Permeability of Skin-Mimicking Cell Coatings by Polymers of Complex Architecture Based on Polyoxazolines
Abstract
1. Introduction
1.1. Experimental Section
1.1.1. Materials
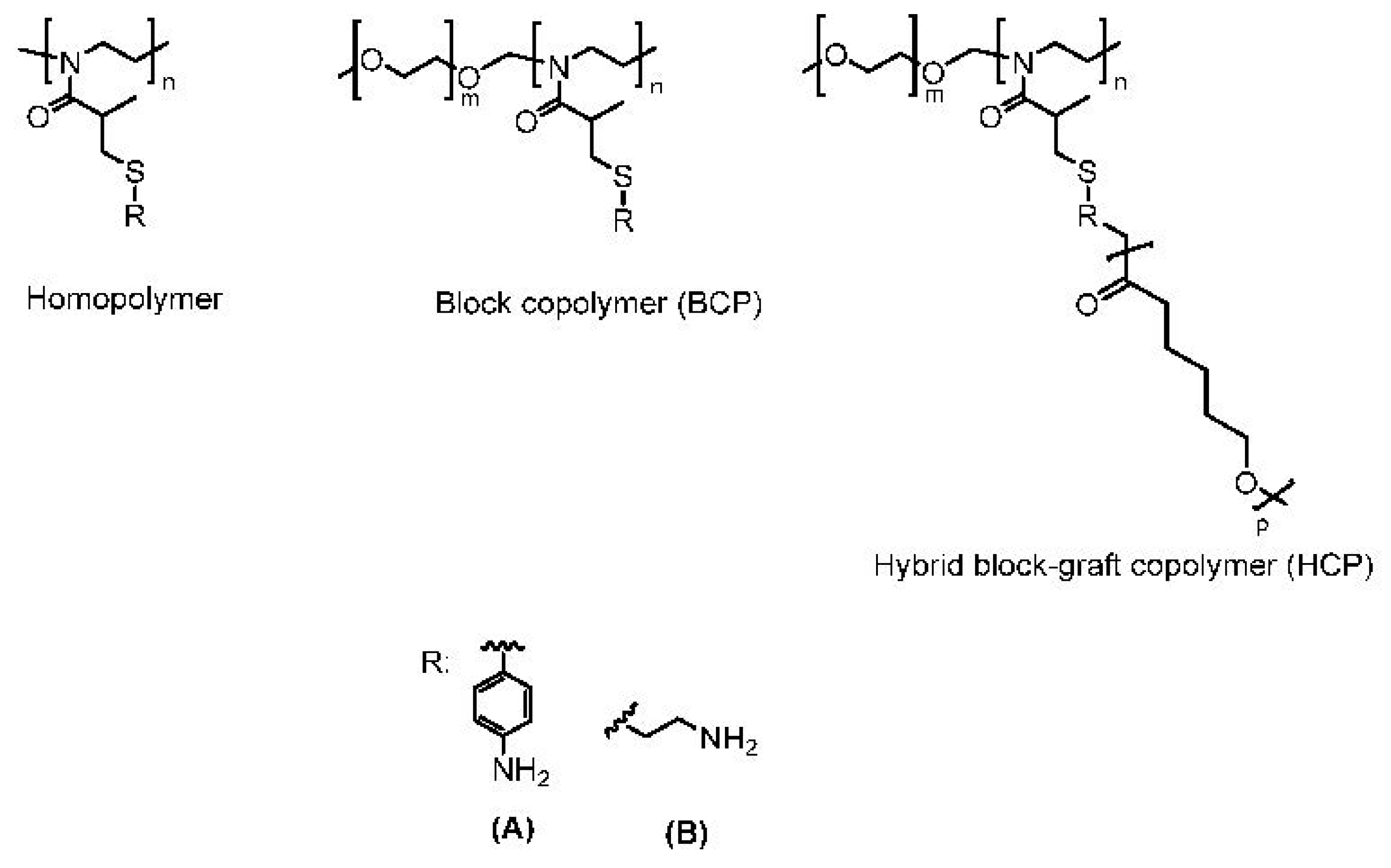
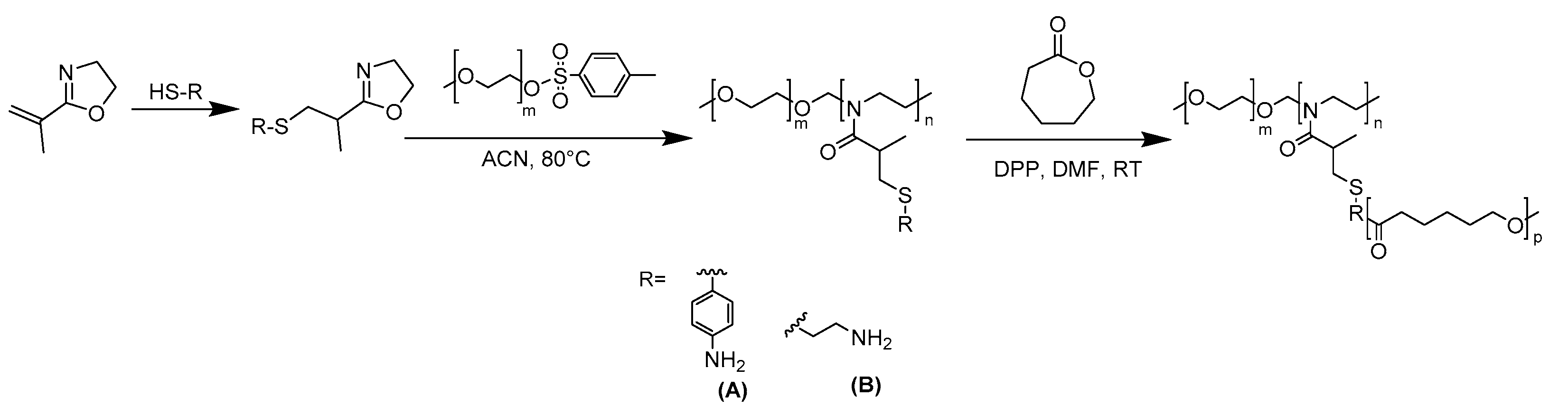
1.1.2. Synthesis of the Hybrid Block–Graft Copolymer
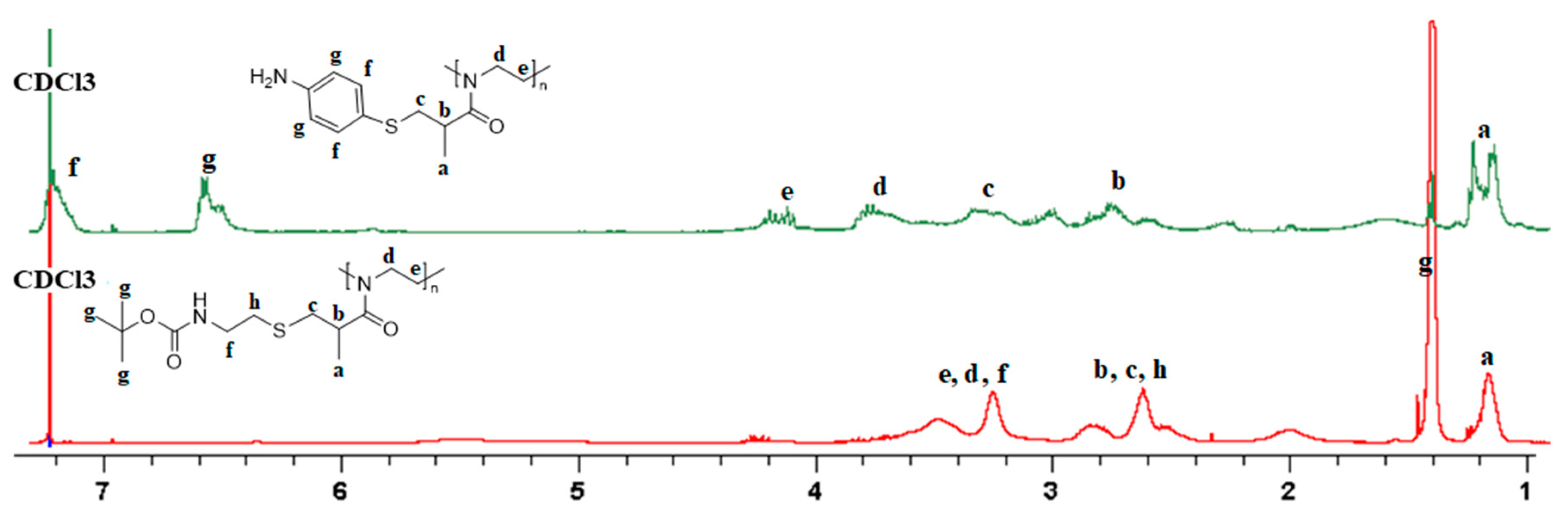
1.1.3. NMR Spectroscopy
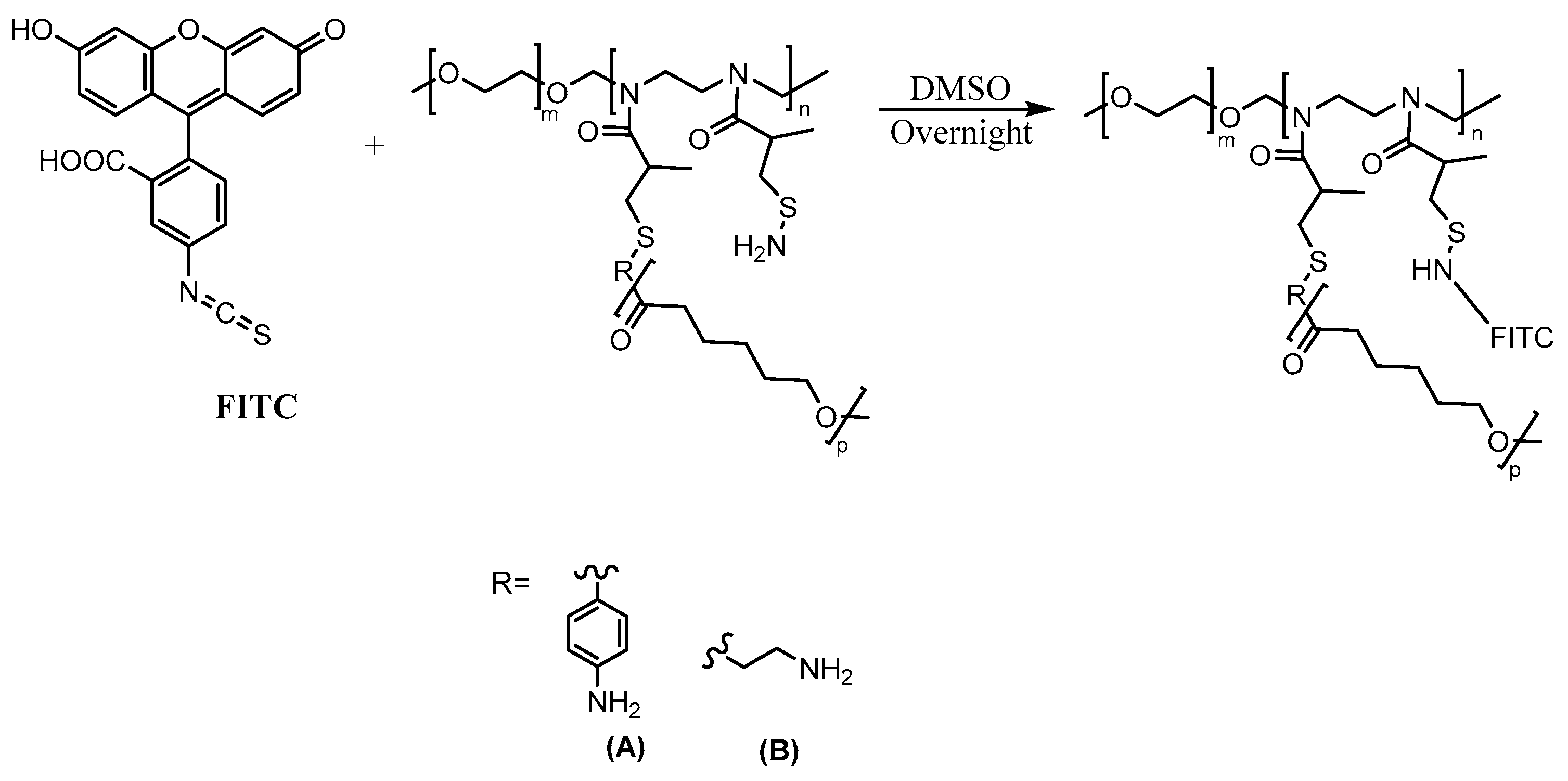
1.1.4. Fluorescently Labeled Polymers
1.1.5. Fluorescence Spectroscopy
1.2. Experimental Methods
1.2.1. Dynamic Light Scattering
1.2.2. Equilibration of EpiDerm™ Tissues
1.2.3. Skin Penetration Studies
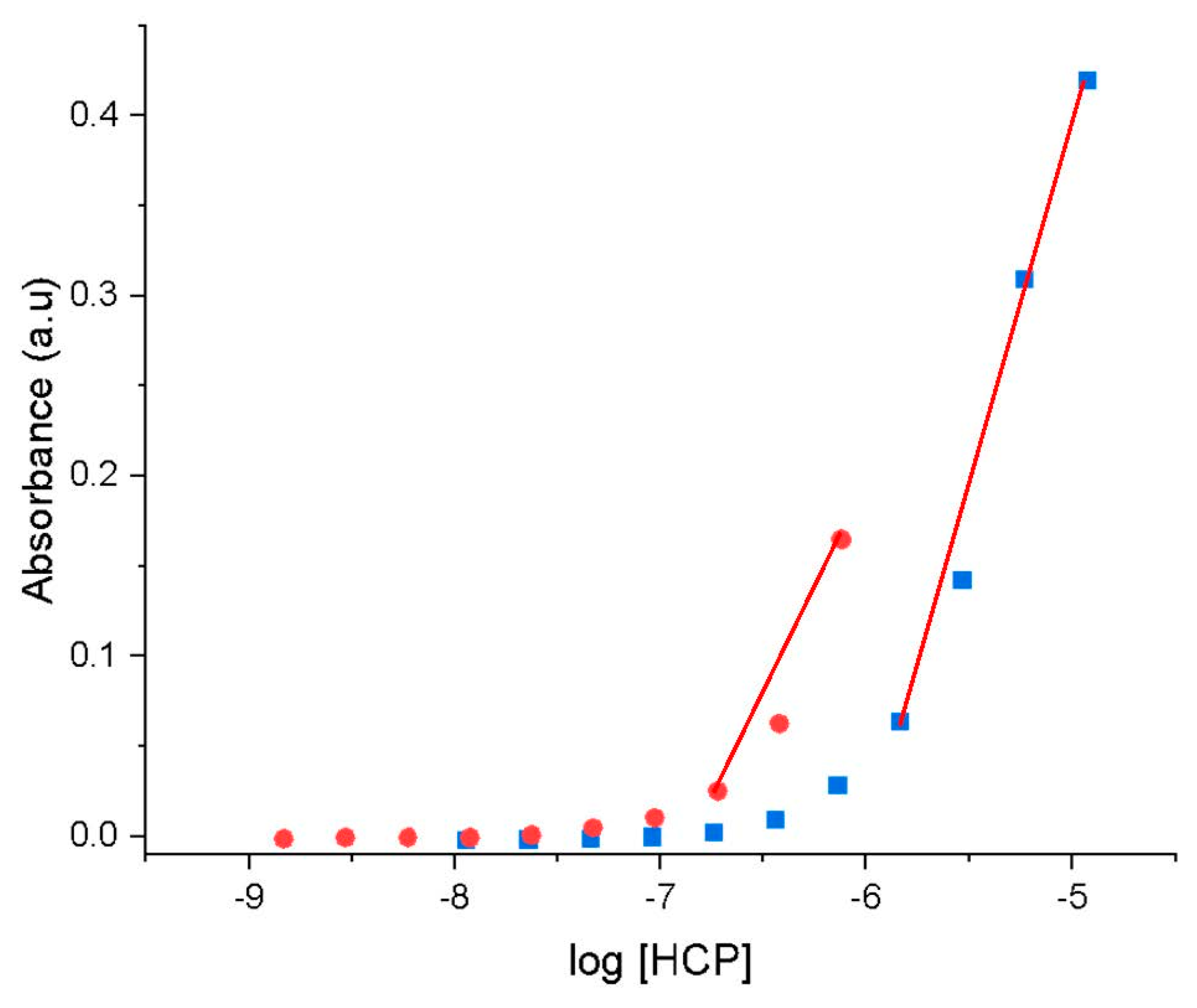
1.2.4. Determination of Critical Micelle Concentration
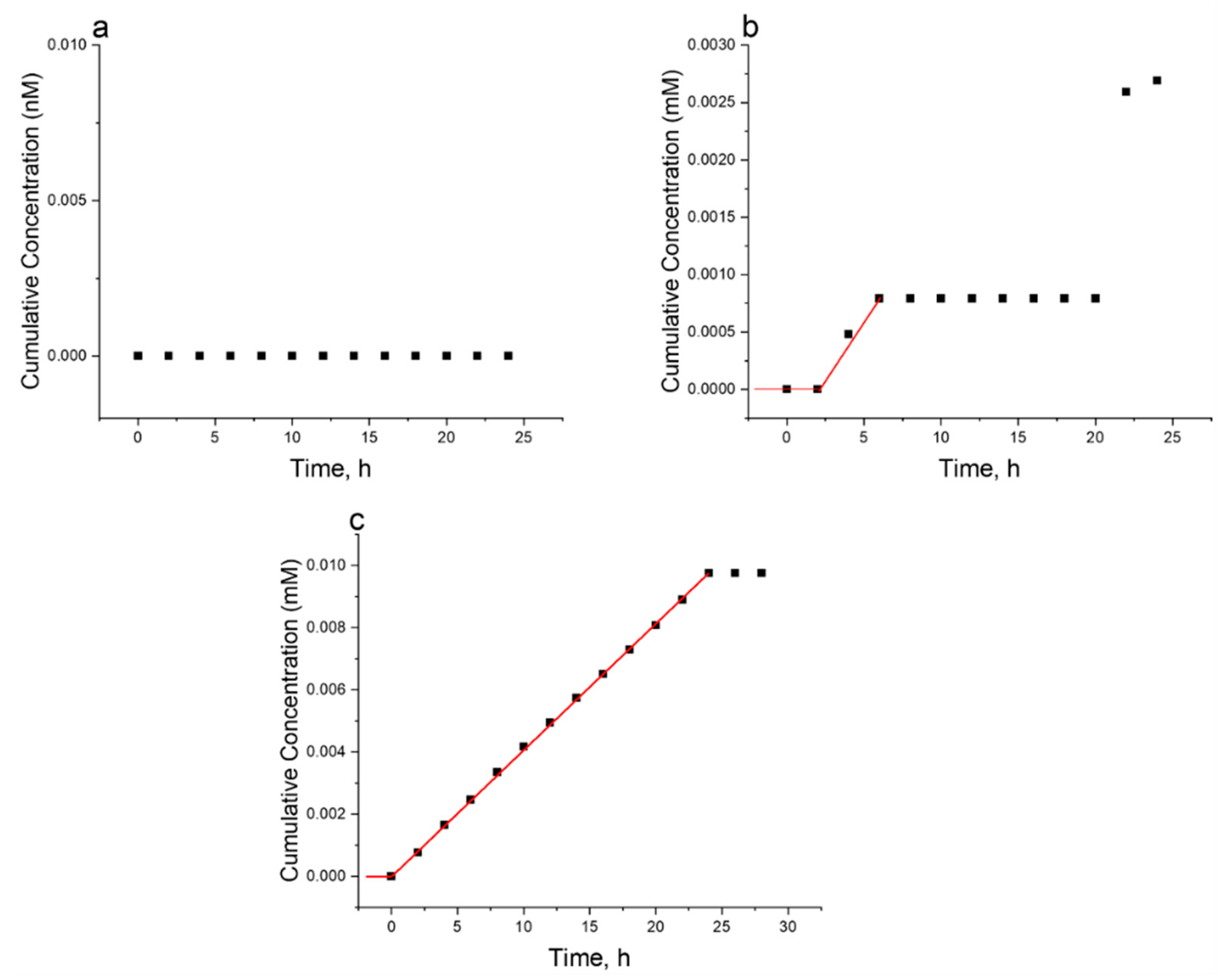
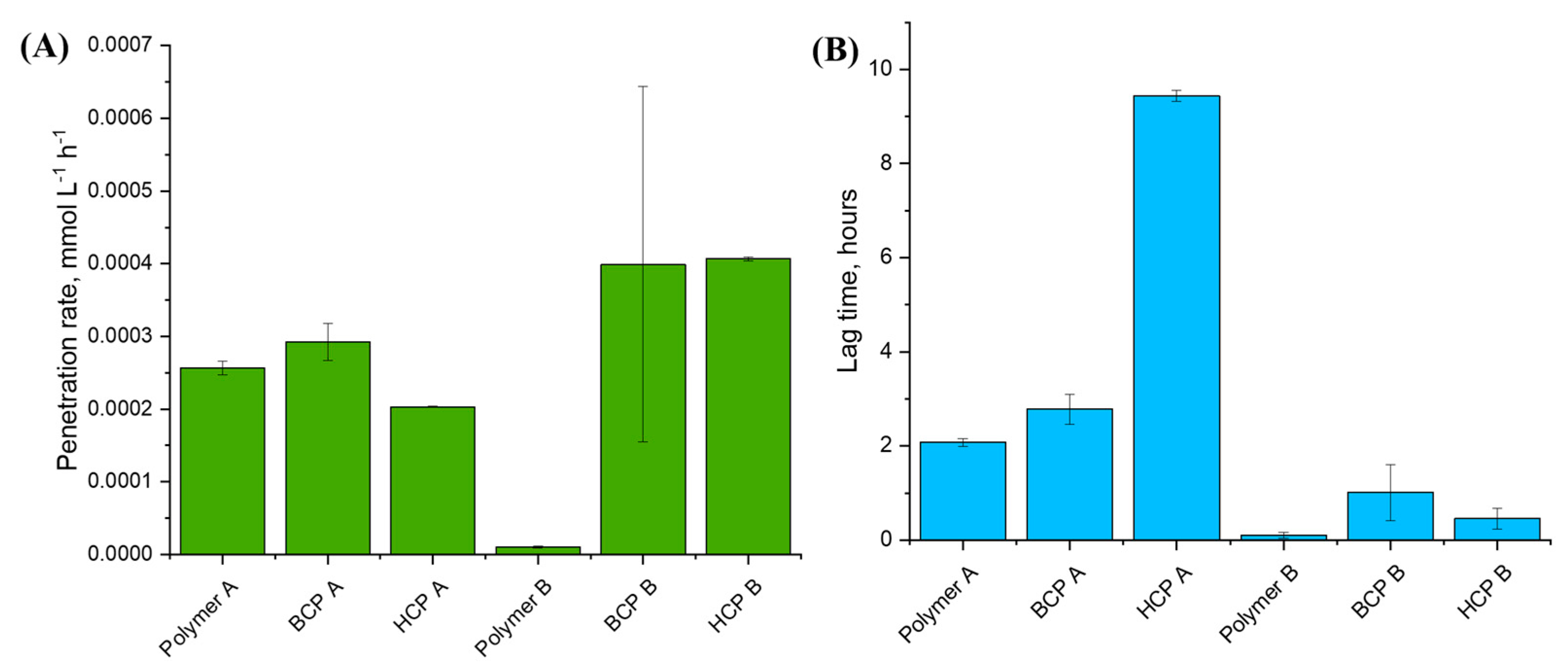
2. Results and Discussion
- Permeation of Aminothiophenol-Functionalized Polymers (A):
- Permeation of Cysteamine-Functionalized Polymers (B):
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Lv, Y.; Qi, J.; Zhu, Q.; Lu, Y.; Wu, W. Overcoming or Circumventing the Stratum Corneum Barrier for Efficient Transcutaneous Immunization. Drug. Discov. Today 2018, 23, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Kapse, A.; Anup, N.; Patel, V.; Saraogi, G.K.; Mishra, D.K.; Tekade, R.K. Polymeric Micelles: A Ray of Hope Among New Drug Delivery Systems. In Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2020; pp. 235–289. [Google Scholar] [CrossRef]
- Arora, A.; Prausnitz, M.R.; Mitragotri, S. Micro-Scale Devices for Transdermal Drug Delivery. Int. J. Pharm. 2008, 364, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Valenta, C.; Auner, B.G. The Use of Polymers for Dermal and Transdermal Delivery. Eur. J. Pharm. Biopharm. 2004, 58, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Cho, H.-E.; Moon, S.H.; Ahn, H.-J.; Bae, S.; Cho, H.-D.; An, S. Transdermal Delivery Systems in Cosmetics. Biomed. Dermatol. 2020, 4, 10. [Google Scholar] [CrossRef]
- Badri, W.; Eddabra, R.; Fessi, H.; Elaissari, A. Biodegradable Polymer Based Nanoparticles: Dermal and Transdermal Drug Delivery. J. Colloid. Sci. Biotechnol. 2014, 3, 141–149. [Google Scholar] [CrossRef]
- Tiwari, N.; Osorio-Blanco, E.R.; Sonzogni, A.; Esporrín-Ubieto, D.; Wang, H.; Calderón, M. Nanocarriers for Skin Applications: Where Do We Stand? Angew. Chem.-Int. Ed. 2022, 61, e202107960. [Google Scholar] [CrossRef] [PubMed]
- Raghavachari, N.; Fahl, W.E. Targeted Gene Delivery to Skin Cells in Vivo: A Comparative Study of Liposomes and Polymers as Delivery Vehicles. J. Pharm. Sci. 2002, 91, 615–622. [Google Scholar] [CrossRef]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for Drug Delivery Systems. Annu. Rev. Chem. Biomol. Eng. 2010, 24, 12. [Google Scholar] [CrossRef]
- Rancan, F.; Volkmann, H.; Giulbudagian, M.; Schumacher, F.; Stanko, J.I.; Kleuser, B.; Blume-Peytavi, U.; Calderón, M.; Vogt, A. Dermal Delivery of the High-Molecular-Weight Drug Tacrolimus by Means of Polyglycerol-Based Nanogels. Pharmaceutics 2019, 11, 394. [Google Scholar] [CrossRef]
- Rancan, F.; Asadian-Birjand, M.; Dogan, S.; Graf, C.; Cuellar, L.; Lommatzsch, S.; Blume-Peytavi, U.; Calderón, M.; Vogt, A. Effects of Thermoresponsivity and Softness on Skin Penetration and Cellular Uptake of Polyglycerol-Based Nanogels. J. Control. Release 2016, 228, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Storti, G.; Jauhola-Straight, R.; Hannigan, J.R.; Sidorenko, A. Design of Amphiphilic, Biodegradable Functionalized Polyoxazoline Hybrid-Block Graft Copolymers Using Click Reactions. Macromolecules 2023, 56, 3538–3549. [Google Scholar] [CrossRef]
- Nemati Mahand, S.; Aliakbarzadeh, S.; Moghaddam, A.; Salehi Moghaddam, A.; Kruppke, B.; Nasrollahzadeh, M.; Khonakdar, H.A. Polyoxazoline: A Review Article from Polymerization to Smart Behaviors and Biomedical Applications. Eur. Polym. J. 2022, 178, 111484. [Google Scholar] [CrossRef]
- Luxenhofer, R.; Han, Y.; Schulz, A.; Tong, J.; He, Z.; Kabanov, A.V.; Jordan, R. Poly(2-Oxazoline)s as Polymer Therapeutics. Macromol. Rapid. Commun. 2012, 33, 1613–1631. [Google Scholar] [CrossRef] [PubMed]
- Salgarella, A.R.; Zahoranová, A.; Šrámková, P.; Majerčíková, M.; Pavlova, E.; Luxenhofer, R.; Kronek, J.; Lacík, I.; Ricotti, L. Investigation of Drug Release Modulation from Poly(2-Oxazoline) Micelle through Ultrasound. Sci. Rep. 2018, 8, 9893. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.A.; Grayson, S.M. Thiol-Ene Click Functionalization and Subsequent Polymerization of 2-Oxazoline Monomers. Macromolecules 2010, 43, 4081–4090. [Google Scholar] [CrossRef]
- Zakharchenko, A.; Xue, Y.; Keeney, S.; Rock, C.A.; Alferiev, I.S.; Stachelek, S.J.; Takano, H.; Thomas, T.; Nagaswami, C.; Krieger, A.M.; et al. Poly-2-Methyl-2-Oxazoline–Modified Bioprosthetic Heart Valve Leaflets Have Enhanced Biocompatibility and Resist Structural Degeneration. Proc. Natl. Acad. Sci. USA 2022, 119, e2120694119. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, F.; Chen, H.; Li, M.; Qiao, F.; Liu, Z.; Hou, Y.; Wu, C.; Fan, Y.; Liu, L.; et al. Selective Antimicrobial Activities and Action Mechanism of Micelles Self-Assembled by Cationic Oligomeric Surfactants. ACS Appl. Mater. Interfaces 2016, 8, 4242–4249. [Google Scholar] [CrossRef]
- Paulovičová, E.; Kroneková, Z.; Paulovičová, L.; Majerčíková, M.; Kronek, J. Cell-Mediated Immunoreactivity of Poly(2-Isopropenyl-2-Oxazoline) as Promising Formulation for Immunomodulation. Materials 2021, 14, 1371. [Google Scholar] [CrossRef]
- Miyata, K.; Christie, R.J.; Kataoka, K. Polymeric Micelles for Nano-Scale Drug Delivery. React. Funct. Polym. 2011, 71, 227–234. [Google Scholar] [CrossRef]
- Adams, N.; Schubert, U.S. Poly(2-Oxazolines) in Biological and Biomedical Application Contexts. Adv. Drug. Deliv. Rev. 2007, 59, 1504–1520. [Google Scholar] [CrossRef]
- Vlassi, E.; Papagiannopoulos, A.; Pispas, S. Amphiphilic Poly(2-Oxazoline) Copolymers as Self-Assembled Carriers for Drug Delivery Applications. Eur. Polym. J. 2017, 88, 516–523. [Google Scholar] [CrossRef]
- Simon, L.; Vincent, M.; Le Saux, S.; Lapinte, V.; Marcotte, N.; Morille, M.; Dorandeu, C.; Devoisselle, J.M.; Bégu, S. Polyoxazolines Based Mixed Micelles as PEG Free Formulations for an Effective Quercetin Antioxidant Topical Delivery. Int. J. Pharm. 2019, 570, 118516. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Jiang, Y.; Cheng, R.; Meng, F.; Zhong, Z. Biodegradable Polymeric Micelles for Targeted and Controlled Anticancer Drug Delivery: Promises, Progress and Prospects. Nano. Today 2012, 7, 467–480. [Google Scholar] [CrossRef]
- Rudmann, D.G.; Alston, J.T.; Hanson, J.C.; Heidel, S. High Molecular Weight Polyethylene Glycol Cellular Distribution and PEG-Associated Cytoplasmic Vacuolation Is Molecular Weight Dependent and Does Not Require Conjugation to Proteins. Toxicol. Pathw. 2013, 41, 970–983. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Kiwada, H. Accelerated Blood Clearance (ABC) Phenomenon upon Repeated Injection of PEGylated Liposomes. Int. J. Pharm. 2008, 354, 56–62. [Google Scholar] [CrossRef]
- Verhoef, J.J.F.; Anchordoquy, T.J. Questioning the Use of PEGylation for Drug Delivery. Drug. Deliv. Transl. Res. 2013, 3, 499–503. [Google Scholar] [CrossRef]
- Einzmann, M.; Binder, W.H. Novel Functional Initiators for Oxazoline Polymerization. Polym. Chem. 2001, 29, 2821–2831. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tokuzawa, T.; Saegusa, T. Cationic Ring-Opening Isomerization Polymerization of 2-[p-(Substituted)Phenyl]-2-Oxazolines. Effects of the Substituent on the Reactivities. MTP Int. Rev. Sci. Phys. Chem. Ser. 1982, 15, 495. [Google Scholar]
- Mattek Company. The EpiDermTM Skin Model; BiCo Group: Gothenburg, Sweden, 2016; Available online: https://www.mattek.com/mattekproduct/epiderm/ (accessed on 20 April 2023).
- Schäfer-Korting, M.; Bock, U.; Diembeck, W.; Düsing, H.J.; Gamer, A.; Haltner-Ukomadu, E.; Hoffmann, C.; Kaca, M.; Kamp, H.; Kersen, S.; et al. The Use of Reconstructed Human Epidermis for Skin Absorption Testing: Results of the Validation Study. ATLA Altern. Lab. Anim. 2008, 36, 161–187. [Google Scholar] [CrossRef]
- Lombardi Borgia, S.; Schlupp, P.; Mehnert, W.; Schäfer-Korting, M. In Vitro Skin Absorption and Drug Release—A Comparison of Six Commercial Prednicarbate Preparations for Topical Use. Eur. J. Pharm. Biopharm. 2008, 68, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Topel, Ö.; Çakir, B.A.; Budama, L.; Hoda, N. Determination of Critical Micelle Concentration of Polybutadiene-Block-Poly(Ethyleneoxide) Diblock Copolymer by Fluorescence Spectroscopy and Dynamic Light Scattering. J. Mol. Liq. 2013, 177, 40–43. [Google Scholar] [CrossRef]
- Sutherland, E.M.; Mercer, S.; Everist, M.G.; Leaist, D. Diffusion in Solutions of Micelles. What Does Dynamic Light Scattering Measure? J. Chem. Amp. Eng. Data 2008, 54, 272–278. [Google Scholar] [CrossRef]
- Okamoto, H.; Taguchi, H.; Iida, K.; Danjo, K. Development of Polymer Film Dosage Forms of Lidocaine for Buccal Administration: I. Penetration Rate and Release Rate. J. Control. Release 2001, 77, 253–260. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Cespi, M.; Lorusso, N.; Palmieri, G.F.; Bonacucina, G.; Blasi, P. Surfactant Self-Assembling and Critical Micelle Concentration: One Approach Fits All? Langmuir 2020, 36, 5745–5753. [Google Scholar] [CrossRef]

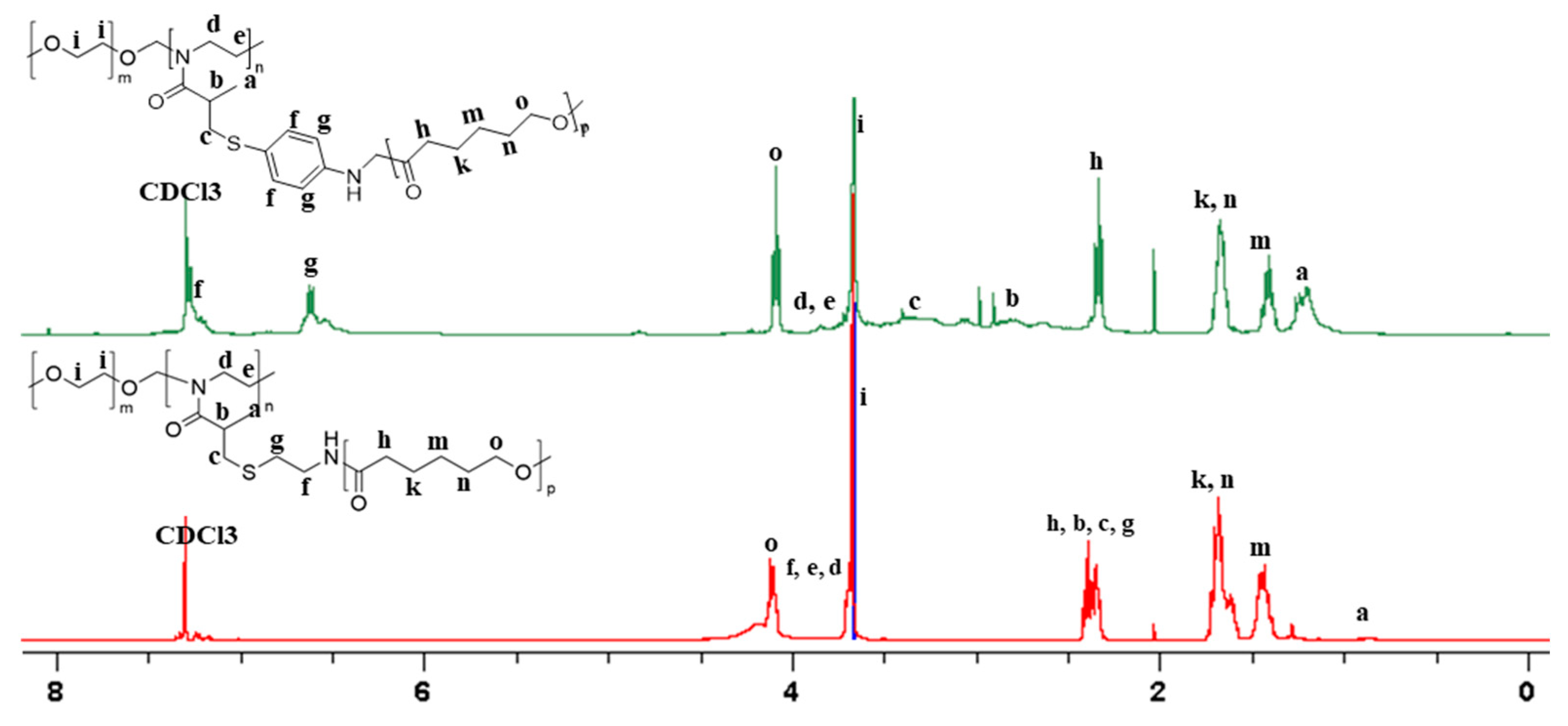
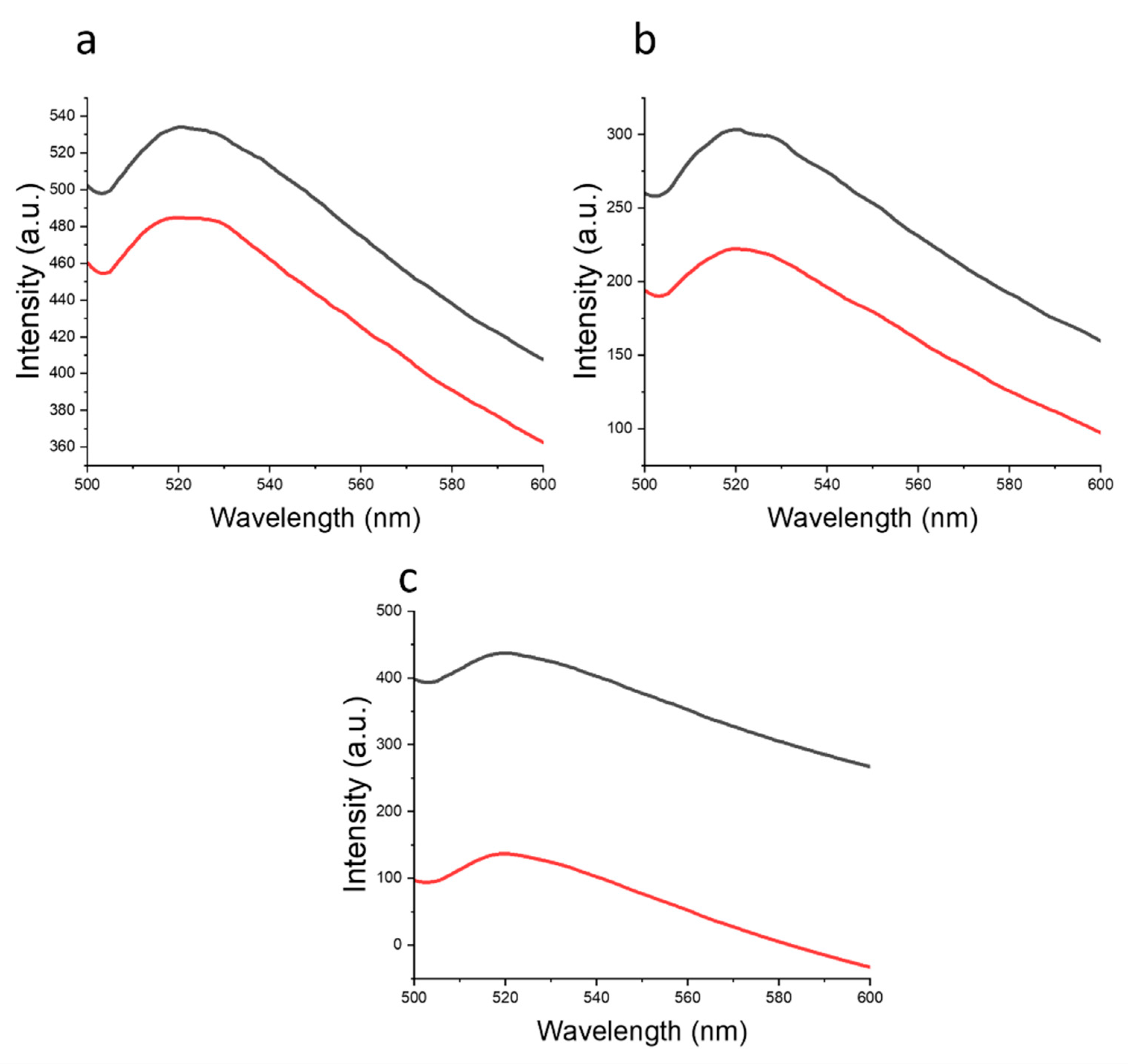
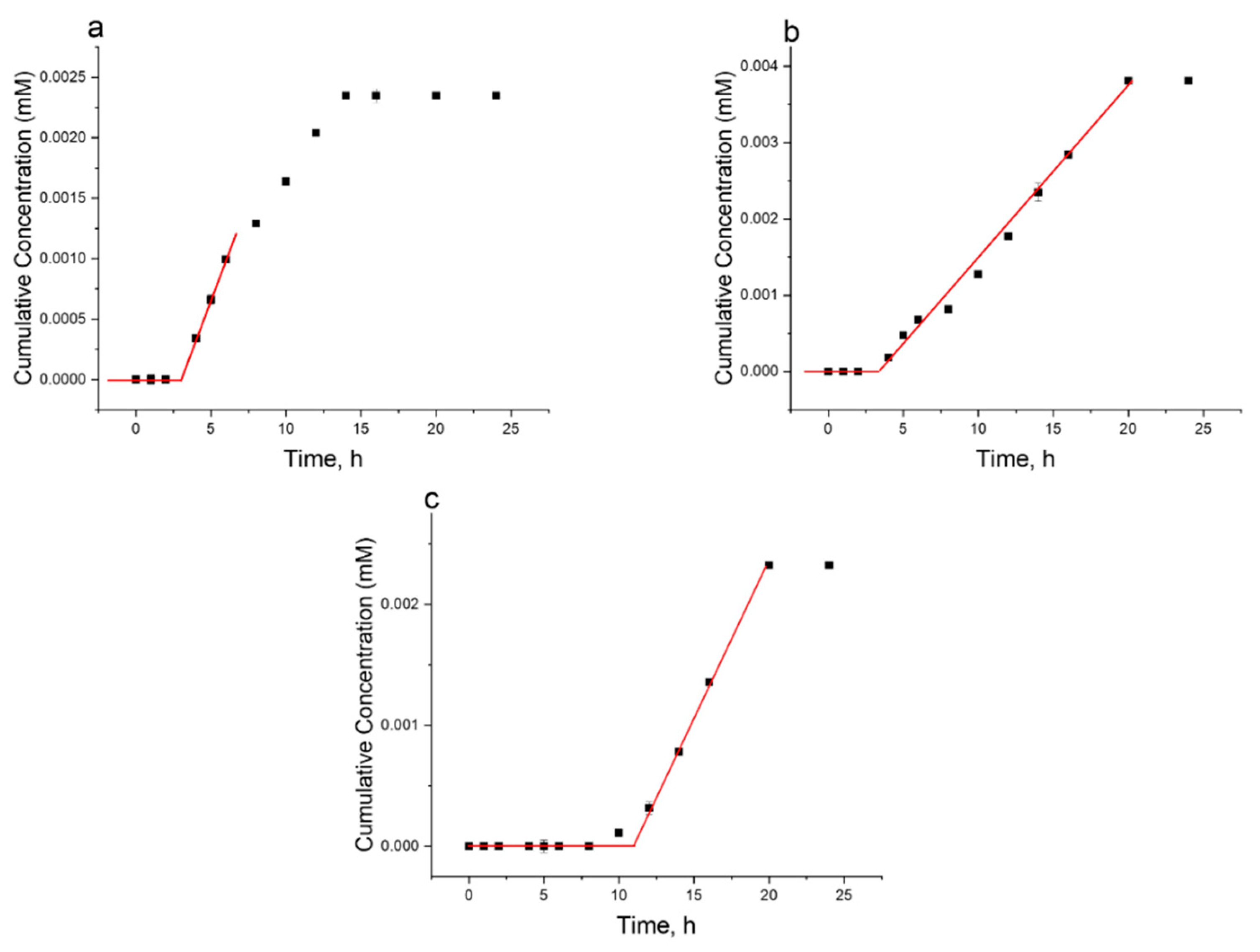
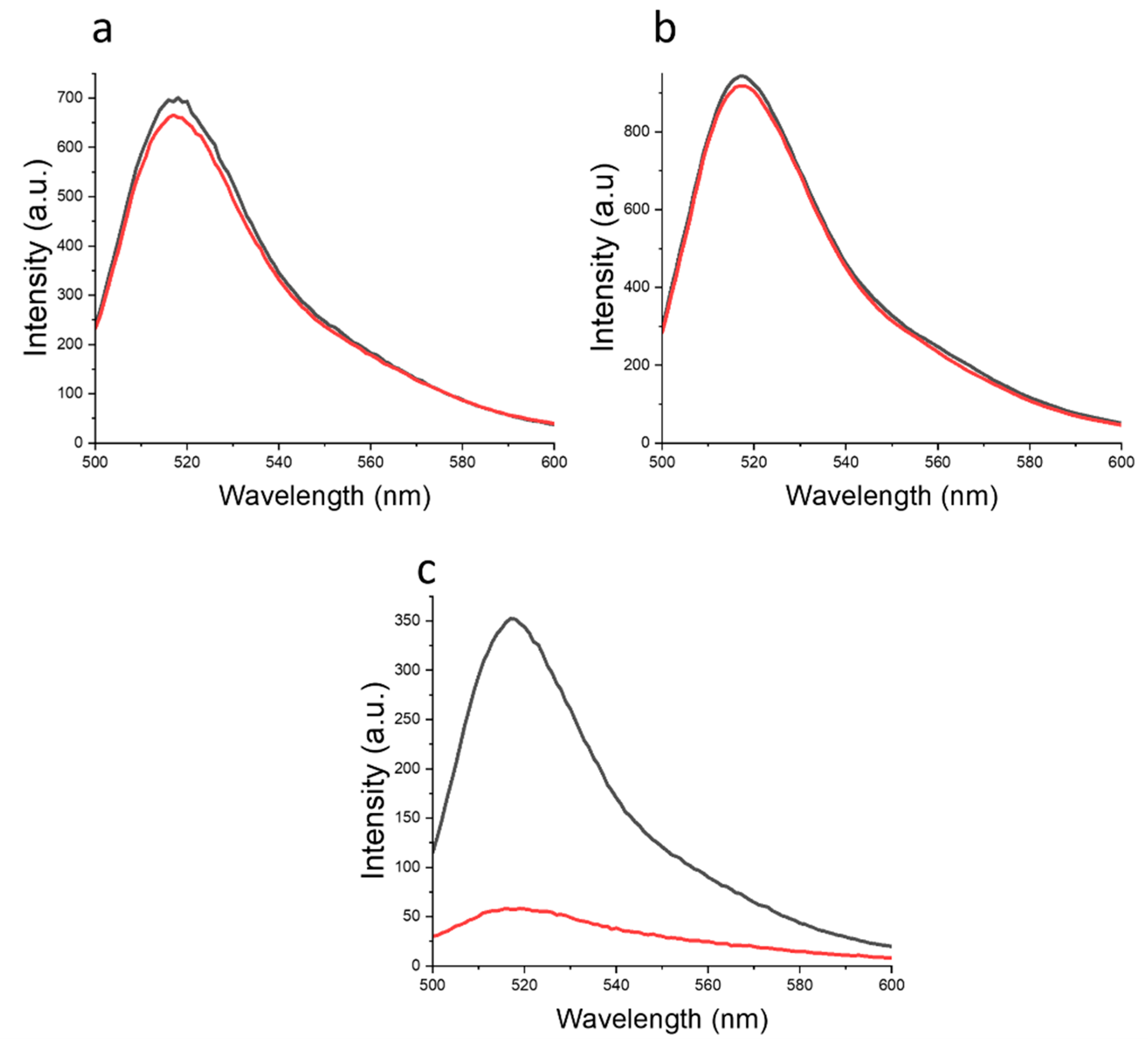
| Mw, kDa | PDI | Fraction | ||
|---|---|---|---|---|
| PEG | PCL | |||
| Homopolymer A | 3.9 | 1.18 | - | - |
| BCP A | 10.1 | 1.15 | 0.20 | - |
| HCP A | 17.1 | 1.21 | 0.12 | 0.41 |
| Homopolymer B | 10.2 | 1.11 | - | - |
| BCP B | 19.2 | 1.12 | 0.10 | - |
| HCP B | 24.4 | 1.11 | 0.08 | 0.21 |
| Micelle Size, nm | ||
|---|---|---|
| 0.1% in DI Water | 0.01% in pH 6.86 Buffer | |
| HCP A | 167.2.2 ± 71.6 | 275.5 ± 81.4 |
| HCP B | 227.9 ± 93.2 | 647.7 ± 119.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Storti, G.; Romano, G.; Gilmore, K.; Sadowski, N.; Tiiara, A.; Luzinov, I.; Sidorenko, A. Permeability of Skin-Mimicking Cell Coatings by Polymers of Complex Architecture Based on Polyoxazolines. Coatings 2023, 13, 1007. https://doi.org/10.3390/coatings13061007
Storti G, Romano G, Gilmore K, Sadowski N, Tiiara A, Luzinov I, Sidorenko A. Permeability of Skin-Mimicking Cell Coatings by Polymers of Complex Architecture Based on Polyoxazolines. Coatings. 2023; 13(6):1007. https://doi.org/10.3390/coatings13061007
Chicago/Turabian StyleStorti, Gia, Giulia Romano, Kristen Gilmore, Nicholas Sadowski, Andrii Tiiara, Igor Luzinov, and Alexander Sidorenko. 2023. "Permeability of Skin-Mimicking Cell Coatings by Polymers of Complex Architecture Based on Polyoxazolines" Coatings 13, no. 6: 1007. https://doi.org/10.3390/coatings13061007
APA StyleStorti, G., Romano, G., Gilmore, K., Sadowski, N., Tiiara, A., Luzinov, I., & Sidorenko, A. (2023). Permeability of Skin-Mimicking Cell Coatings by Polymers of Complex Architecture Based on Polyoxazolines. Coatings, 13(6), 1007. https://doi.org/10.3390/coatings13061007







