Recent Advances in the Plasma-Assisted Synthesis of Silicon-Based Thin Films and Nanostructures
Abstract
1. Introduction
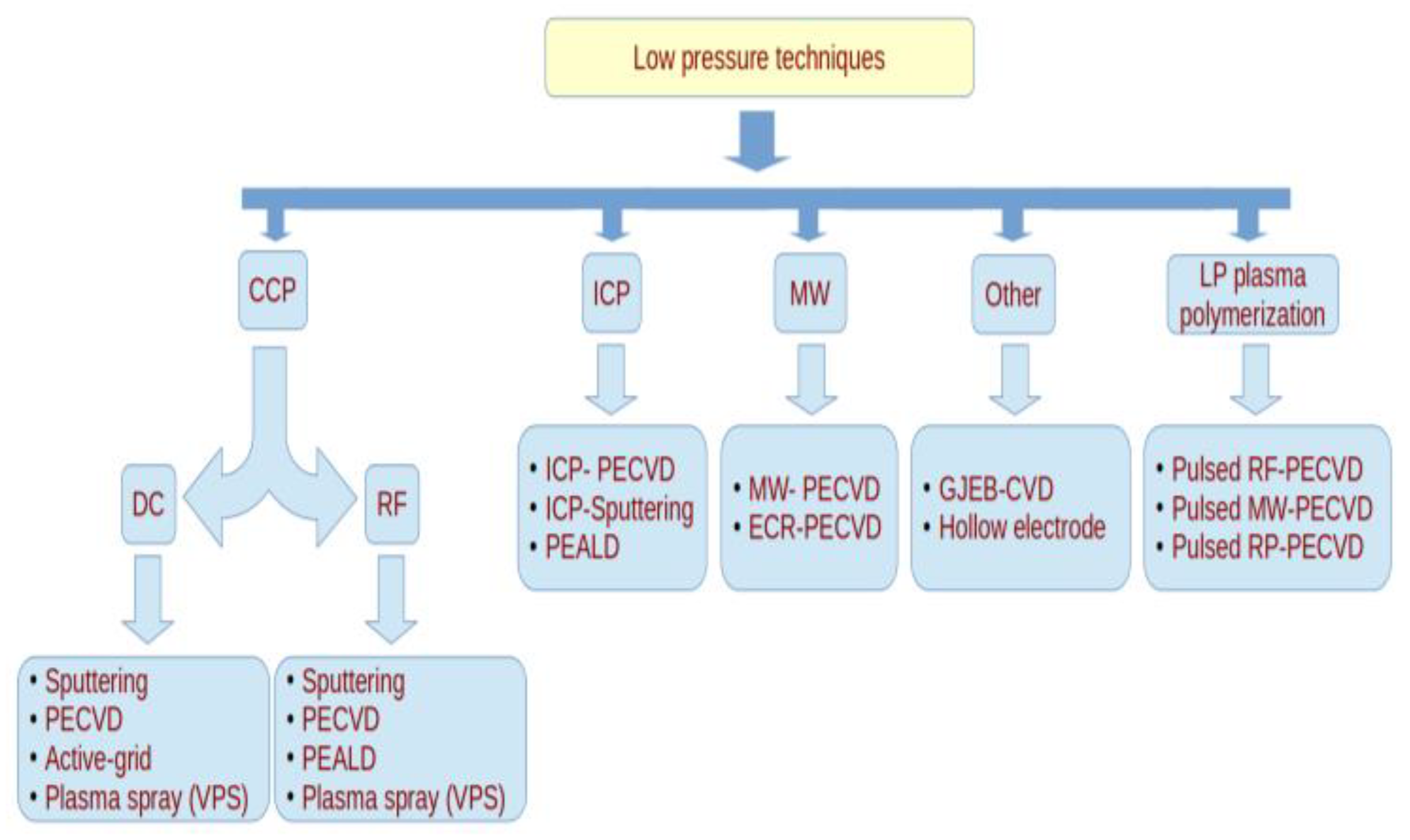
2. Synthesis by Means of Low Pressure Discharges
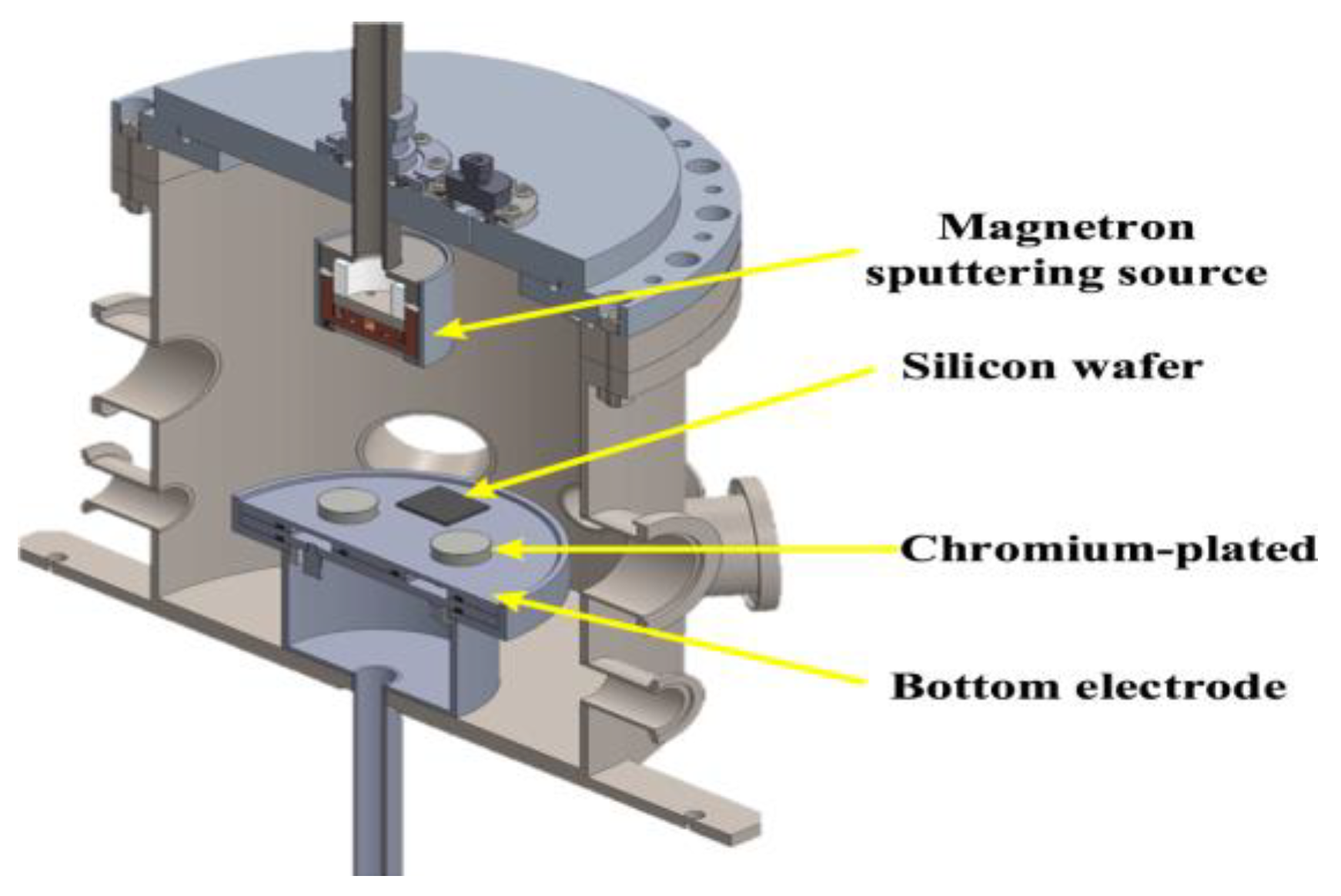
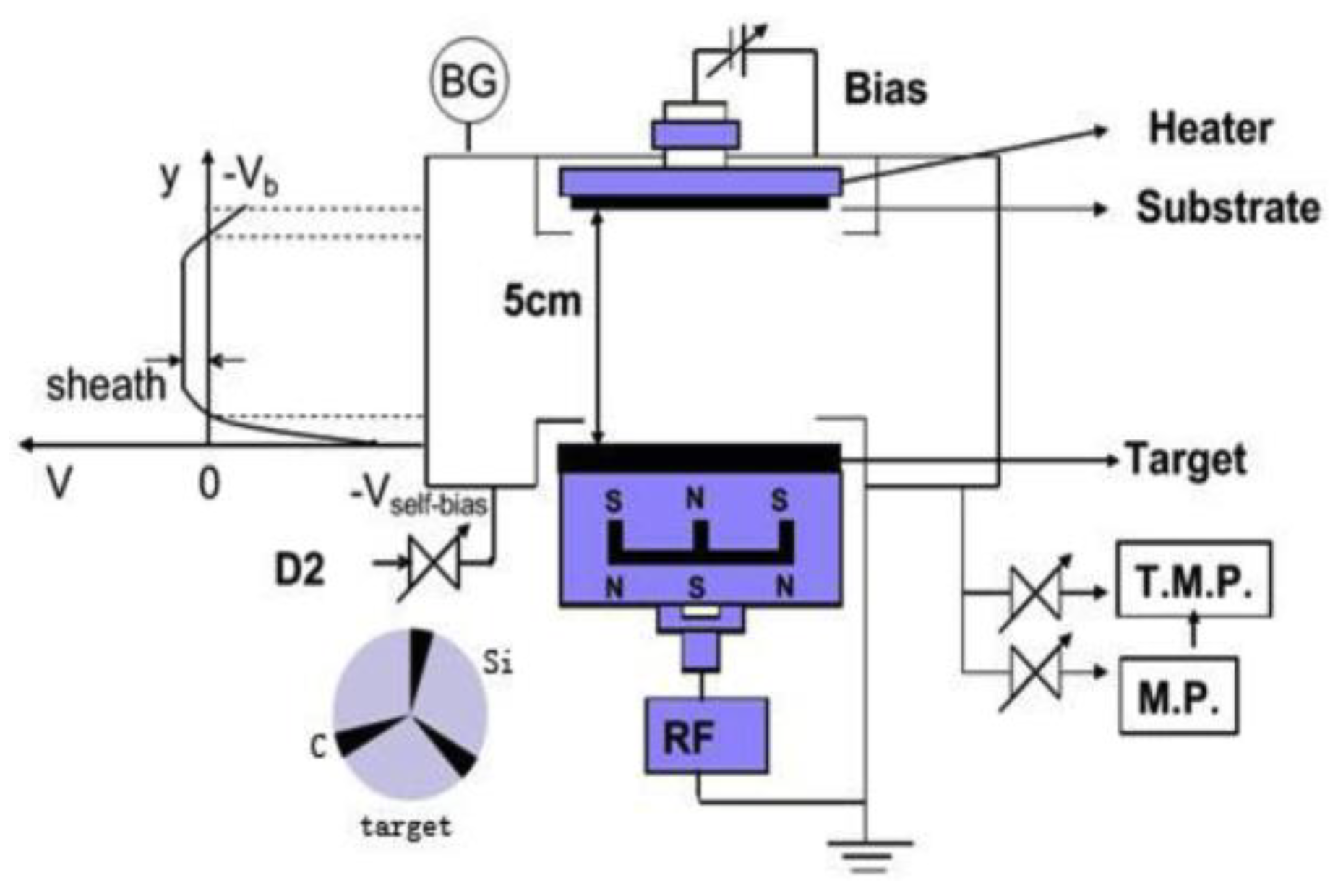
2.1. CCP-DC Discharges
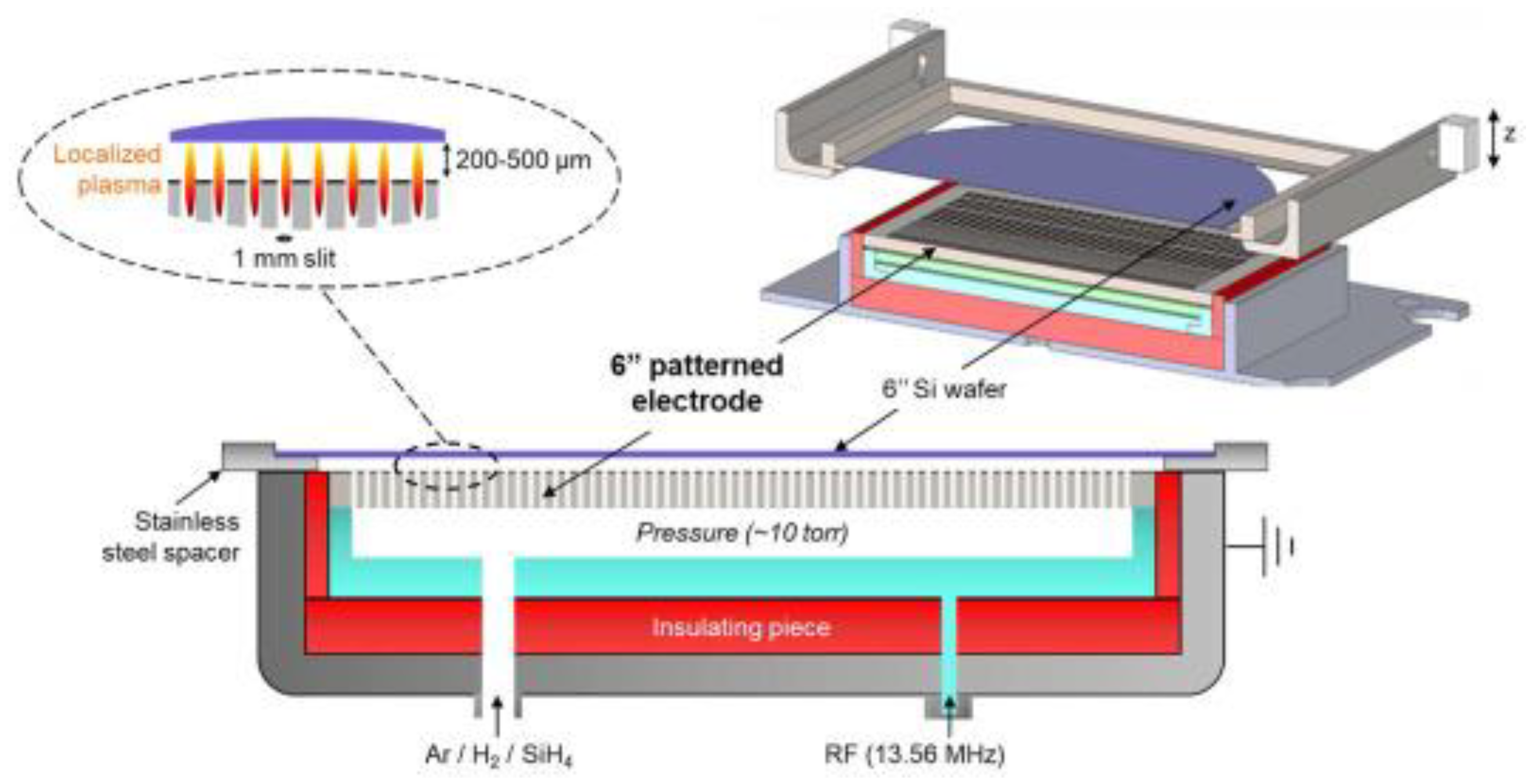
2.2. CCP-RF Discharges
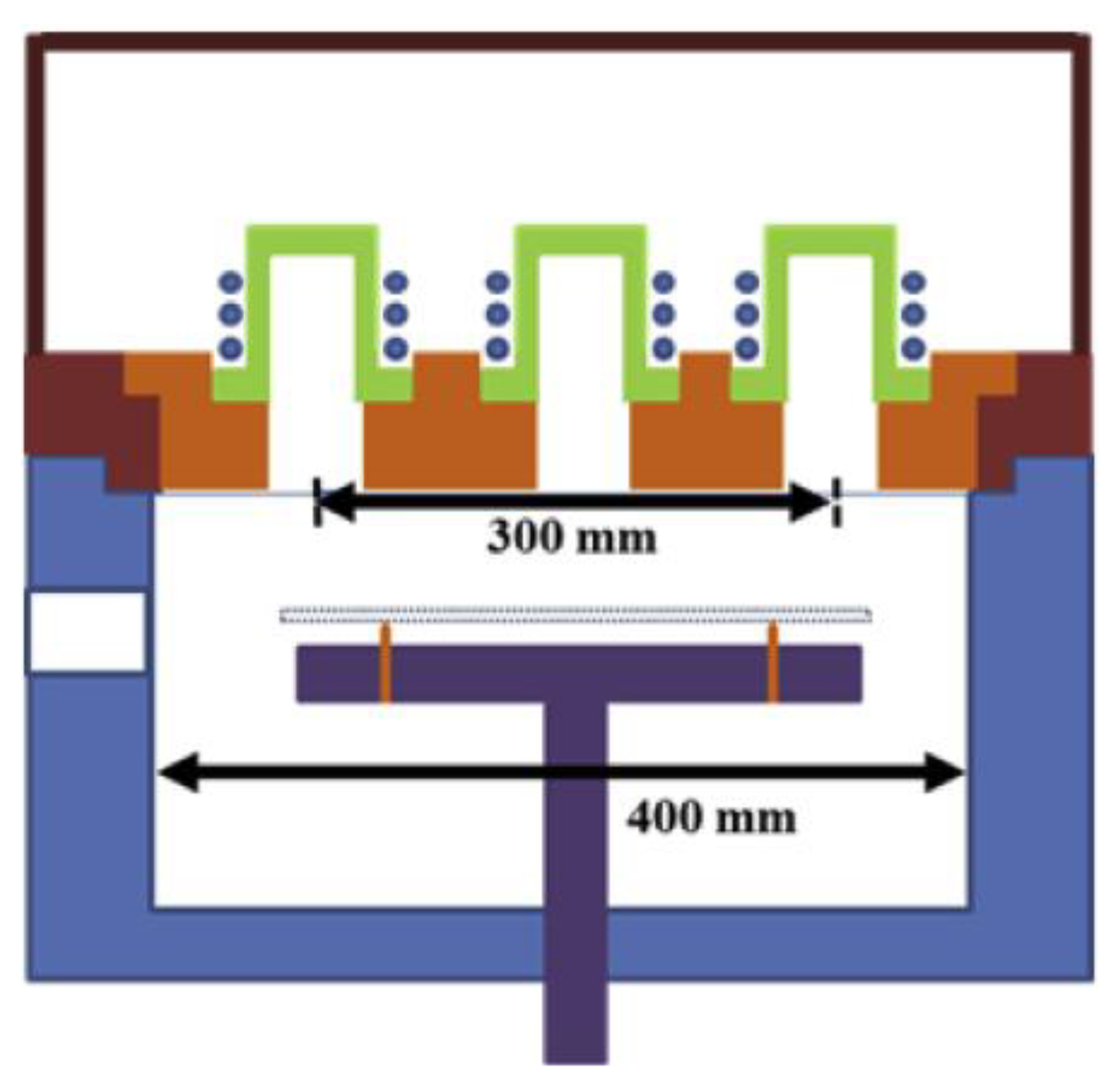
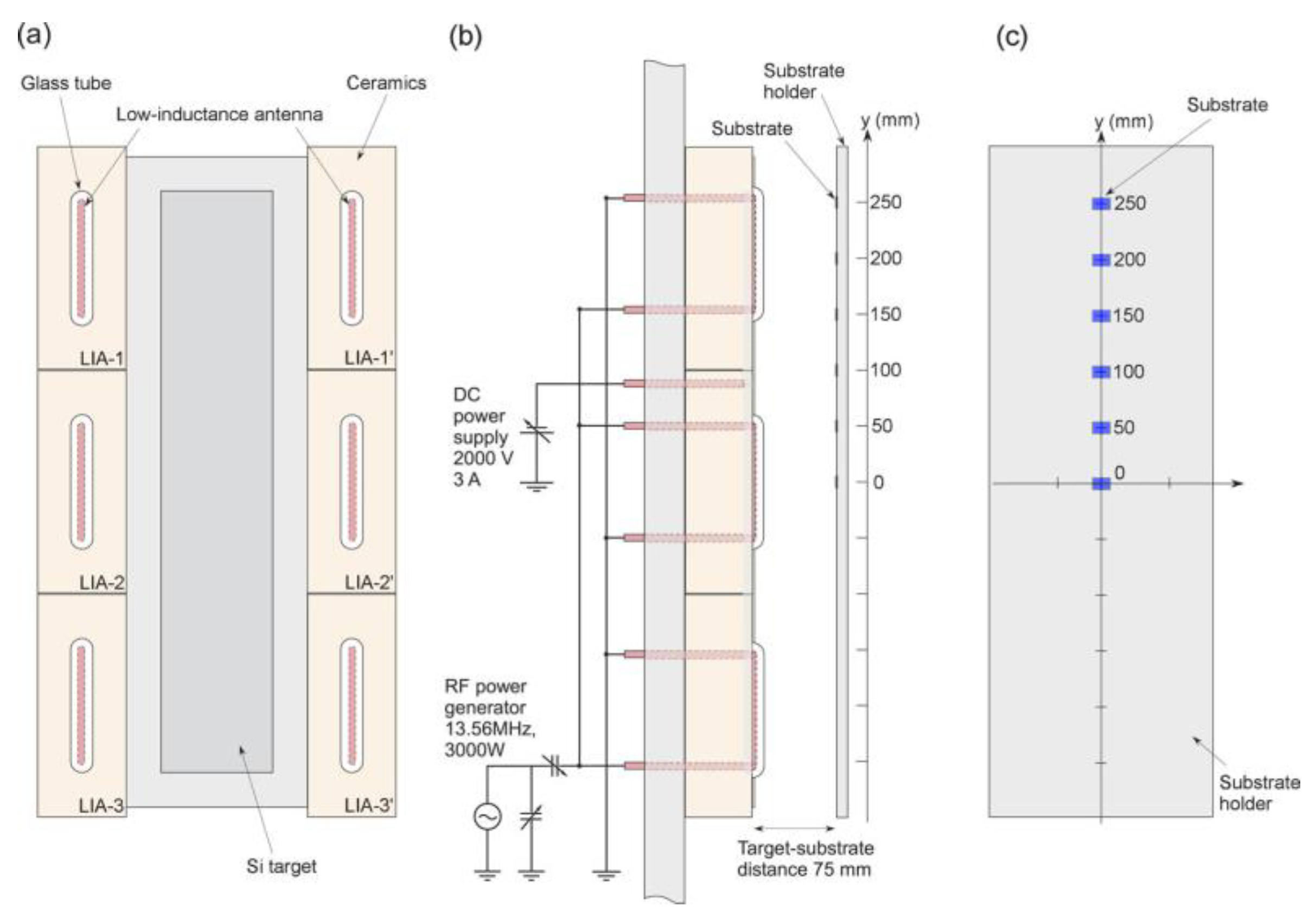
2.3. ICP-RF Discharges
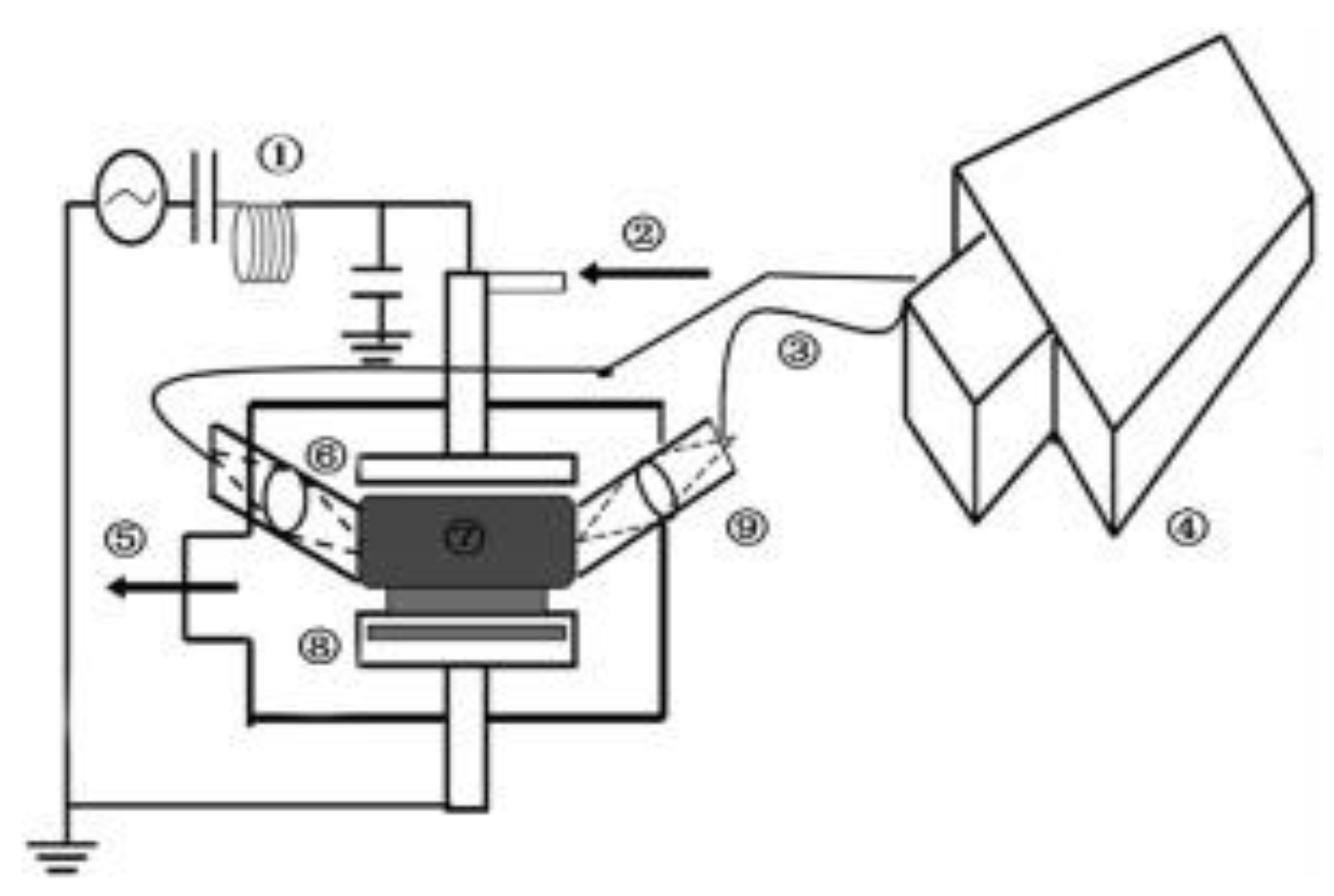
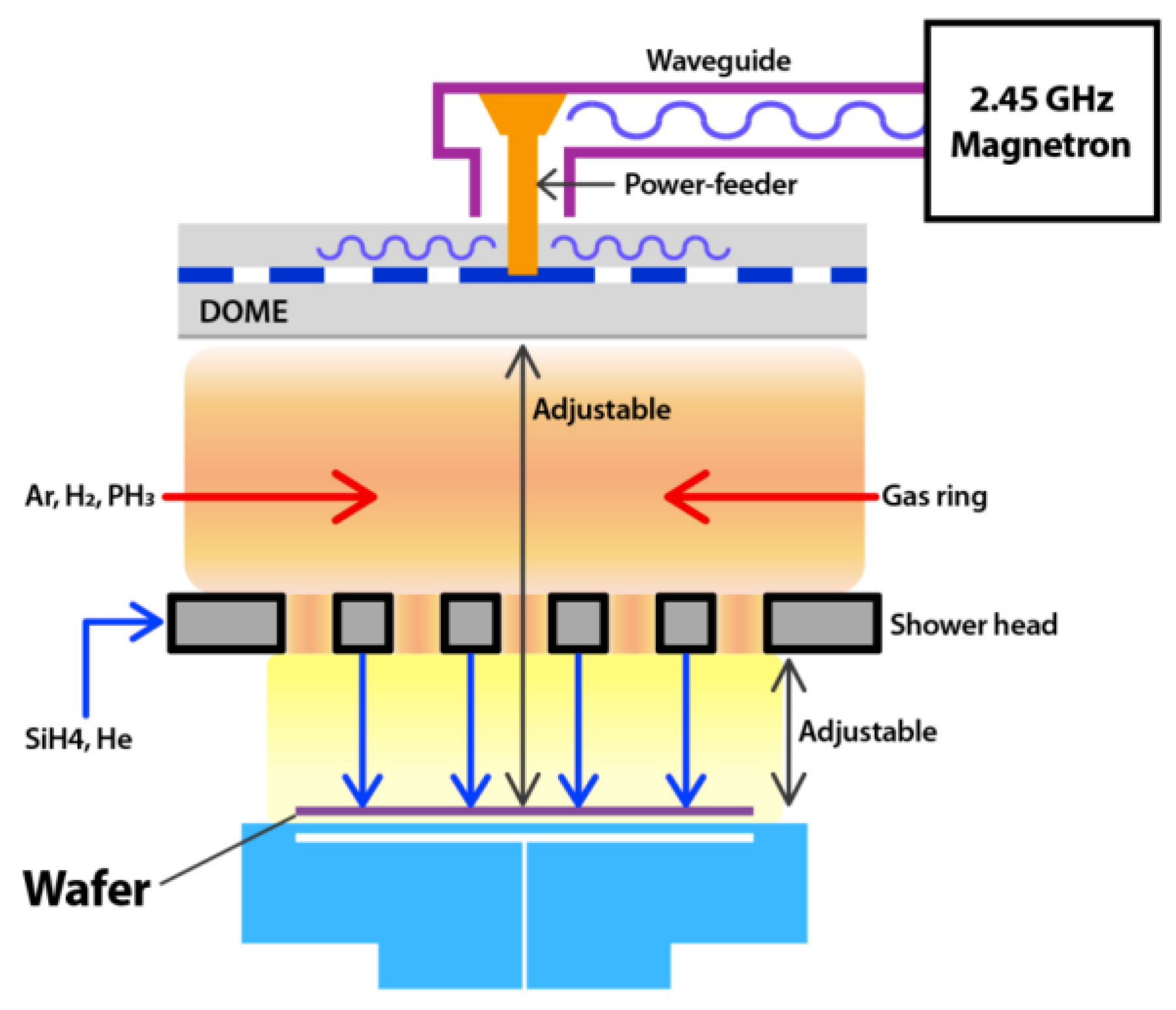
2.4. MW Discharges
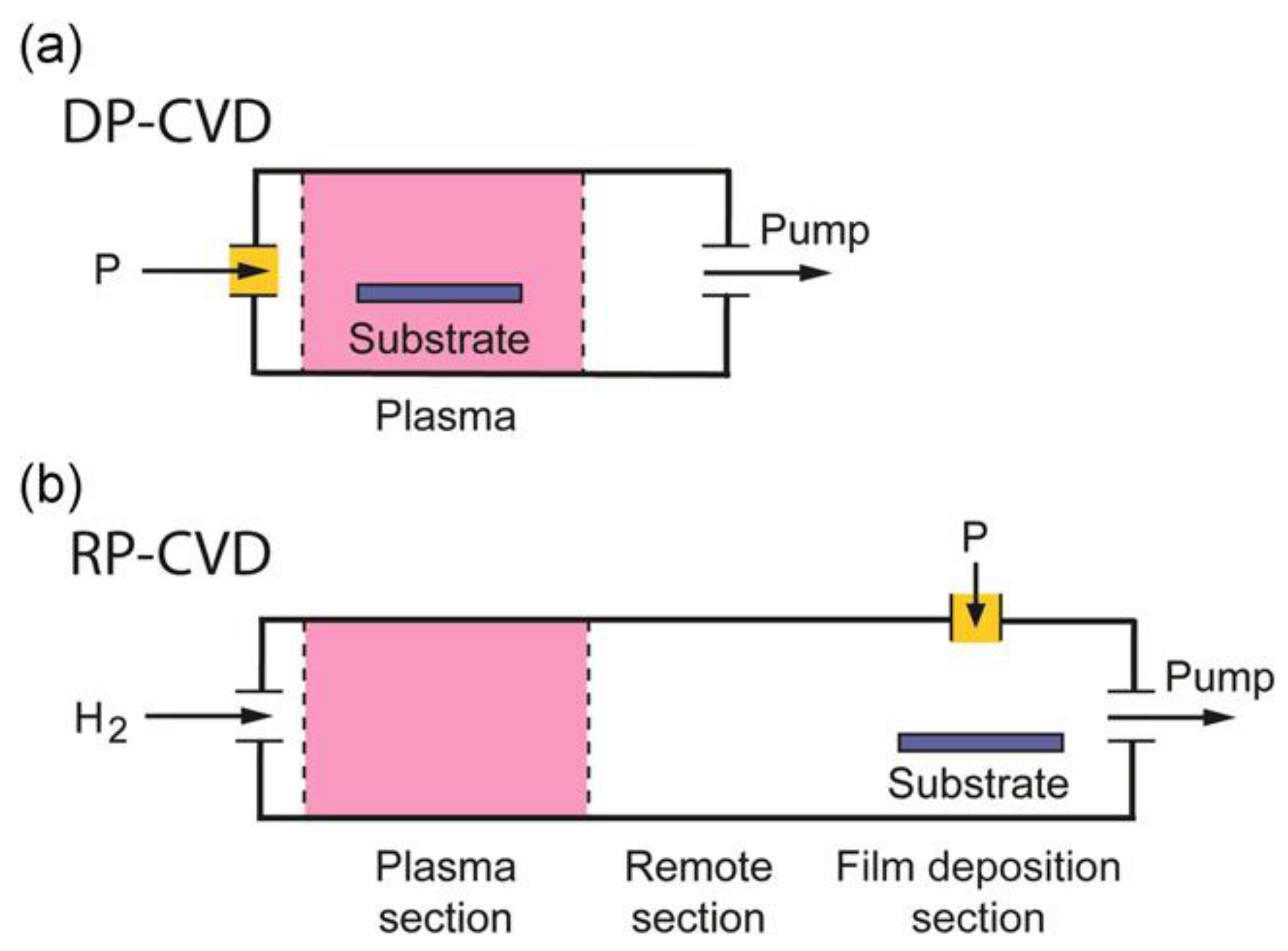
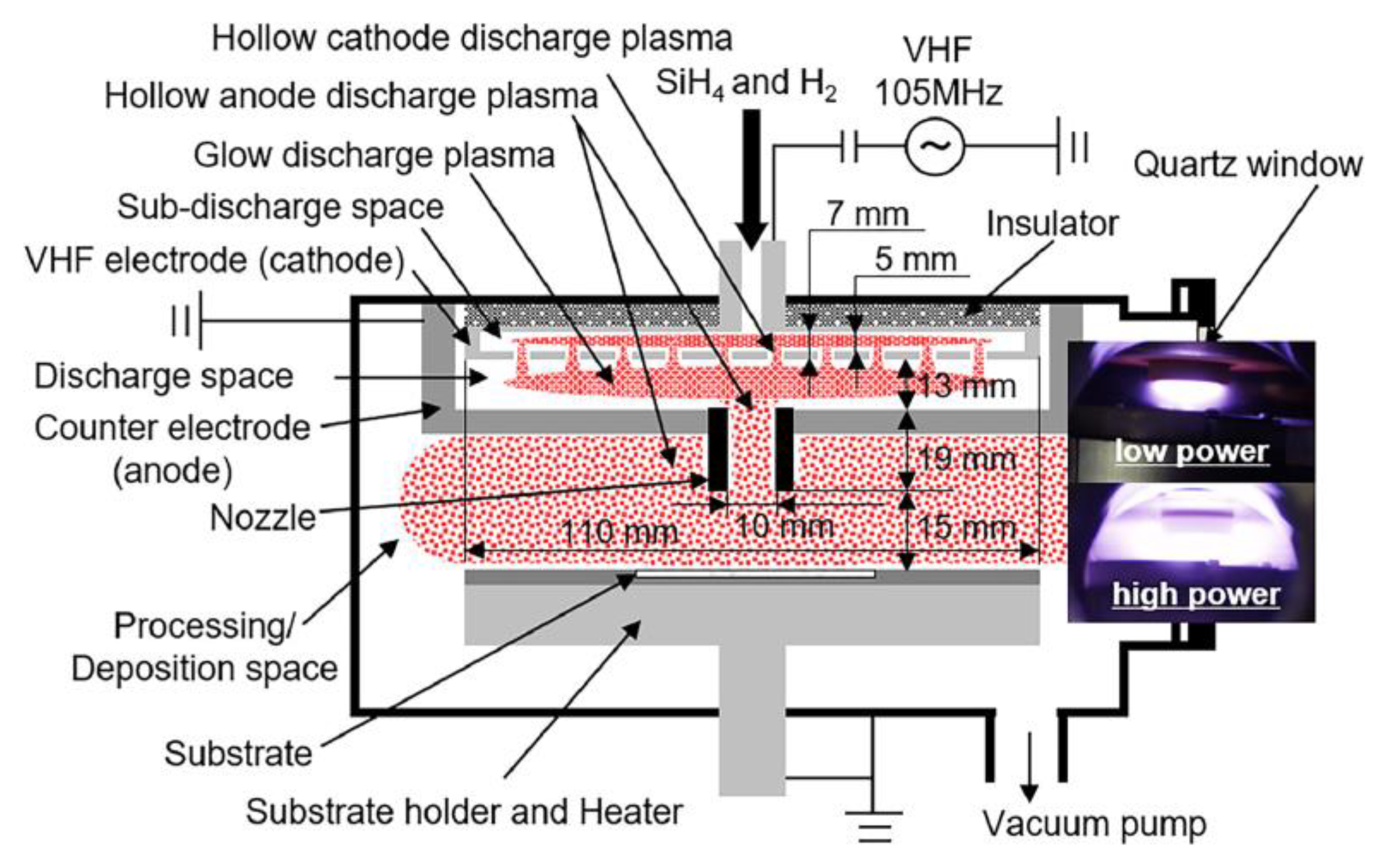
2.5. Other Low Pressure Discharges
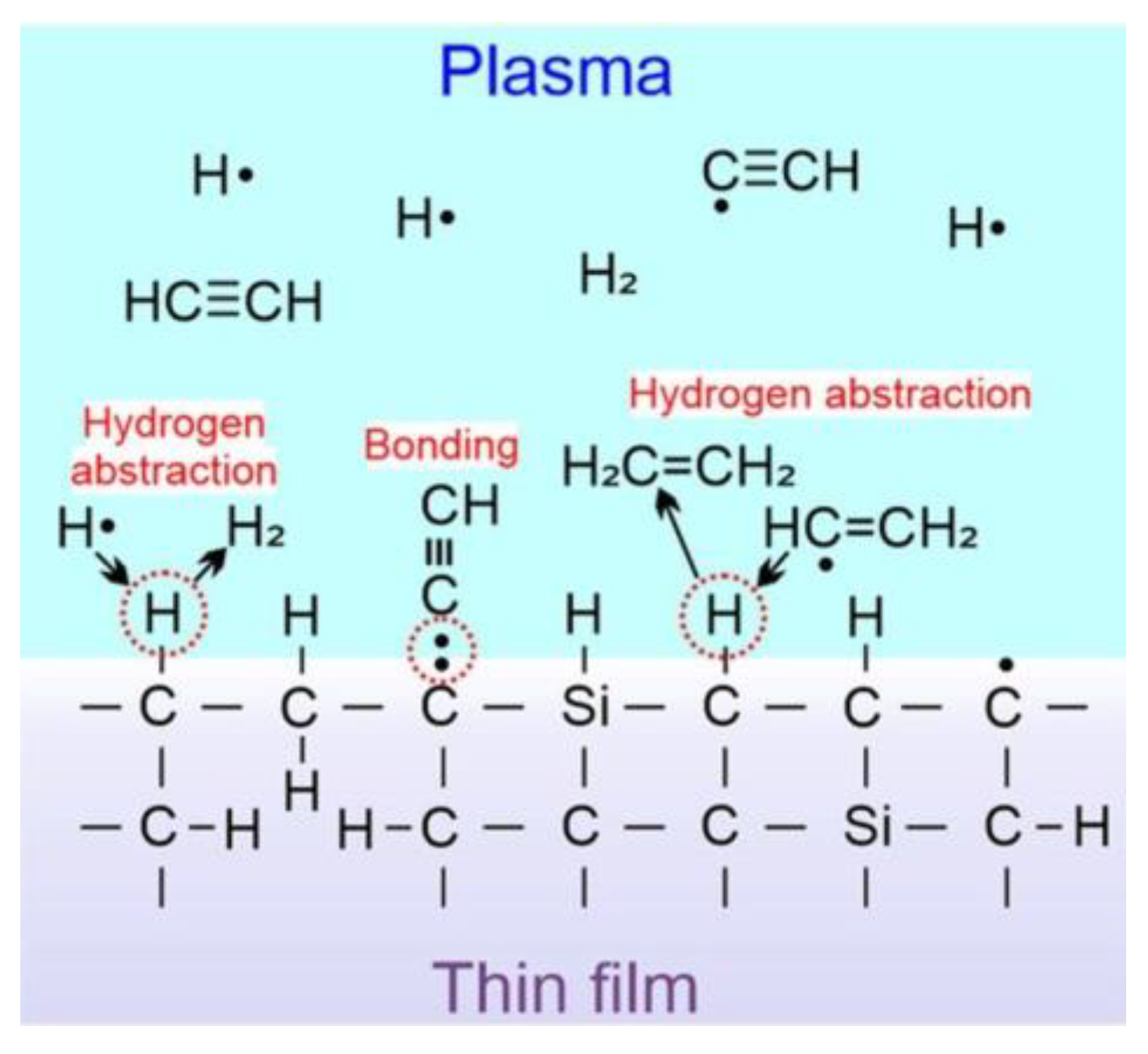
2.6. Low Pressure Plasma Polymerization
2.7. Resume of the Precursors Used at Low Pressure
3. Synthesis by Means of Atmospheric Pressure Discharges
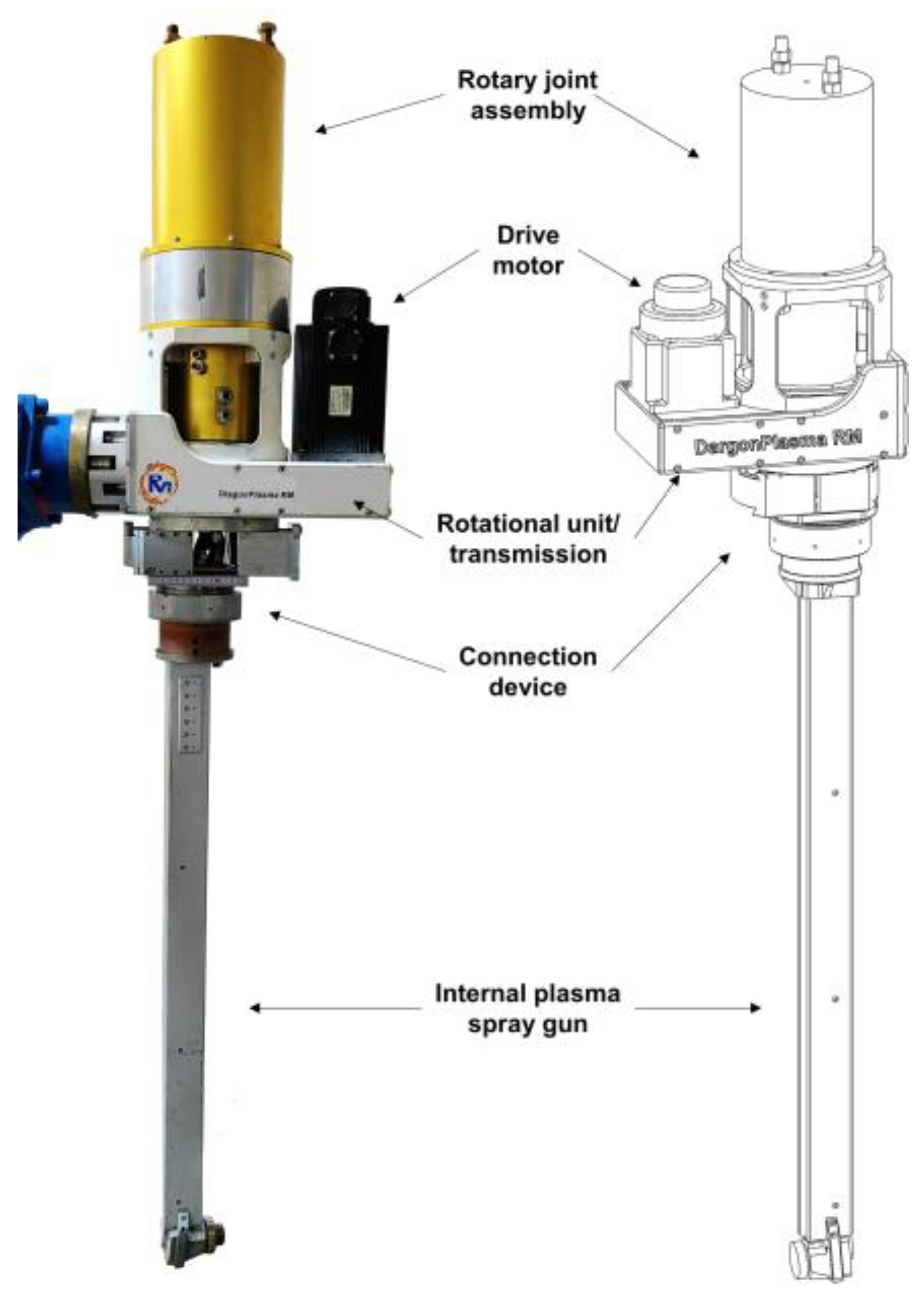
3.1. Plasma Spray
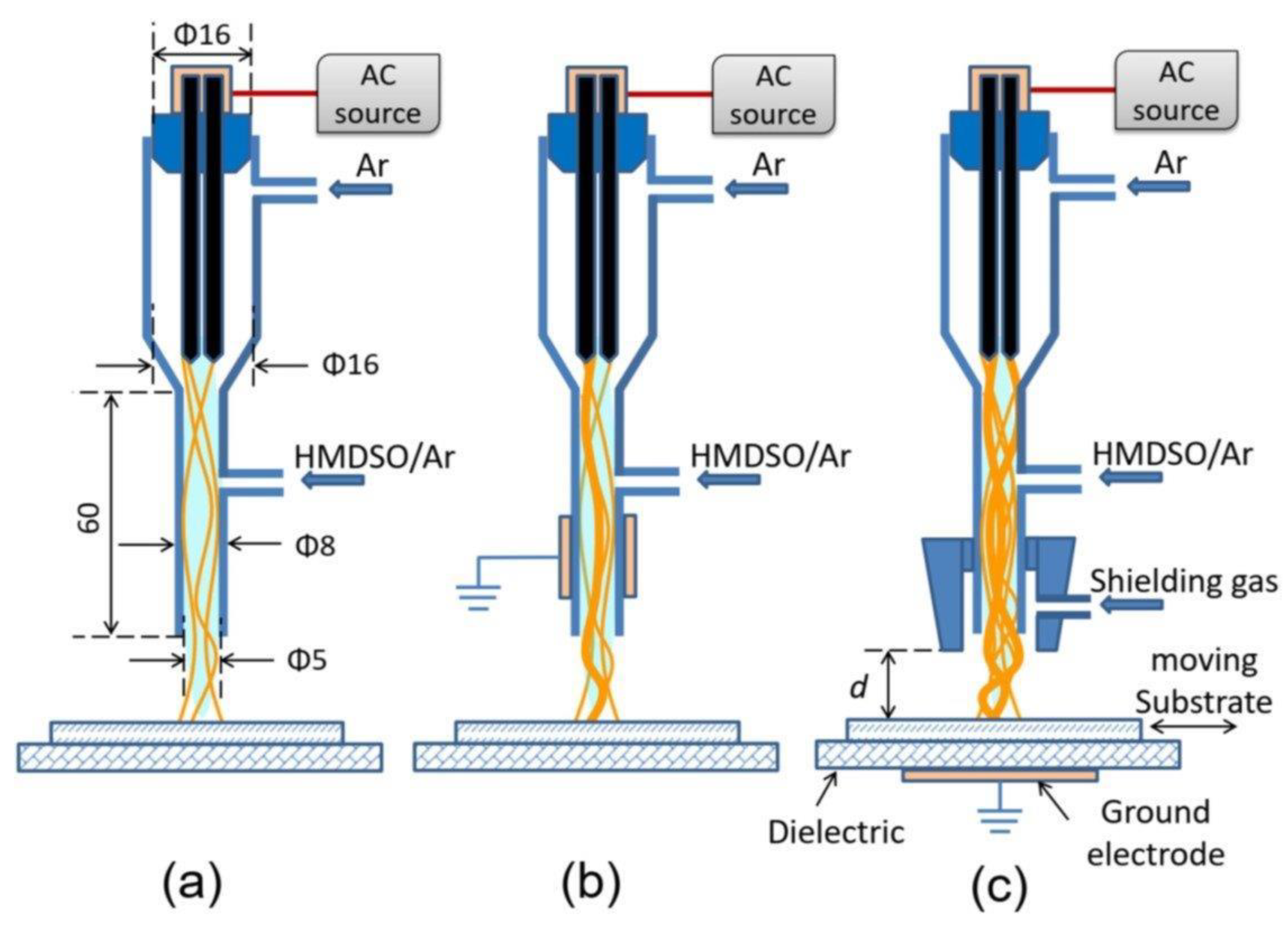
3.2. Plasma Jet
3.3. Other APP Techniques
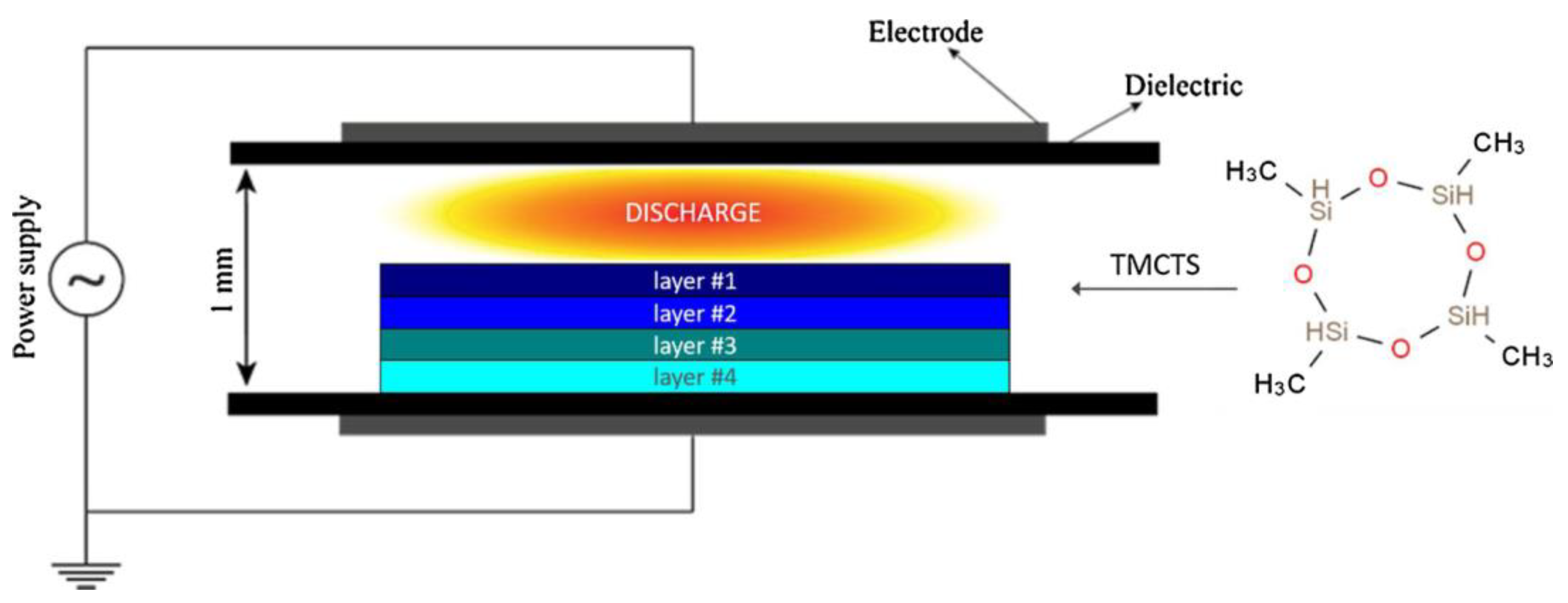
3.4. Plasma Polymerization at Atmospheric Pressure
3.5. Resume of the Precursors Used at Atmospheric Pressure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tayaba, S.; Sethi, H.; Shahid, H.; Malik, R.; Ikram, M.; Ali, S.; Khaliq, S.; Khan, Q.; Maqbool, M. Silicon-Germanium and carbon-based superconductors for electronic, industrial, and medical applications. Mater. Sci. Eng. B 2023, 290, 116332. [Google Scholar] [CrossRef]
- Ballif, C.; Haug, F.-J.; Boccard, M.; Verlinden, P.J.; Hahn, G. Status and perspectives of crystalline silicon photovoltaics in research and industry. Nat. Rev. Mater. 2022, 7, 597–616. [Google Scholar] [CrossRef]
- Mussano, F.; Genova, T.; Laurenti, M.; Munaron, L.; Pirri, C.F.; Rivolo, P.; Carossa, S.; Mandracci, P. Hydrogenated amorphous silicon coatings may modulate gingival cell response. Appl. Surf. Sci. 2018, 436, 603–612. [Google Scholar] [CrossRef]
- Mandracci, P.; Mussano, F.; Ceruti, P.; Pirri, C.F.; Carossa, S. Reduction of bacterial adhesion on dental composite resins by silicon–oxygen thin film coatings. Biomed. Mater. 2015, 10, 015017. [Google Scholar] [CrossRef] [PubMed]
- Osminkina, L.A.; Agafilushkina, S.N.; Kropotkina, E.A.; Saushkin, N.Y.; Bozhev, I.V.; Abramchuk, S.S.; Samsonova, J.V.; Gambaryan, A.S. Antiviral adsorption activity of porous silicon nanoparticles against different pathogenic human viruses. Bioact. Mater. 2022, 7, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Cao, S.; Li, L.; Zhang, X. A review on the mainstream through-silicon via etching methods. Mater. Sci. Semicond. Process. 2022, 137, 106182. [Google Scholar] [CrossRef]
- Daghbouj, N.; Cherkashin, N.; Darras, F.-X.; Paillard, V.; Fnaiech, M.; Claverie, A. Effect of the order of He+ and H+ ion co-implantation on damage generation and thermal evolution of complexes, platelets, and blisters in silicon. J. Appl. Phys. 2016, 119, 135308. [Google Scholar] [CrossRef]
- Cherkashin, N.; Daghbouj, N.; Seine, G.; Claverie, A. Impact of He and H relative depth distributions on the result of sequential He+ and H+ ion implantation and annealing in silicon J. Appl. Phys. 2018, 123, 161556. [Google Scholar] [CrossRef]
- Daghbouj, N.; Li, B.S.; Callisti, M.; Sen, H.S.; Karlik, M.; Polcar, T. Microstructural evolution of helium-irradiated 6H–SiC subjected to different irradiation conditions and annealing temperatures: A multiple characterization study. Acta Mater. 2019, 181, 160–172. [Google Scholar] [CrossRef]
- Martinu, L.; Zabeida, O.; Klemberg-Sapieha, J.E. Plasma-Enhanced Chemical Vapor Deposition of Functional Coatings. In Handbook of Deposition Technologies for Films and Coatings, 3rd ed.; Martin, P.M., Ed.; Elsevier: Oxford, UK, 2010; pp. 392–465. [Google Scholar]
- Vasudev, M.C.; Anderson, K.D.; Bunning, T.J.; Tsukruk, V.V.; Naik, R.R. Exploration of plasma-enhanced chemical vapor deposition as a method for thin-film fabrication with biological applications. ACS Appl. Mater. Interfaces 2013, 5, 3983–3994. [Google Scholar] [CrossRef] [PubMed]
- Huff, M. Recent advances in reactive ion etching and applications of high-aspect-ratio microfabrication. Micromachines 2021, 12, 991. [Google Scholar] [CrossRef] [PubMed]
- Chabert, P.; Tsankov, T.V.; Czarnetzki, U. Foundations of capacitive and inductive radio-frequency discharges. Plasma Sources Sci. Technol. 2021, 30, 024001. [Google Scholar] [CrossRef]
- Delfani-Abbariki, S.; Abdollah-zadeh, A.; Hadavi, S.M.M.; Abedi, M.; Derakhshandeh, S.M.R. Enhancing the adhesion of diamond-like carbon films to steel substrates using silicon-containing interlayers. Surf. Coat. Technol. 2018, 10, 1436–1440. [Google Scholar] [CrossRef]
- Lakhonchai, A.; Chingsungnoen, A.; Poolcharuansin, P.; Chanlek, N.; Tunmee, S.; Rittihong, U. Improvement of corrosion resistance and mechanical properties of chrome plating by diamond-like carbon coating with different silicon-based interlayers. Mater. Res. Express 2022, 9, 055604. [Google Scholar] [CrossRef]
- Sun, S.; Wu, Y.; Wang, Z.; Zhang, Y.; Chen, D.; Chen, J. Plasma enabled in-situ deposition of hybrid structured SiOx/C on polymorphous carbon hosts for superior lithium storage. Carbon 2023, 205, 253–261. [Google Scholar] [CrossRef]
- Grenadyorov, A.S.; Solovyev, A.S.; Oskomov, K.V.; Sypchenko, V.S. Influence of deposition conditions on mechanical properties of a-C:H:SiOx films prepared by plasma-assisted chemical vapor deposition method. Surf. Coat. Technol. 2018, 349, 547–555. [Google Scholar] [CrossRef]
- Grenadyorov, A.S.; Solovyev, A.A.; Oskomov, K.V.; Semenov, V.A.; Zhulkov, M.O.; Sirota, D.A.; Chernyavskiy, A.M.; Karmadonova, N.A.; Malashchenko, V.V.; Litvinova, L.S.; et al. Morphofunctional reaction of leukocytes and platelets in in vitro contact with a-C:H:SiOx-coated Ti–6Al–4V substrate. J. Biomed. Mater. Res. Part A 2023, 111, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Ohta, R.; Fukada, K.; Tashiro, T.; Dougakiuchi, M.; Kambara, M. Effect of PS-PVD production throughput on Si nanoparticles for negative electrode of lithium ion batteries. J. Phys. D 2018, 51, 105501. [Google Scholar] [CrossRef]
- Harder, B.J. Oxidation performance of Si-HfO2 environmental barrier coating bond coats deposited via plasma spray-physical vapor deposition. Surf. Coat. Technol. 2020, 384, 125311. [Google Scholar] [CrossRef]
- Mandracci, P.; Gazia, R.; Virga, A. New Research on the Plasma-Assisted Deposition Of Silicon-Based Amorphous Thin Film Alloys. In Amorphous Materials: New Research, 1st ed.; Mishra, S.B., Ed.; Nova Science Pub Inc.: New York, NY, USA, 2013; pp. 27–50. [Google Scholar]
- Mandracci, P.; Ricciardi, C. Silicon-carbon-oxynitrides grown by plasma-enhanced chemical vapor deposition technique. Thin Solid Film. 2007, 505, 7639–7642. [Google Scholar] [CrossRef]
- Ruan, T.; Qu, M.; Wang, J.; He, Y.; Xu, X.; Yu, C.; Zhang, Y.; Yan, H. Effect of deposition temperature of a-Si:H layer on the performance of silicon heterojunction solar cell. J. Mater. Sci. Mater. Electron. 2019, 30, 13330–13335. [Google Scholar] [CrossRef]
- Özkol, E.; Procel, P.; Zhao, Y.; Mazzarella, L.; Medlin, R.; Šutta, P.; Isabella, O.; Zeman, M. Effective Passivation of Black Silicon Surfaces via Plasma-Enhanced Chemical Vapor Deposition Grown Conformal Hydrogenated Amorphous Silicon Layer. Phys. Status Solidi-Rapid Res. Lett. 2020, 14, 1900087. [Google Scholar] [CrossRef]
- Sai, H.; Hsu, H.-J.; Chen, P.-W.; Chen, P.-L.; Matsui, T. Intrinsic Amorphous Silicon Bilayers for Effective Surface Passivation in Silicon Heterojunction Solar Cells: A Comparative Study of Interfacial Layers. Phys. Status Solidi A 2021, 218, 2000743. [Google Scholar] [CrossRef]
- Wang, J.; Ru, X.; Ruan, T.; Hu, Y.; Zhang, Y.; Yan, H. Performance of heterojunction solar cells with different intrinsic a-Si:H thin layers deposited by RF- and VHF-PECVD. J. Mater. Sci. Mater. Electron. 2021, 32, 25327–25331. [Google Scholar] [CrossRef]
- Pandey, A.; Bhattacharya, S.; Panigrahi, J.; Mandal, S.; Komarala, V.K. Effect of Gas Flow Rate in PECVD of Amorphous Silicon Thin Films for Interface Passivation of Silicon Heterojunction Solar Cells. Phys. Status Solidi A 2022, 219, 2200183. [Google Scholar] [CrossRef]
- Ouaras, K.; Filonovich, S.; Bruneau, B.; Wang, J.; Ghosh, M.; Johnson, E. Maskless interdigitated a-Si:H PECVD process on full M0 c-Si wafer: Homogeneity and passivation assessment. Sol. Energy Mater. Sol. Cells 2022, 246, 111927. [Google Scholar] [CrossRef]
- Chen, W.; Truong, T.N.; Nguyen, H.T.; Samundsett, C.; Pheng Phang, S.; MacDonald, D.; Cuevas, A.; Zhou, L.; Wan, Y.; Yan, D. Influence of PECVD deposition temperature on phosphorus doped poly-silicon passivating contacts. Sol. Energy Mater. Sol. Cells 2020, 206, 110348. [Google Scholar] [CrossRef]
- Truong, T.N.; Yan, D.; Nguyen, C.T.; Kho, T.; Guthrey, H.; Seidel, J.; Al-Jassim, M.; Cuevas, A.; Macdonald, D.; Nguyen, H.T. Morphology, microstructure, and doping behaviour: A comparison between different deposition methods for poly-Si/SiOx passivating contacts. Prog. Photovolt. Res. Appl. 2021, 29, 857–868. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Shen, W. A Convenient and Effective Method to Deposit Low-Defect-Density nc-Si:H Thin Film by PECVD. Nanoscale Res. Lett. 2018, 13, 234. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Chaudhary, D.; Sudhakar, S.; Kumar, S. Intrinsic Sub-Nanocrystalline Silicon Thin Films: Active Layer for Solar Cells. Silicon 2021, 13, 1–7. [Google Scholar] [CrossRef]
- Wang, S.-H.; Chang, H.-E.; Lee, C.-C.; Fuh, Y.-K.; Li, T.T. Evolution of a-Si:H to nc-Si:H transition of hydrogenated silicon films deposited by trichlorosilane using principle component analysis of optical emission spectroscopy. Mater. Chem. Phys. 2020, 240, 122186. [Google Scholar] [CrossRef]
- Ghosh, H.; Mitra, S.; Siddiqui, M.S.; Saxena, A.K.; Chaudhuri, P.; Saha, H.; Banerjee, C. Back scattering involving embedded silicon nitride (SiN) nanoparticles for c-Si solar cells. Opt. Commun. 2018, 413, 63–72. [Google Scholar] [CrossRef]
- Chen, J.; Li, D.; Sun, T.; Han, J.; Zhang, Y.; Li, W.; Xu, J.; Chen, K. Experimental observations on metal-like carrier transport and Mott hopping conduction behaviours in boron-doped Si nanocrystal multilayers. Nanotechnology 2023, 34, 16LT01. [Google Scholar] [CrossRef] [PubMed]
- Shibata, K.; Kato, S.; Kurosawa, M.; Gotoh, K.; Miyamoto, S.; Usami, N.; Kurokawa, Y. Preparation and thermoelectric characterization of boron-doped Si nanocrystals/silicon oxide multilayers. Jpn. J. Appl. Phys. 2023, 62, SC1074. [Google Scholar] [CrossRef]
- Li, X.; Jin, R.; Li, L.; Lu, J.; Gu, Y.; Ren, F.; Huang, J. Effect of deposition rate on the growth mechanism of microcrystalline silicon thin films using very high frequency PECVD. Optik 2019, 180, 104–112. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Fan, Z.; Sun, H.; Shan, F. Low-temperature deposition of large-grain polycrystalline Si thin films on polyethylene terephthalate. Thin Solid Film. 2020, 707, 138065. [Google Scholar] [CrossRef]
- Song, J.; Huang, R.; Zhang, Y.; Lin, Z.; Zhang, W.; Li, H.; Song, C.; Guo, Y.; Lin, Z. Effect of Nitrogen Doping on the Photoluminescence of Amorphous Silicon Oxycarbide Films. Micromachines 2019, 10, 649. [Google Scholar] [CrossRef]
- Hang, L.; Liu, W.; Xu, J.; Yang, C.; Zhou, S. Effects of various substrate materials on microstructural and optical properties of amorphous silicon oxynitride thin films deposited by plasma-enhanced chemical vapor deposition. Thin Solid Film. 2020, 709, 138186. [Google Scholar] [CrossRef]
- Ke, W.; Yang, K.; Zhu, X.D. Investigation on formation of thin Si-B-N films on stainless steel by plasma chemical vapor deposition. Appl. Surf. Sci. 2021, 565, 150583. [Google Scholar] [CrossRef]
- Wang, W.; Ngo, E.; Florea, I.; Foldyna, M.; Roca i Cabarrocas, P.; Maurice, J.-L. Room Temperature Growth of Silica Nanowires on Top of Ultrathin Si Nanowires Synthesized with Sn-Cu Bimetallic Seeds. Phys. Status Solidi A 2021, 218, 2100409. [Google Scholar] [CrossRef]
- Azrak, E.; Xue, Z.; Liu, S.; Chen, W.; Castro, C.; Duguay, S.; Pareige, P.; Yu, L.; Roca i Cabarrocas, P. Ultrahigh Incorporation of Tin in SiSn Nanowires Grown via In-Plane Solid-Liquid-Solid Mechanism. Appl. Surf. Sci. 2023, 618, 156637. [Google Scholar] [CrossRef]
- Baranov, A.I.; Morozov, I.A.; Uvarov, A.V.; Gudovskikh, A.S. Capacitance characterization of GaP/Si superlattice grown by time-modulated PECVD. J. Phys. Conf. Ser. 2019, 1410, 012116. [Google Scholar] [CrossRef]
- Uvarov, A.V.; Gudovskikh, A.S.; Baranov, A.I.; Maksimova, A.A.; Kudryashov, D.A.; Vyacheslavova, E.A.; Yakovlev, G.E.; Zubkov, V.I. Plasma-Deposited Multilayer GaP/Si p-i-n Structure for Tandem Silicon-Based Solar Cells. ACS Appl. Energy Mater. 2021, 5, 5374–5380. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, W.; Su, R.; Tu, H.; Shi, L.; Hu, J. Deuterium trapping in the carbon-silicon co-deposition layers prepared by RF sputtering in D2 atmosphere. J. Nucl. Mater. 2018, 501, 217–223. [Google Scholar] [CrossRef]
- Neßlinger, V.; Welzel, S.; Rieker, F.; Meinderink, D.; Nieken, U.; Grundmeier, G. Thin Organic-Inorganic Anti-Fouling Hybrid-Films for Microreactor Components. Macromol. React. Eng. 2023, 17, 2200043. [Google Scholar] [CrossRef]
- Mostofi Sarkari, N.; Doğan, Ö.; Bat, E.; Mohseni, M.; Ebrahimi, M. Assessing effects of (3-aminopropyl)trimethoxysilane self-assembled layers on surface characteristics of organosilane-grafted moisture-crosslinked polyethylene substrate: A comparative study between chemical vapor deposition and plasma-facilitated in situ grafting methods. Appl. Surf. Sci. 2019, 497, 143751. [Google Scholar]
- Rumyantsev, Y.M.; Chagin, M.N.; Shayapov, V.R.; Yushina, I.V.; Kichai, V.N.; Kosinova, M.L. Synthesis and Properties of Thin Films Formed by Vapor Deposition from Tetramethylsilane in a Radio-Frequency Inductively Coupled Plasma Discharge. Glass Phys. Chem. 2018, 44, 174–182. [Google Scholar] [CrossRef]
- Yang, K.; De Sagazan, O.; Pichon, L.; Salaün, A.-C.; Coulon, N. Inductively Coupled Plasma Chemical Vapor Deposition for Silicon-Based Technology Compatible with Low-Temperature (≤220 °C) Flexible Substrates. Phys. Status Solidi A 2020, 217, 1900556. [Google Scholar] [CrossRef]
- Song, H.; Seo, S.; Chang, H. Study on SiN and SiCN film production using PE-ALD process with high-density multi-ICP source at low temperature. Curr. Appl. Phys. 2018, 18, 1436–1440. [Google Scholar] [CrossRef]
- Jung, C.; Song, S.; Lee, N.; Kim, Y.; Lee, E.J.; Lee, S.G.; Jeon, H. Characteristics of Silicon Oxide Thin Film Deposited via Remote Plasma Atomic Layer Deposition. ECS J. Solid State Sci. Technol. 2021, 10, 043005. [Google Scholar] [CrossRef]
- Takenaka, K.; Setsuhara, Y.; Han, J.G.; Uchida, G.; Ebe, A. Plasma-enhanced reactive linear sputtering source for formation of silicon-based thin films. Rev. Sci. Instrum. 2018, 89, 083902. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, M.; Kim, Y.-J. Selective Growth and Contact Gap-Fill of Low Resistivity Si via Microwave Plasma-Enhanced CVD. Micromachines 2019, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, A.M.; Uznanski, P. Hard silicon carbonitride thin-film coatings produced by remote hydrogen plasma chemical vapor deposition using aminosilane and silazane precursors. 1: Deposition mechanism, chemical structure, and surface morphology. Plasma Process. Polym. 2021, 18, 2000240. [Google Scholar] [CrossRef]
- Wollny, P.; Menser, J.; Engelmann, L.; Sellmann, J.; Schulz, C.; Wiggers, H.; Kempf, A.; Wlokas, I. The role of phase transition by nucleation, condensation, and evaporation for the synthesis of silicon nanoparticles in a microwave plasma reactor—Simulation and experiment. Chem. Eng. J. 2023, 453, 139695. [Google Scholar] [CrossRef]
- Daoudi, K.; Columbus, S.; Falcão, B.P.; Pereira, R.N.; Peripolli, S.B.; Ramachandran, K.; Hadj Kacem, H.; Allagui, A.; Gaidi, M. Label-free DNA detection using silver nanoprism decorated silicon nanoparticles: Effect of silicon nanoparticle size and doping levels. Spectrochim. Acta A 2023, 290, 122262. [Google Scholar] [CrossRef]
- Knipping, J.; Wiggers, H.; Rellinghaus, B.; Roth, P.; Konjhodzic, D.; Meier, C. Synthesis of High Purity Silicon Nanoparticles in a Low Pressure Microwave Reactor. J. Nanosci. Nanotechnol. 2004, 4, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.A.; Lichtemberg, A.J. Principles of Plasma Discharges and Materials Processing, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Miller, J.W.; Khatami, Z.; Wojcik, J.; Bradley, J.D.B.; Mascher, P. Integrated ECR-PECVD and magnetron sputtering system for rare-earth-doped Si-based materials. Surf. Coat. Technol. 2018, 336, 99–105. [Google Scholar] [CrossRef]
- Baranov, E.A.; Khmel, S.Y.; Zamchiy, A.O. Synthesis of amorphous silicon films with high growth rate by gas-jet electron beam plasma chemical vapor deposition method. IEEE Trans. Plasma Sci. 2014, 42, 2794. [Google Scholar] [CrossRef]
- Zamchiy, A.O.; Baranov, E.A.; Khmel, S.Y. Tin-catalyzed oriented array of microropes of silicon oxide nanowires synthesized on different substrates. Vacuum 2018, 147, 99–106. [Google Scholar] [CrossRef]
- Tabuchi, T.; Toyoshima, Y.; Fujimoto, S.; Takashiri, M. Optimized hydrogen concentration within a remotely induced hollow-anode plasma for fast chemical-vapor-deposition of photosensitive and <110>-preferential microcrystalline silicon thin-films. Thin Sol Film. 2020, 694, 137714. [Google Scholar]
- De Freitas, A.S.M.; Maciel, C.C.; Rodrigues, J.S.; Ribeiro, R.P.; Delgado-Silva, A.O.; Rangel, E.C. Organosilicon films deposited in low-pressure plasma from hexamethyldisiloxane—A review. Vacuum 2021, 19, 110556. [Google Scholar] [CrossRef]
- Kleines, L.; Wilski, S.; Alizadeh, P.; Rubner, J.; Wessling, M.; Hopmann, C.; Dahlmann, R. Evaluation of the membrane performance of ultra-smooth silicon organic coatings depending on the process energy density. Thin Solid Film. 2022, 748, 139169. [Google Scholar] [CrossRef]
- Kleines, L.; Wilski, S.; Alizadeh, P.; Rubner, J.; Wessling, M.; Hopmann, C.; Dahlmann, R. Structure and gas separation properties of ultra-smooth PE-CVD silicon organic coated composite membranes. Surf. Coat. Technol. 2021, 42, 127338. [Google Scholar] [CrossRef]
- Mu, H.; Wang, X.; Li, Z.; Xie, Y.; Gao, Y.; Liu, H. Preparation and atomic oxygen erosion resistance of SiOx coating formed on polyimide film by plasma polymer deposition. Vacuum 2019, 165, 7–11. [Google Scholar] [CrossRef]
- Mitschker, F.; Schücke, L.; Hoppe, C.; Jaritz, M.; Dahlmann, R.; De Los Arcos, T.; Hopmann, C.; Grundmeier, G.; Awakowicz, P. Comparative study on the deposition of silicon oxide permeation barrier coatings for polymers using hexamethyldisilazane (HMDSN) and hexamethyldisiloxane (HMDSO). J. Phys. D Appl. Phys. 2018, 51, 235201. [Google Scholar] [CrossRef]
- Cech, V.; Branecky, M. Nonthermal tetravinylsilane plasma used for thin-film deposition: Plasma chemistry controls thin-film chemistry. Plasma Process. Polym. 2022, 19, 2100192. [Google Scholar] [CrossRef]
- Pereira, M.; Baldin, E.K.; Antonini, L.M.; Bernardi, F.; Oliveira, L.; Maurmann, N.; Pranke, P.; Pereira, M.B.; Malfatti, C. de F. TEOS thin films obtained by plasma polymerization on Ti6Al4V alloys: Influence of the deposition pressure on surface properties and cellular response. Appl. Surf. Sci. Adv. 2021, 5, 100123. [Google Scholar] [CrossRef]
- Bulou, S.; Lecoq, E.; Loyer, F.; Frache, G.; Fouquet, T.; Gueye, M.; Belmonte, T.; Choquet, P. Study of a pulsed post-discharge plasma deposition process of APTES: Synthesis of highly organic pp-APTES thin films with NH 2 functionalized polysilsesquioxane evidences. Plasma Process. Polym. 2019, 16, 1800177. [Google Scholar] [CrossRef]
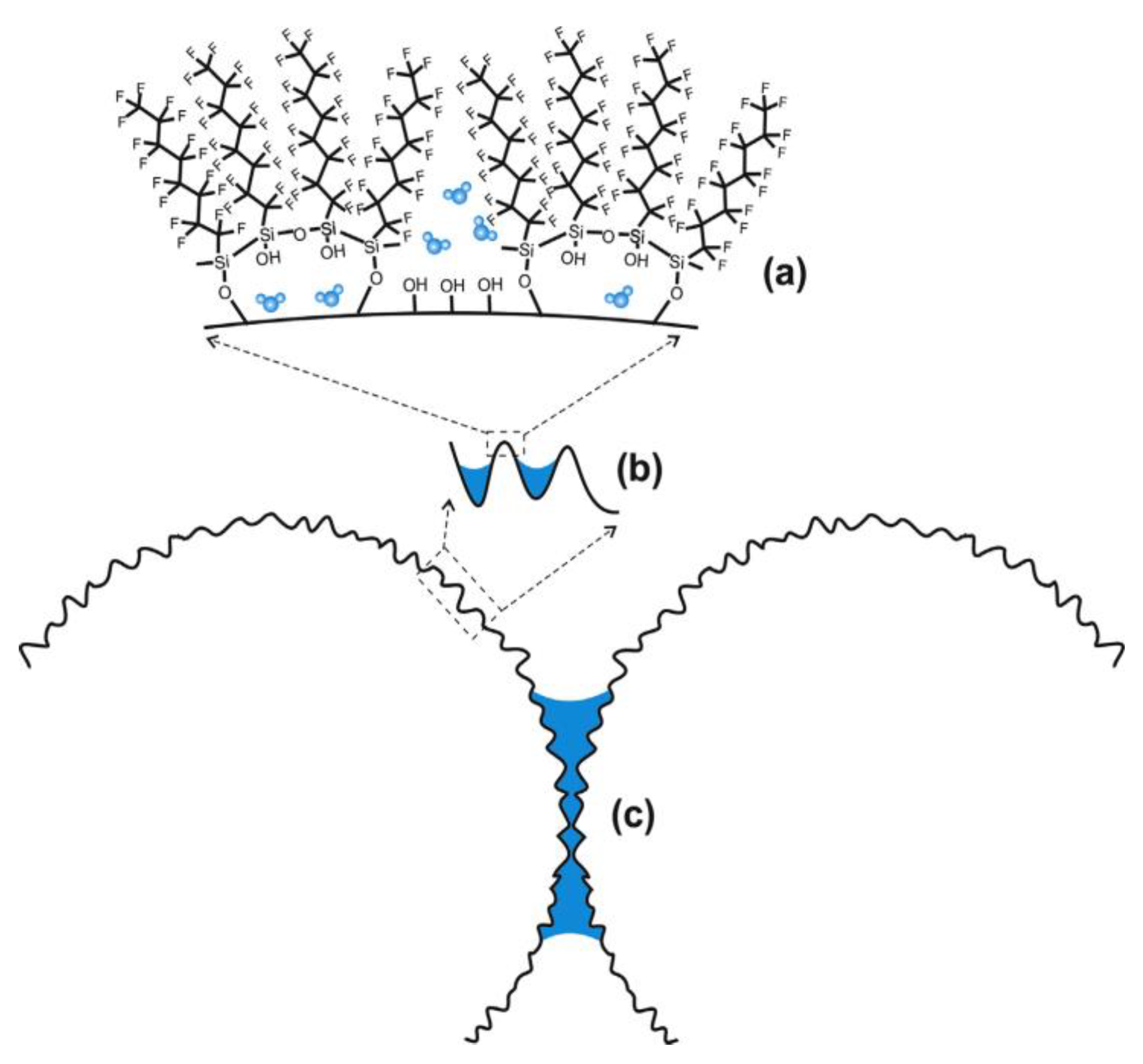
- Giner, I.; Torun, B.; Han, Y.; Duderija, B.; Meinderink, D.; Orive, A.G.; de los Arcos, T.; Weinberger, C.; Tiemann, M.; Schmid, H.J.; et al. Water adsorption and capillary bridge formation on silica micro-particle layers modified with perfluorinated organosilane monolayers. Appl. Surf. Sci. 2019, 475, 873–879. [Google Scholar] [CrossRef]
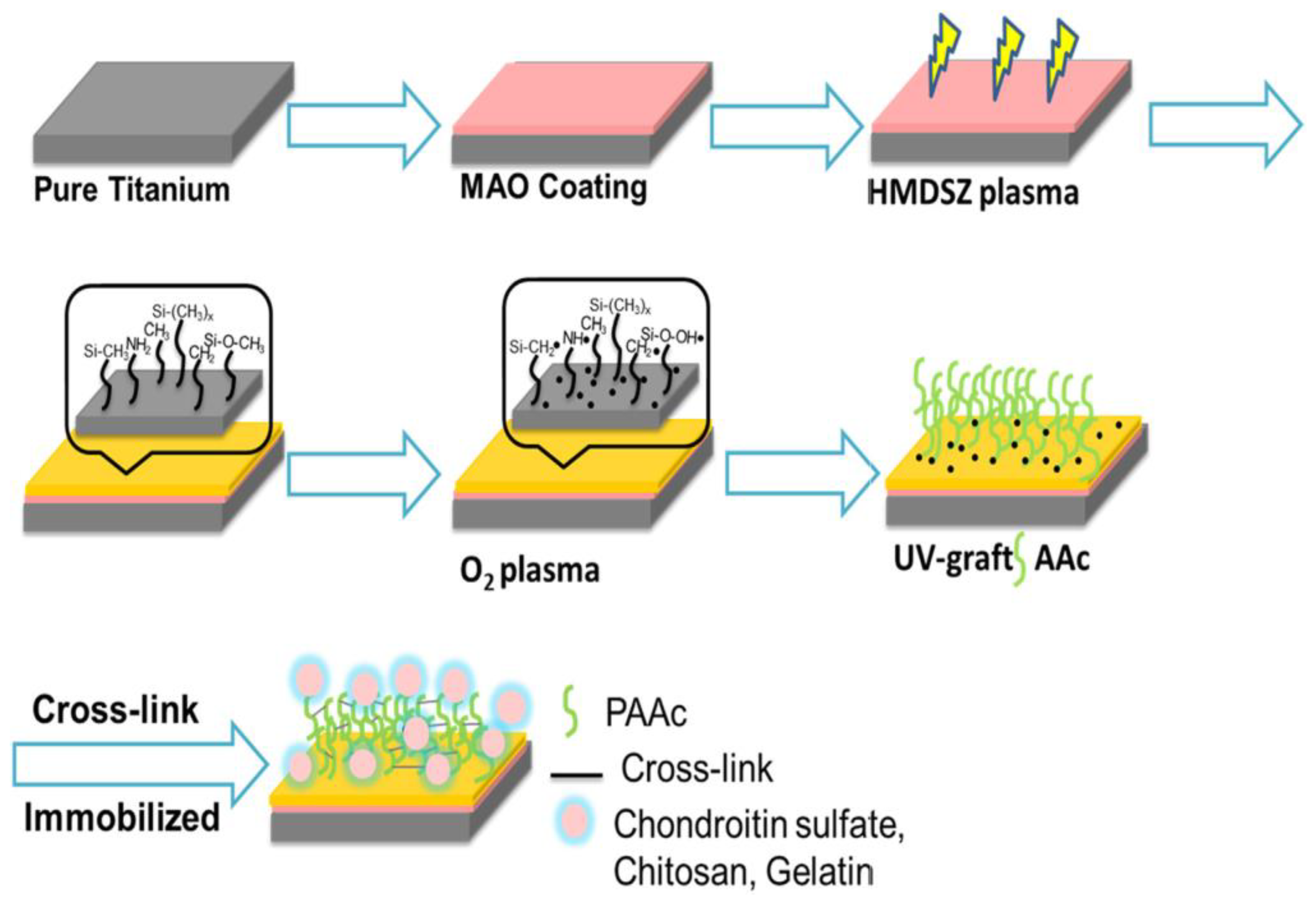
- Liao, S.C.; Chang, C.T.; Chen, C.Y.; Lee, C.H.; Lin, W.L. Functionalization of pure titanium MAO coatings by surface modifications for biomedical applications. Surf. Coat. Technol. 2020, 394, 125812. [Google Scholar] [CrossRef]
- Ho, K.N.; Chen, L.W.; Kuo, T.F.; Chen, K.S.; Lee, S.Y.; Wang, S.F. Surface modification of zirconia ceramics through cold plasma treatment and the graft polymerization of biomolecules. J. Dent. Sci. 2023, 18, 73–80. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta B 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Teli, M.D.; Pandit, P.; Samanta, K.K. Application of atmospheric pressure plasma technology on textile. J. Text. Assoc. 2015, 75, 422–428. [Google Scholar]
- Baniya, H.B.; Guragain, R.P.; Subedi, D.P. Cold atmospheric pressure plasma technology for modifying polymers to enhance adhesion: A critical review. Prog. Adhes. Adhes. 2021, 6, 841–880. [Google Scholar]
- Mariotti, D.; Sankaran, R.M. Perspectives on atmospheric-pressure plasmas for nanofabrication J. Phys. D 2011, 44, 174023. [Google Scholar] [CrossRef]
- Wan, X.; Chen, X.; Yang, S.; Ma, W.; Li, S.; Wei, K. Facile synthesis of silicon nanospheres and nanosheets using DC thermal plasma. Mater. Lett. 2020, 268, 127616. [Google Scholar] [CrossRef]
- Yang, Z.; Dong, Y.; Liu, C.; Feng, X.; Jin, H.; Ma, X.; Ding, F.; Li, B.; Bai, L.; Ouyang, Y.; et al. Design and synthesis of high-silicon silicon suboxide nanowires by radio-frequency thermal plasma for high-performance lithium-ion battery anodes. Appl. Surf. Sci. 2023, 614, 156235. [Google Scholar] [CrossRef]
- Zheng, T.; Xu, B.; Wang, S.; Guo, D.; Zhou, F.; Wang, Y.; Fang, D. Microstructure and nanomechanical properties of plasma-sprayed nanostructured Yb2SiO5 environmental barrier coatings. J. Am. Ceram. Soc. 2023, 106, 2666–2678. [Google Scholar] [CrossRef]
- Ma, G.-Z.; He, P.-F.; Wang, H.-D.; Tian, H.-G.; Zhou, L.; Yong, Q.-S.; Liu, M.; Zhao, H.-C.; He, D.-Y. Promoting bonding strength between internal Al-Si based gradient coating and aluminum alloy cylinder bore by forming homo-epitaxial growth interface. Mater. Des. 2023, 227, 111764. [Google Scholar] [CrossRef]
- Wang, R.; Xia, Z.; Kong, X.; Liang, L.; Ostrikov, K. Etching and annealing treatment to improve the plasma-deposited SiOx film adhesion force. Surf. Coat. Technol. 2021, 427, 127840. [Google Scholar] [CrossRef]
- Wang, R.; Xia, Z.; Kong, X.; Xue, S.; Wang, H. Uniform deposition of silicon oxide film on cylindrical substrate by radially arranged plasma jet array. Surf. Coat. Technol. 2022, 437, 128365. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Xue, S.; Xie, P.; Yang, W. Substrate temperature induced structure transformation in plasma film deposition process. Surf. Coat. Technol. 2022, 451, 129071. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, Y.; Lin, L.; Gao, M.; Huang, Y.; Chu, P.K. Deposition of nanocomposites coating on polyimide films by atmospheric pressure plasma for enhanced thermal conductivity. Surf. Interfaces 2023, 37, 102758. [Google Scholar] [CrossRef]
- Dworschak, M.; Kohlmann, N.; Matějka, F.; Galář, P.; Kienle, L.; Schäfer, J.; Benedikt, J. Silicon nanocrystal synthesis with the atmospheric plasma source HelixJet. Plasma Process. Polym. 2023, 20, 2200129. [Google Scholar] [CrossRef]
- Nijdam, S.; Teunissen, J.; Ebert, U. The physics of streamer discharge phenomena. Plasma Sources Sci. Technol. 2020, 29, 103001. [Google Scholar] [CrossRef]
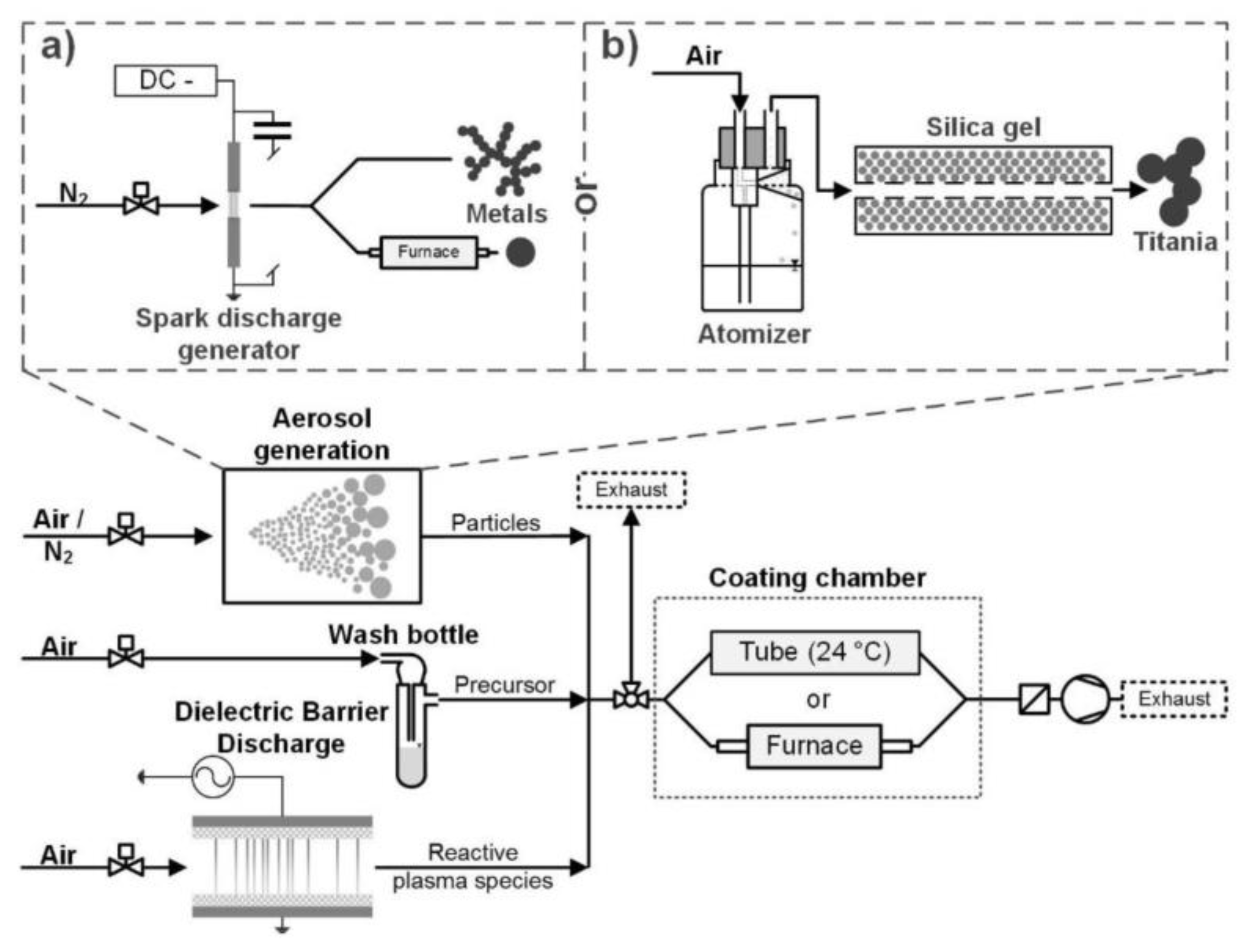
- Post, P.; Wurlitzer, L.; Maus-Friedrichs, W.; Weber, A.P. Characterization and Applications of Nanoparticles Modified in-Flight with Silica or Silica-Organic Coatings. Nanomaterials 2018, 8, 530. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, H.; Ohmi, H.; Yasutake, K. Controllability of structural and electrical properties of silicon films grown in atmospheric-pressure very high-frequency plasma. J. Phys. D 2018, 51, 355203. [Google Scholar] [CrossRef]
- Kakiuchi, H.; Ohmi, H.; Yasutake, K. Pulsed very high-frequency plasma-enhanced chemical vapor deposition of silicon films for low-temperature (120 °C) thin film transistors. J. Phys. D 2020, 53, 15201. [Google Scholar] [CrossRef]
- Sohbatzadeh, F.; Shabannejad, A.; Ghasemi, M.; Mahmoudsani, Z. Deposition of halogen-free flame retardant and water-repellent coatings on firwood surfaces using the new version of DBD. Prog. Org. Coat. 2021, 151, 106070. [Google Scholar] [CrossRef]
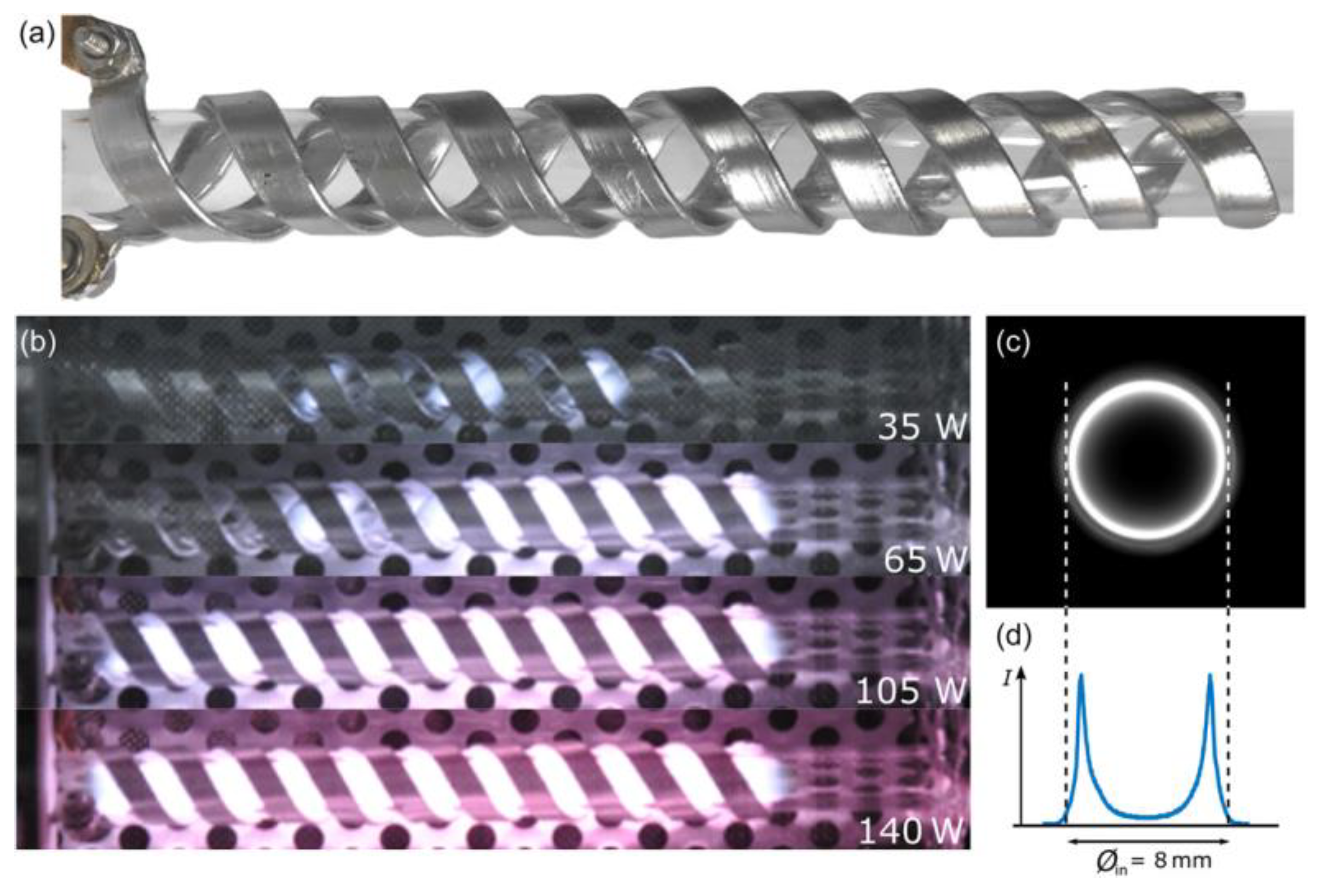
- Trinh, Q.H.; Nguyen, D.B.; Hossain, M.M.; Mok, Y.S. Deposition of superhydrophobic coatings on glass substrates from hexamethyldisiloxane using a kHz-powered plasma jet. Surf. Coat. Technol. 2019, 361, 377–385. [Google Scholar] [CrossRef]
- Silva, L.L.G.; Kodaira, F.V.P.; Fagundes, P.V.M.; Quade, A.; Kostov, K.G. Study of Organosilicon Films Deposited on SAE 1020 Steel by Atmospheric Plasma Jet for Corrosion Protection. Braz. J. Phys. 2022, 5, 114. [Google Scholar] [CrossRef]
- Profili, J.; Asadollahi, S.; Vinchon, P.; Dorris, A.; Beck, S.; Sarkassian, A.; Stafford, L. Recent progress on organosilicon coatings deposited on bleached unrefined Kraft paper by non-thermal plasma process at atmospheric pressure. Prog. Org. Coat. 2020, 147, 105865. [Google Scholar] [CrossRef]






| Refs. | Materials | Formula | Precursor name |
|---|---|---|---|
| [23,24,25,26,27,28,29,30,31,32,34,35,36,37,38,39,40,41] [42,43,44,45,50,54,56,57,58,61,63] | a-Si:H, nc-Si:H, mc-Si, c-Si, a-SiN:H, a-SiO2 SiCxOy:N, a-SiOxNy a-SiBxNy, Si/SiSn nanowires, Si nanoparticles | SiH4 | Silane |
| [25] | a-Si:H | Si2H6 | Disilane |
| [14] | a-Si, a-SiC, a-SiN | SiCl4 | Silicon tetrachloride |
| [33] | a-Si, nc-Si | SiHCl3 | Trichlorosilane (TCS) |
| [49] | a-SiCx:H, a-SiCxNy:H | Si(CH3)4 | Tetramethylsilane |
| [55] | a-SiCxNy | C6H18N2Si | (Dimethylamino)dimethylsilane (DMADMS) |
| [55] | a-SiCxNy | C5H16N2Si | Bis(dimethylamino)methylsilane (BDMAMS) |
| [55] | a-SiCxNy | C7H21N3Si | Tris(dimethylamino)silane (TDMAS) |
| [55] | a-SiCxNy | C4H13NSi2 | 1,1,3,3-Tetramethyldisilazane (TMDSN) |
| [55] | a-SiCxNy | C8H24N2Si4 | 1,3-Bis(dimethylsilyl)-2,2,4,4-tetramethylcyclodisilazane (BSCDSN) |
| [14] [39] | a-SiC, DLC, SiCxOy:N | CH4 | Methane |
| [15] | a-C:H, a-SiC:H | C2H2 | Acetylene |
| [17,18] | a-C:H:SiOx | CH3[Si(CH3)2O]nSi(CH3)3 | Polyphenylmethylsiloxane (PPMS) |
| [52] | SiO2 | C8H22N2Si | bis(tertiary-butylamino)silane (BTBAS) |
| [47,72] | FOTS-grafted-SiOx | C14H19F13O3Si | 1H,1H,2H,2H-Perfluorooctyltriethoxysilane (FOTS) |
| [48] | APTMS-grafted-SiOx | C6H17NO3Si | (3-Aminopropyl)trimethoxysilane (APTMS) |
| [71] | Silsesquioxanes, cyclosiloxanes, amino groups | C9H23NO3Si | (3-Aminopropyl)triethoxysilane (APTES) |
| [68,73,74] | SiOxCyHz | C6H19NSi2 | Hexamethyldisilazane (HMDSZ) or (HDMSN) |
| [64,65,66,67,68] | SiOxCyHz | C6H18OSi2 | Hexamethyldisiloxane (HDMSO) |
| [69] | SiCxHy | C8H12Si | Tetravinylsilane (TVS) |
| [70] | SiO2 | SiC8H20O4 | Tetraethoxysilane (TEOS) |
| [41,49,51] | a-SiBxNy, a-SiCxNy:H | N2 | Nitrogen ** |
| [34] [39,40] | a-SiN:H, SiCxOy:N, a-SiOxNy | NH3 | Ammonia |
| [40] | a-SiOxNy | N2O | Nitrous oxide |
| [39,50] | a-SiO2, a-SiCxOy:N | O2 | Oxygen |
| [36] | a-SiO2 | CO2 | Carbon dioxide |
| [41] | a-SiBxNy | B2H6 | Diborane * |
| Refs. | Materials | Formula | Precursor Name |
|---|---|---|---|
| [83,84,85,89] | a-SiOx Si/SiO2 coated nanoparticles | SiC8H20O4 | Tetraethylorthosilicate (TEOS) |
| [89,92,93,94] | Si/SiO2 coated nanoparticles; SiOx coating | Si2C6H18O | Hexamethyldisiloxane (HMDSO) |
| [95] | SiOx | C4H12O4Si4 | 2,4,6,8-Tetramethylcyclotetrasiloxane (TMCTS) |
| [87,90,91] | Si nanocrystals, a-Si | SiH4 | Silane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandracci, P.; Rivolo, P. Recent Advances in the Plasma-Assisted Synthesis of Silicon-Based Thin Films and Nanostructures. Coatings 2023, 13, 1075. https://doi.org/10.3390/coatings13061075
Mandracci P, Rivolo P. Recent Advances in the Plasma-Assisted Synthesis of Silicon-Based Thin Films and Nanostructures. Coatings. 2023; 13(6):1075. https://doi.org/10.3390/coatings13061075
Chicago/Turabian StyleMandracci, Pietro, and Paola Rivolo. 2023. "Recent Advances in the Plasma-Assisted Synthesis of Silicon-Based Thin Films and Nanostructures" Coatings 13, no. 6: 1075. https://doi.org/10.3390/coatings13061075
APA StyleMandracci, P., & Rivolo, P. (2023). Recent Advances in the Plasma-Assisted Synthesis of Silicon-Based Thin Films and Nanostructures. Coatings, 13(6), 1075. https://doi.org/10.3390/coatings13061075







