Preparation, Characterization, Release and Antibacterial Properties of Cinnamon Essential Oil Microcapsules
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Encapsulation Process
2.4. Physical and Chemical Characterizations of CMS
2.4.1. Encapsulation Efficiency
2.4.2. Particle Size and Scanning Electron Microscopy (SEM)
2.4.3. Zeta Potential
2.4.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.4.5. Thermal Gravity–Differential Thermal Analysis (TG-DTA)
2.4.6. Differential Scanning Calorimetry (DSC)
2.5. In Vitro Release Studies
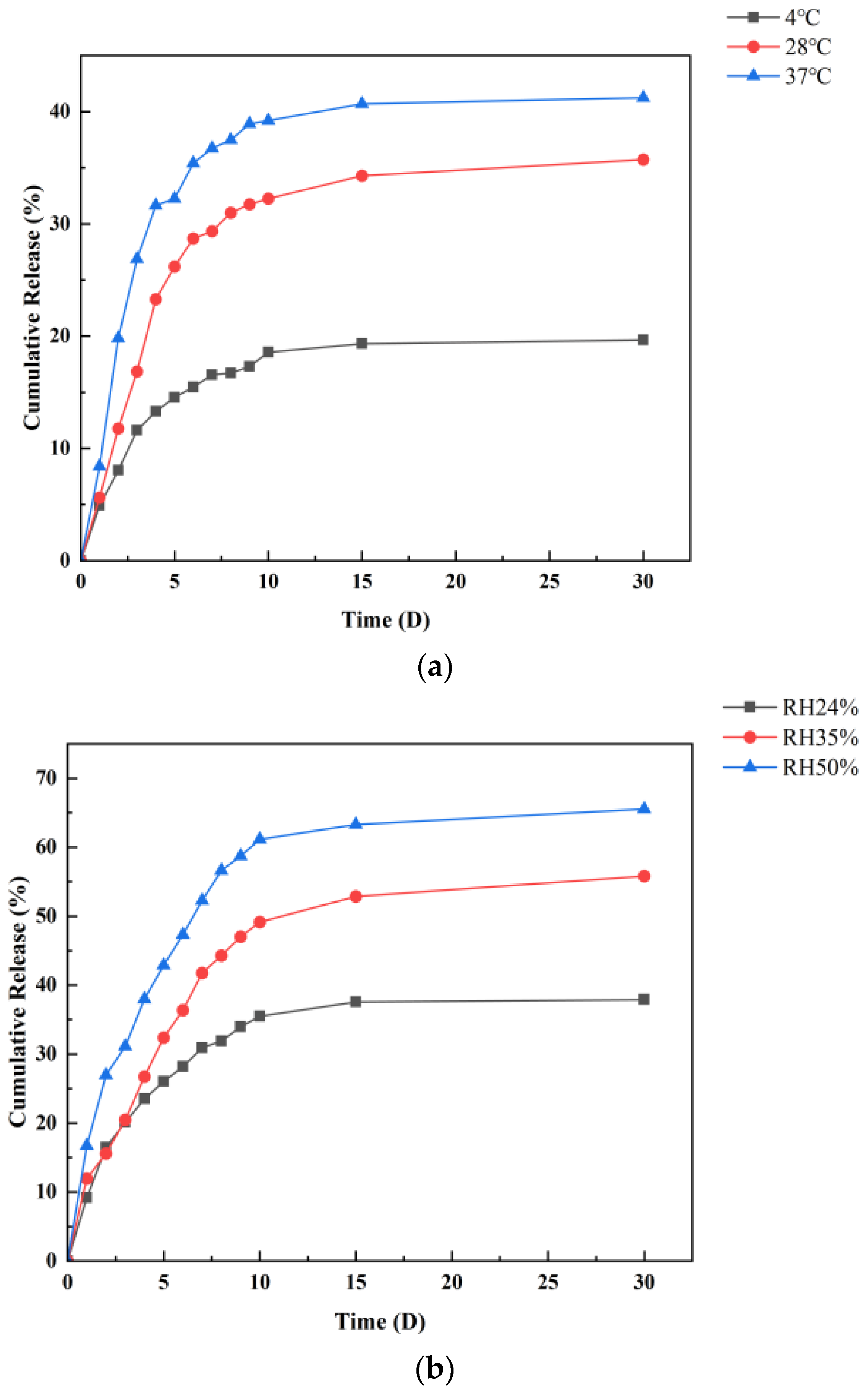
2.5.1. Effect of Temperature on the Release of Cinnamon Oil and Microcapsule
2.5.2. Effect of Relative Humidity on the Release of Cinnamon Oil and Microcapsule
2.6. Antimicrobial Properties
2.6.1. Antimicrobial Activity of Cinnamon Oil and Microcapsule
2.6.2. Effect of Temperature on Antibacterial Stability
2.6.3. Effect of pH on Antibacterial Stability
2.7. Statistical Analysis
3. Results and Discussion
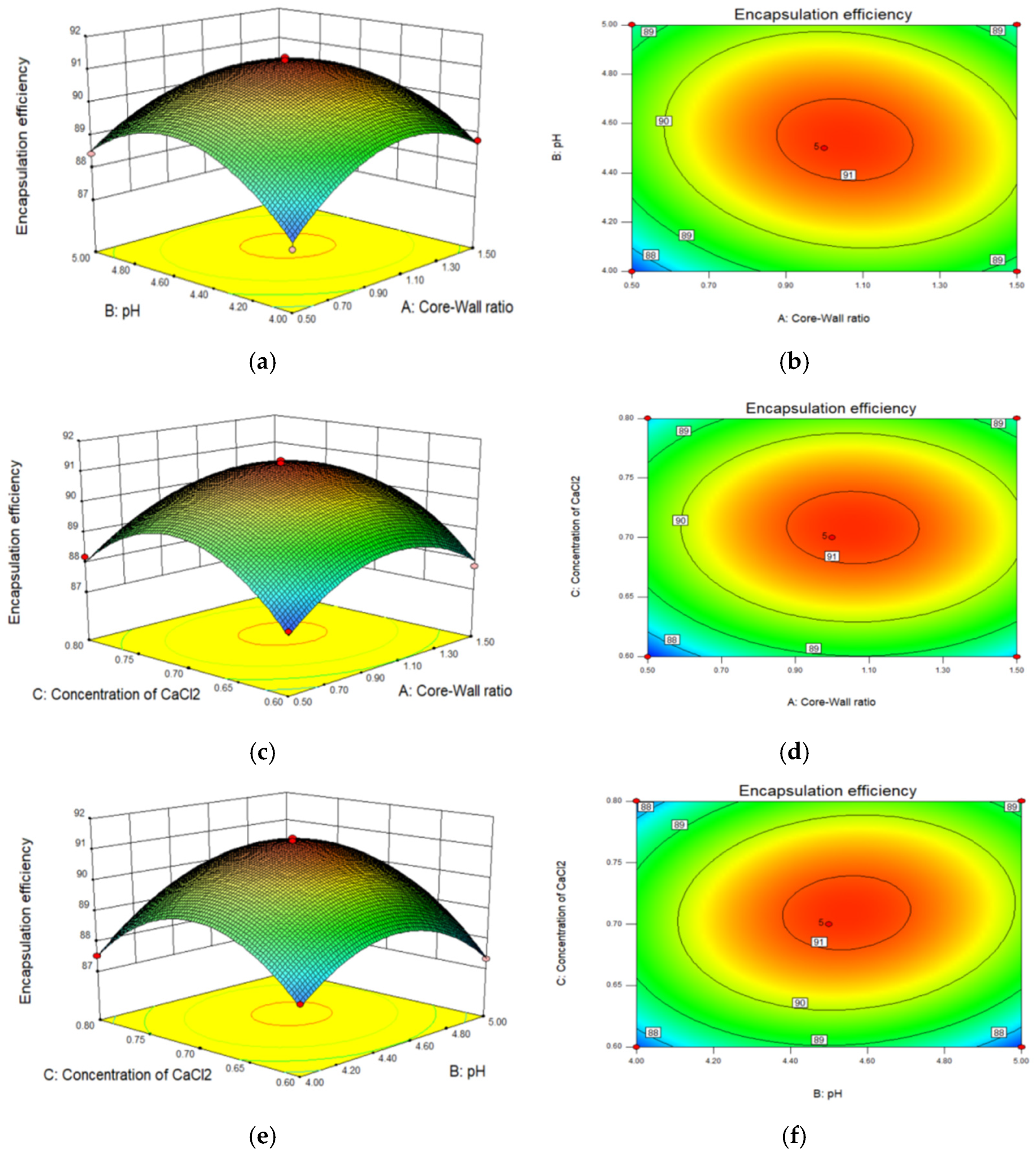
3.1. Optimization of CEO Microcapsules by RSM
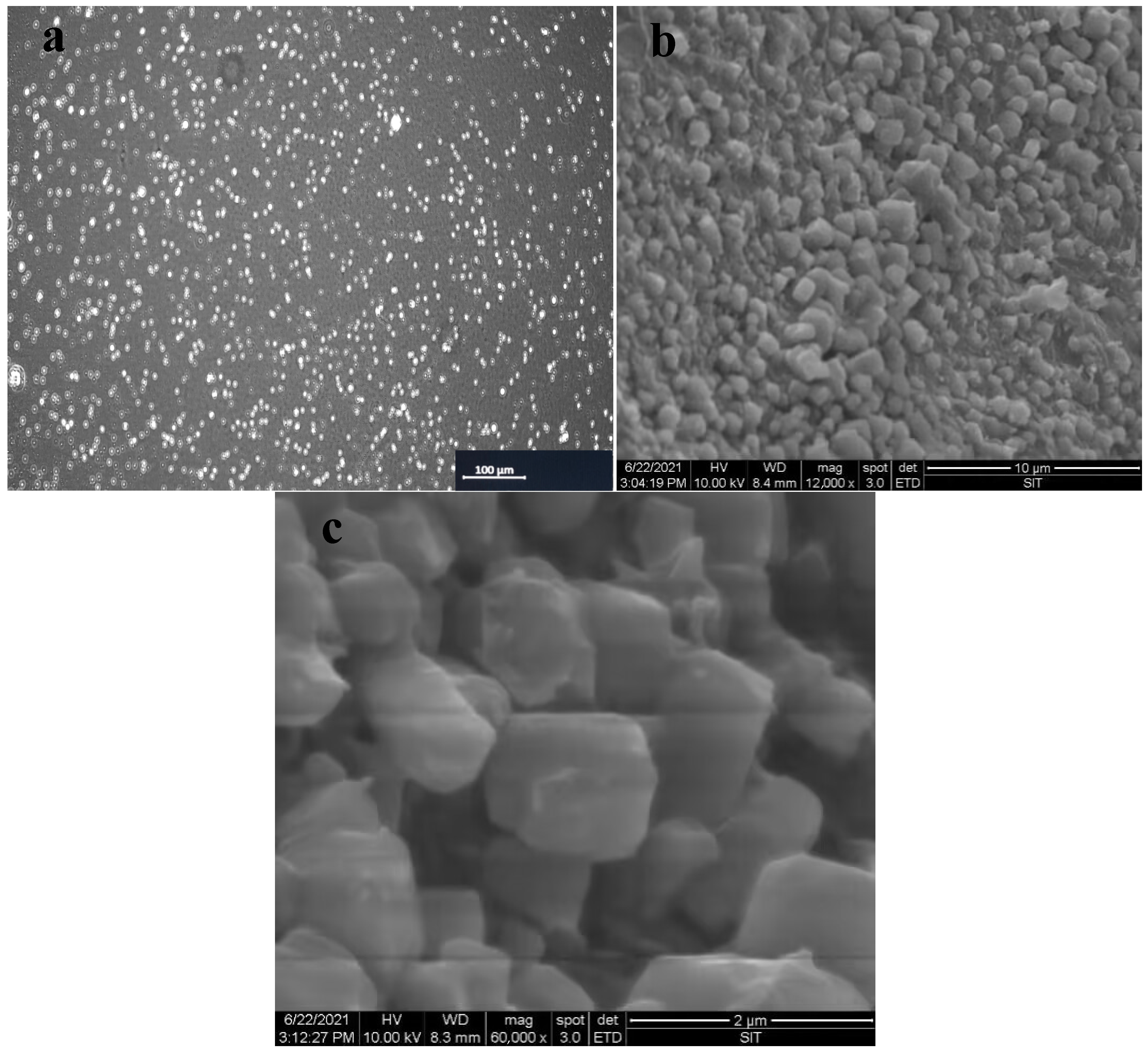
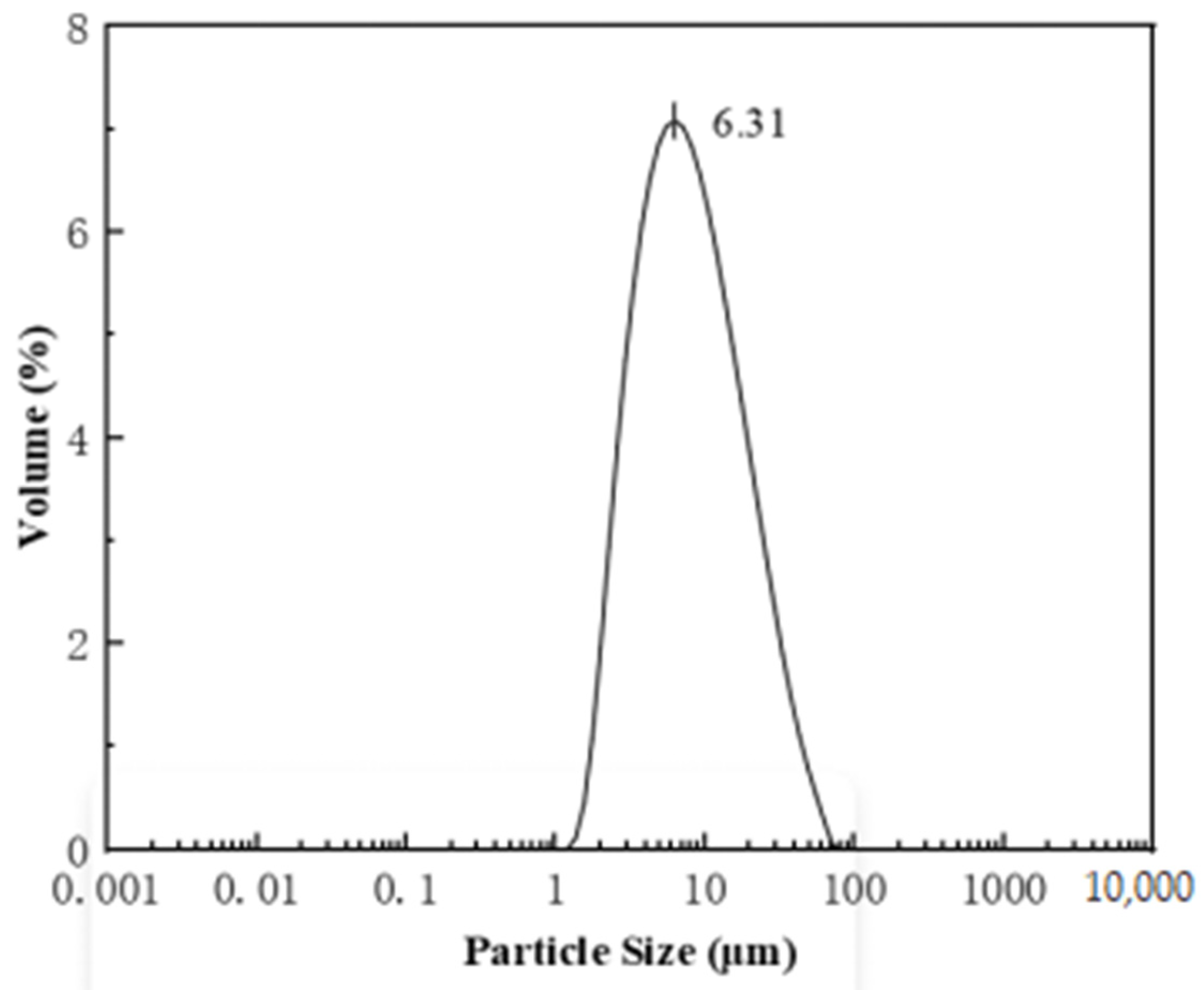
3.2. Morphology and Particle Size Distribution of CMS
3.3. Zeta Potential
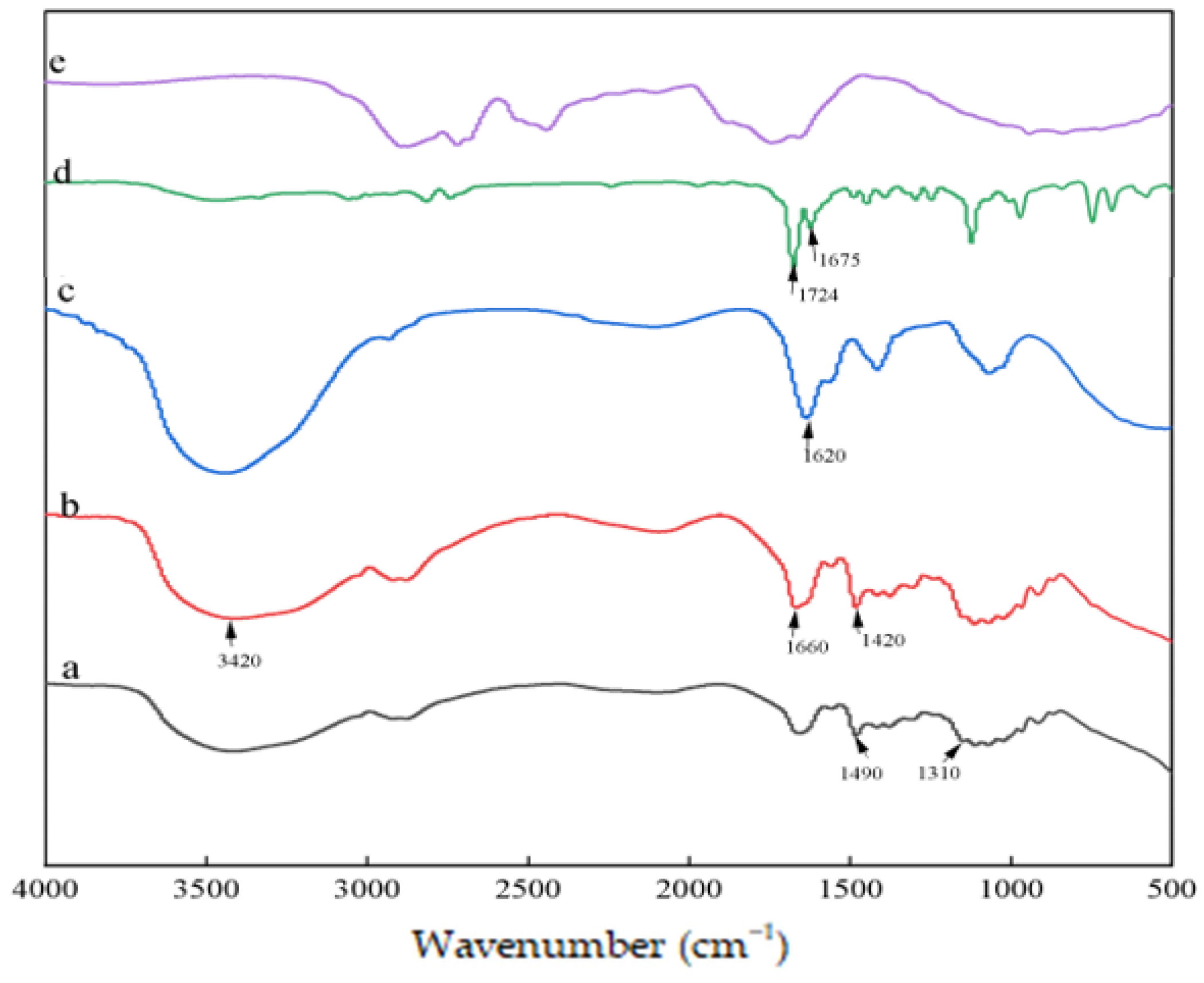
3.4. FTIR Determination of CMS
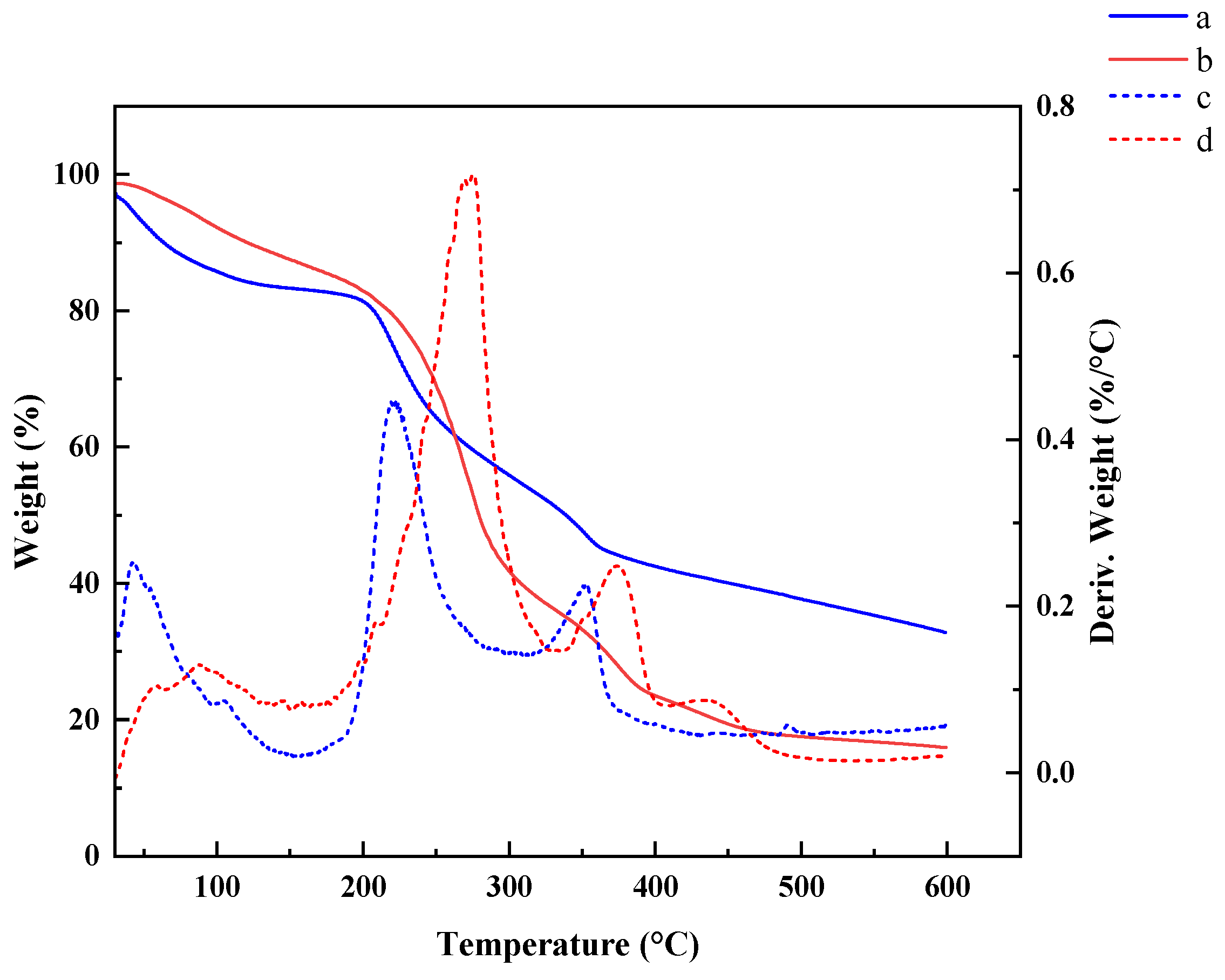
3.5. Thermal Stability Analysis of CMS
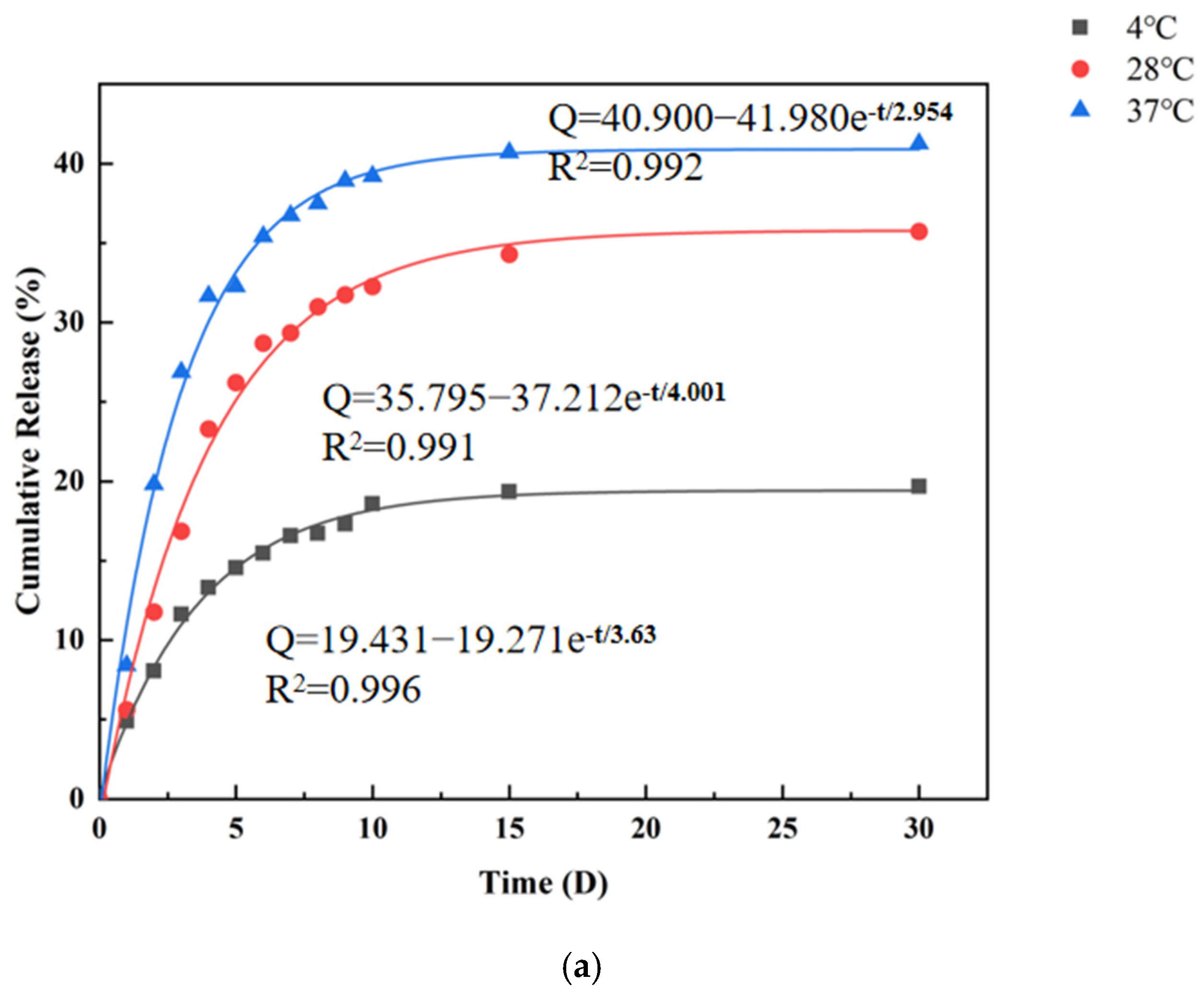
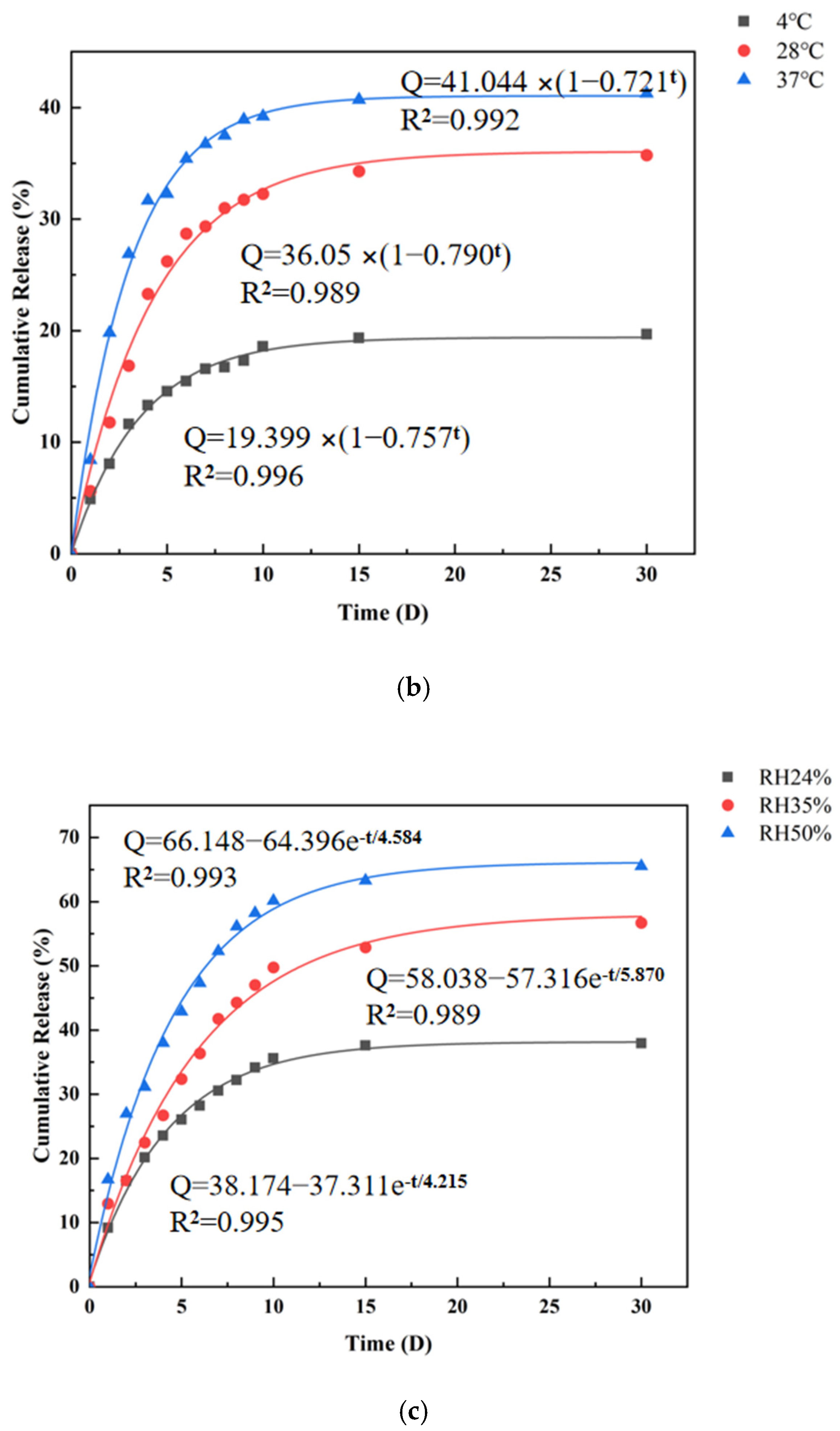
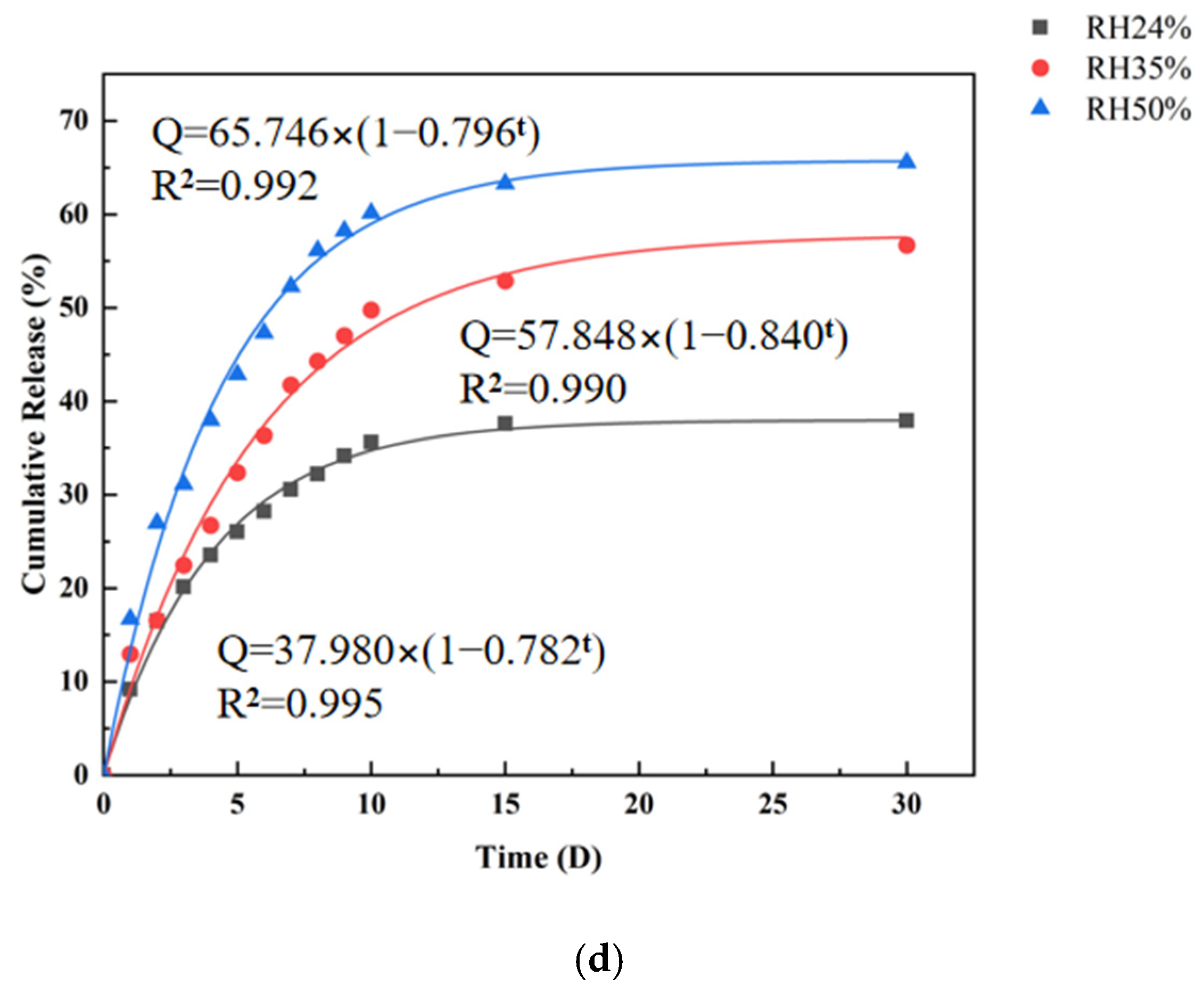
3.6. In Vitro Release Study of CMS
3.7. The Antibacterial Activities of CMS
3.7.1. Antimicrobial Activity of CEO and Microcapsule
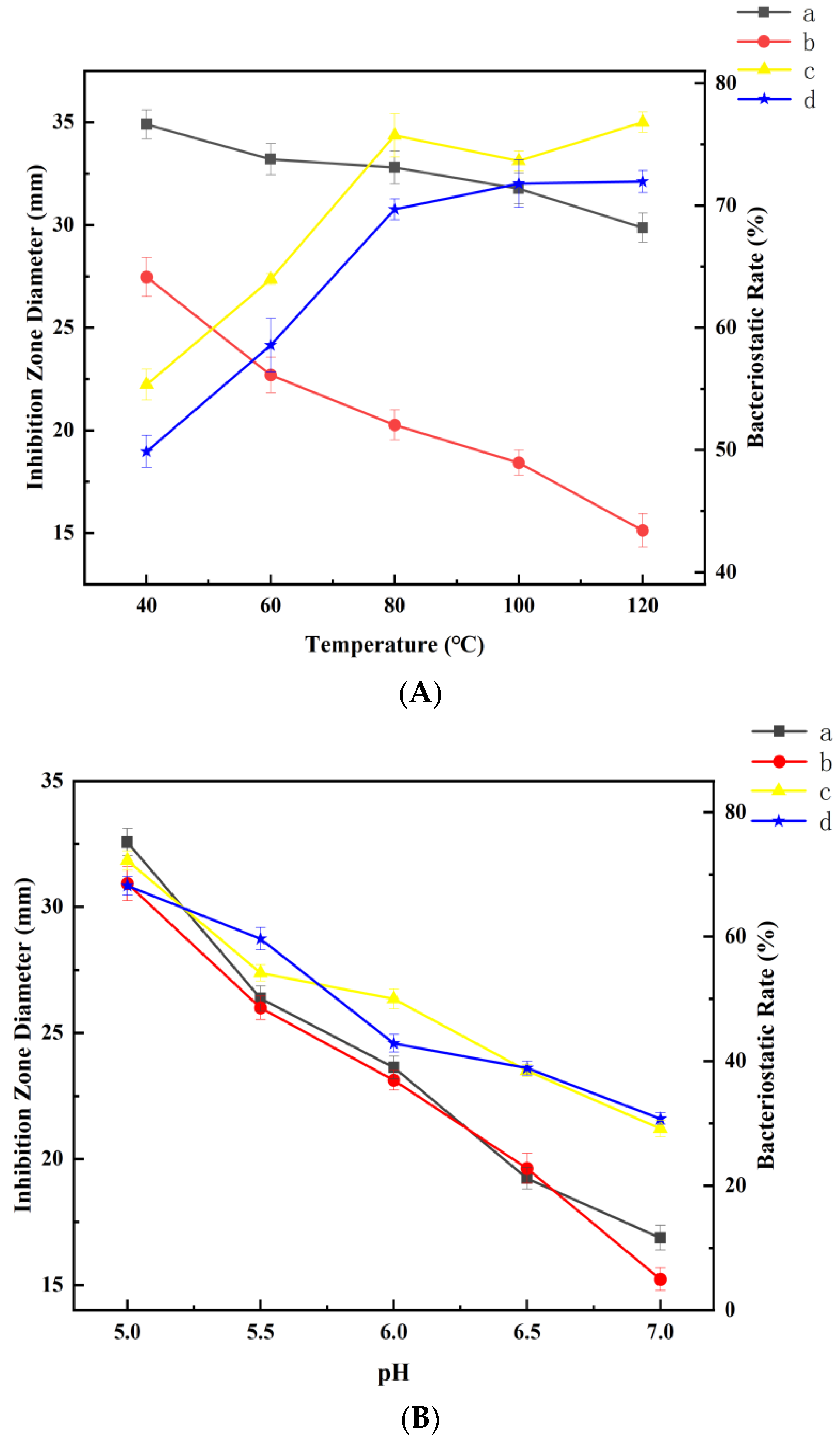
3.7.2. Effect of Temperature on Antibacterial Stability
3.7.3. Effect of pH on Antibacterial Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Felix, P.H.C.; Birchal, V.S.; Botrel, D.A.; Marques, G.R.; Borges, S.V. Physicochemical and thermal stability of microcapsules of cinnamon essential oil by spray drying. J. Food Process. Preserv. 2017, 41, 9. [Google Scholar] [CrossRef]
- Shan, B.; Cai, Y.-Z.; Brooks, J.D.; Corke, H. Antibacterial Properties and Major Bioactive Components of Cinnamon Stick (Cinnamomum burmannii): Activity against Foodborne Pathogenic Bacteria. J. Agric. Food Chem. 2007, 55, 5484–5490. [Google Scholar] [CrossRef]
- Li, L.; Zhang, W.; Peng, J.; Xue, B.; Liu, Z.; Luo, Z.; Lu, D.; Zhao, X. A Novel Shell Material-Highland Barley Starch for Microencapsulation of Cinnamon Essential Oil with Different Preparation Methods. Materials 2020, 13, 1192. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, J.; He, X.; Kong, D.; Hu, Y. Preparation of Cinnamon Oil-Loaded Antibacterial Composite Microcapsules by In Situ Polymerization of Pickering Emulsion Templates. Macromol. Mater. Eng. 2020, 305, 1900851. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Xiao, Z.; Bi, W. Effect of chitosan nanoparticles loaded with cinnamon essential oil on the quality of chilled pork. LWT-Food Sci. Technol. 2015, 63, 519–526. [Google Scholar] [CrossRef]
- Maia, G.A.; de Carvalho, W.M.; Alexandrino, A.C.; Rodrigues, M.D.P.; Brasil, I.M. Freshness retention of minimally processed melon using different packages and multilayered edible coating containing microencapsulated essential oil. Int. J. Food Sci. Technol. 2014, 49, 2192–2203. [Google Scholar]
- Chaib, S.; Benali, N.; Arhab, R.; Ajmi, I.S.; Bendaoued, H.; Romdhane, M. Preparation of Thymus vulgaris Essential Oil Microcapsules by Complex Coacervation and Direct Emulsion: Synthesis, Characterization and Controlled Release Properties. Arab. J. Sci. Eng. 2021, 46, 5429–5446. [Google Scholar] [CrossRef]
- Noghabi, M.S.; Molaveisi, M. Microencapsulation optimization of cinnamon essential oil in the matrices of gum Arabic, maltodextrin, and inulin by spray-drying using mixture design. J. Food Process Eng. 2020, 43, e13341. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, B.A.; Han, X.; Xu, L.N.; Qi, Y.; Yin, L.H.; Xu, Y.W.; Zhao, Y.Y.; Liu, K.X.; Peng, J.Y. Protection of the flavonoid fraction from Rosa laevigata Michx fruit against carbon tetrachloride-induced acute liver injury in mice. Food Chem. Toxicol. 2019, 132, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, C.H.; Wu, T.T.; Wang, L.P.; Chen, S.G.; Ding, T.; Hu, Y.Q. Preparation and characterization of citrus essential oils loaded in chitosan microcapsules by using different emulsifiers. J. Food Eng. 2018, 217, 108–114. [Google Scholar] [CrossRef]
- Sun, Y.J.; Fan, S.Y.; Liang, R.; Ni, X.J.; Du, Y.B.; Wang, J.; Yang, C. Design and characterization of starch/solid lipids hybrid microcapsules and their thermal stability with menthol. Food Hydrocoll. 2021, 116, 106631. [Google Scholar] [CrossRef]
- Ma, Y.B.; Lv, S.S.; Yao, X.R.; Zhang, Y.H.; Gu, J.Y. Preparation of isocyanate microcapsules as a high-performance adhesive for PLA/WF. Constr. Build. Mater. 2020, 260, 120483. [Google Scholar] [CrossRef]
- Zhang, T.; Luo, Y.; Wang, M.X.; Chen, F.; Liu, J.K.; Meng, K.; Zhao, H.J. Double-Layered Microcapsules Significantly Improve the Long-Term Effectiveness of Essential Oil. Polymers 2020, 12, 1651. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.G.; Gupta, R.; Timilsena, Y.P.; Adhikari, B. Optimisation of the complex coacervation between canola protein isolate and chitosan. J. Food Eng. 2016, 191, 58–66. [Google Scholar] [CrossRef]
- Yang, X.; Gao, N.; Hu, L.D.; Li, J.L.; Sun, Y.B. Development and evaluation of novel microcapsules containing poppy-seed oil using complex coacervation. J. Food Eng. 2015, 161, 87–93. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Siauruseviciute, A.; Morkuniene, R.; Lazauskas, R.; Bernatoniene, J. Influence of Technological Factors on the Quality of Chitosan Microcapsules with Boswellia serata L. Essential Oil. Pharmaceutics 2022, 14, 1259. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhang, Y.D.; Xiao, Z.B.; Wang, X.G. Preparation and properties of cinnamon-thyme-ginger composite essential oil nanocapsules. Ind. Crops Prod. 2018, 122, 85–92. [Google Scholar] [CrossRef]
- Li, D.H.; Liu, L.M.; Tian, K.L.; Liu, J.C.; Fan, X.Q. Synthesis, biodegradability and cytotoxicity of water-soluble isobutylchitosan. Carbohydr. Polym. 2007, 67, 40–45. [Google Scholar] [CrossRef]
- Tan, H.; Ma, R.; Lin, C.; Liu, Z. Quaternized Chitosan as an Antimicrobial Agent: Antimicrobial Activity, Mechanism of Action and Biomedical Applications in Orthopedics. Int. J. Mol. Sci. 2013, 14, 1854–1869. [Google Scholar] [CrossRef]
- Agrawal, A.K.; Urimi, D.; Jain, S. Multifunctional Polymeric Nano-Carriers in Targeted Drug Delivery. In Targeted Drug Delivery: Concepts and Design; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Hu, Y.; Yang, Z.M.; Liu, J.Y.; Cheng, J.H.; Yang, Z.H. Preparation and Controlled Release Analysis of Patchouli Oil Complex Microcapsules. Adv. Mater. Res. 2011, 236–238, 2669–2672. [Google Scholar] [CrossRef]
- Li, H.; Xing, Z.; Li, R.; Zhang, M.X.; Wang, M.L.; Tan, Y.Z.; Wu, G.Z. Preparation of Cu2+-Chitosan Quaternary Ammonium Salt-g-Poly(acrylicacid) with Efficient Antibacterial Activity. Chem. J. Chin. Univ. Chin. 2017, 38, 902–906. [Google Scholar] [CrossRef]
- Argin, S.; Kofinas, P.; Lo, Y.M. The cell release kinetics and the swelling behavior of physically crosslinked xanthan-chitosan hydrogels in simulated gastrointestinal conditions. Food Hydrocoll. 2014, 40, 138–144. [Google Scholar] [CrossRef]
- Avadi, M.R.; Sadeghi, A.; Mohammadpour, N.; Abedin, S.; Atyabi, F.; Dinarvand, R.; Rafiee-Tehrani, M. Preparation and characterization of insulin nanoparticles using chitosan and Arabic gum with ionic gelation method. Nanomedicine 2010, 6, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.C.; Ma, R.J.; Duan, M.W.; Lu, D. Preparation and antimicrobial activity of thyme essential oil microcapsules prepared with gum arabic. Rsc. Adv. 2019, 9, 19740–19747. [Google Scholar] [CrossRef]
- Li, C.; Li, B.G.; Zhu, C.H.; Meng, X. Modeling and optimization of tea polyphenol-alginate/chitosan magnetic microcapsules. J. Mol. Struct. 2020, 1208, 127827. [Google Scholar] [CrossRef]
- Zhao, D.; Jiao, X.; Zhang, M.M.; Ye, K.; Shi, X.D.; Lu, X.H.; Qiu, G.; Shea, K.J. Preparation of high encapsulation efficiency fragrance microcapsules and their application in textiles. Rsc. Adv. 2016, 6, 80924–80933. [Google Scholar] [CrossRef]
- Nayak, A.K.; Pal, D.; Santra, K. Development of calcium pectinate-tamarind seed polysaccharide mucoadhesive beads containing metformin HCl. Carbohydr. Polym. 2014, 101, 220–230. [Google Scholar] [CrossRef]
- Chen, M.J.; Hu, Y.; Zhou, J.; Xie, Y.R.; Wu, H.; Yuan, T.; Yang, Z.H. Facile fabrication of tea tree oil-loaded antibacterial microcapsules by complex coacervation of sodium alginate/quaternary ammonium salt of chitosan. Rsc. Adv. 2016, 6, 13032–13039. [Google Scholar] [CrossRef]
- Li, D.H.; Wu, H.J.; Huang, W.; Guo, L.; Dou, H.T. Microcapsule of Sweet Orange Essential Oil Encapsulated in Beta-Cyclodextrin Improves the Release Behaviors In Vitro and In Vivo. Eur. J. Lipid Sci. Technol. 2018, 120, 1700521. [Google Scholar] [CrossRef]
- Yang, Y.H.; Li, X.Z.; Zhang, S. Preparation methods and release kinetics of Litsea cubeba essential oil microcapsules. Rsc. Adv. 2018, 8, 29980–29987. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Tappi, S.; Wang, C.F.; Yu, Y.; Zhu, S.M.; Dalla Rosa, M.; Rocculi, P. Effect of High Hydrostatic Pressure (HHP) on the Antioxidant and Volatile Properties of Candied Wumei Fruit (Prunus mume) During Osmotic Dehydration. Food Bioprocess Technol. 2019, 12, 98–109. [Google Scholar] [CrossRef]
- Lucia, A.; Toloza, A.; Guzman, E.; Ortega, F.; Rubio, R.G. Novel polymeric micelles for insect pest control: Encapsulation of essential oil monoterpenes inside a triblock copolymer shell for head lice control. PEERJ 2017, 5, e3171. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; He, N.N.; Yang, T.; Cai, S.Y.; Zhang, Y.; Lin, J.J.; Huang, M.Q.; Chen, W.Z.; Zhang, Y.P.; Hong, Z.A. Fucoxanthin Loaded in Palm Stearin- and Cholesterol-Based Solid Lipid Nanoparticle-Microcapsules, with Improved Stability and Bioavailability In Vivo. Mar. Drugs 2022, 20, 237. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.B.; Kang, Y.X.; Hou, W.J.; Niu, Y.W.; Kou, X.R. Microcapsules based on octenyl succinic anhydride (OSA)-modified starch and maltodextrins changing the composition and release property of rose essential oil. Int. J. Biol. Macromol. 2019, 137, 132–138. [Google Scholar] [CrossRef]
- Yang, Z.M.; Peng, Z.; Li, J.H.; Li, S.D.; Kong, L.X.; Li, P.W.; Wang, Q.H. Development and evaluation of novel flavour microcapsules containing vanilla oil using complex coacervation approach. Food Chem. 2014, 145, 272–277. [Google Scholar] [CrossRef]
- Franca, D.; Medina, A.F.; Messa, L.L.; Souza, C.F.; Faez, R. Chitosan spray-dried microcapsule and microsphere as fertilizer host for swellable—Controlled release materials. Carbohydr. Polym. 2018, 196, 47–55. [Google Scholar] [CrossRef]
- Bohora, A.A.; Kokate, S.R.; Khedkar, S.; Vankudre, A. Antimicrobial activity of probiotics against endodontic pathogens:- A preliminary study. Indian J. Med. Microbiol. 2019, 37, 5–11. [Google Scholar] [CrossRef]
- Golus, J.; Sawicki, R.; Widelski, J.; Ginalska, G. The agar microdilution method—A new method for antimicrobial susceptibility testing for essential oils and plant extracts. J. Appl. Microbiol. 2016, 121, 1291–1299. [Google Scholar] [CrossRef]
- Bugiereck, A.M.; Pereira, A.T.; Gussati, E.; Magro, J.D.; Mello, J.; Dalcanton, F.; Ternus, R.Z.; Soares, C.; Riella, H.G. Microencapsulation of eugenol molecules by β-cyclodextrine as a thermal protection method of antibacterial action. Mater. Sci. Eng. C 2017, 75, 259–271. [Google Scholar]
- Tu, Q.B.; Wang, P.Y.; Sheng, S.; Xu, Y.; Wang, J.Z.; You, S.; Zhu, A.H.; Wang, J.; Wu, F.A. Microencapsulation and Antimicrobial Activity of Plant Essential Oil Against Ralstonia solanacearum. Waste Biomass Valorization 2020, 11, 5273–5282. [Google Scholar] [CrossRef]
- Odeku, O.A.; Okunlola, A.; Lamprecht, A. Formulation and In vitro Evaluation of Natural Gum-Based Microbeads for Delivery of Ibuprofen. Trop. J. Pharm. Res. 2014, 13, 1577–1583. [Google Scholar] [CrossRef]
- Leng, P.F.; Zhang, Z.M.; Li, Q.; Zhao, M.J.; Pan, G.T. Microemulsion Formulation of Carbendazim and Its In Vitro Antifungal Activities Evaluation. PLoS ONE 2014, 9, e109580. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Moreno, S.; Osorio-Revilla, G.; Gallardo-Velazquez, T.; Cardenas-Bailon, F.; Meza-Marquez, G. Effect of the cross-linking agent and drying method on encapsulation efficiency of orange essential oil by complex coacervation using whey protein isolate with different polysaccharides. J. Microencapsul. 2018, 35, 165–180. [Google Scholar] [CrossRef]
- Lee, A.R.; Han, C.H.; Yi, E. Preparation and Characterization of Melamine-formaldehyde Microcapsules Containing Citrus unshiu Essential Oil. Fibers Polym. 2014, 15, 35–40. [Google Scholar] [CrossRef]
- Zhu, Z.M.; Hu, J.F.; Zhong, Z.C. Preparation and characterization of long-term antibacterial and pH-responsive Polylactic acid/Octenyl succinic anhydride-chitosan @ tea tree oil microcapsules. Int. J. Biol. Macromol. 2022, 220, 1318–1328. [Google Scholar] [CrossRef]
- Ferreira, I.V.W.; Focke, W.W.; du Toit, E.L. Spontaneous microencapsulation of geraniol by zein. Express Polym. Lett. 2018, 12, 986–995. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Sun, B.Y.; Muhoza, B. Influence of processing conditions on the physical properties, retention rate, and antimicrobial activity of cinnamaldehyde loaded in gelatin/pectin complex coacervates. Food Biophys. 2022, 17, 289–301. [Google Scholar] [CrossRef]
- Butstraen, C.; Salaun, F. Preparation of microcapsules by complex coacervation of gum Arabic and chitosan. Carbohydr. Polym. 2014, 99, 608–616. [Google Scholar] [CrossRef]
- Wang, J.G.; Li, X.; Chen, M.J.; Chen, Z.M.; Wu, H.; Zhang, P.; Yuan, T.; Yang, Z.H.; Hu, Y. Fabrication of sustained-release and antibacterial citronella oil-loaded composite microcapsules based on Pickering emulsion templates. J. Appl. Polym. Sci. 2018, 135, 46386. [Google Scholar] [CrossRef]
- Lb, A.; Nk, B.; Ss, A.; Sk, B.; Ml, A. Correlation between chemical composition and antimicrobial properties of essential oils against most common food pathogens and spoilers: In-vitro efficacy and predictive modelling—ScienceDirect. Microb. Pathog. 2020, 147, 104212. [Google Scholar]
- Yu, D.W.; Xu, Y.S.; Jiang, Q.X.; Xia, W.S. Effects of chitosan coating combined with essential oils on quality and antioxidant enzyme activities of grass carp (Ctenopharyngodon idellus) fillets stored at 4 degrees C. Int. J. Food Sci. Technol. 2017, 52, 404–412. [Google Scholar] [CrossRef]
- Torini, L.; Argillier, J.F.; Zydowicz, N. Interfacial Polycondensation Encapsulation in Miniemulsion. Macromolecules 2005, 38, 995–1004. [Google Scholar] [CrossRef]
- Yang, K.; Liu, A.P.; Hu, A.X.; Li, J.X.; Zen, Z.; Liu, Y.T.; Tang, S.Y.; Li, C. Preparation and characterization of cinnamon essential oil nanocapsules and comparison of volatile components and antibacterial ability of cinnamon essential oil before and after encapsulation. Food Control 2021, 123, 107783. [Google Scholar] [CrossRef]
- Xiao, Z.; Hou, W.; Kang, Y.; Niu, Y.; Kou, X. Encapsulation and sustained release properties of watermelon flavor and its characteristic aroma compounds from γ-cyclodextrin inclusion complexes. Food Hydrocoll. 2019, 97, 105202. [Google Scholar] [CrossRef]
- Correa, L.C.; Moldao-Martins, M.; Alves, V.D. Advances in the Application of Microcapsules as Carriers of Functional Compounds for Food Products. Appl. Sci. 2019, 9, 571. [Google Scholar] [CrossRef]
- Hui-Qing, W.U.; Qing-Ping, W.U.; Shi, L.S.; Cheng-Si, L.I. Study on Inhibitory Effects of Plant Essential Oils against Microorganisms. Food Sci. 2008, 29, 83–86. [Google Scholar]


| Factors | Levels | ||
|---|---|---|---|
| −1 | 0 | −1 | |
| Core than wall (X1) | 1:2 | 1:1 | 3:2 |
| pH (X2) | 4.0 | 4.5 | 5.0 |
| The concentration of CaCl2 (X3) | 0.6 | 0.7 | 0.8 |
| Run | Independent Variables | Response Variables | ||
|---|---|---|---|---|
| No | X1 | X2 | X3 (%) | EE (%) |
| 1 | 1.50 | 4.00 | 0.70 | 88.76 |
| 2 | 1.00 | 4.00 | 0.60 | 87.56 |
| 3 | 1.00 | 4.50 | 0.70 | 91.35 |
| 4 | 0.50 | 5.00 | 0.70 | 88.44 |
| 5 | 1.00 | 5.00 | 0.80 | 88.53 |
| 6 | 1.00 | 4.00 | 0.80 | 87.52 |
| 7 | 0.50 | 4.00 | 0.70 | 87.15 |
| 8 | 0.50 | 4.50 | 0.60 | 87.34 |
| 9 | 1.00 | 4.50 | 0.70 | 91.13 |
| 10 | 0.50 | 4.50 | 0.80 | 88.20 |
| 11 | 1.50 | 5.00 | 0.70 | 88.68 |
| 12 | 1.50 | 4.50 | 0.80 | 88.36 |
| 13 | 1.00 | 5.00 | 0.60 | 87.32 |
| 14 | 1.50 | 4.50 | 0.60 | 87.78 |
| 15 | 1.00 | 4.50 | 0.70 | 91.24 |
| 16 | 1.00 | 4.50 | 0.70 | 90.89 |
| 17 | 1.00 | 4.50 | 0.70 | 91.10 |
| Source | Sum of Squares | Df | Mean Square | F-Value | p-Value | Significant |
|---|---|---|---|---|---|---|
| Model | 39.05 | 9 | 4.34 | 87.55 | <0.0001 | ** |
| X1 | 0.75 | 1 | 0.75 | 15.14 | 0.0060 | ** |
| X2 | 0.49 | 1 | 0.49 | 9.89 | 0.0163 | * |
| X3 | 0.85 | 1 | 0.85 | 17.18 | 0.0043 | ** |
| X1X2 | 0.47 | 1 | 0.47 | 9.47 | 0.0179 | * |
| X1X3 | 0.020 | 1 | 0.020 | 0.40 | 0.5494 | |
| X2X3 | 0.39 | 1 | 0.39 | 7.88 | 0.0262 | * |
| X12 | 7.66 | 1 | 7.66 | 154.50 | <0.0001 | ** |
| X22 | 9.93 | 1 | 9.93 | 200.45 | <0.0001 | ** |
| X32 | 14.78 | 1 | 14.78 | 298.22 | <0.0001 | ** |
| Residual | 0.35 | 7 | 0.050 | |||
| Lack of fit | 0.23 | 3 | 0.076 | 2.58 | 0.1914 | |
| Pure error | 0.12 | 4 | 0.030 | |||
| Cor total | 39.40 | 16 | ||||
| R2 = 0.9912; Radj2 = 0.9799; Adeq Precision = 22.985 | ||||||
| Mathematical Model | RH24% | RH35% | RH50% |
|---|---|---|---|
| ExpDec1 | Q = 38.174 − 37.311e−t/4.215 R2 = 0.995 | Q = 58.038 − 57.316e−t/5.870 R2 = 0.989 | Q = 66.148 − 64.396e−t/4.584 R2 = 0.993 |
| BoxLucas1Mod | Q = 37.980 × (1 − 0.782t) R2 = 0.995 | Q = 57.848 × (1 − 0.840t) R2 = 0.990 | Q = 65.746 × (1 − 0.796t) R2 = 0.992 |
| Mathematical model | 4 °C | 28 °C | 37 °C |
| ExpDec1 | Q = 40.900 − 41.980e−t/2.954 R2 = 0.996 | Q = 35.795 − 37.212e−t/4.001 R2 = 0.991 | Q = 40.900 − 41.980e−t/2.954 R2 = 0.992 |
| BoxLucas1Mod | Q = 41.044 × (1 − 0.721t) R2 = 0.996 | Q = 36.05 × (1 − 0.790t) R2 = 0.989 | Q = 41.044 × (1 − 0.721t) R2 = 0.992 |
| Active Ingredient | Inhibition Zone Diameter (mm) | ||||
|---|---|---|---|---|---|
| S. aureus | E. coli | P. aeruginosa | C. albicans | A. niger | |
| HACC | 9.4 | 8.5 | 10.3 | 11.4 | 9.1 |
| GA | - | - | - | - | - |
| CEO | 31.8 | 22.7 | 25.1 | 28.7 | 18.9 |
| CEO-microcapsule | 19.5 | 17.5 | 18.1 | 18.7 | 16.3 |
| Active Ingredient | Minimum Inhibitory Concentration (μL/mL) | ||||
|---|---|---|---|---|---|
| S. aureus | E. coli | P. aeruginosa | C. albicans | A. niger | |
| HACC | 8 | 2 | 0.5 | 0.25 | 4 |
| GA | - | - | - | - | - |
| CEO | 0.39 | 0.39 | 0.78 | 0.1 | 0.2 |
| CEO-microcapsule | 7 | 14 | 7 | 7 | 14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhao, Z.; Xu, W.; Cheng, M.; Chen, Y.; Xun, M.; Liu, Q.; Wang, W. Preparation, Characterization, Release and Antibacterial Properties of Cinnamon Essential Oil Microcapsules. Coatings 2023, 13, 973. https://doi.org/10.3390/coatings13060973
Liu H, Zhao Z, Xu W, Cheng M, Chen Y, Xun M, Liu Q, Wang W. Preparation, Characterization, Release and Antibacterial Properties of Cinnamon Essential Oil Microcapsules. Coatings. 2023; 13(6):973. https://doi.org/10.3390/coatings13060973
Chicago/Turabian StyleLiu, Huimin, Zhiwei Zhao, Wenying Xu, Mingyan Cheng, Yinfeng Chen, Menghan Xun, Qinglei Liu, and Wei Wang. 2023. "Preparation, Characterization, Release and Antibacterial Properties of Cinnamon Essential Oil Microcapsules" Coatings 13, no. 6: 973. https://doi.org/10.3390/coatings13060973
APA StyleLiu, H., Zhao, Z., Xu, W., Cheng, M., Chen, Y., Xun, M., Liu, Q., & Wang, W. (2023). Preparation, Characterization, Release and Antibacterial Properties of Cinnamon Essential Oil Microcapsules. Coatings, 13(6), 973. https://doi.org/10.3390/coatings13060973






