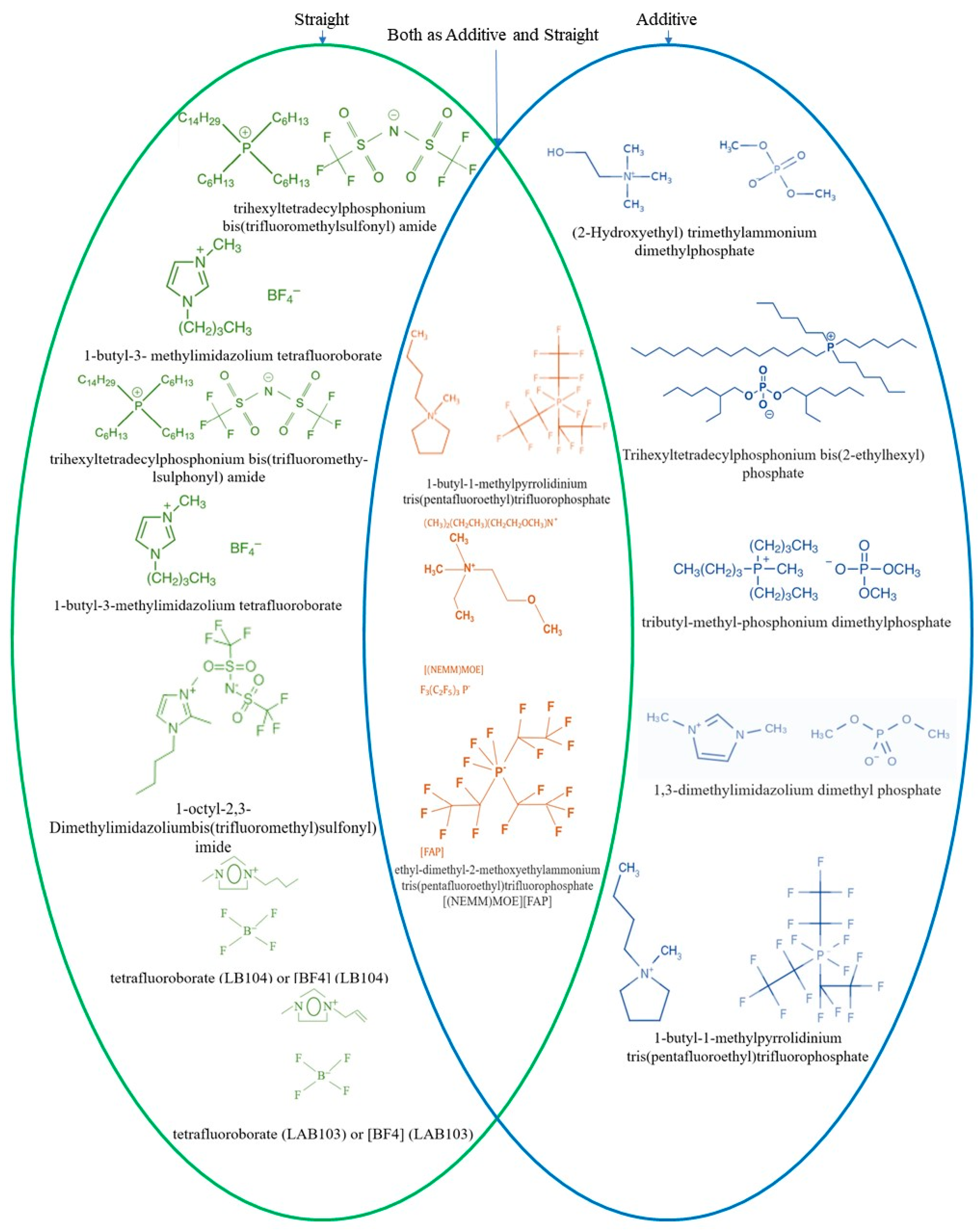
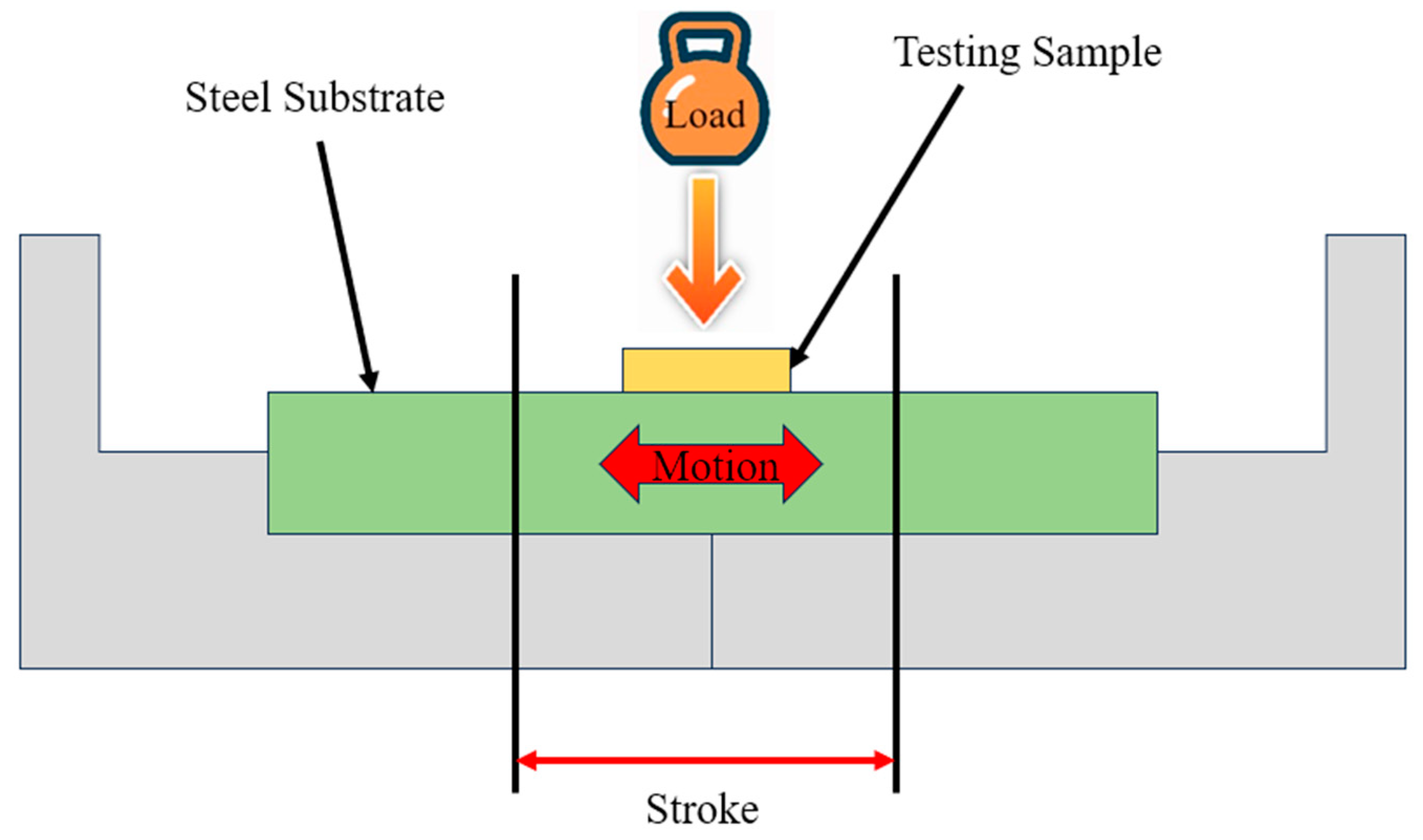
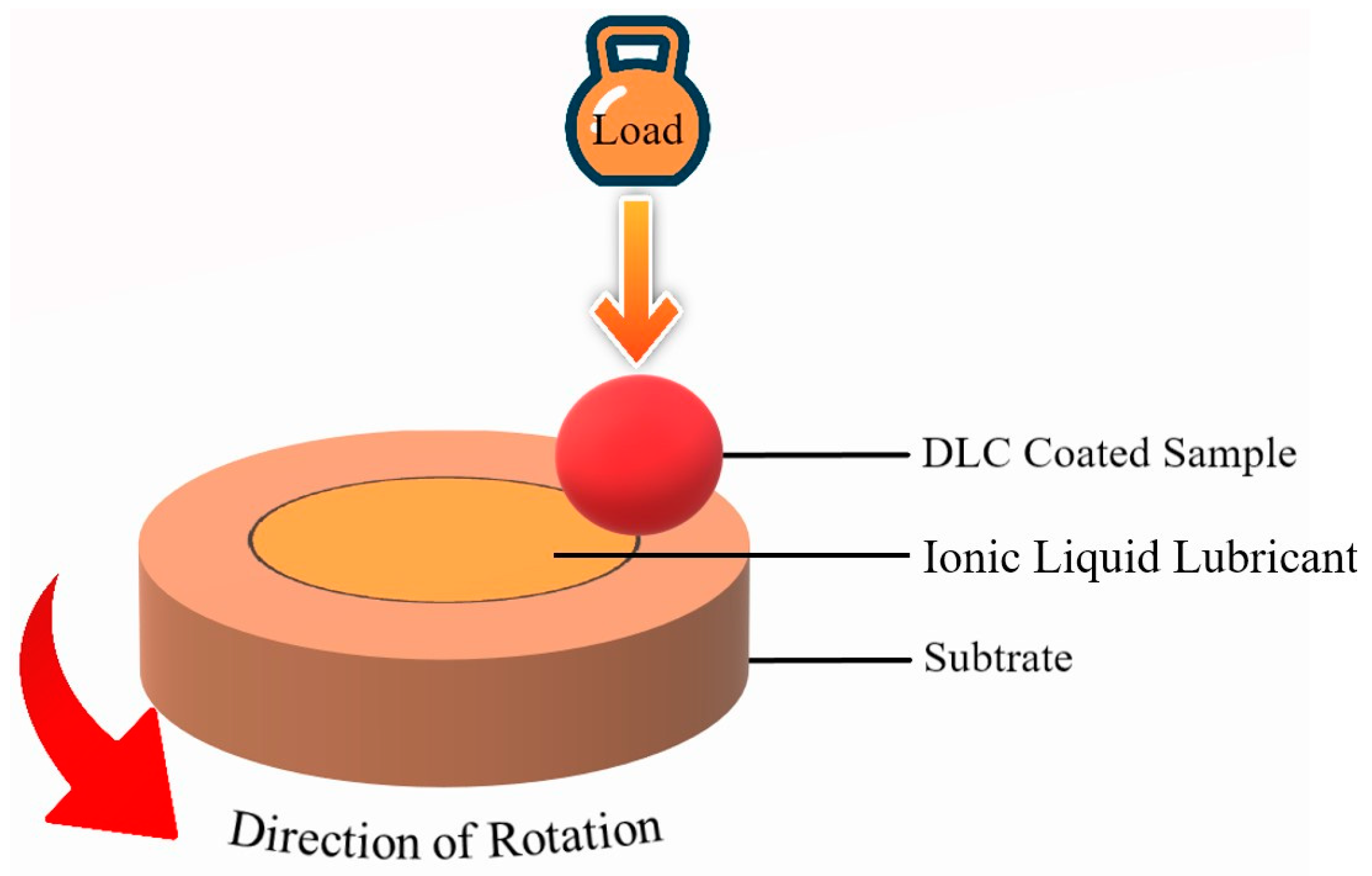
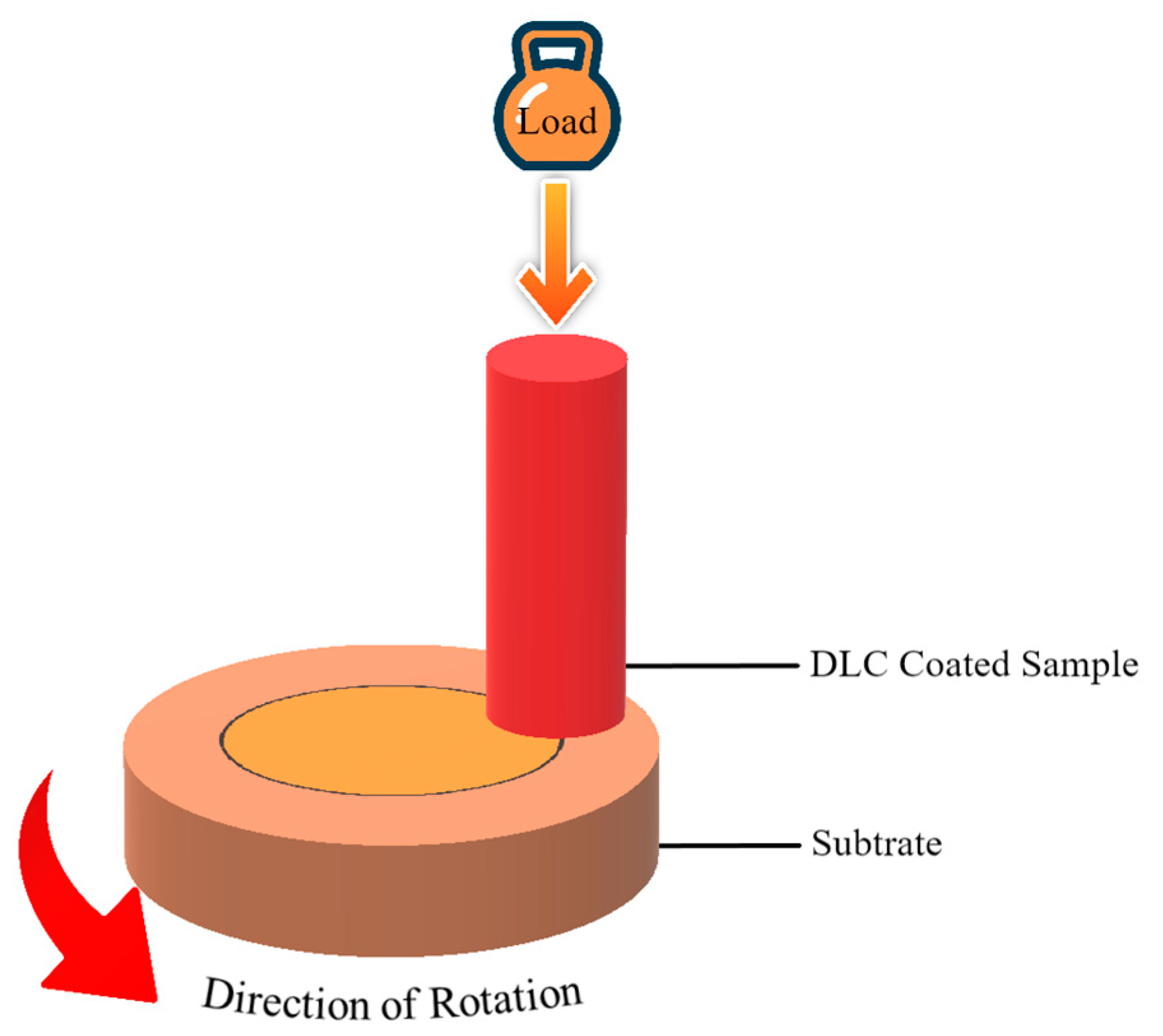
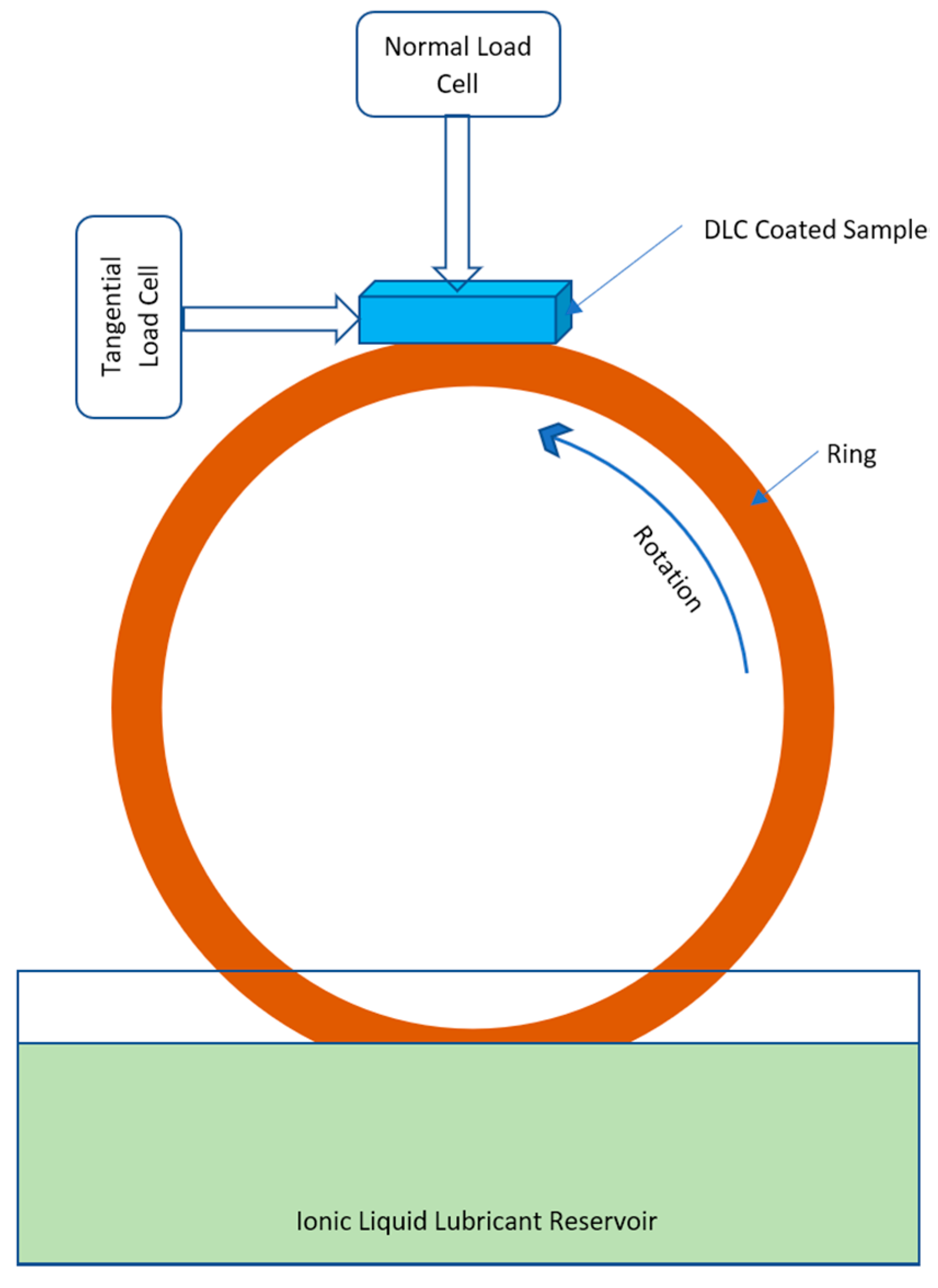
| Name of ILs | Tribometer | Additive or Main Lubricant? | Ref. |
|---|---|---|---|
| 1-octyl-2,3-Dimethylimidazolium bis(trifluoromethyl)sulfonyl | UMT-3 tribometer (ball-on-plate) | Used as main lubricant (100 wt.%) | [169] |
| 1-butyl-1-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate | CETR-UMT-3 micro-tribometer (ball-on-plate) | Used as both additive in PAO 6 (1 wt.% additive) and main lubricant (for comparison) | [170] |
| ethyl-dimethyl-2-methoxyethylammonium tris(pentafluoroethyl)trifluorophosphate | UMT-3 microtribometer (ball-on-plate) | Used as both additive in PAO 6 (1 wt.%) and main lubricant (for comparison) | [171] |
| The synthesized ionic liquids of functionalized borate esters (The specific names are not mentioned but molecular structure is explained in the article) | Reciprocating friction and wear tester (ball-on-disk) | Used as 2 wt.% additives to polyalphaolefin (PAO 6) and compared with 2 wt.% ZDDP as a reference additive | [172] |
| (1) 1-butyl-3-methylimidazolium tetrafluoroborate And (2) trihexyltetradecylphosphonium bis(trifluoromethy-lsulphonyl) amide | T-01M tester (ball-on-disc configuration) | Used as main lubricant (100 wt.%) | [173] |
| Name of Ionic Liquids | Tribometer | Doped DLC Type | Ref. |
|---|---|---|---|
| dialkyloimidazolium tetrafluoroborates (Used as main lubricant, i.e., 100 wt.%) | T-01M tribometer (ball-on-disc configuration) | W-doped DLC | [174] |
| tributylmethylphosphonium dimethylphosphate (PP) (used as additive with 1 wt.%) and 1,3-dimethylimidazolium dimethylphosphate (IM) (used as additive with 1 wt.%) and 1-butyl-1-methylpyrrolidinium tris(pentafluoroethyl)trifluorophosphate (BMP) (used as additive with 1 wt.%) | TE 38-Phoenix Tribometer (reciprocating pin-on-disc tribometer) | W-doped DLC, and Ag-doped DLC | [23] |
| 1,3-dimethylimidazolium dimethylphosphate (used as additive with 1 wt.%) | UMT-2 tribometer with (ball-on-flat-disc geometry) | W-doped DLC | [175] |
| (1) 1-butyl-1-methylpyrrolidinium tris(pentafluoroethyl) trifluorophosphate ([BMP][FAP]) (used as additive with 1 wt.%) and (2) tributyl-methyl-phosphonium dimethylphosphate (PP) (used as additive with 1 wt.%) and (3) (2-hydroxyethyl) trimethylammonium dimethylphosphate (AM) (used as additive with 1 wt.%) | UMT-2 tribometer (reciprocating motion with a ball-on-flat-disc geometry) | W-doped DLC | [176] |
| (1) tetrafluoroborate (LB104) or [BF4] (LB104) (used as main lubricant, i.e., 100 wt.%) And (2) tetrafluoroborate (LAB103) or [BF4] (LAB103) (used as main lubricant, i.e., 100 wt.%) | CSM Switzerland tribometer (ball-on-disc) | Cr-doped GLC and Cr-doped DLC | [26] |
| trihexyltetradecylphosphonium bis (2-ethylhexyl) phosphate [P_66614] [DEHP] (used as additive with 1 wt.%) | Block-on-Ring configuration | Gd-DLC and Eu-DLC | [177] |
| trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl) amide (used as main lubricant, i.e., 100 wt.%) | Anton Paar TRB tribometer (Ball-on-disc configuration) | Si-DLC | [178] |
| 1-alkyl-3-octylimidazolium hexafluorophosphate (L-P801) (used as main lubricant, i.e., 100 wt.%) And 1-alkyl-3-octylimidazolium hexafluorophosphate (L-P804) (used as main lubricant, i.e., 100 wt.%) | UMT-2MT (reciprocating ball-on-disk) | Ti-DLC | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaikh, S.; Sadeghi, M.; Cruz, S.; Ferreira, F. Recent Progress on the Tribology of Pure/Doped Diamond-like Carbon Coatings and Ionic Liquids. Coatings 2024, 14, 71. https://doi.org/10.3390/coatings14010071
Shaikh S, Sadeghi M, Cruz S, Ferreira F. Recent Progress on the Tribology of Pure/Doped Diamond-like Carbon Coatings and Ionic Liquids. Coatings. 2024; 14(1):71. https://doi.org/10.3390/coatings14010071
Chicago/Turabian StyleShaikh, Shahsharif, Mohammadamin Sadeghi, Sandra Cruz, and Fábio Ferreira. 2024. "Recent Progress on the Tribology of Pure/Doped Diamond-like Carbon Coatings and Ionic Liquids" Coatings 14, no. 1: 71. https://doi.org/10.3390/coatings14010071
APA StyleShaikh, S., Sadeghi, M., Cruz, S., & Ferreira, F. (2024). Recent Progress on the Tribology of Pure/Doped Diamond-like Carbon Coatings and Ionic Liquids. Coatings, 14(1), 71. https://doi.org/10.3390/coatings14010071









