ZnO Matrices as a Platform for Tunable Localized Surface Plasmon Resonances of Silver Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Details
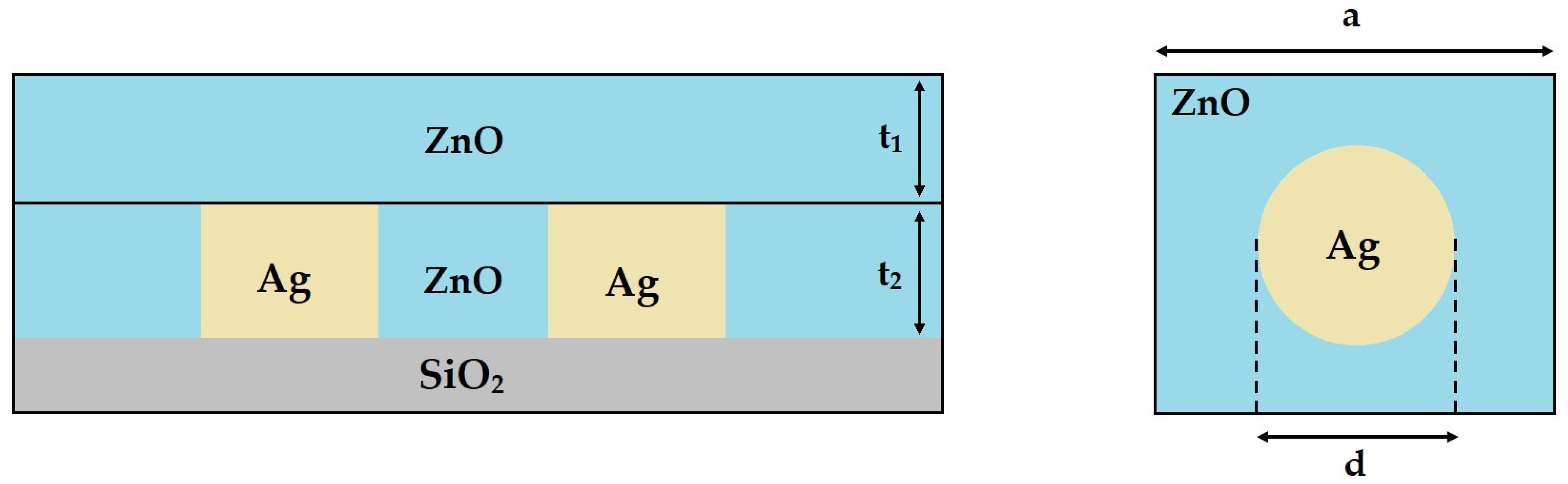
2.2. Theoretical Model
3. Results
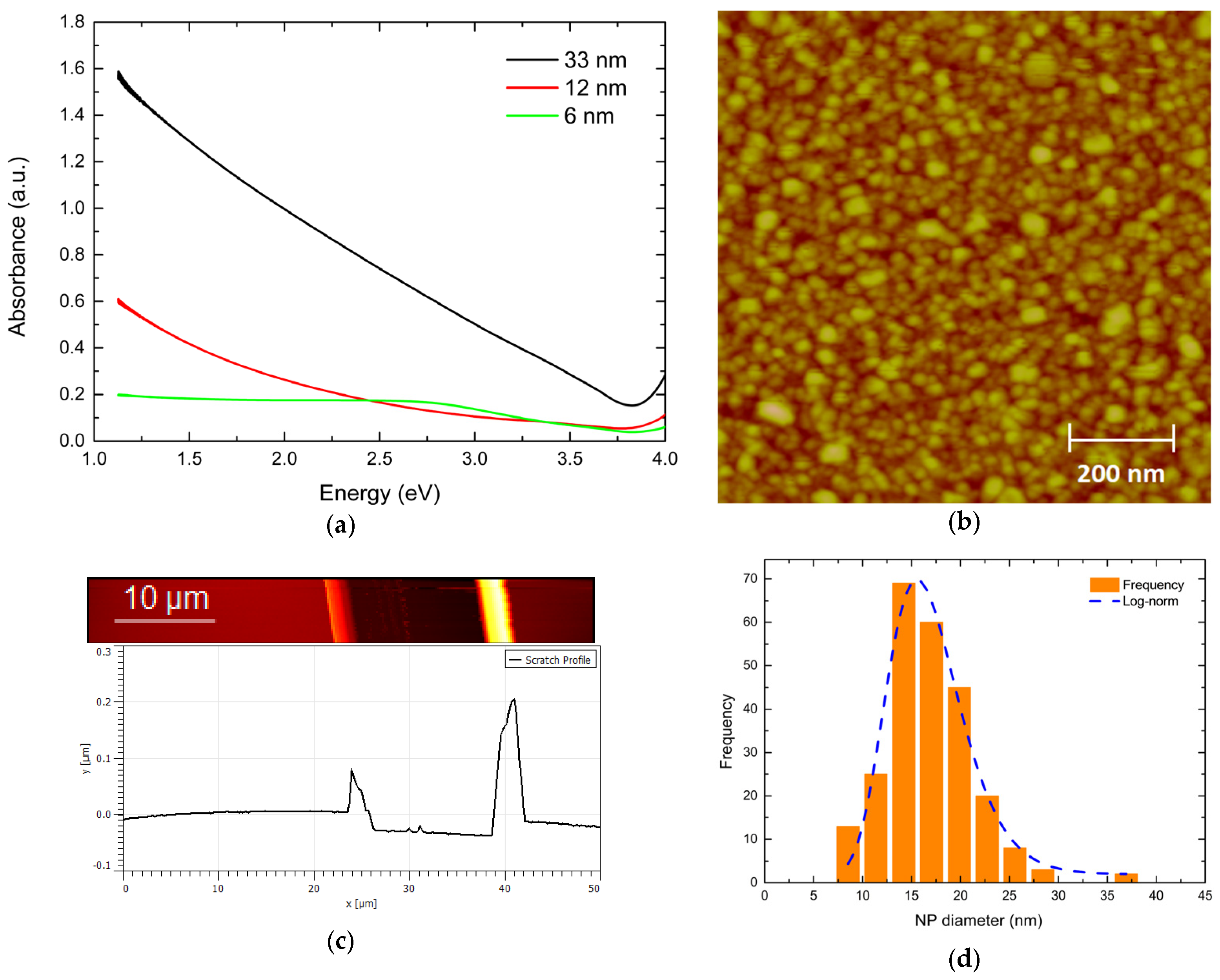
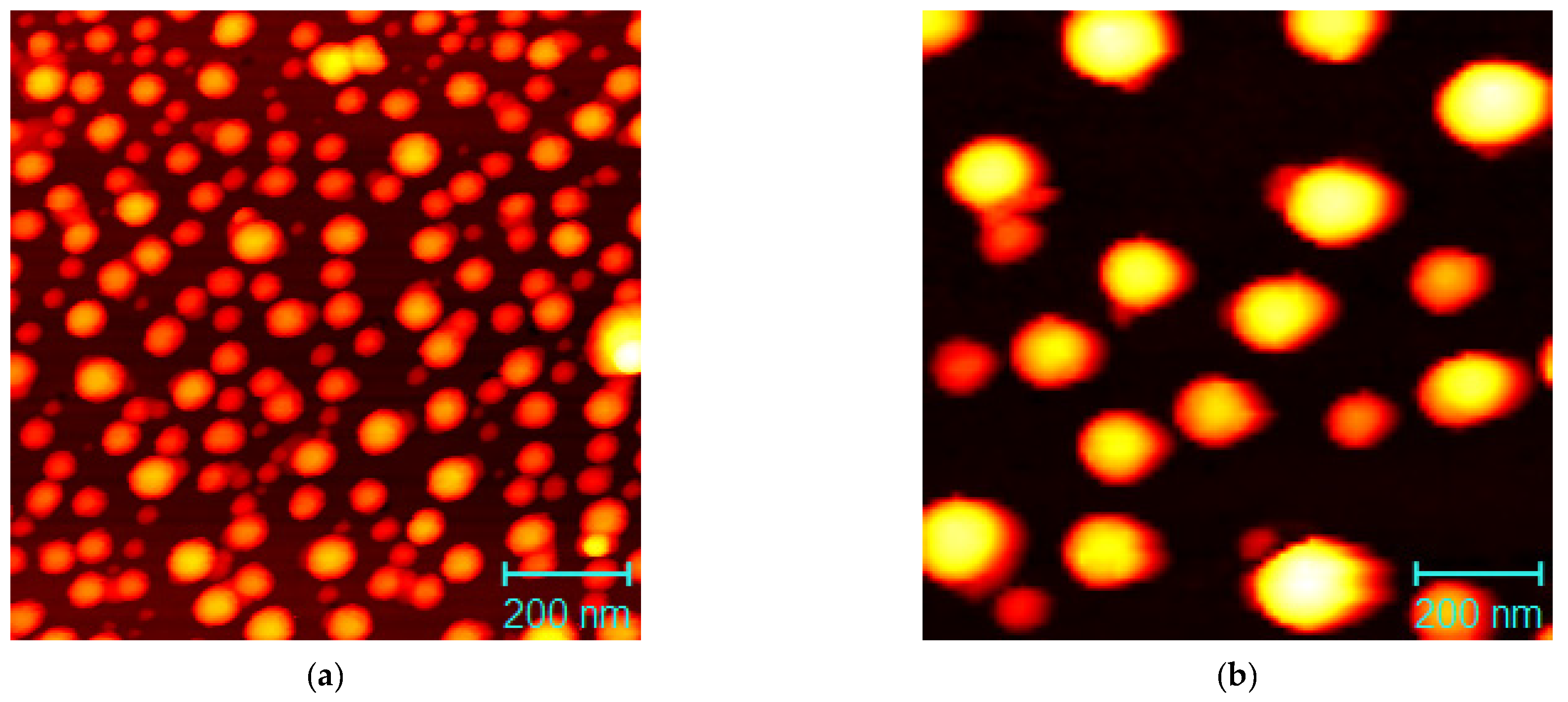
3.1. Experimental
UV-Vis and Microstructure Analysis
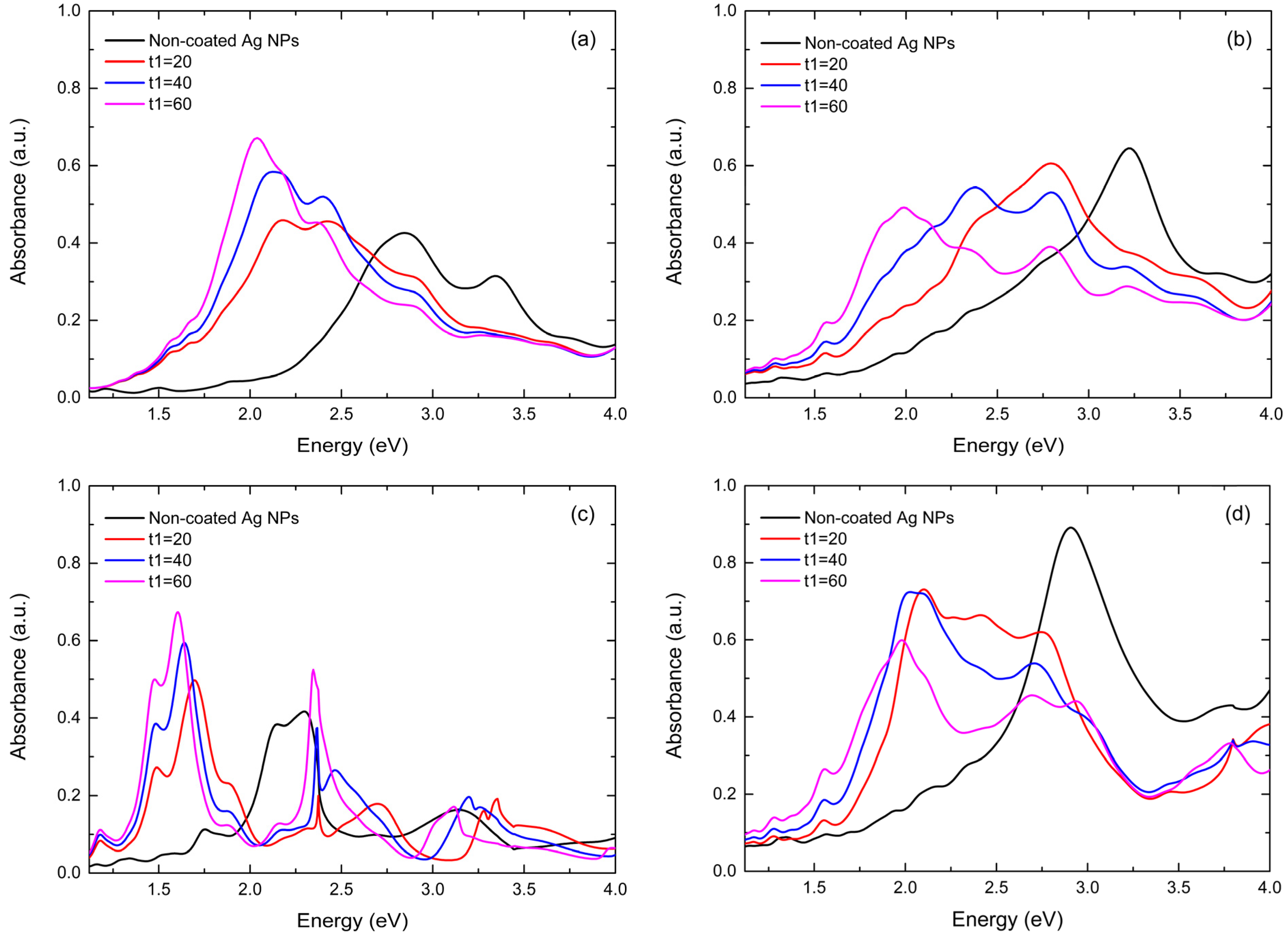
3.2. Theoretical Results
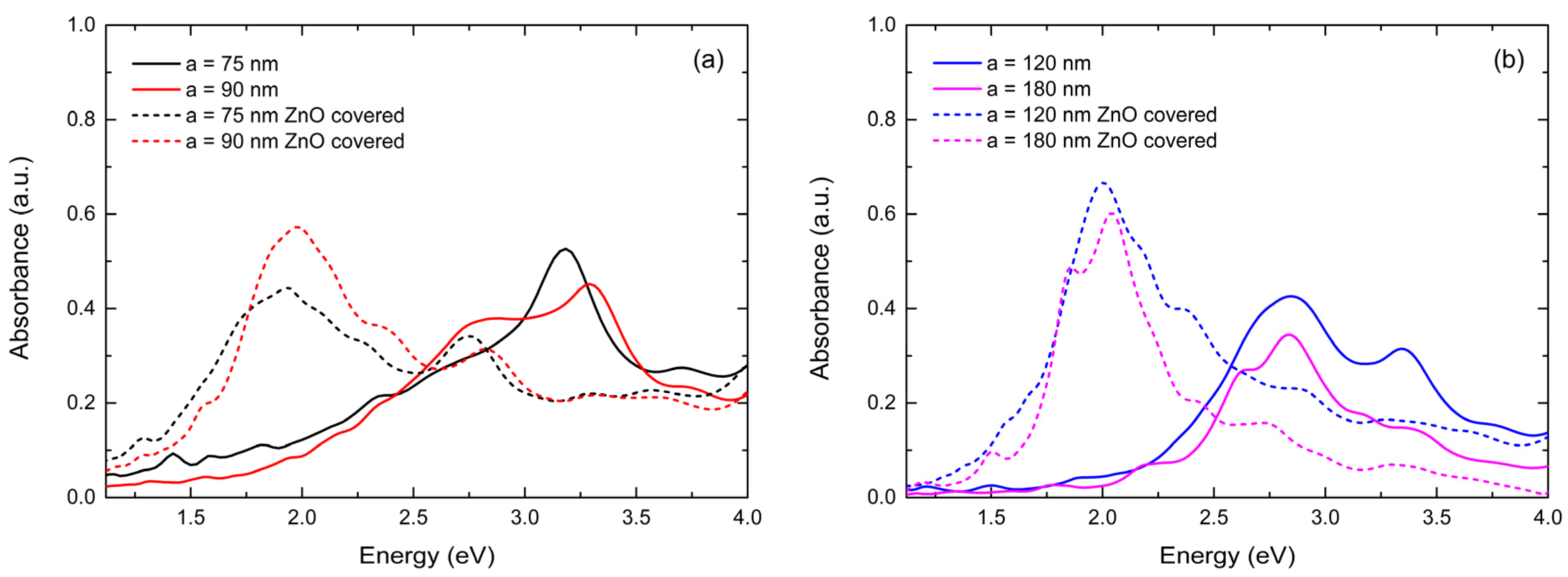
3.2.1. Comparative Plasmonic Behavior of ZnO-Coated and Non-Coated Ag NPs of Various Dimensions and Distributions
3.2.2. Influence of ZnO Thickness on Plasmonic Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Le-The, H.; Berenschot, E.; Tiggelaar, R.M.; Tas, N.R.; Van Den Berg, A.; Eijkel, J.C.T. Large-Scale Fabrication of Highly Ordered Sub-20 Nm Noble Metal Nanoparticles on Silica Substrates without Metallic Adhesion Layers. Microsyst. Nanoeng. 2018, 4, 4. [Google Scholar] [CrossRef] [PubMed]
- Ossi, P.M.; Neri, F.; Santo, N.; Trusso, S. Noble Metal Nanoparticles Produced by Nanosecond Laser Ablation. Appl. Phys. A 2011, 104, 829–837. [Google Scholar] [CrossRef]
- Wang, H.; Qiao, X.; Chen, J.; Ding, S. Preparation of Silver Nanoparticles by Chemical Reduction Method. Colloids Surf. A Physicochem. Eng. Asp. 2005, 256, 111–115. [Google Scholar] [CrossRef]
- Wolf, J.B.; Stawski, T.M.; Smales, G.J.; Thünemann, A.F.; Emmerling, F. Towards Automation of the Polyol Process for the Synthesis of Silver Nanoparticles. Sci. Rep. 2022, 12, 5769. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.T.; Yonezawa, T. Sputtering onto a Liquid: Interesting Physical Preparation Method for Multi-Metallic Nanoparticles. Sci. Technol. Adv. Mater. 2018, 19, 883–898. [Google Scholar] [CrossRef]
- Grammatikopoulos, S.; Pappas, S.; Dracopoulos, V.; Poulopoulos, P.; Fumagalli, P.; Velgakis, M.; Politis, C. Self-Assembled Au Nanoparticles on Heated Corning Glass by Dc Magnetron Sputtering: Size-Dependent Surface Plasmon Resonance Tuning. J. Nanopart. Res. 2013, 15, 1446. [Google Scholar] [CrossRef]
- Ntemogiannis, D.; Floropoulos, P.; Karoutsos, V.; Grammatikopoulos, S.; Poulopoulos, P.; Alexandropoulos, D. Plasmonic Nanostructuring by Means of Industrial-Friendly Laser Techniques. Photonics 2023, 10, 384. [Google Scholar] [CrossRef]
- Chronis, A.G.; Stamatelatos, A.; Grammatikopoulos, S.; Sigalas, M.M.; Karoutsos, V.; Maratos, D.M.; Lysandrou, S.P.; Trachylis, D.; Politis, C.; Poulopoulos, P. Microstructure and Plasmonic Behavior of Self-Assembled Silver Nanoparticles and Nanorings. J. Appl. Phys. 2019, 125, 023106. [Google Scholar] [CrossRef]
- Chutrakulwong, F.; Thamaphat, K. Green Synthesis of Silver Nanoparticles Using Durian Rind Extract and Optical Characteristics of Surface Plasmon Resonance-Based Optical Sensor for the Detection of Hydrogen Peroxide. Green Process. Synth. 2023, 12, 20230070. [Google Scholar] [CrossRef]
- Hutter, E.; Fendler, J.H. Exploitation of Localized Surface Plasmon Resonance. Adv. Mater. 2004, 16, 1685–1706. [Google Scholar] [CrossRef]
- Habibullah, G.; Viktorova, J.; Ruml, T. Current Strategies for Noble Metal Nanoparticle Synthesis. Nanoscale Res. Lett. 2021, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Nalwa, H.S. (Ed.) Encyclopedia of Nanoscience and Nanotechnology. v. 1-10; American Scientific Publishers: Stevenson Ranch, CA, USA, 2004; Volume 8, ISBN 978-1-58883-001-2. [Google Scholar]
- Haes, A.J.; Hall, W.P.; Chang, L.; Klein, W.L.; Van Duyne, R.P. A Localized Surface Plasmon Resonance Biosensor: First Steps toward an Assay for Alzheimer’s Disease. Nano Lett. 2004, 4, 1029–1034. [Google Scholar] [CrossRef]
- Fei Guo, C.; Sun, T.; Cao, F.; Liu, Q.; Ren, Z. Metallic Nanostructures for Light Trapping in Energy-Harvesting Devices. Light Sci. Appl. 2014, 3, e161. [Google Scholar] [CrossRef]
- Zeng, S.; Baillargeat, D.; Ho, H.-P.; Yong, K.-T. Nanomaterials Enhanced Surface Plasmon Resonance for Biological and Chemical Sensing Applications. Chem. Soc. Rev. 2014, 43, 3426. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; Bakr, E.A.; El-Attar, H.G. Pt@Ag and Pd@Ag Core/Shell Nanoparticles for Catalytic Degradation of Congo Red in Aqueous Solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Nazemi, M.; Soule, L.; Liu, M.; El-Sayed, M.A. Ambient Ammonia Electrosynthesis from Nitrogen and Water by Incorporating Palladium in Bimetallic Gold–Silver Nanocages. J. Electrochem. Soc. 2020, 167, 054511. [Google Scholar] [CrossRef]
- Henley, S.J.; Carey, J.D.; Silva, S.R.P. Laser-Nanostructured Ag Films as Substrates for Surface-Enhanced Raman Spectroscopy. Appl. Phys. Lett. 2006, 88, 081904. [Google Scholar] [CrossRef]
- Pan, C.; Dong, L.; Zhu, G.; Niu, S.; Yu, R.; Yang, Q.; Liu, Y.; Wang, Z.L. High-Resolution Electroluminescent Imaging of Pressure Distribution Using a Piezoelectric Nanowire LED Array. Nat. Photon 2013, 7, 752–758. [Google Scholar] [CrossRef]
- Austin, L.A.; Mackey, M.A.; Dreaden, E.C.; El-Sayed, M.A. The Optical, Photothermal, and Facile Surface Chemical Properties of Gold and Silver Nanoparticles in Biodiagnostics, Therapy, and Drug Delivery. Arch. Toxicol. 2014, 88, 1391–1417. [Google Scholar] [CrossRef]
- Kvítek, O.; Siegel, J.; Hnatowicz, V.; Švorčík, V. Noble Metal Nanostructures Influence of Structure and Environment on Their Optical Properties. J. Nanomater. 2013, 2013, 743684. [Google Scholar] [CrossRef]
- Hezam, A.; Drmosh, Q.A.; Ponnamma, D.; Bajiri, M.A.; Qamar, M.; Namratha, K.; Zare, M.; Nayan, M.B.; Onaizi, S.A.; Byrappa, K. Strategies to Enhance ZnO Photocatalyst’s Performance for Water Treatment: A Comprehensive Review. Chem. Rec. 2022, 22, e202100299. [Google Scholar] [CrossRef] [PubMed]
- Munawar, K.; Mansoor, M.A.; Olmstead, M.M.; Zaharinie, T.; Mohd Zubir, M.N.; Haniffa, M.; Basirun, W.J.; Mazhar, M. Fabrication of Ag-ZnO Composite Thin Films for Plasmonic Enhanced Water Splitting. Mater. Chem. Phys. 2020, 255, 123220. [Google Scholar] [CrossRef]
- Doustkhah, E.; Esmat, M.; Fukata, N.; Ide, Y.; Hanaor, D.A.H.; Assadi, M.H.N. MOF-Derived Nanocrystalline ZnO with Controlled Orientation and Photocatalytic Activity. Chemosphere 2022, 303, 134932. [Google Scholar] [CrossRef] [PubMed]
- Hessien, M. Recent Progress in Zinc Oxide Nanomaterials and Nanocomposites: From Synthesis to Applications. Ceram. Int. 2022, 48, 22609–22628. [Google Scholar] [CrossRef]
- Liu, H.-Q.; Yao, C.-B.; Cai, Y.; Yin, H.-T. Synthesis, Photoluminescence and Photocatalytic Characteristics of Ag–ZnO Sandwich Structures. J. Phys. Chem. Solids 2022, 165, 110697. [Google Scholar] [CrossRef]
- Rashid, J.; Barakat, M.A.; Salah, N.; Habib, S.S. Ag/ZnO Nanoparticles Thin Films as Visible Light Photocatalysts. RSC Adv. 2014, 4, 56892–56899. [Google Scholar] [CrossRef]
- Alrebdi, T.A.; Ahmed, H.A.; Alkallas, F.H.; Pashameah, R.A.; Alrefaee, S.H.; Alsubhe, E.; Mostafa, A.M.; Mwafy, E.A. Ag/ZnO Thin Film Nanocomposite Membrane Prepared by Laser-Assisted Method for Catalytic Degradation of 4-Nitrophenol. Membranes 2022, 12, 732. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Liao, J.; Cai, H.; Sun, H.; Xue, Z.; Guo, P.; Wu, M. Photocatalytic Property of Silver Enhanced Ag/ZnO Composite Catalyst. Chem. Phys. Lett. 2021, 768, 138394. [Google Scholar] [CrossRef]
- Singh, S. Natural Sunlight Driven Photocatalytic Performance of Ag/ZnO Nanocrystals. Mater. Today Commun. 2022, 33, 104438. [Google Scholar] [CrossRef]
- Wang, T.-J.; Huang, Y.-T.; Liu, Z.-Y.; Barveen, N.R. Photochemical Synthesis of ZnO/Ag Heterogeneous Nanostructure on Chemically Patterned Ferroelectric Crystals for High Performance SERS Detection. J. Alloys Compd. 2021, 864, 158120. [Google Scholar] [CrossRef]
- Koleva, M.E.; Nedyalkov, N.N.; Atanasov, P.A.; Gerlach, J.W.; Hirsch, D.; Prager, A.; Rauschenbach, B.; Fukata, N.; Jevasuwan, W. Porous Plasmonic Nanocomposites for SERS Substrates Fabricated by Two-Step Laser Method. J. Alloys Compd. 2016, 665, 282–287. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, M.; Wen, X.; Ho, H.-P.; Lu, H. Nanostructured ZnO/Ag Film Prepared by Magnetron Sputtering Method for Fast Response of Ammonia Gas Detection. Molecules 2020, 25, 1899. [Google Scholar] [CrossRef] [PubMed]
- Dare, E.; Adanu-Ogbole, B.; Oladoyinbo, F.; Makinde, F.; Uzosike, A.O. Synthesis and Characterization of Silver–Zinc Oxide Nanocomposites for Humidity Sensing. Nano Select 2023, 4, 255–262. [Google Scholar] [CrossRef]
- Kanimozhi, G.; Vinoth, S.; Kumar, H.; Srinadhu, E.S.; Satyanarayana, N. Electrospun Nanocomposite Ag–ZnO Nanofibrous Photoanode for Better Performance of Dye-Sensitized Solar Cells. J. Electron. Mater. 2019, 48, 4389–4399. [Google Scholar] [CrossRef]
- Tran, T.H.; Tran, T.N.A.; Bach, T.C.; Sai, C.D.; Pham, N.H.; Tran, V.T.; Nguyen, T.B.; Nguyen, Q.H.; Pham, V.T.; Doan, Q.K.; et al. Effect of Annealing on the Properties of Transparent Conducting Ag Doped ZnO Thin Films Prepared by r.f. Magnetron Sputtering Method. Micro Nanostruct. 2022, 166, 207219. [Google Scholar] [CrossRef]
- Sarkar, A.; Gogurla, N.; Bhaktha, B.N.S.; Ray, S.K. Plasmonic Enhanced Optical Characteristics of Ag Nanostructured ZnO Thin Films. Mater. Res. Express 2016, 3, 046403. [Google Scholar] [CrossRef]
- Xu, L.; Miao, J.; Chen, Y.; Su, J.; Yang, M.; Zhang, L.; Zhao, L.; Ding, S. Characterization of Ag-Doped ZnO Thin Film for Its Potential Applications in Optoelectronic Devices. Optik 2018, 170, 484–491. [Google Scholar] [CrossRef]
- Rajkumar, P.; Barman, D.; Kashyap, A.; Sarma, B.K. Realization of ZnO Microrods and Ag Nanoparticles on Glass and Si Substrates by Magnetron Sputtering and near Band Edge Photoluminescence Enhancement from the Exciton-Plasmon System. Mater. Lett. 2022, 325, 132898. [Google Scholar] [CrossRef]
- Da Silva, D.J.; Duran, A.; Cabral, A.D.; Fonseca, F.L.A.; Bueno, R.F.; Rosa, D.S. Questioning ZnO, Ag, and Ag/ZnO Nanoparticles as Antimicrobial Agents for Textiles: Do They Guarantee Total Protection against Bacteria and SARS-CoV-2? J. Photochem. Photobiol. B Biol. 2022, 234, 112538. [Google Scholar] [CrossRef]
- Mtavangu, S.G.; Machunda, R.L.; Van Der Bruggen, B.; Njau, K.N. In Situ Facile Green Synthesis of Ag–ZnO Nanocomposites Using Tetradenia Riperia Leaf Extract and Its Antimicrobial Efficacy on Water Disinfection. Sci. Rep. 2022, 12, 15359. [Google Scholar] [CrossRef]
- Karunakaran, C.; Rajeswari, V.; Gomathisankar, P. Antibacterial and Photocatalytic Activities of Sonochemically Prepared ZnO and Ag–ZnO. J. Alloys Compd. 2010, 508, 587–591. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Muthuraman, P.; Tettey, C.O.; Yoo, K.; Shim, J. In Vitro Anticancer Activity of Eco-Friendly Synthesized ZnO/Ag Nanocomposites. Ceram. Int. 2021, 47, 34940–34948. [Google Scholar] [CrossRef]
- Rafique, S.; Bashir, S.; Akram, R.; Jawaid, S.; Bashir, M.; Aftab, A.; Attique, A.; Awan, S.U. In Vitro Anticancer Activity and Comparative Green Synthesis of ZnO/Ag Nanoparticles by Moringa Oleifera, Mentha Piperita, and Citrus Lemon. Ceram. Int. 2023, 49, 5613–5620. [Google Scholar] [CrossRef]
- Praveena, R.; Sameera, V.S.; Mohiddon, M.A.; Krishna, M.G. Surface Plasmon Resonance, Photoluminescence and Surface Enhanced Raman Scattering Behaviour of Ag/ZnO, ZnO/Ag and ZnO/Ag/ZnO Thin Films. Phys. B Condens. Matter 2019, 555, 118–124. [Google Scholar] [CrossRef]
- Roy, R.K.; Bandyopadhyaya, S.; Pal, A.K. Surface Plasmon Resonance in Nanocrystalline Silver in a ZnO Matrix. Eur. Phys. J. B 2004, 39, 491–498. [Google Scholar] [CrossRef]
- Garoufalis, C.S.; Poulopoulos, P.; Bouropoulos, N.; Barnasas, A.; Baskoutas, S. Growth and Optical Properties of Fe2O3 Thin Films: A Study of Quantum Confinement Effects by Experiment and Theory. Phys. E Low-Dimens. Syst. Nanostruct. 2017, 89, 67–71. [Google Scholar] [CrossRef]
- Moharam, M.G.; Gaylord, T.K. Rigorous Coupled-Wave Analysis of Metallic Surface-Relief Gratings. J. Opt. Soc. Am. A 1986, 3, 1780. [Google Scholar] [CrossRef]
- Stamatelatos, A.; Tsarmpopoulou, M.; Chronis, A.G.; Kanistras, N.; Anyfantis, D.I.; Violatzi, E.; Geralis, D.; Sigalas, M.M.; Poulopoulos, P.; Grammatikopoulos, S. Optical Interpretation for Plasmonic Adjustment of Nanostructured Ag-NiO Thin Films. Int. J. Mod. Phys. B 2021, 35, 2150093. [Google Scholar] [CrossRef]
- Grammatikopoulos, S.; Stamatelatos, A.; Delimitis, A.; Sousanis, A.; Chrisanthopoulou, A.; Trachylis, D.; Politis, C.; Poulopoulos, P. Growth of Au Nanoparticles in NiO via Short Annealing of Precursor Material Thin Film and Optimization of Plasmonics. Phys. Status Solidi A 2017, 214, 1700303. [Google Scholar] [CrossRef]
- Tsarmpopoulou, M.; Chronis, A.G.; Sigalas, M.; Stamatelatos, A.; Poulopoulos, P.; Grammatikopoulos, S. Calculation of the Localized Surface Plasmon Resonances of Au Nanoparticles Embedded in NiO. Solids 2022, 3, 55–65. [Google Scholar] [CrossRef]
- Stamatelatos, A.; Tsarmpopoulou, M.; Geralis, D.; Chronis, A.G.; Karoutsos, V.; Ntemogiannis, D.; Maratos, D.M.; Grammatikopoulos, S.; Sigalas, M.; Poulopoulos, P. Interpretation of Localized Surface Plasmonic Resonances of Gold Nanoparticles Covered by Polymeric Coatings. Photonics 2023, 10, 408. [Google Scholar] [CrossRef]
- Kravets, V.G.; Kabashin, A.V.; Barnes, W.L.; Grigorenko, A.N. Plasmonic Surface Lattice Resonances: A Review of Properties and Applications. Chem. Rev. 2018, 118, 5912–5951. [Google Scholar] [CrossRef] [PubMed]
- Wokaun, A.; Gordon, J.P.; Liao, P.F. Radiation Damping in Surface-Enhanced Raman Scattering. Phys. Rev. Lett. 1982, 48, 957–960. [Google Scholar] [CrossRef]






| Film—Thickness | Growth Method |
|---|---|
| Ag #1, 33 nm | RF magnetron sputtering |
| Ag #2, 6 nm | RF magnetron sputtering and post-deposition annealing @ 440 °C for 5′ |
| Ag #3, 12 nm | RF magnetron sputtering and post-deposition annealing @ 440 °C for 30′ |
| Ag #4, 18 nm | DC magnetron sputtering on heated substrate @ 440 °C |
| ZnO #1, 70 nm | RF magnetron sputtering |
| AgZnO #2, 70 nm | RF magnetron sputtering (on Ag #2 NPs) |
| AgZnO #3, 70 nm | RF magnetron sputtering (on Ag #3 NPs) |
| AgZnO #4, 70 nm | RF magnetron sputtering (on Ag #4 NPs) |
| Specimen—Thickness | Thermal Treatment | LSPR Position (eV) | LSPR Amplitude (a.u.) | FWHM (eV) | LSPR Position (eV) | LSPR Amplitude (a.u.) | FWHM (eV) |
|---|---|---|---|---|---|---|---|
| 1st peak | 2nd peak | ||||||
| Ag #2 (6 nm) | Post-deposition annealing @ 440 °C for 5′ | 2.774 | 0.587 | 0.521 | 3.503 | 0.147 | 0.384 |
| Ag #3 (12 nm) | Post-deposition annealing @ 440 °C for 30′ | 2.460 | 0.518 | 0.737 | 3.425 | 0.280 | 0.449 |
| Ag #4 (18 nm) | Deposition on heated substrate @ 440 °C | 2.505 | 1.104 | 0.955 | 3.522 | 0.220 | 0.299 |
| AgZnO #2 (6 nm) | - | 2.403 | 0.644 | 0.655 | - | - | - |
| AgZnO #3 (12 nm) | - | 1.887 | 0.473 | 0.695 | 2.465 | 0.3668 | 0.422 |
| AgZnO #4 (18 nm) | - | 2.029 | 1.487 | 0.862 | 2.737 | 0.788 | 0.612 |
| Case | Diameter (d) | Lattice Constant (a) | Dielectric Medium Thickness (t1) | NP Height (t2) |
|---|---|---|---|---|
| (i) | d = 60 nm | 75 nm | 70 nm | 30 nm |
| 90 nm | ||||
| 120 nm | ||||
| 150 nm | ||||
| (ii) | d = 90 nm | 113 nm | 70 nm | 45 nm |
| 135 nm | ||||
| 180 nm | ||||
| 270 nm | ||||
| (iii) | d = 120 nm | 144 nm | 70 nm | 60 nm |
| 180 nm | ||||
| 240 nm | ||||
| 360 nm | ||||
| (iv) | d = 150 nm | 188 nm | 70 nm | 75 nm |
| 225 nm | ||||
| 300 nm | ||||
| 450 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntemogiannis, D.; Tsarmpopoulou, M.; Stamatelatos, A.; Grammatikopoulos, S.; Karoutsos, V.; Anyfantis, D.I.; Barnasas, A.; Alexopoulos, V.; Giantzelidis, K.; Ndoj, E.A.; et al. ZnO Matrices as a Platform for Tunable Localized Surface Plasmon Resonances of Silver Nanoparticles. Coatings 2024, 14, 69. https://doi.org/10.3390/coatings14010069
Ntemogiannis D, Tsarmpopoulou M, Stamatelatos A, Grammatikopoulos S, Karoutsos V, Anyfantis DI, Barnasas A, Alexopoulos V, Giantzelidis K, Ndoj EA, et al. ZnO Matrices as a Platform for Tunable Localized Surface Plasmon Resonances of Silver Nanoparticles. Coatings. 2024; 14(1):69. https://doi.org/10.3390/coatings14010069
Chicago/Turabian StyleNtemogiannis, Dimitrios, Maria Tsarmpopoulou, Alkeos Stamatelatos, Spyridon Grammatikopoulos, Vagelis Karoutsos, Dimitrios I. Anyfantis, Alexandros Barnasas, Vasilis Alexopoulos, Konstantinos Giantzelidis, Emanuel A. Ndoj, and et al. 2024. "ZnO Matrices as a Platform for Tunable Localized Surface Plasmon Resonances of Silver Nanoparticles" Coatings 14, no. 1: 69. https://doi.org/10.3390/coatings14010069
APA StyleNtemogiannis, D., Tsarmpopoulou, M., Stamatelatos, A., Grammatikopoulos, S., Karoutsos, V., Anyfantis, D. I., Barnasas, A., Alexopoulos, V., Giantzelidis, K., Ndoj, E. A., Sigalas, M., & Poulopoulos, P. (2024). ZnO Matrices as a Platform for Tunable Localized Surface Plasmon Resonances of Silver Nanoparticles. Coatings, 14(1), 69. https://doi.org/10.3390/coatings14010069











