Corrosion Behavior of Alkyd-Resin-Coated Carbon Steel under Cathodic Polarization in Both Static and Flowing Seawater
Abstract
1. Introduction
2. Experimental
2.1. Electrode and Solution Preparation
2.2. Cathodic Polarization
2.3. EIS Measurement
2.4. Current Density Test
2.5. Surface Morphology Analysis
2.6. FT-IR Analysis
3. Results and Discussion
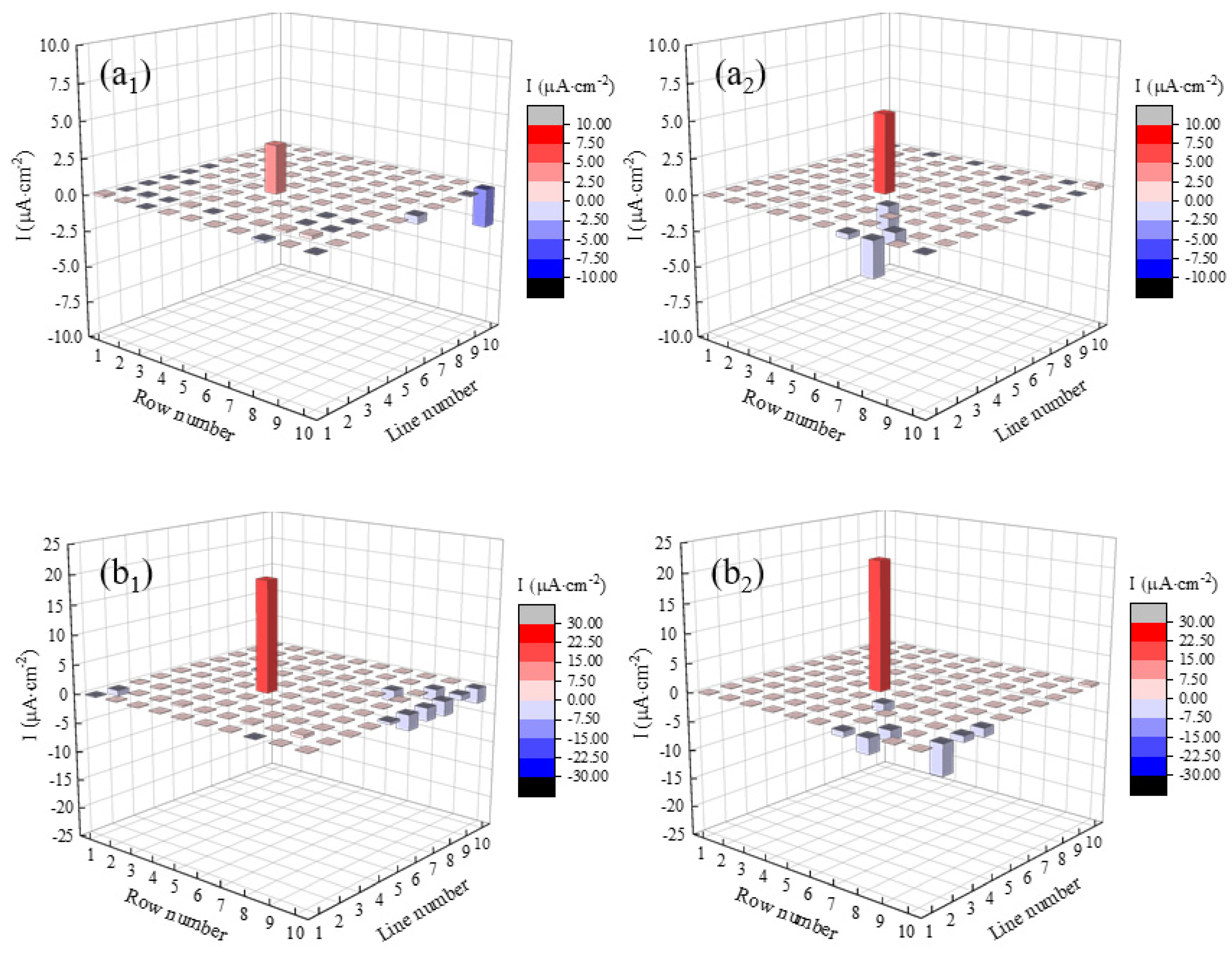
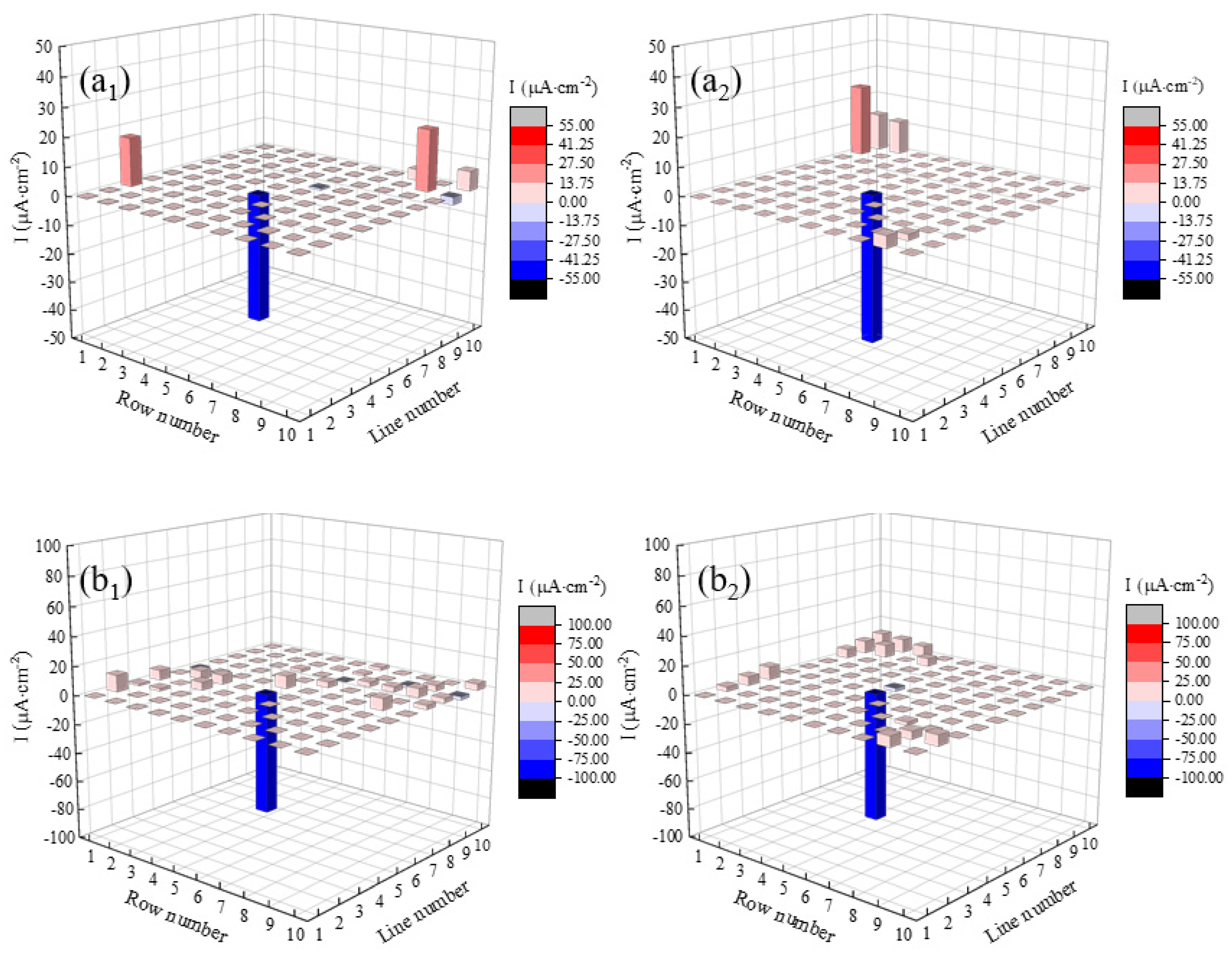
3.1. Current Density Distributions of the WBE
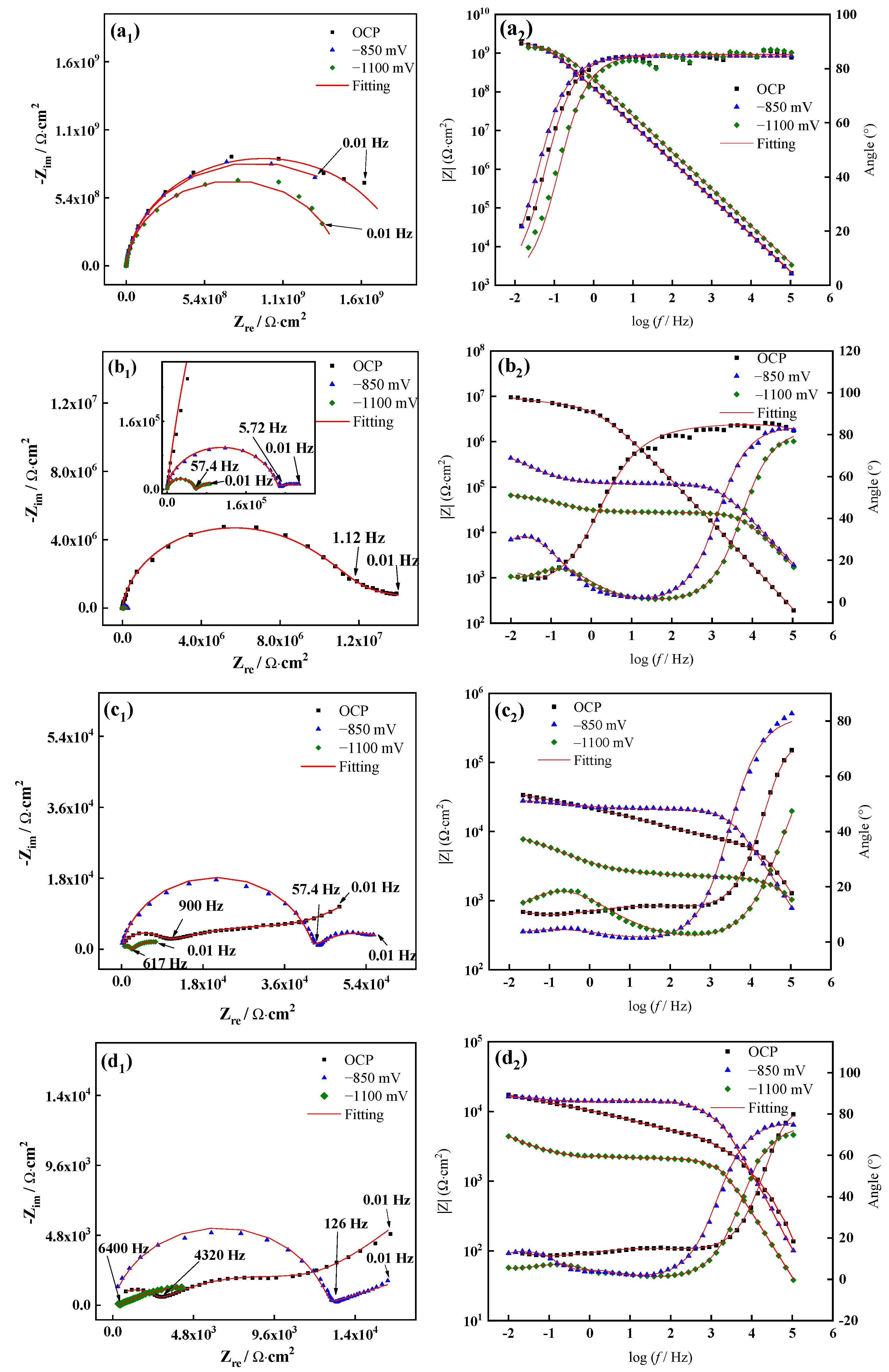
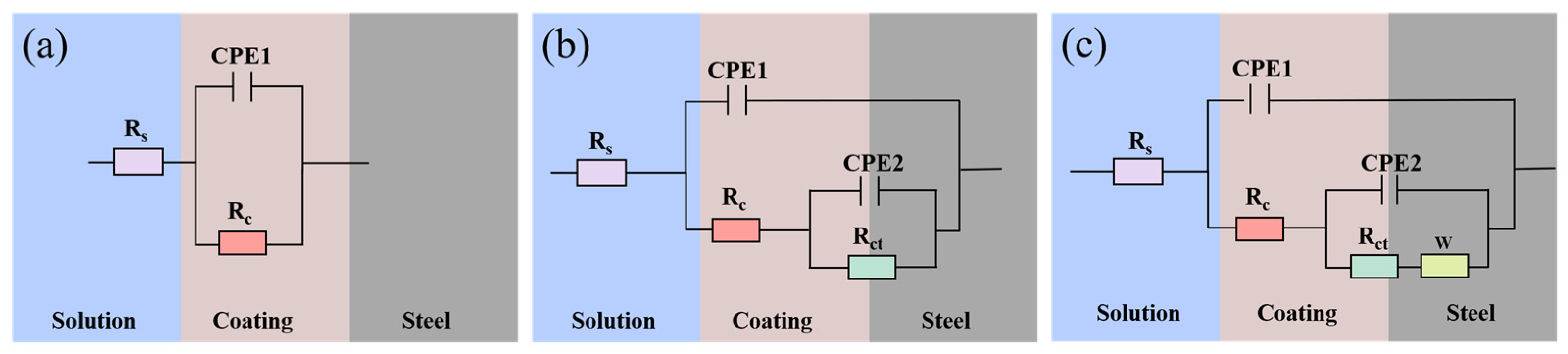
3.2. EIS Characteristics of the WBE
3.3. Morphology of Organic Coating
3.4. Development Process of Cathodic Delamination
3.5. Morphology and Composition of the Coating in the Delamination Area
4. Conclusions
- (1).
- The SEM and EIS results showed that the cathodic reaction with the metal matrix, as well as the degradation of the organic coating, could be accelerated by cathodic polarization in both static and flowing seawater. This phenomenon became serious with the negative shifting of polarization potential; the impedance under −1100 mV was one order of magnitude lower than that under the OCP.
- (2).
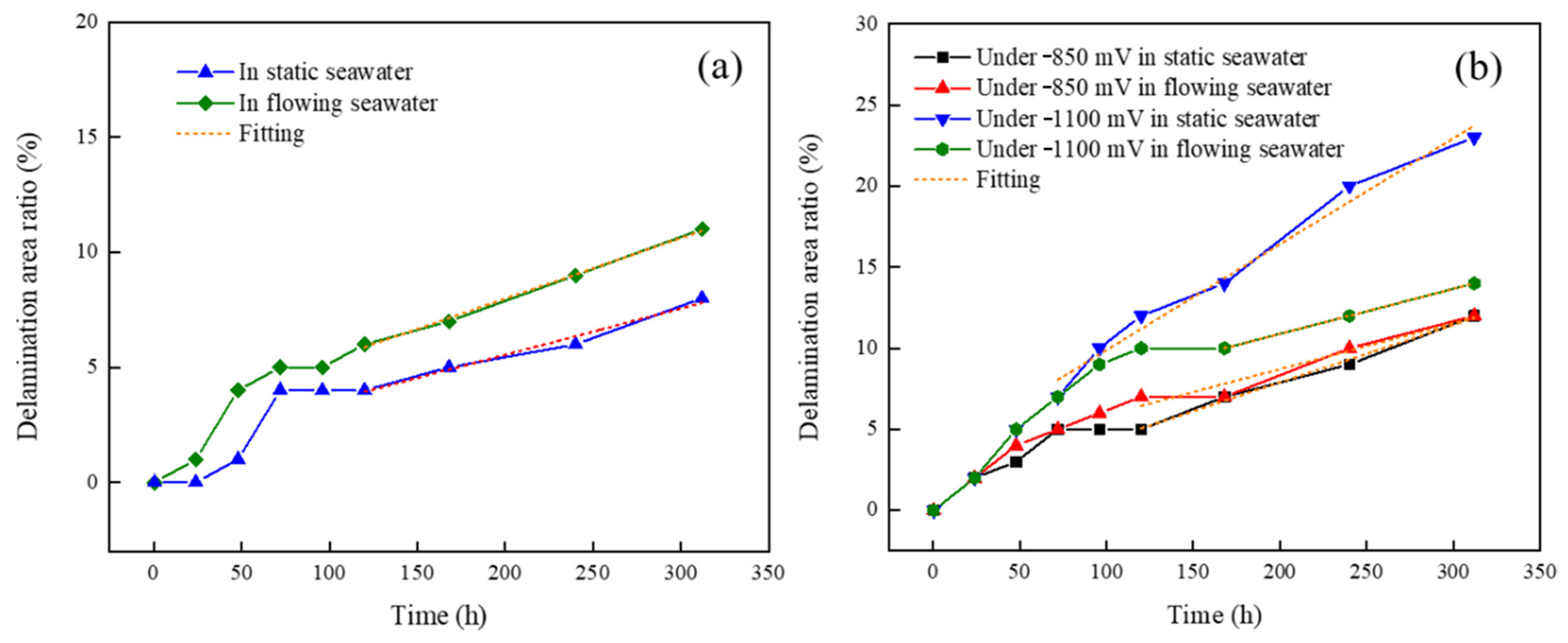
- Under the condition of OCP, the delamination area ratios in static and flowing seawater were 8% and 11%, respectively. The diffusion of corrosive particles into the coating could be accelerated by the flowing seawater, and the dissolved oxygen consumed by the cathodic reaction at the interface could be replenished in time, resulting in a faster electrochemical reaction speed, promoting the cathodic delamination of the coating.
- (3).
- Under the same cathodic polarization potential, the delamination rate in flowing seawater was first higher than that in static seawater and then lower. The reason was that the anodic dissolution reaction of the metal matrix in flowing seawater enhanced with the increasing cathodic delamination area. Then, the cathodic polarization, cathodic reaction, and cathodic delamination were weakened accordingly.
- (4).
- The FT-IR results showed that alcohols and carbonyl species were formed during the chemical degradation process of the coating in the two seawater environments. A more negative polarization potential could significantly affect the chemical structure of the coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.P.; Zhang, L.W.; Lu, Z.H.; Ding, R.; Zhao, X.D.; Wang, B.; Cui, H.T.; Liu, J. Studies on waterline corrosion processes and corrosion product characteristics of carbon steel in 3.5 wt.% NaCl solution. Mater. Corros. 2020, 72, 732–742. [Google Scholar] [CrossRef]
- Mughal, K.; Mughal, M.P.; Farooq, M.U.; Anwar, S.; Ammarullah, M.I. Using nano−fluids minimum quantity lubrication (NF−MQL) to improve tool wear characteristics for efficient machining of CFRP/Ti6Al4V aeronautical structural composite. Processes 2023, 11, 1540. [Google Scholar] [CrossRef]
- Naderi, R.; Attar, M.M. The role of zinc aluminum phosphate anticorrosive pigment in Protective Performance and cathodic disbondment of epoxy coating. Corros. Sci. 2010, 52, 1291–1296. [Google Scholar] [CrossRef]
- Narozny, M.; Zakowski, K.; Darowicki, K. Application of electrochemical impedance spectroscopy to evaluate cathodically protected coated steel in seawater. Constr. Build. Mater. 2018, 181, 721–726. [Google Scholar] [CrossRef]
- Shreepathi, S. Physicochemical parameters influencing the testing of cathodic delamination resistance of high build pigmented epoxy coating. Prog. Org. Coat. 2016, 90, 438–447. [Google Scholar] [CrossRef]
- Zhu, C.F.; Xie, R.; Xue, J.H.; Song, L.L. Studies of the impedance models and water transport behaviors of cathodically polarized coating. Electrochim. Acta. 2011, 56, 5828–5835. [Google Scholar] [CrossRef]
- Henry, L.; Wendy, W.; Lars, I. The mechanism for the cathodic delamination of organic coating from a metal surface. Prog. Org. Coat. 1983, 11, 19–40. [Google Scholar]
- Lvar, S.J.; Unni, S. Cathodic disbonding of paint films−transport of charge. Corros. Sci. 1993, 35, 1385–1389. [Google Scholar]
- Steinsmo, U.; Skar, J.I. Factors influencing the rate of cathodic disbonding of coating. Corrosion 1994, 50, 934–939. [Google Scholar] [CrossRef]
- Calovi, M.; Rossi, S.; Deflorian, F.; Dirè, S.; Ceccato, R.; Guo, X.L.; Frankel, G.S. Effects of graphene–based fillers on cathodic delamination and abrasion resistance of cataphoretic organic coatings. Coatings 2020, 10, 602. [Google Scholar] [CrossRef]
- Leng, A.; Streckel, H.; Stratmann, M. The delamination of polymeric coating from steel. Part 1: Calibration of the Kelvinprobe and basic delamination mechanism. Corros. Sci. 1998, 41, 547–578. [Google Scholar] [CrossRef]
- Dong, C.F.; Fu, A.Q.; Li, X.G.; Cheng, Y.F. Localized EIS characterization of corrosion of steel at coating defect under cathodic protection. Electrochim. Acta 2008, 54, 628–633. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.G.; Du, C.W.; Cheng, Y.F. Effect of cathodic protection on corrosion of pipeline steel under disbonded coating. Corros. Sci. 2009, 51, 2242–2245. [Google Scholar] [CrossRef]
- Wint, N.; Griffiths, C.M.; Richards, C.J.; Williams, G.; McMurray, H.N. The role of benzotriazole modified zinc phosphate in preventing corrosion–driven organic coating disbondment on galvanised steel. Corros. Sci. 2020, 174, 108839. [Google Scholar] [CrossRef]
- Naderi, R.; Attar, M.M. Cathodic disbondment of epoxy coating with zinc aluminum polyphosphate as a modified zinc phosphate anticorrosion pigment. Prog. Org. Coat. 2010, 69, 392–395. [Google Scholar] [CrossRef]
- Perdomo, J.J.; Song, I. Chemical and electrochemical conditions on steel under disbonded coating: The effect of applied potential, solution resistivity, crevice thickness and holiday size. Corros. Sci. 2000, 42, 1389–1415. [Google Scholar] [CrossRef]
- Watts, J.F.; Castle, J.E. The application of X–ray photoelectron spectroscopy to the study of polymer–to–metal adhesion Part 2. The cathodic disbondment of epoxy coated mild steel. J. Mater. Sci. 1984, 19, 2259–2272. [Google Scholar] [CrossRef]
- Koehler, E.L. The mechanism of cathodic disbondment of protective organic coating–aqueous displacement at elevated Ph. Corrosion 1984, 40, 5–8. [Google Scholar] [CrossRef]
- Nakache, M.; Aragon, E.; Belec, L.; Perrin, F.X.; Roux, G.; Gac, P.Y.L. Degradation of rubber to metals bonds during its cathodic delamination: Validation of an artificial ageing test. Prog. Org. Coat. 2011, 72, 279–286. [Google Scholar] [CrossRef]
- Bi, H.C.; Sykes, J. Cathodic disbonding of an unpigmented epoxy coating on mild steel under semi– and full–immersion conditions. Corros. Sci. 2011, 53, 3416–3425. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Dam–Johansen, K.; Weinell, C.E.; Kiil, S. Cathodic delamination: Quantification of ionic transport rates along coating–steel interfaces. Prog. Org. Coat. 2010, 68, 70–78. [Google Scholar] [CrossRef]
- Sørensen, P.A.; Dam–Johansen, K.; Weinell, C.E.; Kiil, S. Cathodic delamination of seawater–immersed anticorrosive coating: Mapping of parameters affecting the rate. Prog. Org. Coat. 2010, 68, 283–292. [Google Scholar] [CrossRef]
- Bi, H.; Sykes, J. Cathodic delamination of unpigmented and pigmented epoxy coating from mild steel. Prog. Org. Coat. 2016, 90, 114–125. [Google Scholar] [CrossRef]
- Muhammad, I.A.; Gatot, S.; Sugiharto, S.; Toto, S.; Ojo, K.; Mohammad, T.; Tri, I.W.; Jamari, J. Tresca stress study of CoCrMo–on–CoCrMo bearings based on body mass index using 2D computational model. J. Tribologi. 2022, 33, 31–38. [Google Scholar]
- Xie, J.; Lu, Z.H.; Zhou, K.; Li, C.P.; Ma, J.Y.; Wang, B.; Xu, K.S.; Cui, H.T.; Liu, J. Researches on corrosion behaviors of carbon steel/copper alloy couple under organic coating in both static and flowing seawater. Prog. Org. Coat. 2022, 166, 106793. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Z.H.; Zhang, L.W.; Li, C.P.; Ding, R. Studies of corrosion behaviors of a carbon steel/copper–nickel alloy couple under epoxy coating with artificial defect in 3.5 wt.% NaCl solution using the WBE and EIS techniques. Prog. Org. Coat. 2020, 148, 105909. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.W.; Mu, X.L.; Zhang, P.Q. Studies of electrochemical corrosion of low alloy steel under epoxy coating exposed to natural seawater using the WBE and EIS techniques. Prog. Org. Coat. 2017, 111, 315–321. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, W.; Wang, J. A novel device for the wire beam electrode method and its application in the ennoblement study. Corros. Sci. 2009, 51, 1475–1479. [Google Scholar] [CrossRef]
- Tan, Y.J.; Aung, N.N.; Liu, T. Novel corrosion experiments using the wire beam electrode. (I) Studying electrochemical noise signatures from localised corrosion processes. Corros. Sci. 2004, 48, 23–38. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Z.N.; Qiao, L.J.; Su, Y.J.; Yan, Y. Role of protein in crevice corrosion of CoCrMo alloy: An investigation using wire beam electrodes. Corros. Sci. 2023, 215, 111028. [Google Scholar] [CrossRef]
- Meng, F.D.; Zhang, T.; Liu, L.; Cui, Y.; Wang, F.H. Failure behaviour of an epoxy coating with polyaniline modified graphene oxide under marine alternating hydrostatic pressure. Surf. Coat. Tech. 2019, 361, 188–195. [Google Scholar] [CrossRef]
- Zheng, H.P.; Liu, L.; Meng, F.D.; Cui, Y.; Wang, F.H. Etched basalt scales wrapped in self–assembled poly (urea–formaldehyde) for robust anticorrosive coatings. Prog. Org. Coat. 2021, 153, 106160. [Google Scholar] [CrossRef]
- Meng, F.D.; Liu, L.; Tian, W.L.; Wu, H.; Li, Y.; Zhang, T.; Wang, F.H. The influence of the chemically bonded interface between fillers and binder on the failure behaviour of an epoxy coating under marine alternating hydrostatic pressure. Corros. Sci. 2015, 101, 139–154. [Google Scholar] [CrossRef]
- Budi, S.; Sri, H.; Muhammad, I.A.; Muhammad, D.B.; Armin, S. Power and Energy Optimization of Carbon Based Lithium-Ion Battery from Water Spinach (Ipomoea Aquatica). J. Ecol. Eng. 2023, 24, 213–223. [Google Scholar]
- Liu, J.; Li, X.B.; Wang, J.; Luo, T.Y.; Wang, X.M. Studies of impedance models and water transport behaviours of epoxy coating at hydrostatic pressure of seawater. Prog. Org. Coat. 2013, 76, 1075–1081. [Google Scholar]
- Barlow, B.C.; Situm, A.; Guo, B.; Guo, X.X.; Grosvenor, A.P.; Burgess, L.J. X–ray microprobe characterization of corrosion at the buried polymer–steel interface. Corros. Sci. 2018, 144, 198–206. [Google Scholar] [CrossRef]
- Mahdavi, F.; Forsyth, M.; Tan, M.Y.J. Understanding the effects of applied cathodic protection potential and environmental conditions on the rate of cathodic disbondment of coating by means of local electrochemical measurements on a multi–electrode array. Prog. Org. Coat. 2017, 103, 83–92. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, S.B.; Li, Z.; Liu, J. Degradation processes of organic coating with artificial defects in flowing seawater. Eq. Environ. Eng. 2018, 15, 102–108. [Google Scholar]
- Liu, Y.W.; Zhou, X.R.; Lyon, S.B.; Emad, R.; Hashimoto, T. An organic coating pigmented with strontium aluminium polyphosphate for corrosion protection of zinc alloy coated steel. Prog. Org. Coat. 2017, 102, 29–36. [Google Scholar] [CrossRef]
- Cristoforetti, A.; Rossi, S.; Deflorian, F.; Fedel, M. On the limits of the EIS low–frequency impedance modulus as a tool to describe the protection properties of organic coatings exposed to accelerated aging tests. Coatings 2023, 13, 598. [Google Scholar] [CrossRef]
- Lashgari, S.M.; Yari, H.; Mahdavian, M.; Ramezanzadeh, B.; Bahlakeh, G.; Ramezanzadeh, M. Application of nanoporous cobalt–based ZIF–67 metal–organic framework (MOF) for construction of an epoxy–composite coating with superior anti–corrosion properties. Corros. Sci. 2021, 178, 109099. [Google Scholar] [CrossRef]
- Zhang, J.T.; Hu, J.M.; Zhang, J.Q.; Cao, C.N. Studies of water transport behavior and impedance models of epoxy–coated metals in NaCl solution by EIS. Prog. Org. Coat. 2004, 51, 145–151. [Google Scholar] [CrossRef]
- Zhang, J.T.; Hu, J.M.; Zhang, J.Q.; Cao, C.N. Studies of impedance models and water transport behaviors of polypropylene coated metals in NaCl solution. Prog. Org. Coat. 2004, 49, 293–301. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Wang, J. Evaluation of the deterioration of epoxy coating by EIS and WBE techniques. Mater. Sci. Tech. 2013, 21, 33–39. [Google Scholar]
- Peng, X.; Wang, J.; Shan, C.; Wang, H.J.; Liu, Z.J. Corrosion behavior of long–time immersed rusted carbon steel in flowing seawater. Acta. Metall. Sin. 2012, 48, 1260–1266. [Google Scholar] [CrossRef]
- Wang, H.J.; Wang, J.; Wang, W.; Zhang, W. The study of the varying characteristics of cathodic regions for defective coating in 3.5% sodium chloride solution by EIS and WBE. J. Ocean Univ. China 2015, 14, 269–276. [Google Scholar] [CrossRef]
- Thu, Q.L.; Bonnet, G.; Compere, C.; Trong, H.L.; Touzain, S. Modified wire beam electrode: A useful tool to evaluate compatibility between organic coating and cathodic protection. Prog. Org. Coat. 2005, 52, 118–125. [Google Scholar] [CrossRef]
- Duce, C.; Della, P.V.; Tiné, M.R.; Spepi, A.; Ghezzi, L.; Colombini, M.P.; Bramanti, E. FTIR study of ageing of fast drying oil colour (FDOC) alkyd paint replicas. Spectrochim. Acta A 2014, 130, 214–221. [Google Scholar] [CrossRef]
- Cakić, S.M.; Ristić, L.S.; Jašo, V.M.; Radičević, R.Ž.; Ilić, O.Z.; Simendić, J.K.B. Investigation of the curing kinetics of alkyd–melamine–epoxy resin system. Prog. Org. Coat. 2012, 73, 415–424. [Google Scholar] [CrossRef]
- Gan, S.N.; Tan, B.Y. FTIR studies of the curing reactions of palm oil alkyd–melamine enamels. J. Appl. Polym. Sci. 2001, 80, 2309–2315. [Google Scholar] [CrossRef]






| Chemical Composition | C | Si | Mn | P | S | Fe |
|---|---|---|---|---|---|---|
| Q235 carbon steel | 0.18 | 0.30 | 0.32 | 0.04 | 0.03 | Balance |
| Potential | Time (h) | Rc (Ω∙cm2) | CPE1 (S·sn1·cm−2) | n1 | Rct (Ω∙cm2) | CPE2 (S·sn2·cm−2) | n2 | W (S·s0.5·cm−2) | Chi-Squared Value | Sum-of-Squares Value |
|---|---|---|---|---|---|---|---|---|---|---|
| OCP (−214~−216 mV) | 0.5 | 1.89 × 109 | 1.43 × 10−9 | 0.95 | - | - | - | - | 1.88 × 10−3 | 1.39 × 102 |
| 48 | 1.61 × 107 | 2.59 × 10−9 | 0.90 | 2.88 × 106 | 5.43 × 10−7 | 0.72 | - | 1.05 × 10−3 | 1.18 × 102 | |
| 168 | 1.41 × 104 | 3.84 × 10−9 | 0.85 | 5.06 × 104 | 6.42 × 10−6 | 0.39 | 1.53 × 10−4 | 6.17 × 10−4 | 1.28 × 101 | |
| 312 | 3.72 × 103 | 3.90 × 10−9 | 0.86 | 1.75 × 104 | 3.61 × 10−5 | 0.41 | 3.59 × 10−4 | 7.17 × 10−4 | 1.20 × 101 | |
| −850 mV vs. SCE | 0.5 | 1.82 × 109 | 1.45 × 10−9 | 0.95 | - | - | - | - | 1.84 × 10−3 | 7.02 × 10−2 |
| 48 | 2.21 × 105 | 1.77 × 10−9 | 0.94 | 6.75 × 104 | 1.71 × 10−5 | 0.45 | - | 7.13 × 10−4 | 2.69 × 101 | |
| 168 | 3.09 × 104 | 4.14 × 10−9 | 0.91 | 1.83 × 104 | 8.57 × 10−5 | 0.40 | - | 5.77 × 10−4 | 1.84 × 101 | |
| 312 | 1.23 × 104 | 5.48 × 10−9 | 0.88 | 9.13 × 103 | 4.56 × 10−4 | 0.35 | - | 3.52 × 10−4 | 1.26 × 101 | |
| −1100 mV vs. SCE | 0.5 | 1.47 × 109 | 8.24 × 10−10 | 0.95 | - | - | - | - | 2.12 × 10−3 | 2.23 × 102 |
| 48 | 1.29 × 104 | 2.88 × 10−9 | 0.91 | 2.53 × 104 | 1.40 × 10−4 | 0.55 | - | 2.25 × 10−4 | 1.97 × 101 | |
| 168 | 2.25 × 103 | 1.61 × 10−8 | 0.80 | 9.24 × 103 | 2.24 × 10−4 | 0.51 | - | 2.76 × 10−4 | 1.89 × 101 | |
| 312 | 3.80 × 102 | 1.73 × 10−8 | 0.80 | 8.34 × 103 | 3.08 × 10−4 | 0.36 | - | 3.75 × 10−4 | 2.56 × 101 |
| Potential | Time (h) | Rc (Ω∙cm2) | CPE1 (S·sn1·cm−2) | n1 | Rct (Ω∙cm2) | CPE2 (S·sn2·cm−2) | n2 | W (S·s0.5·cm−2) | Chi-Squared Value | Sum-of-Squares Value |
|---|---|---|---|---|---|---|---|---|---|---|
| OCP (−218~−223 mV) | 0.5 | 1.99 × 109 | 1.84 × 10−9 | 0.83 | - | - | - | - | 1.02 × 10−3 | 1.15 × 102 |
| 48 | 1.75 × 105 | 2.49 × 10−9 | 0.95 | 7.56 × 106 | 2.73 × 10−8 | 0.50 | 1.44 × 10−6 | 2.32 × 10−3 | 3.42 × 102 | |
| 168 | 3.06 × 104 | 6.59 × 10−9 | 0.94 | 7.27 × 103 | 1.75 × 10−6 | 0.59 | 4.17 × 10−4 | 6.38 × 10−4 | 1.41 × 102 | |
| 312 | 1.32 × 104 | 3.51 × 10−9 | 0.93 | 1.82 × 103 | 2.54 × 10−5 | 0.63 | 7.16 × 10−4 | 2.13 × 10−4 | 1.25 × 102 | |
| −850 mV vs. SCE | 0.5 | 2.10 × 109 | 1.38 × 10−9 | 0.83 | - | - | - | - | 1.21 × 10−3 | 7.58 × 102 |
| 48 | 2.74 × 104 | 2.54 × 10−9 | 0.92 | 3.56 × 104 | 3.67 × 10−5 | 0.73 | 3.33 × 10−4 | 2.47 × 10−4 | 8.69 × 102 | |
| 168 | 1.88 × 104 | 2.51 × 10−9 | 0.88 | 1.65 × 104 | 8.28 × 10−5 | 0.62 | 3.84 × 10−4 | 3.71 × 10−4 | 1.19 × 102 | |
| 312 | 2.37 × 104 | 1.34 × 10−8 | 0.85 | 1.74 × 103 | 5.47 × 10−4 | 0.59 | 2.55 × 10−3 | 6.12 × 10−4 | 1.17 × 102 | |
| −1100 mV vs. SCE | 0.5 | 1.86 × 109 | 2.06 × 10−9 | 0.94 | - | - | - | - | 4.19 × 10−5 | 1.06 × 101 |
| 48 | 1.46 × 104 | 1.34 × 10−8 | 0.94 | 1.04 × 105 | 1.11 × 10−4 | 0.67 | - | 5.73 × 10−4 | 3.26 × 102 | |
| 168 | 2.97 × 103 | 2.02 × 10−8 | 0.87 | 3.70 × 103 | 4.41 × 10−4 | 0.55 | - | 1.43 × 10−4 | 1.30 × 102 | |
| 312 | 4.80 × 103 | 2.75 × 10−8 | 0.83 | 1.16 × 102 | 8.44 × 10−4 | 0.78 | 2.65 × 10−4 | 1.39 × 10−4 | 1.49 × 101 |
| Condition | Slope | Variance (%) |
|---|---|---|
| static seawater | 0.0201 | 98.0 |
| flowing seawater | 0.0264 | 99.5 |
| Potential | Condition | Slope | Variance (%) |
|---|---|---|---|
| −850 mV vs. SCE | static seawater | 0.0354 | 99.3 |
| flowing seawater | 0.0283 | 94.4 | |
| −1100 mV vs. SCE | static seawater | 0.0653 | 98.1 |
| flowing seawater | 0.0278 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, H.; Zhou, K.; Feng, Z.; Li, C.; Xie, J.; Ma, J.; Zhang, X.; Wang, X.; Xu, K.; Li, C.; et al. Corrosion Behavior of Alkyd-Resin-Coated Carbon Steel under Cathodic Polarization in Both Static and Flowing Seawater. Coatings 2023, 13, 1296. https://doi.org/10.3390/coatings13071296
Guo H, Zhou K, Feng Z, Li C, Xie J, Ma J, Zhang X, Wang X, Xu K, Li C, et al. Corrosion Behavior of Alkyd-Resin-Coated Carbon Steel under Cathodic Polarization in Both Static and Flowing Seawater. Coatings. 2023; 13(7):1296. https://doi.org/10.3390/coatings13071296
Chicago/Turabian StyleGuo, Hui, Kun Zhou, Zhenliang Feng, Chengjie Li, Jie Xie, Jiyuan Ma, Xinyue Zhang, Xiaohui Wang, Kunshan Xu, Chuanpeng Li, and et al. 2023. "Corrosion Behavior of Alkyd-Resin-Coated Carbon Steel under Cathodic Polarization in Both Static and Flowing Seawater" Coatings 13, no. 7: 1296. https://doi.org/10.3390/coatings13071296
APA StyleGuo, H., Zhou, K., Feng, Z., Li, C., Xie, J., Ma, J., Zhang, X., Wang, X., Xu, K., Li, C., & Liu, J. (2023). Corrosion Behavior of Alkyd-Resin-Coated Carbon Steel under Cathodic Polarization in Both Static and Flowing Seawater. Coatings, 13(7), 1296. https://doi.org/10.3390/coatings13071296







