Transparent Self-Cleaning Coatings: A Review
Abstract
1. Introduction
2. Basic Theories of Wetting Ability and Self-Cleaning Coatings
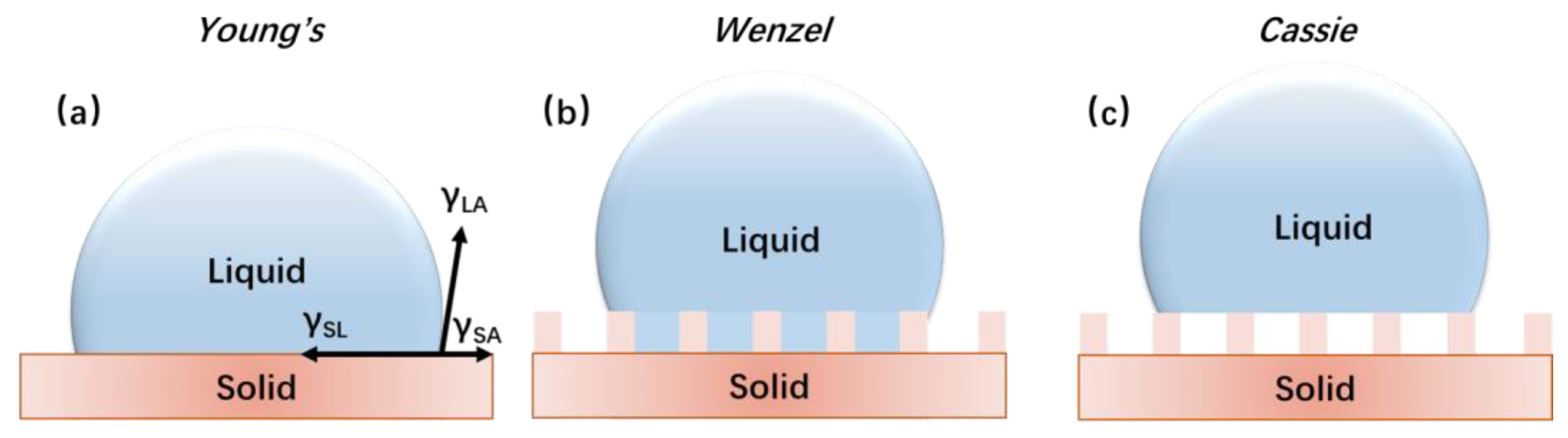
2.1. Basic Theories of Wetting Ability
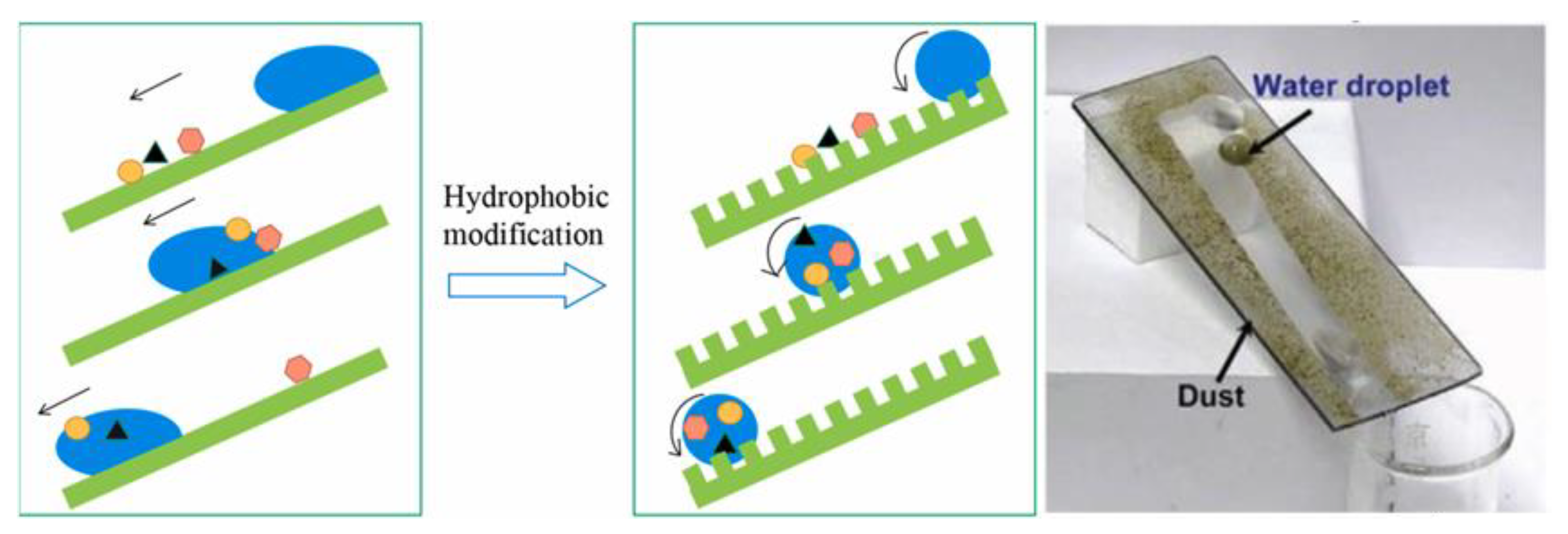
2.2. Hydrophobic Self-Cleaning Surface
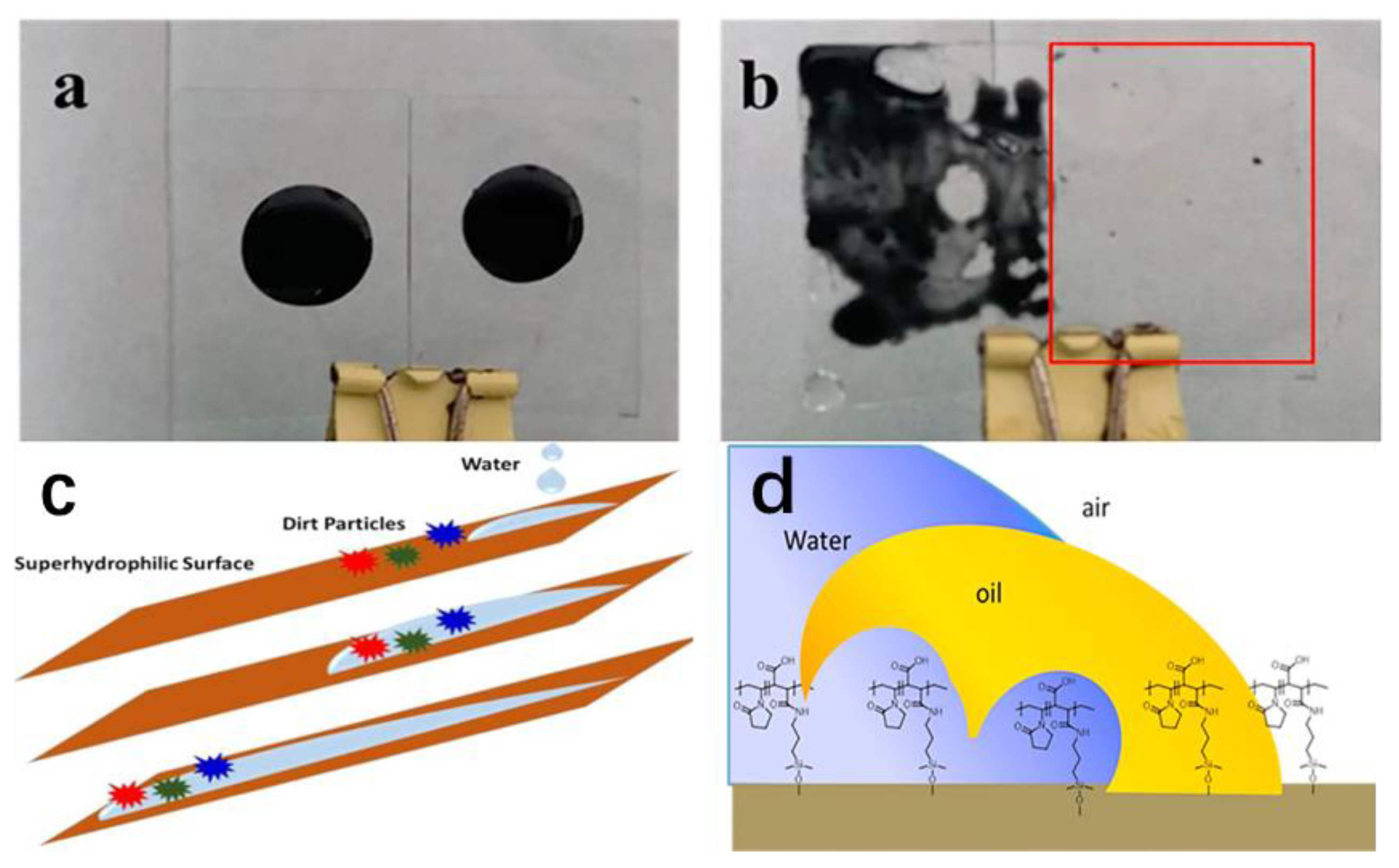
2.3. Hydrophilic Self-Cleaning Coatings
3. Desired Performance of Transparent Self-Cleaning Coatings and Measurement Methods
3.1. Mechanical Performance
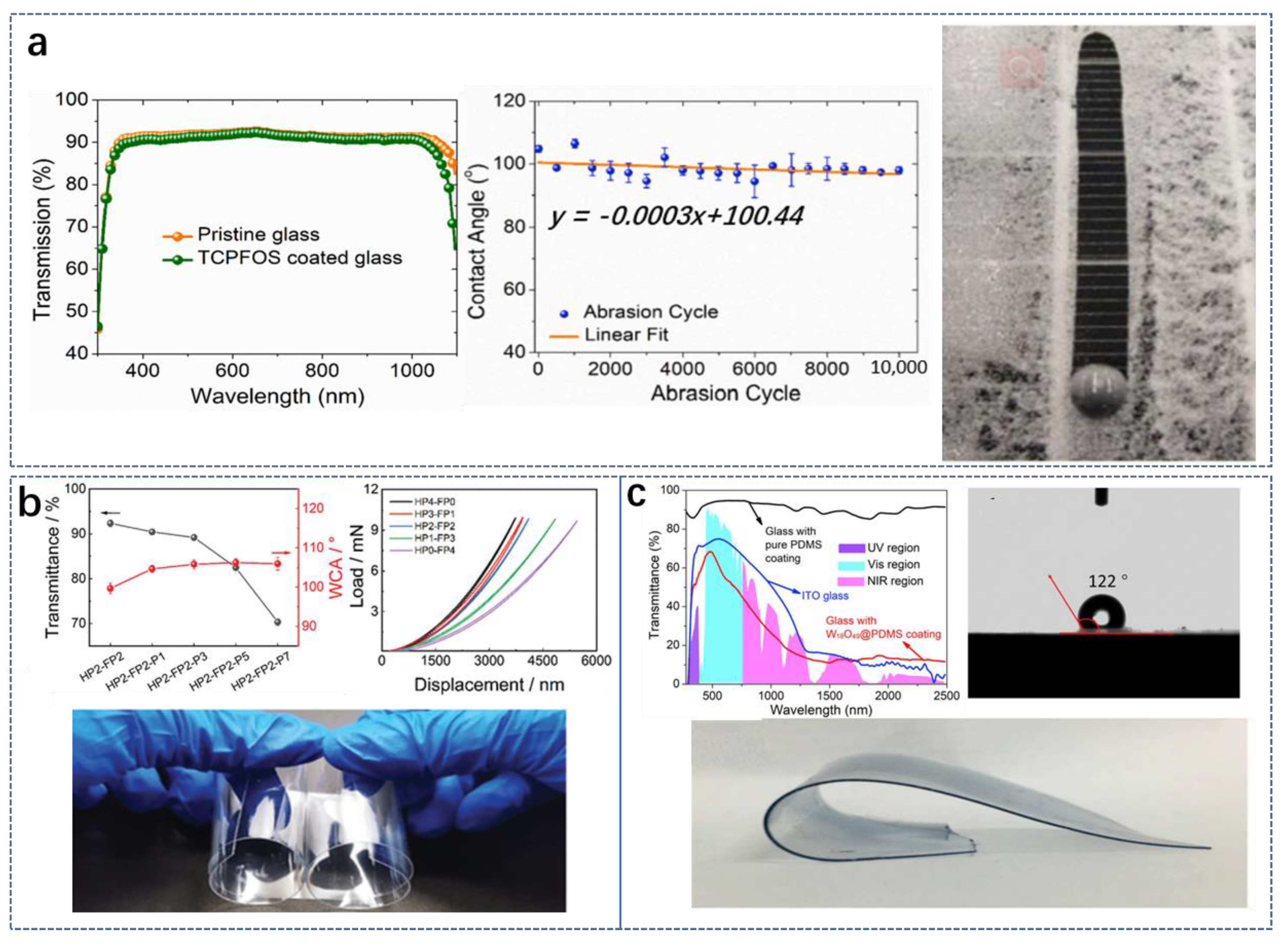
3.1.1. Hardness and Flexibility
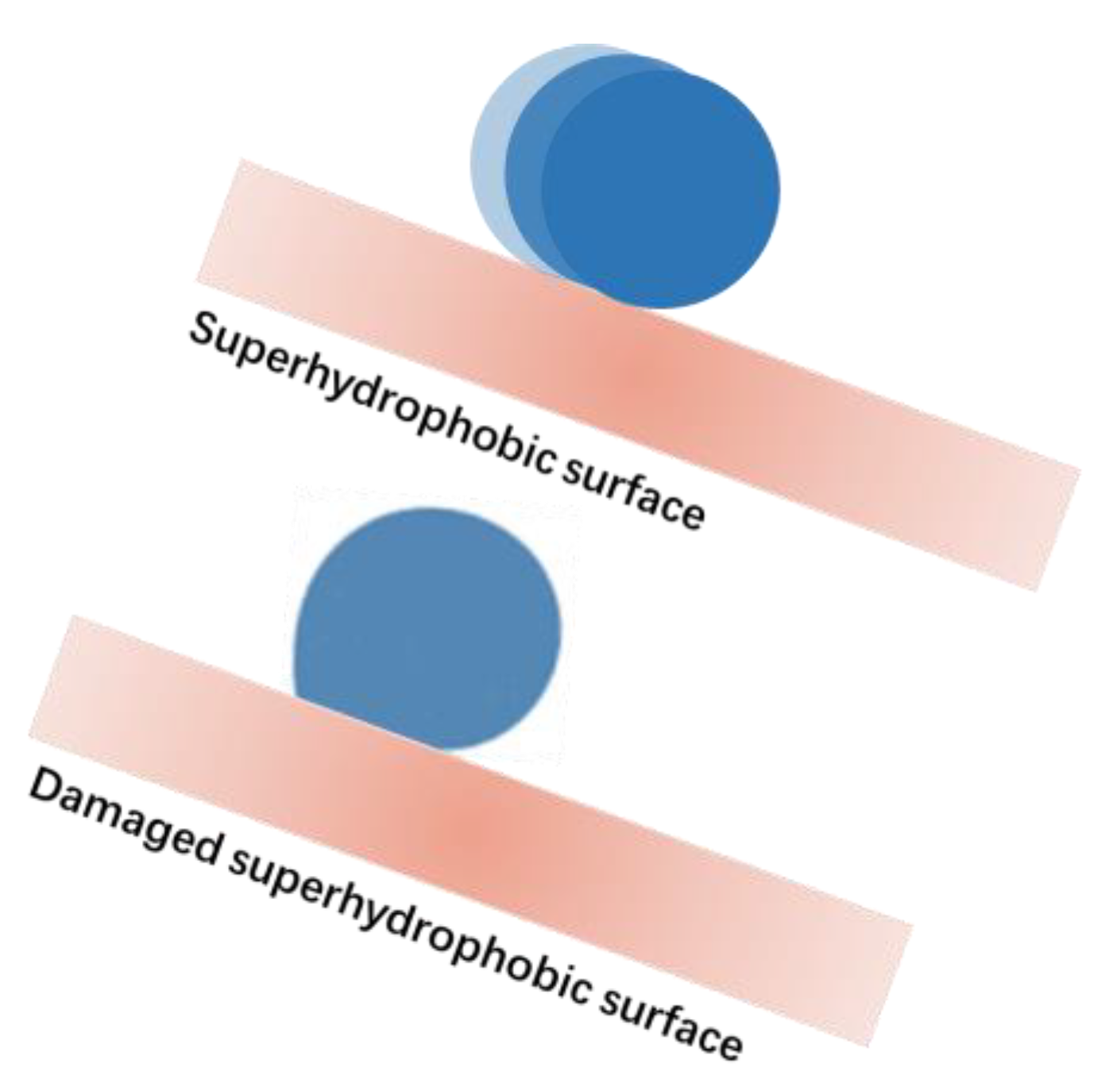
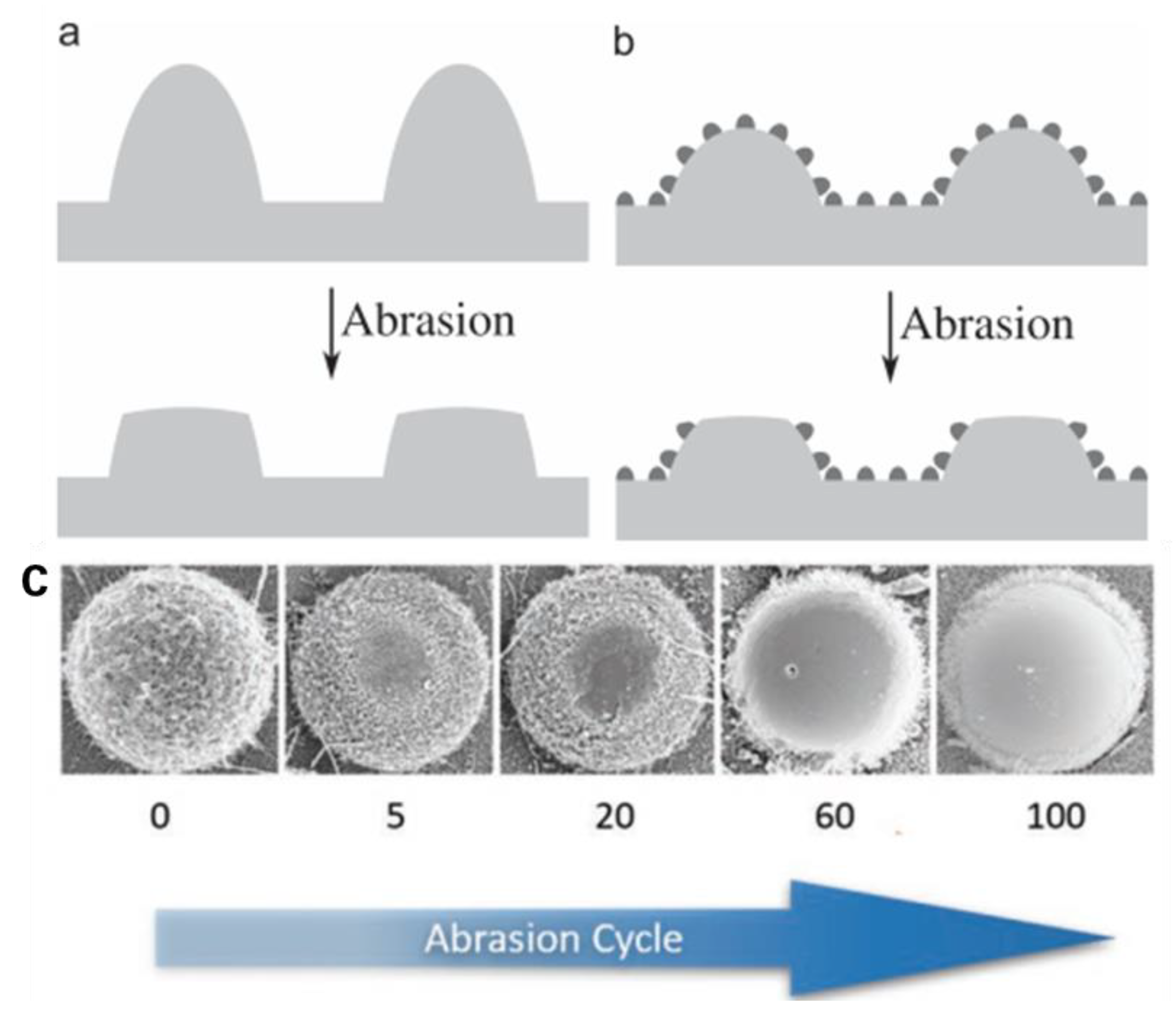
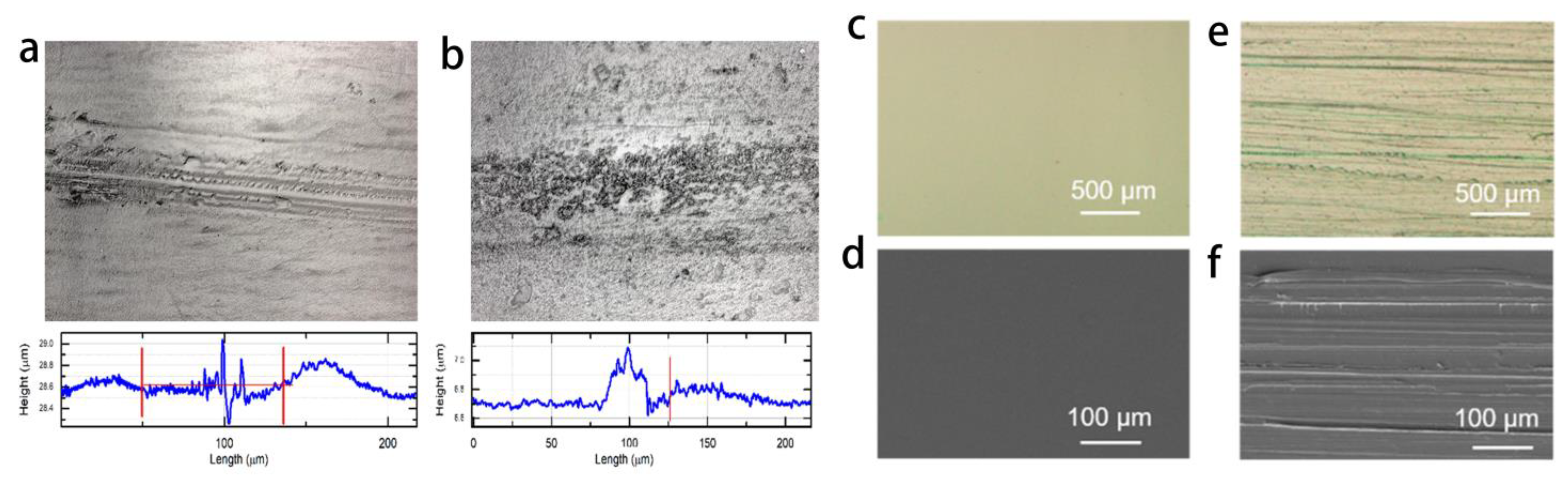
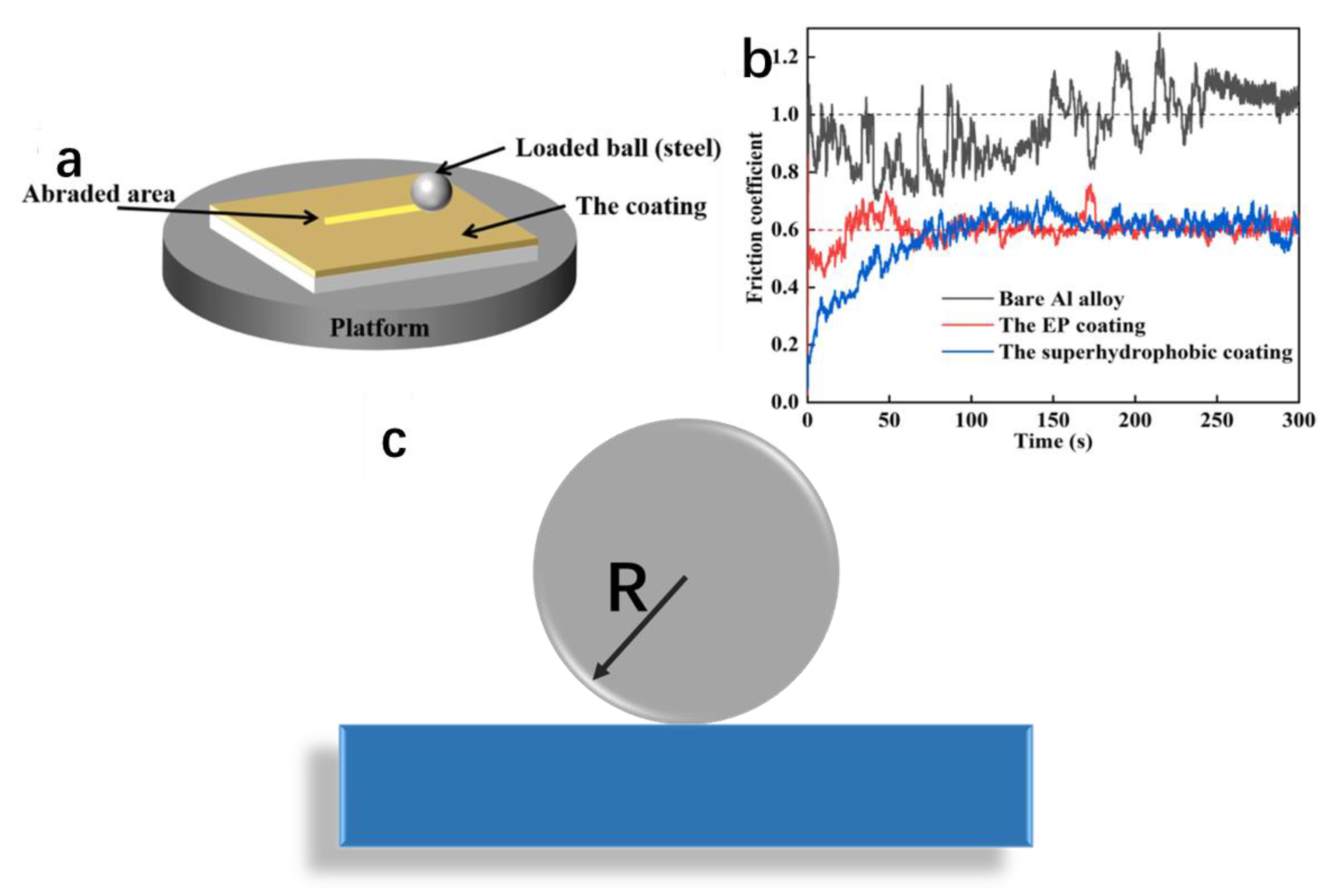
3.1.2. Mechanical Durability
3.1.3. Adhesion to the Substrate
3.2. Optical and Self-Cleaning Properties
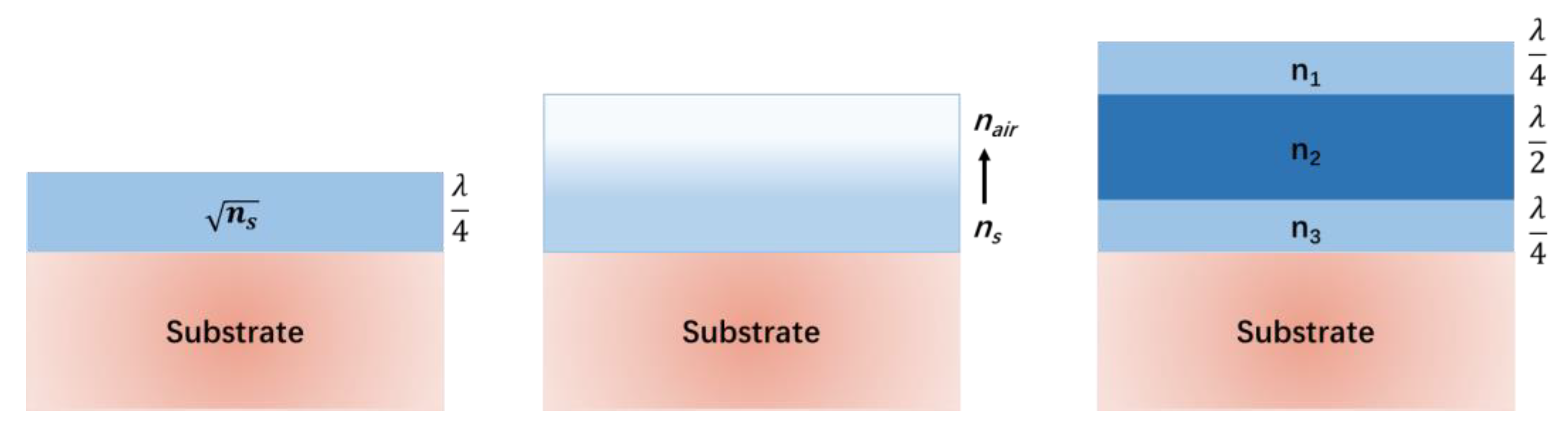
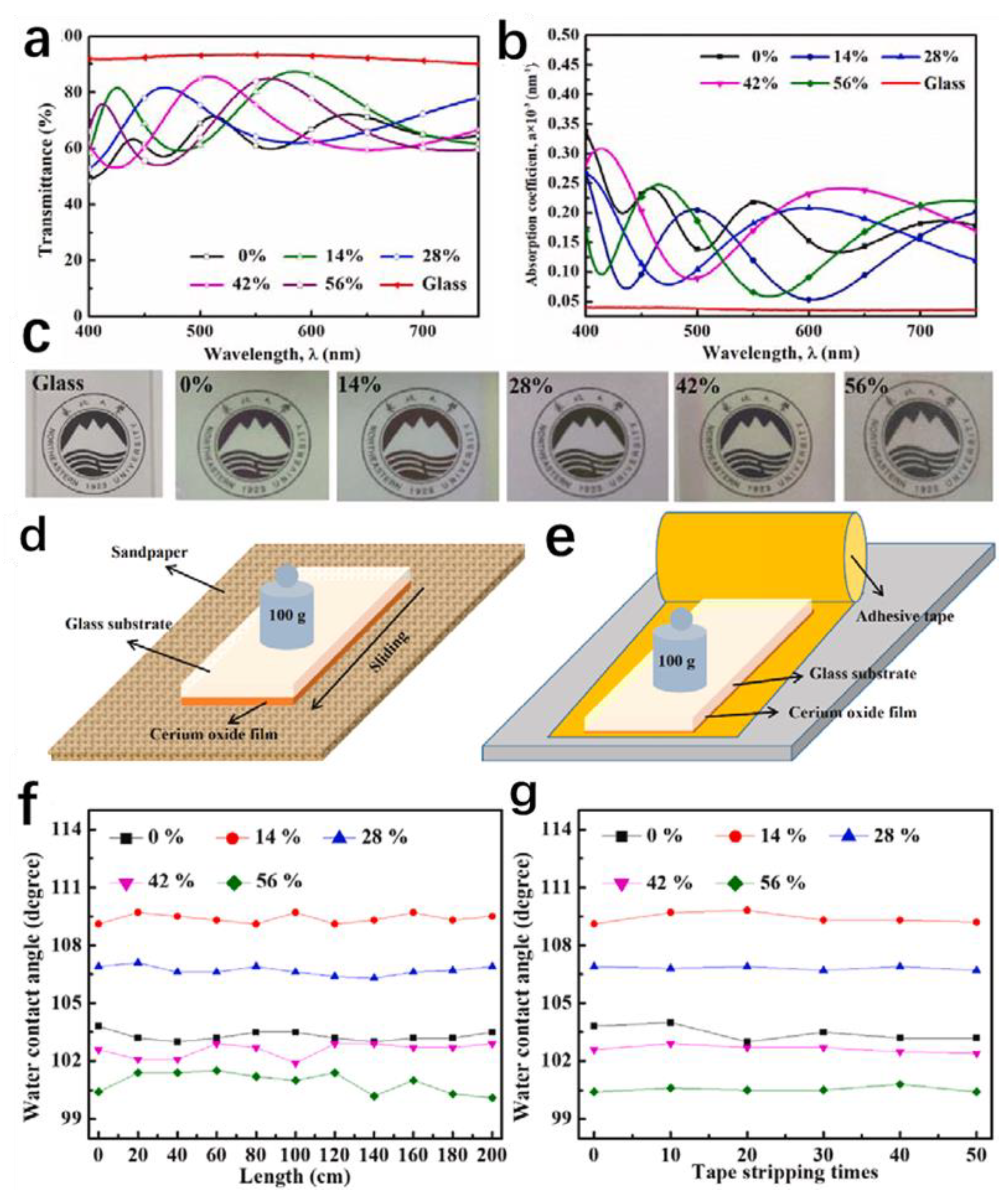
3.2.1. Optical Properties
3.2.2. Self-Cleaning Properties of Hydrophobic Coatings
3.2.3. Self-Cleaning and Photocatalytic Properties of Hydrophilic Coatings
4. Fabrication Methods and Materials for Multifunctional Coatings
4.1. Coatings Prepared by the Sol–Gel Method
4.2. Methods for Uniform Coatings
4.3. CVD Method
5. MS-Made Multifunctional Coatings
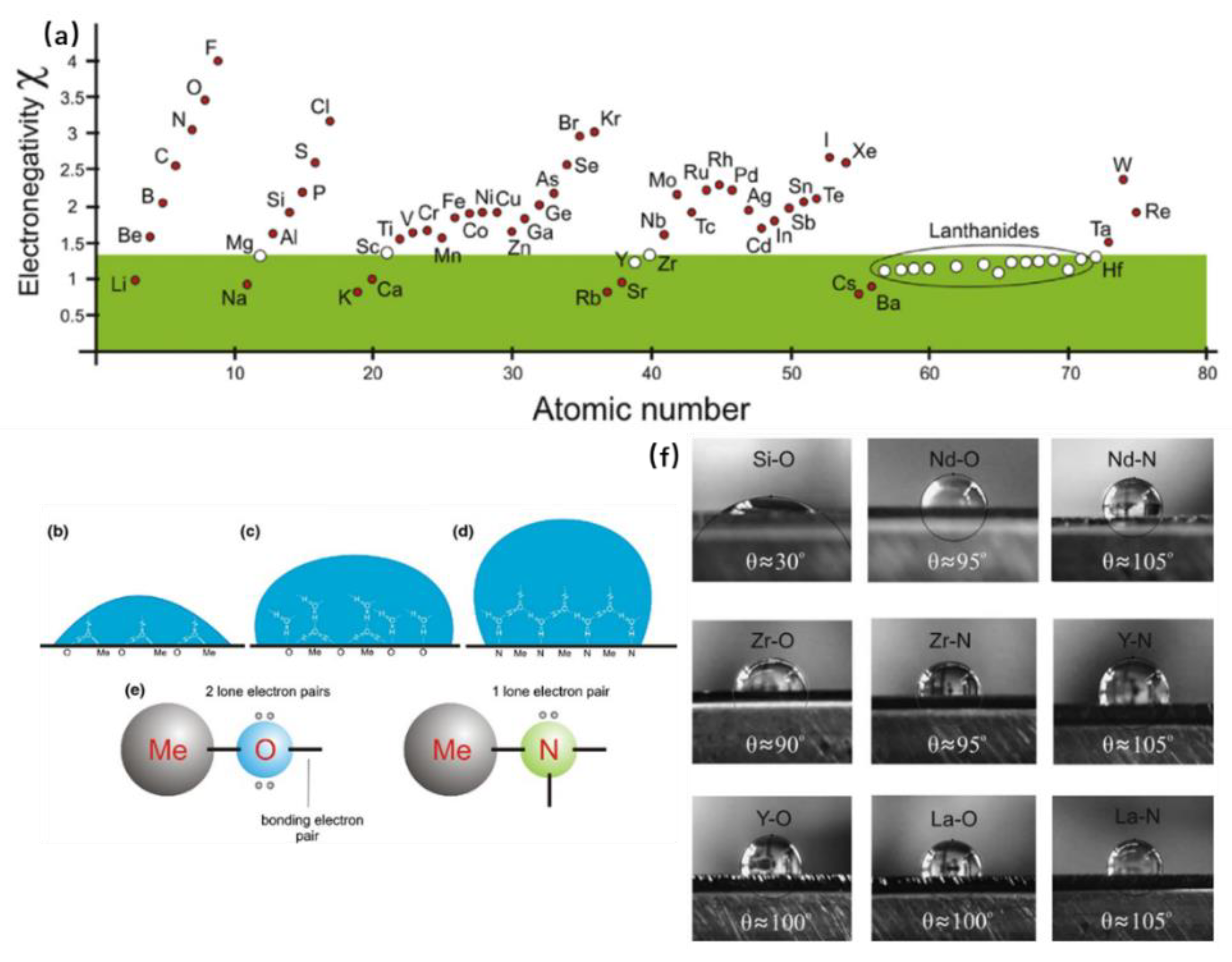
5.1. MS-Made Hydrophobic Multifunctional Coatings
5.2. MS-Made Photocatalytic Multifunctional Coatings
5.3. Advances in Multifunctional Coatings in Multilayer Structures
6. Conclusions and Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, X.; Li, H.-T.; Nie, M.-X.; Fu, S.-R.; Li, Y.; Zhang, Q.; Chen, F.; Han, D.; Fu, Q. Engineering the Properties of Transparent Hybrid Coating toward High Hardness, Excellent Flexibility, and Multifunction. ACS Appl. Mater. Interfaces 2022, 14, 39432–39440. [Google Scholar] [CrossRef] [PubMed]
- Dalawai, S.P.; Saad Aly, M.A.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.-S.; Kumar Sadasivuni, K.; Liu, S. Recent Advances in Durability of Superhydrophobic Self-Cleaning Technology: A Critical Review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, H.; Shao, Y.; Zhang, H.; Zhu, J. Recent Progresses of Superhydrophobic Coatings in Different Application Fields: An Overview. Coatings 2021, 11, 116. [Google Scholar] [CrossRef]
- Guo, H.; Xu, T.; Zhang, J.; Zhao, W.; Zhang, J.; Lin, C.; Zhang, L. A Multifunctional Anti-Fog, Antibacterial, and Self-Cleaning Surface Coating Based on Poly(NVP-Co-MA). Chem. Eng. J. 2018, 351, 409–417. [Google Scholar] [CrossRef]
- Howarter, J.A.; Youngblood, J.P. Self-Cleaning and Anti-Fog Surfaces via Stimuli-Responsive Polymer Brushes. Adv. Mater. 2007, 19, 3838–3843. [Google Scholar] [CrossRef]
- Parkin, I.P.; Palgrave, R.G. Self-Cleaning Coatings. J. Mater. Chem. 2005, 15, 1689. [Google Scholar] [CrossRef]
- Ellinas, K.; Tserepi, A.; Gogolides, E. Durable Superhydrophobic and Superamphiphobic Polymeric Surfaces and Their Applications: A Review. Adv. Colloid Interface Sci. 2017, 250, 132–157. [Google Scholar] [CrossRef]
- Mortazavi, V.; Khonsari, M.M. On the Degradation of Superhydrophobic Surfaces: A Review. Wear 2017, 372–373, 145–157. [Google Scholar] [CrossRef]
- Ritchie, R.O. The Conflicts between Strength and Toughness. Nat. Mater. 2011, 10, 817–822. [Google Scholar] [CrossRef]
- Garner, S.; Glaesemann, S.; Li, X. Ultra-Slim Flexible Glass for Roll-to-Roll Electronic Device Fabrication. Appl. Phys. A 2014, 116, 403–407. [Google Scholar] [CrossRef]
- Auch, M.D.J.; Soo, O.K.; Ewald, G.; Soo-Jin, C. Ultrathin Glass for Flexible OLED Application. Thin Solid Films 2002, 417, 47–50. [Google Scholar] [CrossRef]
- Demetriou, M.D.; Launey, M.E.; Garrett, G.; Schramm, J.P.; Hofmann, D.C.; Johnson, W.L.; Ritchie, R.O. A Damage-Tolerant Glass. Nat. Mater. 2011, 10, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lee, H.; Im, H.-G.; Jo, W.; Choi, G.-M.; Kim, T.-S.; Jang, J.; Bae, B.-S. Transparent and Flexible Hybrid Cover Window Film: Hard Coating/Substrate All-in-One Composite Film for Reliable Foldable Display. Compos. Part B Eng. 2022, 247, 110336. [Google Scholar] [CrossRef]
- Kumar, D.; Wu, X.; Fu, Q.; Ho, J.W.C.; Kanhere, P.D.; Li, L.; Chen, Z. Hydrophobic Sol–Gel Coatings Based on Polydimethylsiloxane for Self-Cleaning Applications. Mater. Des. 2015, 86, 855–862. [Google Scholar] [CrossRef]
- Chen, R.; Xie, Q.; Zeng, H.; Ma, C.; Zhang, G. Non-Elastic Glassy Coating with Fouling Release and Resistance Abilities. J. Mater. Chem. A 2020, 8, 380–387. [Google Scholar] [CrossRef]
- Bae, G.; Choi, G.-M.; Ahn, C.; Kim, S.-M.; Kim, W.; Choi, Y.; Park, D.; Jang, D.; Hong, J.-W.; Han, S.M.; et al. Flexible Protective Film: Ultrahard, Yet Flexible Hybrid Nanocomposite Reinforced by 3D Inorganic Nanoshell Structures. Adv. Funct. Mater. 2021, 31, 2010254. [Google Scholar] [CrossRef]
- Xu, C.-L.; Song, F.; Wang, X.-L.; Wang, Y.-Z. Surface Modification with Hierarchical CuO Arrays toward a Flexible, Durable Superhydrophobic and Self-Cleaning Material. Chem. Eng. J. 2017, 313, 1328–1334. [Google Scholar] [CrossRef]
- Kumar, D.; Li, L.; Chen, Z. Mechanically Robust Polyvinylidene Fluoride (PVDF) Based Superhydrophobic Coatings for Self-Cleaning Applications. Prog. Org. Coat. 2016, 101, 385–390. [Google Scholar] [CrossRef]
- Chen, X.; Wang, M.; Xin, Y.; Huang, Y. One-Step Fabrication of Self-Cleaning Superhydrophobic Surfaces: A Combined Experimental and Molecular Dynamics Study. Surf. Interfaces 2022, 31, 102022. [Google Scholar] [CrossRef]
- Jiang, L.; Hou, P.; He, S.; Han, M.; Xiang, P.; Xiao, T.; Tan, X. The Robust Superhydrophobic SiO2/Diatomite/PDMS/KH-570/Me-MQ Composite Coating for Self-Cleaning Application of Building Surface. Colloids Surf. Physicochem. Eng. Asp. 2022, 634, 127936. [Google Scholar] [CrossRef]
- Cully, P.; Karasu, F.; Müller, L.; Jauzein, T.; Leterrier, Y. Self-Cleaning and Wear-Resistant Polymer Nanocomposite Surfaces. Surf. Coat. Technol. 2018, 348, 111–120. [Google Scholar] [CrossRef]
- Deng, W.; Long, M.; Miao, X.; Wen, N.; Deng, W. Eco-Friendly Preparation of Robust Superhydrophobic Cu(OH)2 Coating for Self-Cleaning, Oil-Water Separation and Oil Sorption. Surf. Coat. Technol. 2017, 325, 14–21. [Google Scholar] [CrossRef]
- Krella, A. Resistance of PVD Coatings to Erosive and Wear Processes: A Review. Coatings 2020, 10, 921. [Google Scholar] [CrossRef]
- Penkov, O.V.; Khadem, M.; Lee, J.-S.; Kheradmandfard, M.; Kim, C.-L.; Cho, S.-W.; Kim, D.-E. Highly Durable and Biocompatible Periodical Si/DLC Nanocomposite Coatings. Nanoscale 2018, 10, 4852–4860. [Google Scholar] [CrossRef]
- Gilewicz, A.; Warcholinski, B.; Szymanski, W.; Grimm, W. CrCN/CrN+ta-C Multilayer Coating for Applications in Wood Processing. Tribol. Int. 2013, 57, 1–7. [Google Scholar] [CrossRef]
- Čolović, B.; Kisić, D.; Jokanović, B.; Rakočević, Z.; Nasov, I.; Petkoska, A.T.; Jokanović, V. Wetting Properties of Titanium Oxides, Oxynitrides and Nitrides Obtained by DC and Pulsed Magnetron Sputtering and Cathodic Arc Evaporation. Mater. Sci. Pol. 2019, 37, 173–181. [Google Scholar] [CrossRef]
- Dobromir, M.; Apetrei, R.P.; Rebegea, S.; Manole, A.V.; Nica, V.; Luca, D. Synthesis and Characterization of RF Sputtered WO3/TiO2 Bilayers. Surf. Coat. Technol. 2016, 285, 197–202. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Qian, L.Q.; Guo, C.; Jia, X.; Wang, J.W.; Tang, W.H. Natural Superhydrophilic TiO2/SiO2 Composite Thin Films Deposited by Radio Frequency Magnetron Sputtering. J. Alloys Compd. 2009, 479, 532–535. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, Z.; Cui, X.; Neil, E.; Li, Y.; Kasap, S.; Yang, Q. Reversible Superhydrophilicity and Hydrophobicity Switching of V2O5 Thin Films Deposited by Magnetron Sputtering. Appl. Surf. Sci. 2018, 433, 1094–1099. [Google Scholar] [CrossRef]
- Gao, W.; Ma, F.; Yin, Y.; Li, J. Robust and Durable Transparent Superhydrophobic F-TNTs/TiN Coating Fabricated by Structure Tuning on Surface of TiN Hard Coating. Appl. Surf. Sci. 2023, 613, 155967. [Google Scholar] [CrossRef]
- Kratochvíl, J.; Kuzminova, A.; Solař, P.; Hanuš, J.; Kylián, O.; Biederman, H. Wetting and Drying on Gradient-Nanostructured C:F Surfaces Synthesized Using a Gas Aggregation Source of Nanoparticles Combined with Magnetron Sputtering of Polytetrafluoroethylene. Vacuum 2019, 166, 50–56. [Google Scholar] [CrossRef]
- Yuan, Y.; Duan, Y.; Zuo, Z.; Yang, L.; Liao, R. Novel, Stable and Durable Superhydrophobic Film on Glass Prepared by RF Magnetron Sputtering. Mater. Lett. 2017, 199, 97–100. [Google Scholar] [CrossRef]
- Luo, B.; Deng, Y.; Wang, Y.; Shi, Y.; Cao, L.; Zhu, W. Magnetron Sputtering Based Direct Fabrication of Three Dimensional CdTe Hierarchical Nanotrees Exhibiting Stable Superhydrophobic Property. Appl. Surf. Sci. 2013, 280, 550–555. [Google Scholar] [CrossRef]
- Veziroglu, S.; Röder, K.; Gronenberg, O.; Vahl, A.; Polonskyi, O.; Strunskus, T.; Rubahn, H.-G.; Kienle, L.; Adam, J.; Fiutowski, J.; et al. Cauliflower-like CeO2–TiO2 Hybrid Nanostructures with Extreme Photocatalytic and Self-Cleaning Properties. Nanoscale 2019, 11, 9840–9844. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Li, H.; Gao, J.; Wang, Z.; Zhou, K.; Yu, S. Large-Scale Fabrication of Decoupling Coatings with Promising Robustness and Superhydrophobicity for Antifouling, Drag Reduction, and Organic Photodegradation. Friction 2023, 11, 716–736. [Google Scholar] [CrossRef]
- Khadem, M.; Penkov, O.V.; Yang, H.-K.; Kim, D.-E. Tribology of Multilayer Coatings for Wear Reduction: A Review. Friction 2017, 5, 248–262. [Google Scholar] [CrossRef]
- Extremely Durable Biofouling-Resistant Metallic Surfaces Based on Electrodeposited Nanoporous Tungstite Films on Steel|Nature Communications. Available online: https://www.nature.com/articles/ncomms9649 (accessed on 19 March 2023).
- Li, G.-X.; Liu, Y.; Wang, B.; Song, X.-M.; Li, E.; Yan, H. Preparation of Transparent BN Films with Superhydrophobic Surface. Appl. Surf. Sci. 2008, 254, 5299–5303. [Google Scholar] [CrossRef]
- Sambasivam, S.; Maram, P.S.; Muralee Gopi, C.V.V.; Obaidat, I.M. Effect of Erbium on the Structural, Morphological, and Optical Properties of SnO2 Thin Films Deposited by Spray Pyrolysis. Optik 2020, 202, 163596. [Google Scholar] [CrossRef]
- Wu, Y.; Du, J.; Liu, G.; Ma, D.; Jia, F.; Klemeš, J.J.; Wang, J. A Review of Self-Cleaning Technology to Reduce Dust and Ice Accumulation in Photovoltaic Power Generation Using Superhydrophobic Coating. Renew. Energy 2022, 185, 1034–1061. [Google Scholar] [CrossRef]
- Bao, Y.; Chang, J.; Zhang, Y.; Chen, L. Robust Superhydrophobic Coating with Hollow SiO2/PAA-b-PS Janus Microspheres for Self-Cleaning and Oil–Water Separation. Chem. Eng. J. 2022, 446, 136959. [Google Scholar] [CrossRef]
- Xiao, S.; Hao, X.; Yang, Y.; Li, L.; He, N.; Li, H. Feasible Fabrication of a Wear-Resistant Hydrophobic Surface. Appl. Surf. Sci. 2019, 463, 923–930. [Google Scholar] [CrossRef]
- Young, T., III. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1997, 95, 65–87. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of Porous Surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Yao, L.; He, J. Recent Progress in Antireflection and Self-Cleaning Technology—From Surface Engineering to Functional Surfaces. Prog. Mater. Sci. 2014, 61, 94–143. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, X.; Chen, S.; Lin, H.; Li, Z.; Lin, X. Hydrophobic or Superhydrophobic Modification of Cement-Based Materials: A Systematic Review. Compos. Part B Eng. 2022, 243, 110104. [Google Scholar] [CrossRef]
- Niu, W.; Chen, G.Y.; Xu, H.; Liu, X.; Sun, J. Highly Transparent and Self-Healable Solar Thermal Anti-/Deicing Surfaces: When Ultrathin MXene Multilayers Marry a Solid Slippery Self-Cleaning Coating. Adv. Mater. 2022, 34, 2108232. [Google Scholar] [CrossRef] [PubMed]
- Garlisi, C.; Trepci, E.; Li, X.; Al Sakkaf, R.; Al-Ali, K.; Nogueira, R.P.; Zheng, L.; Azar, E.; Palmisano, G. Multilayer Thin Film Structures for Multifunctional Glass: Self-Cleaning, Antireflective and Energy-Saving Properties. Appl. Energy 2020, 264, 114697. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Zheng, H.; Chen, R.; Ma, C.; Zhang, G. Multifunctional Hard Yet Flexible Coatings Fabricated Using a Universal Step-by-Step Strategy. Adv. Sci. 2022, 9, 2200268. [Google Scholar] [CrossRef]
- Li, X.-M.; Reinhoudt, D.; Crego-Calama, M. What Do We Need for a Superhydrophobic Surface? A Review on the Recent Progress in the Preparation of Superhydrophobic Surfaces. Chem. Soc. Rev. 2007, 36, 1350–1368. [Google Scholar] [CrossRef]
- Zhang, L.; Uzoma, P.C.; Xiaoyang, C.; Penkov, O.V.; Hu, H. Bio-Inspired Hierarchical Micro/Nanostructured Surfaces for Superhydrophobic and Anti-Ice Applications. Front. Bioeng. Biotechnol. 2022, 10, 872268. [Google Scholar] [CrossRef]
- Verho, T.; Bower, C.; Andrew, P.; Franssila, S.; Ikkala, O.; Ras, R.H.A. Mechanically Durable Superhydrophobic Surfaces. Adv. Mater. 2011, 23, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Y.; Xiao, F.; Hess, D.W.; Wong, C.P. Superhydrophobic Optically Transparent Silica Films Formed with a Eutectic Liquid. Thin Solid Films 2009, 517, 1610–1615. [Google Scholar] [CrossRef]
- Zhu, D.; Tan, X.; Ji, L.; Shi, Z.; Zhang, X. Preparation of Transparent and Hydrophobic Cerium Oxide Films with Stable Mechanical Properties by Magnetron Sputtering. Vacuum 2021, 184, 109888. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.; Lee, S.W.; Kang, S.-M.; Kim, Y.H.; Jo, W.; Kim, T.-S.; Jang, J.; Bae, B.-S. Elongation Improvement of Transparent and Flexible Surface Protective Coating Using Polydimethylsiloxane-Anchored Epoxy-Functionalized Siloxane Hybrid Composite for Reliable out-Foldable Displays. Compos. Part B Eng. 2021, 225, 109313. [Google Scholar] [CrossRef]
- Maharjan, S.; Liao, K.-S.; Wang, A.J.; Barton, K.; Haldar, A.; Alley, N.J.; Byrne, H.J.; Curran, S.A. Self-Cleaning Hydrophobic Nanocoating on Glass: A Scalable Manufacturing Process. Mater. Chem. Phys. 2020, 239, 122000. [Google Scholar] [CrossRef]
- Zhong, Q.; Macharia, D.K.; Zhong, W.; Liu, Z.; Chen, Z. Synthesis of Hydrophobic W18O49 Nanorods for Constructing UV/NIR-Shielding and Self-Cleaning Film. Ceram. Int. 2020, 46, 11898–11904. [Google Scholar] [CrossRef]
- Sarkın, A.S.; Ekren, N.; Sağlam, Ş. A Review of Anti-Reflection and Self-Cleaning Coatings on Photovoltaic Panels. Sol. Energy 2020, 199, 63–73. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, X. Special Oleophobic and Hydrophilic Surfaces: Approaches, Mechanisms, and Applications. J. Mater. Chem. A 2017, 5, 3759–3773. [Google Scholar] [CrossRef]
- England, M.W.; Urata, C.; Dunderdale, G.J.; Hozumi, A. Anti-Fogging/Self-Healing Properties of Clay-Containing Transparent Nanocomposite Thin Films. ACS Appl. Mater. Interfaces 2016, 8, 4318–4322. [Google Scholar] [CrossRef]
- Ratova, M.; Klaysri, R.; Praserthdam, P.; Kelly, P.J. Visible Light Active Photocatalytic C-Doped Titanium Dioxide Films Deposited via Reactive Pulsed DC Magnetron Co-Sputtering: Properties and Photocatalytic Activity. Vacuum 2018, 149, 214–224. [Google Scholar] [CrossRef]
- Zhao, W.; Lu, H. Self-Cleaning Performance of Super-Hydrophilic Coatings for Dust Deposition Reduction on Solar Photovoltaic Cells. Coatings 2021, 11, 1059. [Google Scholar] [CrossRef]
- Anandan, S.; Narasinga Rao, T.; Sathish, M.; Rangappa, D.; Honma, I.; Miyauchi, M. Superhydrophilic Graphene-Loaded TiO2 Thin Film for Self-Cleaning Applications. ACS Appl. Mater. Interfaces 2013, 5, 207–212. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-Cleaning Applications of TiO2 by Photo-Induced Hydrophilicity and Photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 396–428. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Light-Induced Amphiphilic Surfaces. Nature 1997, 388, 431–432. [Google Scholar] [CrossRef]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Photogeneration of Highly Amphiphilic TiO2 Surfaces. Adv. Mater. 1998, 10, 135–138. [Google Scholar] [CrossRef]
- Su, X.; Li, H.; Lai, X.; Chen, Z.; Zeng, X. Highly Stretchable and Conductive Superhydrophobic Coating for Flexible Electronics. ACS Appl. Mater. Interfaces 2018, 10, 10587–10597. [Google Scholar] [CrossRef] [PubMed]
- Ge, C.; Cretu, E. A Sacrificial-Layer-Free Fabrication Technology for MEMS Transducer on Flexible Substrate. Sens. Actuators Phys. 2019, 286, 202–210. [Google Scholar] [CrossRef]
- Musil, J. Flexible Hard Nanocomposite Coatings. RSC Adv. 2015, 5, 60482–60495. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, S.; Wang, J.; Liu, G. Transparent Organic/Silica Nanocomposite Coating That Is Flexible, Omniphobic, and Harder than a 9H Pencil. Chem. Eng. J. 2020, 396, 125211. [Google Scholar] [CrossRef]
- Mehanna, Y.A.; Crick, C.R. Image Analysis Methodology for a Quantitative Evaluation of Coating Abrasion Resistance. Appl. Mater. Today 2021, 25, 101203. [Google Scholar] [CrossRef]
- Amazon.Com: MXBAOHENG QTX Paint Film Flexibility Tester Elastic Film Tester GB GB/T1731-93: Tools & Home Improvement. Available online: https://www.amazon.com/-/zh_TW/HQ-1317/dp/B077FX74VG (accessed on 19 May 2023).
- Yanagisawa, T.; Nakajima, A.; Sakai, M.; Kameshima, Y.; Okada, K. Preparation and Abrasion Resistance of Transparent Super-Hydrophobic Coating by Combining Crater-like Silica Films with Acicular Boehmite Powder. Mater. Sci. Eng. B 2009, 161, 36–39. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Salim, A.; Seeger, S. Hierarchical Structured Multifunctional Self-Cleaning Material with Durable Superhydrophobicity and Photocatalytic Functionalities. Small 2019, 15, 1901822. [Google Scholar] [CrossRef] [PubMed]
- Kustandi, T.S.; Loh, W.W.; Shen, L.; Low, H.Y. Reversible Recovery of Nanoimprinted Polymer Structures. Langmuir 2013, 29, 10498–10504. [Google Scholar] [CrossRef] [PubMed]
- Ionov, L.; Synytska, A. Self-Healing Superhydrophobic Materials. Phys. Chem. Chem. Phys. 2012, 14, 10497–10502. [Google Scholar] [CrossRef] [PubMed]
- Penkov, O.V.; Khadem, M.; Kim, D.-E. Hard, Flexible, and Transparent Nanolayered SiNx/BN Periodical Coatings. ACS Appl. Mater. Interfaces 2019, 11, 9685–9690. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Z.; Yang, C.; Zhang, D.; Tian, Y.; Liu, X. The Investigation of Mechanical and Thermal Properties of Super-Hydrophobic Nitinol Surfaces Fabricated by Hybrid Methods of Laser Irradiation and Carbon Ion Implantation. Appl. Surf. Sci. 2020, 527, 146889. [Google Scholar] [CrossRef]
- Li, H.; Xin, L.; Zhang, K.; Yin, X.; Yu, S. Fluorine-Free Fabrication of Robust Self-Cleaning and Anti-Corrosion Superhydrophobic Coating with Photocatalytic Function for Enhanced Anti-Biofouling Property. Surf. Coat. Technol. 2022, 438, 128406. [Google Scholar] [CrossRef]
- Penkov, O.V.; Devizenko, A.Y.; Khadem, M.; Zubarev, E.N.; Kondratenko, V.V.; Kim, D.-E. Toward Zero Micro/Macro-Scale Wear Using Periodic Nano-Layered Coatings. ACS Appl. Mater. Interfaces 2015, 7, 18136–18144. [Google Scholar] [CrossRef]
- Straffelini, G. Friction and Wear: Methodologies for Design and Control; Springer Tracts in Mechanical Engineering; Springer International Publishing: Cham, Switzerland, 2015; ISBN 978-3-319-05893-1. [Google Scholar]
- Wu, Y.; Tan, X.; Wang, Y.; Tao, F.; Yu, M.; Chen, X. Nonfluorinated, Transparent, and Antireflective Hydrophobic Coating with Self-Cleaning Function. Colloids Surf. Physicochem. Eng. Asp. 2022, 634, 127919. [Google Scholar] [CrossRef]
- Kumar, D.; Wu, X.; Fu, Q.; Ho, J.W.C.; Kanhere, P.D.; Li, L.; Chen, Z. Development of Durable Self-Cleaning Coatings Using Organic–Inorganic Hybrid Sol–Gel Method. Appl. Surf. Sci. 2015, 344, 205–212. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, J.; Cao, W.; Huang, Y.; Zhou, Z.; Huang, Y.-X. Fabrication of Transparent Wear-Resistant Superhydrophobic SiO2 Film via Phase Separation and Chemical Vapor Deposition Methods. Ceram. Int. 2022, 48, 32143–32151. [Google Scholar] [CrossRef]
- Adachi, T.; Latthe, S.S.; Gosavi, S.W.; Roy, N.; Suzuki, N.; Ikari, H.; Kato, K.; Katsumata, K.; Nakata, K.; Furudate, M.; et al. Photocatalytic, Superhydrophilic, Self-Cleaning TiO2 Coating on Cheap, Light-Weight, Flexible Polycarbonate Substrates. Appl. Surf. Sci. 2018, 458, 917–923. [Google Scholar] [CrossRef]
- Mazur, M.; Wojcieszak, D.; Kaczmarek, D.; Domaradzki, J.; Song, S.; Gibson, D.; Placido, F.; Mazur, P.; Kalisz, M.; Poniedzialek, A. Functional Photocatalytically Active and Scratch Resistant Antireflective Coating Based on TiO2 and SiO2. Appl. Surf. Sci. 2016, 380, 165–171. [Google Scholar] [CrossRef]
- Cheng, W.; Ren, H.; Chen, Y.; Wu, D.; Li, X.; Yu, C.; Li, F. Fabrication of Cu/Cu2O/CuO@WO3 Composite Films with Antireflective, Hydrophilic, and Photocatalytic Properties by Magnetron Sputtering. Appl. Surf. Sci. 2022, 585, 152714. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, G.; Pan, J.; Ma, C.; Zhang, G. Non-Isocyanate Polyurethane Coating with High Hardness, Superior Flexibility, and Strong Substrate Adhesion. ACS Appl. Mater. Interfaces 2023, 15, 5998–6004. [Google Scholar] [CrossRef]
- Sun, Y.; Singh Rawat, R.; Chen, Z. Mechanically Robust Multifunctional Antifogging Coating on Transparent Plastic Substrates. Appl. Surf. Sci. 2022, 580, 152307. [Google Scholar] [CrossRef]
- Zhang, J.; Singh, V.; Huang, W.; Mandal, P.; Tiwari, M.K. Self-Healing, Robust, Liquid-Repellent Coatings Exploiting the Donor–Acceptor Self-Assembly. ACS Appl. Mater. Interfaces 2023, 15, 8699–8708. [Google Scholar] [CrossRef]
- Hanson, B.; Hofmann, J.; Pasquinelli, M.A. Influence of Copolyester Composition on Adhesion to Soda-Lime Glass via Molecular Dynamics Simulations. ACS Appl. Mater. Interfaces 2016, 8, 13583–13589. [Google Scholar] [CrossRef]
- Rezaee, M.; Tsai, L.-C.; Haider, M.I.; Yazdi, A.; Sanatizadeh, E.; Salowitz, N.P. Quantitative Peel Test for Thin Films/Layers Based on a Coupled Parametric and Statistical Study. Sci. Rep. 2019, 9, 19805. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Y.; Wang, C.-L.; Meng, S. Experimental and Finite Element Analysis of Flexural Performance of Steel-Timber Composite Beams Connected by Hybrid-Anchored Screws. Eng. Struct. 2023, 292, 116503. [Google Scholar] [CrossRef]
- Motamedi, M.; Warkiani, M.E.; Taylor, R.A. Transparent Surfaces Inspired by Nature. Adv. Opt. Mater. 2018, 6, 1800091. [Google Scholar] [CrossRef]
- Cai, J.; Qi, L. Recent Advances in Antireflective Surfaces Based on Nanostructure Arrays. Mater. Horiz. 2014, 2, 37–53. [Google Scholar] [CrossRef]
- Schulz, U. Review of Modern Techniques to Generate Antireflective Properties on Thermoplastic Polymers. Appl. Opt. 2006, 45, 1608–1618. [Google Scholar] [CrossRef] [PubMed]
- Ruud, C.J.; Cleri, A.; Maria, J.-P.; Giebink, N.C. Ultralow Index SiO 2 Antireflection Coatings Produced via Magnetron Sputtering. Nano Lett. 2022, 22, 7358–7362. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Z.; Zhang, X.; Zhang, Z.; Chen, J. (0 0 1) Facets Optimized Surface Oxygen Vacancies in TiO2 Films to Enhance Photocatalytic Antibacterial and Hydrophilic Properties. Appl. Surf. Sci. 2023, 616, 156571. [Google Scholar] [CrossRef]
- Zuo, Z.; Gao, J.; Liao, R.; Zhao, X.; Yuan, Y. A Novel and Facile Way to Fabricate Transparent Superhydrophobic Film on Glass with Self-Cleaning and Stability. Mater. Lett. 2019, 239, 48–51. [Google Scholar] [CrossRef]
- Xu, Z.; Guo, Y.; Liu, Y.; Jia, B.; Sha, P.; Li, L.; Yu, Z.; Zhang, Z.; Ren, L. An Extremely Efficiency Method to Achieve Stable Superhydrophobicity on the Surface of Additive Manufactured NiTi Alloys: “Ultrasonic Fluorination”. Appl. Surf. Sci. 2023, 612, 155947. [Google Scholar] [CrossRef]
- Qian, B.; Shen, Z. Fabrication of Superhydrophobic Surfaces by Dislocation-Selective Chemical Etching on Aluminum, Copper, and Zinc Substrates. Langmuir 2005, 21, 9007–9009. [Google Scholar] [CrossRef]
- Balu, B.; Breedveld, V.; Hess, D.W. Fabrication of “Roll-off” and “Sticky” Superhydrophobic Cellulose Surfaces via Plasma Processing. Langmuir 2008, 24, 4785–4790. [Google Scholar] [CrossRef]
- Fresno, F.; González, M.U.; Martínez, L.; Fernández-Castro, M.; Barawi, M.; Villar-García, I.J.; Soler-Morala, J.; Reñones, P.; Luna, M.; Huttel, Y.; et al. Photo-Induced Self-Cleaning and Wettability in TiO2 Nanocolumn Arrays Obtained by Glancing-Angle Deposition with Sputtering. Adv. Sustain. Syst. 2021, 5, 2100071. [Google Scholar] [CrossRef]
- Lukong, V.T.; Ukoba, K.; Jen, T.-C. Review of Self-Cleaning TiO2 Thin Films Deposited with Spin Coating. Int. J. Adv. Manuf. Technol. 2022, 122, 3525–3546. [Google Scholar] [CrossRef]
- Xia, X.; Liu, J.; Liu, Y.; Lei, Z.; Han, Y.; Zheng, Z.; Yin, J. Preparation and Characterization of Biomimetic SiO2-TiO2-PDMS Composite Hydrophobic Coating with Self-Cleaning Properties for Wall Protection Applications. Coatings 2023, 13, 224. [Google Scholar] [CrossRef]
- Seifi, A.; Salari, D.; Khataee, A.; Çoşut, B.; Arslan, L.Ç.; Niaei, A. Enhanced Photocatalytic Activity of Highly Transparent Superhydrophilic Doped TiO2 Thin Films for Improving the Self-Cleaning Property of Solar Panel Covers. Ceram. Int. 2023, 49, 1678–1689. [Google Scholar] [CrossRef]
- Ismail, A.A.; Al-Hajji, L.; Azad, I.S.; Al-Yaqoot, A.; Habibi, N.; Alseidi, M.; Ahmed, S. Self-Cleaning Application of Mesoporous ZnO, TiO2 and Fe2O3 Films with the Accommodation of Silver Nanoparticles for Antibacterial Activity. J. Taiwan Inst. Chem. Eng. 2023, 142, 104627. [Google Scholar] [CrossRef]
- Xue, F.; Shi, X.; Bai, W.; Li, J. Enhanced Durability and Versatile Superhydrophobic Coatings via Facile One-Step Spraying Technique. Colloids Surf. Physicochem. Eng. Asp. 2022, 640, 128411–128420. [Google Scholar] [CrossRef]
- Huang, Z.; Gurney, R.S.; Wang, T.; Liu, D. Environmentally Durable Superhydrophobic Surfaces with Robust Photocatalytic Self-Cleaning and Self-Healing Properties Prepared via Versatile Film Deposition Methods. J. Colloid Interface Sci. 2018, 527, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Wang, Y.; Huang, Z.; Sabin, S.; Xiao, T.; Jiang, L.; Chen, X. Facile Fabrication of a Mechanical, Chemical, Thermal, and Long-Term Outdoor Durable Fluorine-Free Superhydrophobic Coating. Adv. Mater. Interfaces 2021, 8, 2002209. [Google Scholar] [CrossRef]
- Lasprilla-Botero, J.; Torres-Giner, S.; Pardo-Figuerez, M.; Álvarez-Láinez, M.M.; Lagaron, J. Superhydrophobic Bilayer Coating Based on Annealed Electrospun Ultrathin Poly(ε-Caprolactone) Fibers and Electrosprayed Nanostructured Silica Microparticles for Easy Emptying Packaging Applications. Coatings 2018, 8, 173. [Google Scholar] [CrossRef]
- Raut, H.K.; Ganesh, V.A.; Nair, A.S.; Ramakrishna, S. Anti-Reflective Coatings: A Critical, in-Depth Review. Energy Environ. Sci. 2011, 4, 3779–3804. [Google Scholar] [CrossRef]
- Butt, M.A. Thin-Film Coating Methods: A Successful Marriage of High-Quality and Cost-Effectiveness—A Brief Exploration. Coatings 2022, 12, 1115. [Google Scholar] [CrossRef]
- Park, S.; Huo, J.; Shin, J.; Heo, K.J.; Kalmoni, J.J.; Sathasivam, S.; Hwang, G.B.; Carmalt, C.J. Production of an EP/PDMS/SA/AlZnO Coated Superhydrophobic Surface through an Aerosol-Assisted Chemical Vapor Deposition Process. Langmuir 2022, 38, 7825–7832. [Google Scholar] [CrossRef] [PubMed]
- Mauchauffé, R.; Kang, S.; Moon, S.Y. Fast Formation of Amorphous Titanium Dioxide Thin Films Using a Liquid-Assisted Plasma-Enhanced Deposition Process in Open Air. Surf. Coat. Technol. 2019, 376, 84–89. [Google Scholar] [CrossRef]
- Weihao, L.; Shengnan, C.; Jianxun, W.; Shaohui, J.; Chenlu, S.; Yong, L.; Gaorong, H. Study on Structural, Optical and Hydrophilic Properties of FTO/TiO2 Tandem Thin Film Prepared by Aerosol-Assisted Chemical Vapor Deposition Method. Surf. Coat. Technol. 2019, 358, 715–720. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, C.-H.; Cao, M.; Zhang, M.-M.; Li, M.; Ma, J.-Z. Highly Transparent Fluorine-Free Superhydrophobic Silica Nanotube Coatings. Chem. Eng. J. 2017, 320, 244–252. [Google Scholar] [CrossRef]
- Tan, X.-Q.; Liu, J.-Y.; Niu, J.-R.; Liu, J.-Y.; Tian, J.-Y. Recent Progress in Magnetron Sputtering Technology Used on Fabrics. Materials 2018, 11, 1953. [Google Scholar] [CrossRef]
- Yang, M.K. Optical Properties of Teflon® AF Amorphous Fluoropolymers. J. Micro Nanolithogr. MEMS MOEMS 2008, 7, 033010. [Google Scholar] [CrossRef]
- Sohn, S.; Kim, H.-M.; Jang, J. Super-Hydrophobicity of PTFE Films Coated on an Etched Al Surface by Using a RF-Magnetron Sputtering Method. J. Korean Phys. Soc. 2010, 57, 1281–1284. [Google Scholar] [CrossRef]
- Tripathi, S.; Haque, S.M.; Rao, K.D.; De, R.; Shripathi, T.; Deshpande, U.; Ganesan, V.; Sahoo, N.K. Investigation of Optical and Microstructural Properties of RF Magnetron Sputtered PTFE Films for Hydrophobic Applications. Appl. Surf. Sci. 2016, 385, 289–298. [Google Scholar] [CrossRef]
- Zenkin, S.; Kos, Š.; Musil, J. Hydrophobicity of Thin Films of Compounds of Low-Electronegativity Metals. J. Am. Ceram. Soc. 2014, 97, 2713–2717. [Google Scholar] [CrossRef]
- Musil, J.; Zenkin, S.; Kos, Š.; Čerstvý, R.; Haviar, S. Flexible Hydrophobic ZrN Nitride Films. Vacuum 2016, 131, 34–38. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, L.; Qian, H.; Li, X. Superhydrophobic Surfaces for Corrosion Protection: A Review of Recent Progresses and Future Directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef]
- PalDey, S.; Deevi, S.C. Single Layer and Multilayer Wear Resistant Coatings of (Ti, Al)N: A Review. Mater. Sci. Eng. A 2003, 342, 58–79. [Google Scholar] [CrossRef]
- Karuppasamy, A. Enhanced Photocatalysis and Photohydrophilicity in TiO2–W5O14 Nanocomposite Thin Films Grown by in-Lay Sputtering. Mater. Chem. Phys. 2023, 301, 127580. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, C.; Wang, L.; Fan, X.; Wang, Q.; Liu, J. Preparation and Optical Properties of TiO2/SiO2 Bilayer Antireflection Film. Opt. Mater. 2021, 121, 111594. [Google Scholar] [CrossRef]
- Lukong, V.T.; Mouchou, R.T.; Enebe, G.C.; Ukoba, K.; Jen, T.C. Deposition and Characterization of Self-Cleaning TiO2 Thin Films for Photovoltaic Application. Mater. Today Proc. 2022, 62, S63–S72. [Google Scholar] [CrossRef]
- Khan, S.B.; Zhang, Z.; Lee, S.L. Single Component: Bilayer TiO2 as a Durable Antireflective Coating. J. Alloys Compd. 2020, 834, 155137. [Google Scholar] [CrossRef]
- Miao, D.; Hu, H.; Li, A.; Jiang, S.; Shang, S. Fabrication of Porous and Amorphous TiO2 Thin Films on Flexible Textile Substrates. Ceram. Int. 2015, 41, 9177–9182. [Google Scholar] [CrossRef]
- Lelis, M.; Tuckute, S.; Varnagiris, S.; Urbonavicius, M.; Laukaitis, G.; Bockute, K. Tailoring of TiO2 Film Microstructure by Pulsed-DC and RF Magnetron Co-Sputtering. Surf. Coat. Technol. 2019, 377, 124906. [Google Scholar] [CrossRef]
- Sabbah, H. Effect of Sputtering Parameters on the Self-Cleaning Properties of Amorphous Titanium Dioxide Thin Films. J. Coat. Technol. Res. 2017, 14, 1423–1433. [Google Scholar] [CrossRef]
- Feng, J.; Yan, B.; Lai, M.O.; Li, L. Design and Fabrication of an All-Solid-State Thin-Film Li-Ion Microbattery with Amorphous TiO2 as the Anode. Energy Technol. 2014, 2, 397–400. [Google Scholar] [CrossRef]
- Stamate, M.D. On the Dielectric Properties of Dc Magnetron TiO2 Thin Films. Appl. Surf. Sci. 2003, 218, 318–323. [Google Scholar] [CrossRef]
- Twu, M.J.; Chiou, A.H.; Hu, C.C.; Hsu, C.Y.; Kuo, C.G. Properties of TiO2 Films Deposited on Flexible Substrates Using Direct Current Magnetron Sputtering and Using High Power Impulse Magnetron Sputtering. Polym. Degrad. Stab. 2015, 117, 1–7. [Google Scholar] [CrossRef]
- Arun, J.; Nachiappan, S.; Rangarajan, G.; Alagappan, R.P. Synthesis and Application of Titanium Dioxide Photocatalysis for Energy, Decontamination and Viral Disinfection: A Review. Environ. Chem. Lett. 2023, 21, 339–362. [Google Scholar] [CrossRef] [PubMed]
- Abidi, M.; Assadi, A.A.; Bouzaza, A.; Hajjaji, A.; Bessais, B.; Rtimi, S. Photocatalytic Indoor/Outdoor Air Treatment and Bacterial Inactivation on CuxO/TiO2 Prepared by HiPIMS on Polyester Cloth under Low Intensity Visible Light. Appl. Catal. B Environ. 2019, 259, 118074. [Google Scholar] [CrossRef]
- Fouad, S.S.; Baradács, E.; Nabil, M.; Parditka, B.; Negm, S.; Erdélyi, Z. Microstructural and Optical Duality of TiO2/Cu/TiO2 Trilayer Films Grown by Atomic Layer Deposition and DC Magnetron Sputtering. Inorg. Chem. Commun. 2022, 145, 110017. [Google Scholar] [CrossRef]
- Khadem, M.; Penkov, O.V.; Jais, J.; Bae, S.-M.; Dhandapani, V.S.; Kang, B.; Kim, D.-E. Formation of Discrete Periodic Nanolayered Coatings through Tailoring of Nanointerfaces—Toward Zero Macroscale Wear. Sci. Adv. 2021, 7, eabk1224. [Google Scholar] [CrossRef]
- Penkov, O.V.; Kopylets, I.A.; Kondratenko, V.V.; Khadem, M. Synthesis and Structural Analysis of Mo/B Periodical Multilayer X-ray Mirrors for beyond Extreme Ultraviolet Optics. Mater. Des. 2021, 198, 109318. [Google Scholar] [CrossRef]


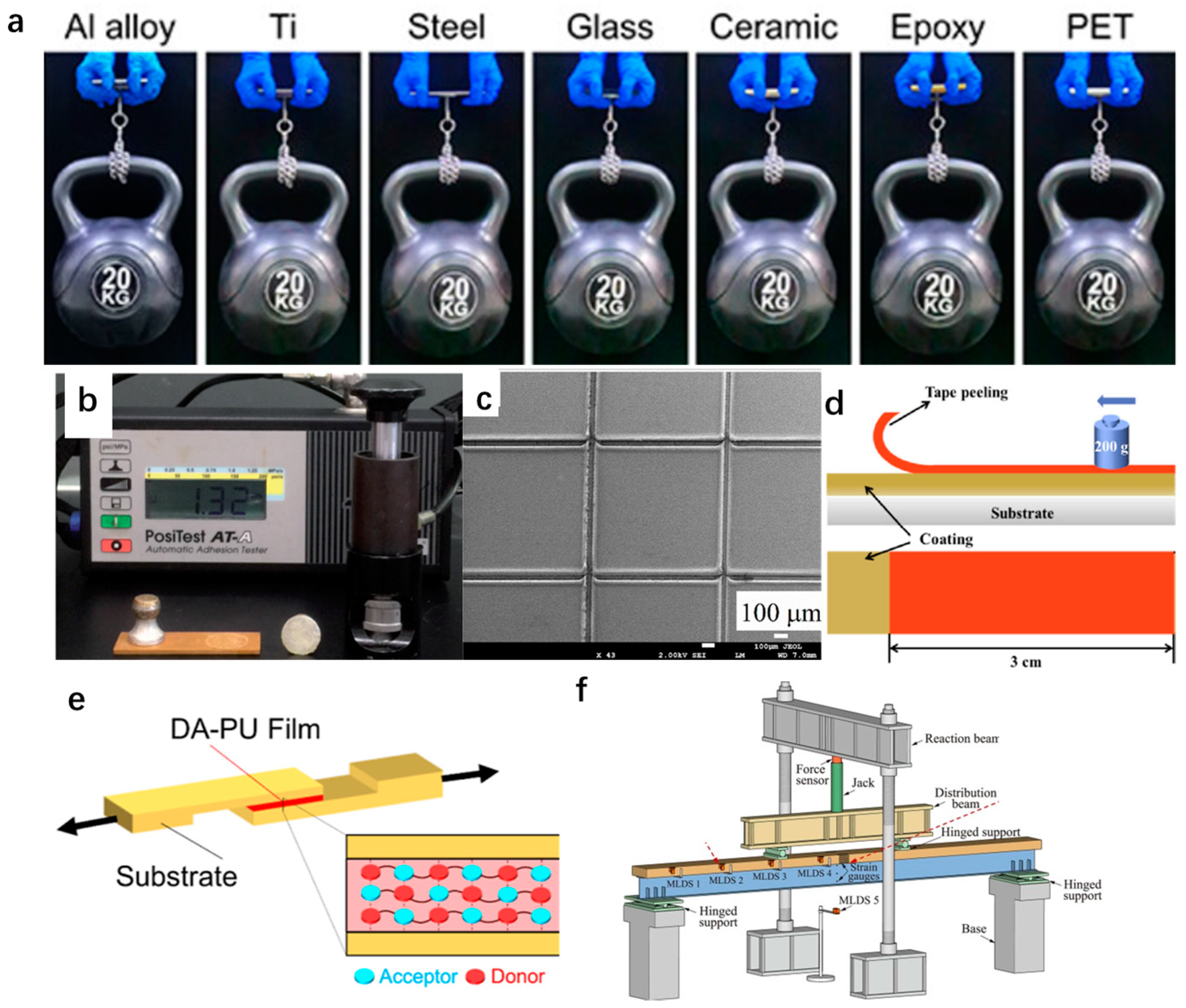



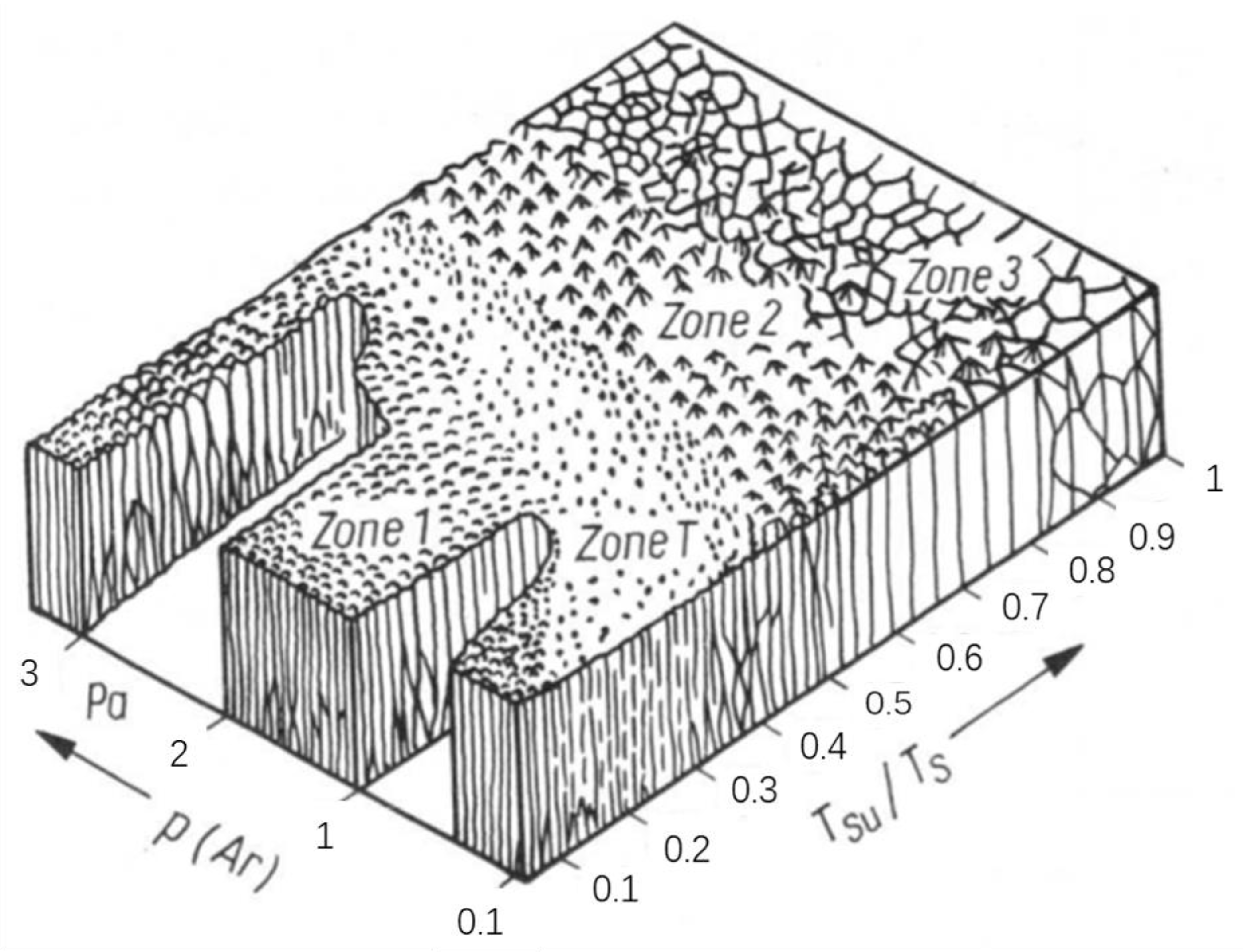
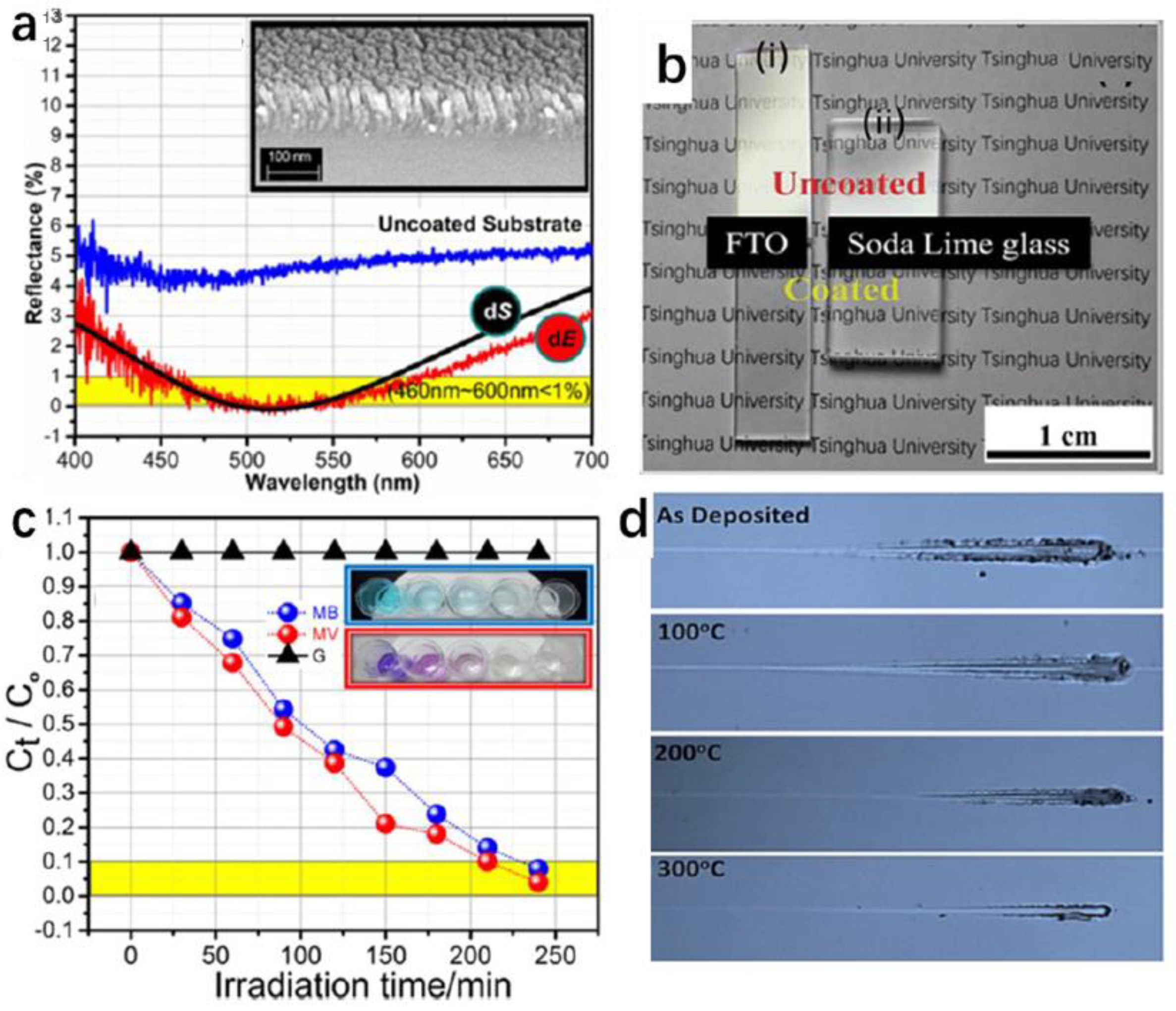
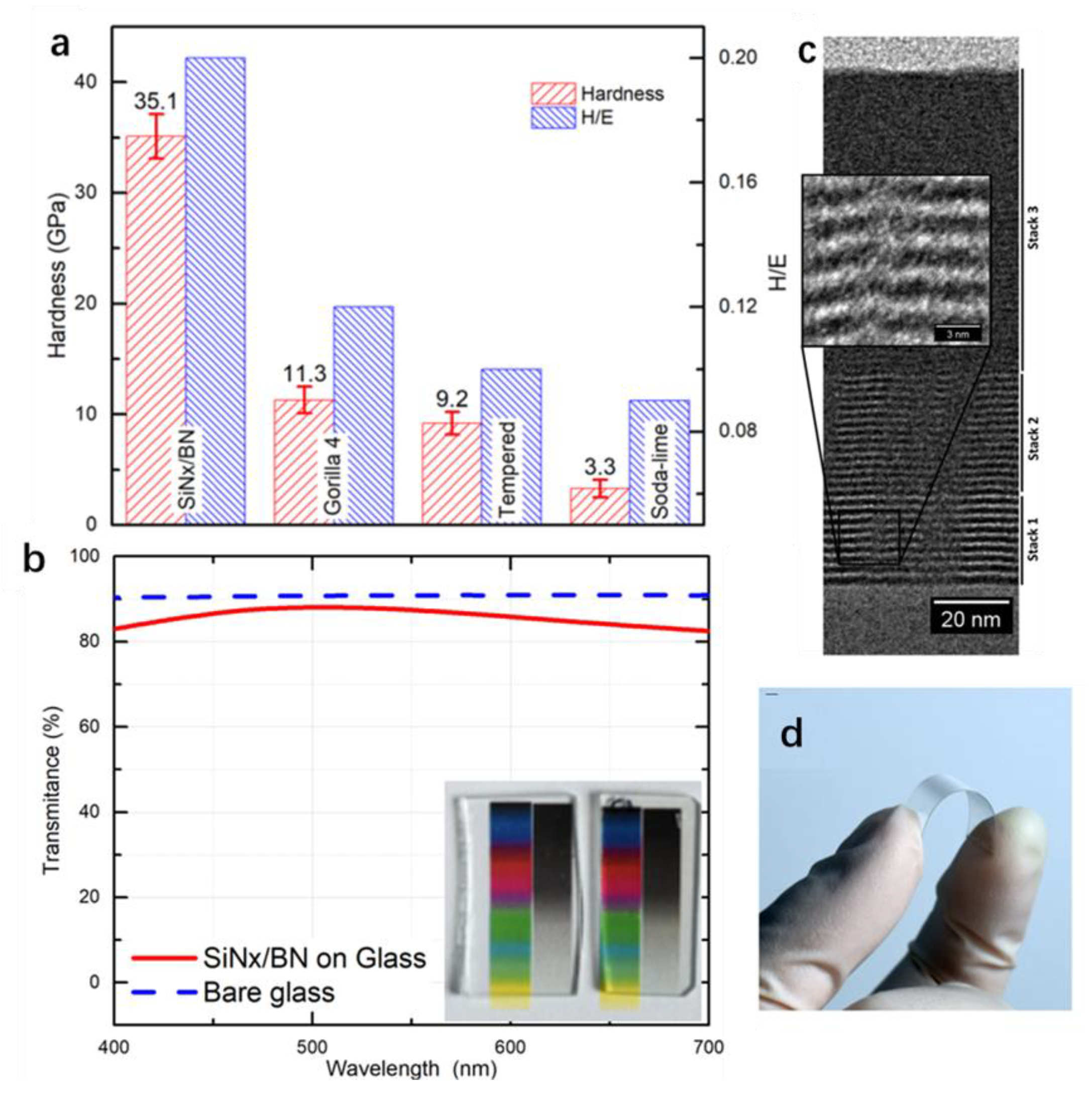
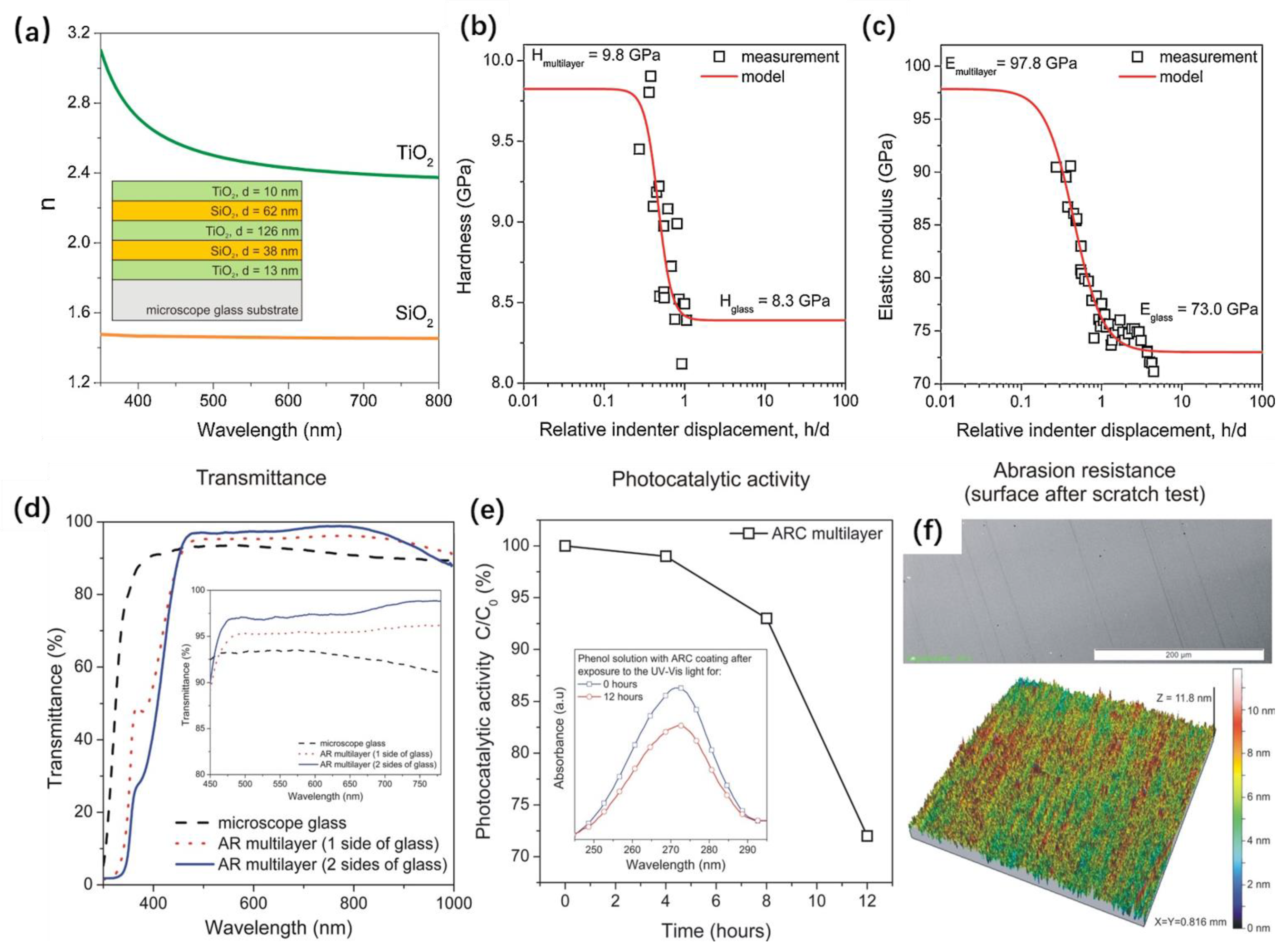
| Method | Sample | Property | Test Method for Mechanical Durability | Application | Ref. |
|---|---|---|---|---|---|
| Sol–gel with spray coating | Micro-SiO2 and nano-TiO2 | Super hydrophobic, transparent, photocatalytic | Sandpaper abrasion, Linear wear test | Self-cleaning, drag reduction, antifouling | [35] |
| Sol–gel | Epoxy–siloxane | Hydrophobic, transparent, flexible, hard (7H) | Linear wear test | Foldable displays, marine industries | [47] |
| Sol–gel with dip coating | Nano-SiO2 and Me-MQ | Hydrophobic, transparent, antireflective | Sand impact test | Self-cleaning, anti-ultraviolet | [102] |
| Sol–gel with dip coating | TEOS and glycidoxypropyltriethox ysilane | Super hydrophobic, transparent | Sandpaper abrasion | Self-cleaning, outdoor applications | [94] |
| Spin coating and CVD | Nano-SiO2 | Super hydrophobic, transparent | Linear wear test | Self-cleaning, anti-icing | [103] |
| Sol–gel with dip coating | SiO2/TiO2 | Super hydrophilic, photocatalytic, transparent, flexible | Taber abrasion tests | Microscopes, eyeglasses, solar cell panel covers | [104] |
| Sol–gel with spin coating | polyvinylpyrrolidone and aminopropyl-functionalized, nanoscale clay platelets | Hydrophilic, transparent, self-healing | N/A | Anti-fogging applications | [60] |
| Sol–gel | Poly (NVP-co-MA) | Hydrophilic, transparent | N/A | Lenses, ocean exploration equipment | [4] |
| Sputtering | CeO2—TiO2 | Hydrophilic, photocatalytic, transparent | N/A | Photocatalytic and self-cleaning outdoor and indoor applications | [34] |
| Sputtering | SiO2/TiO2 | Photocatalytic, antireflective, hard (9.8 GPa) | Scratch test, Linear wear test. | Displays, solar cells | [93] |
| Sputtering | CeO2 | Hydrophobic, transparent | Linear abrasion test | Protective coating | [54] |
| Sputtering | Cu/Cu2O/CuO@WO3 | Hydrophilic, photocatalytic, transparent | N/A | Solar panels | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Xue, Z.; Yu, T.; Penkov, O.V. Transparent Self-Cleaning Coatings: A Review. Coatings 2023, 13, 1270. https://doi.org/10.3390/coatings13071270
Wu P, Xue Z, Yu T, Penkov OV. Transparent Self-Cleaning Coatings: A Review. Coatings. 2023; 13(7):1270. https://doi.org/10.3390/coatings13071270
Chicago/Turabian StyleWu, Pengyuan, Zhuanzhuan Xue, Tianxiang Yu, and Oleksiy V. Penkov. 2023. "Transparent Self-Cleaning Coatings: A Review" Coatings 13, no. 7: 1270. https://doi.org/10.3390/coatings13071270
APA StyleWu, P., Xue, Z., Yu, T., & Penkov, O. V. (2023). Transparent Self-Cleaning Coatings: A Review. Coatings, 13(7), 1270. https://doi.org/10.3390/coatings13071270







