Atomic Layer Deposition of Chlorine Containing Titanium–Zinc Oxide Nanofilms Using the Supercycle Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Atomic Layer Deposition of TiO2, ZnO, and ZTO Nanocoatings
2.2. Samples Characterization
2.3. Antibacterial Activity
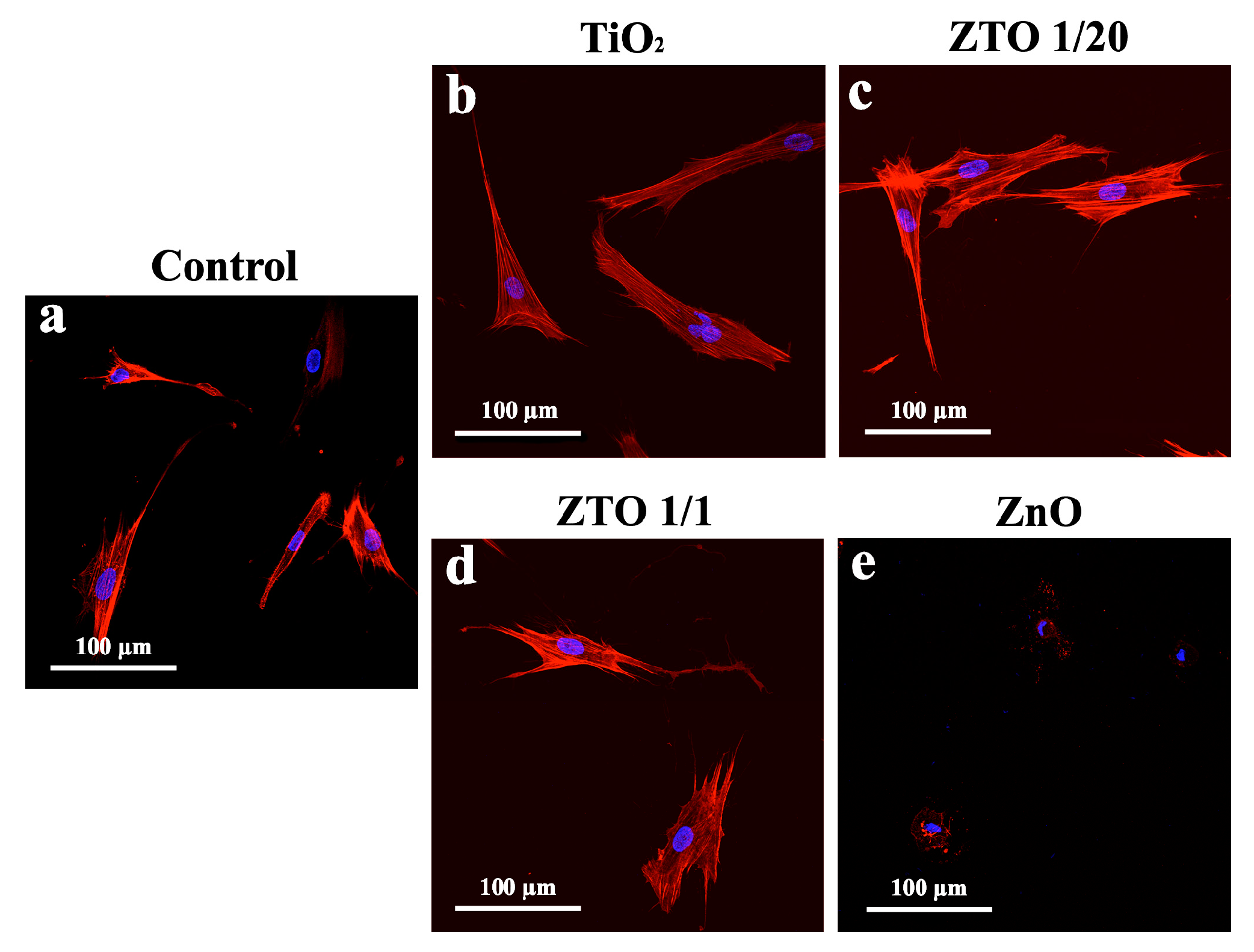
2.4. FetMSC Cells Adhesion
3. Results
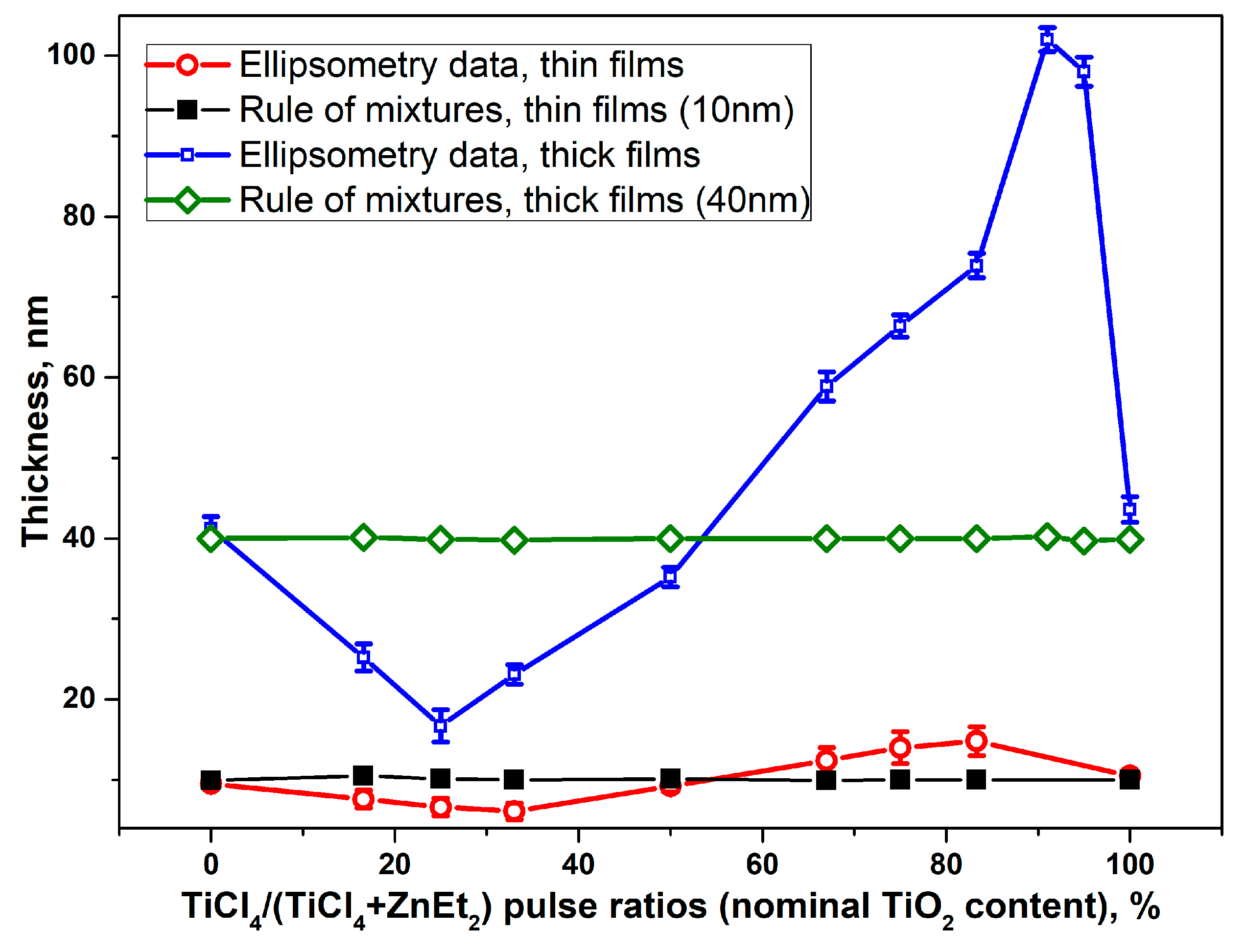
3.1. Atomic Layer Deposition
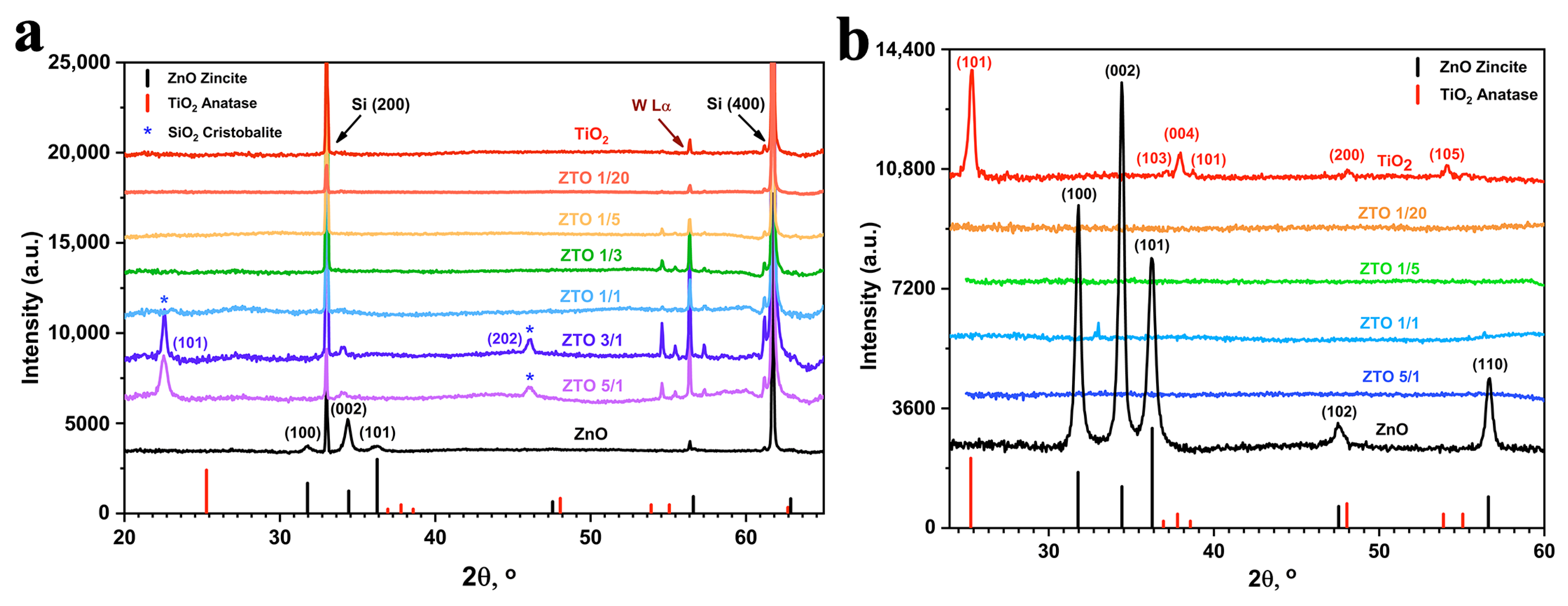
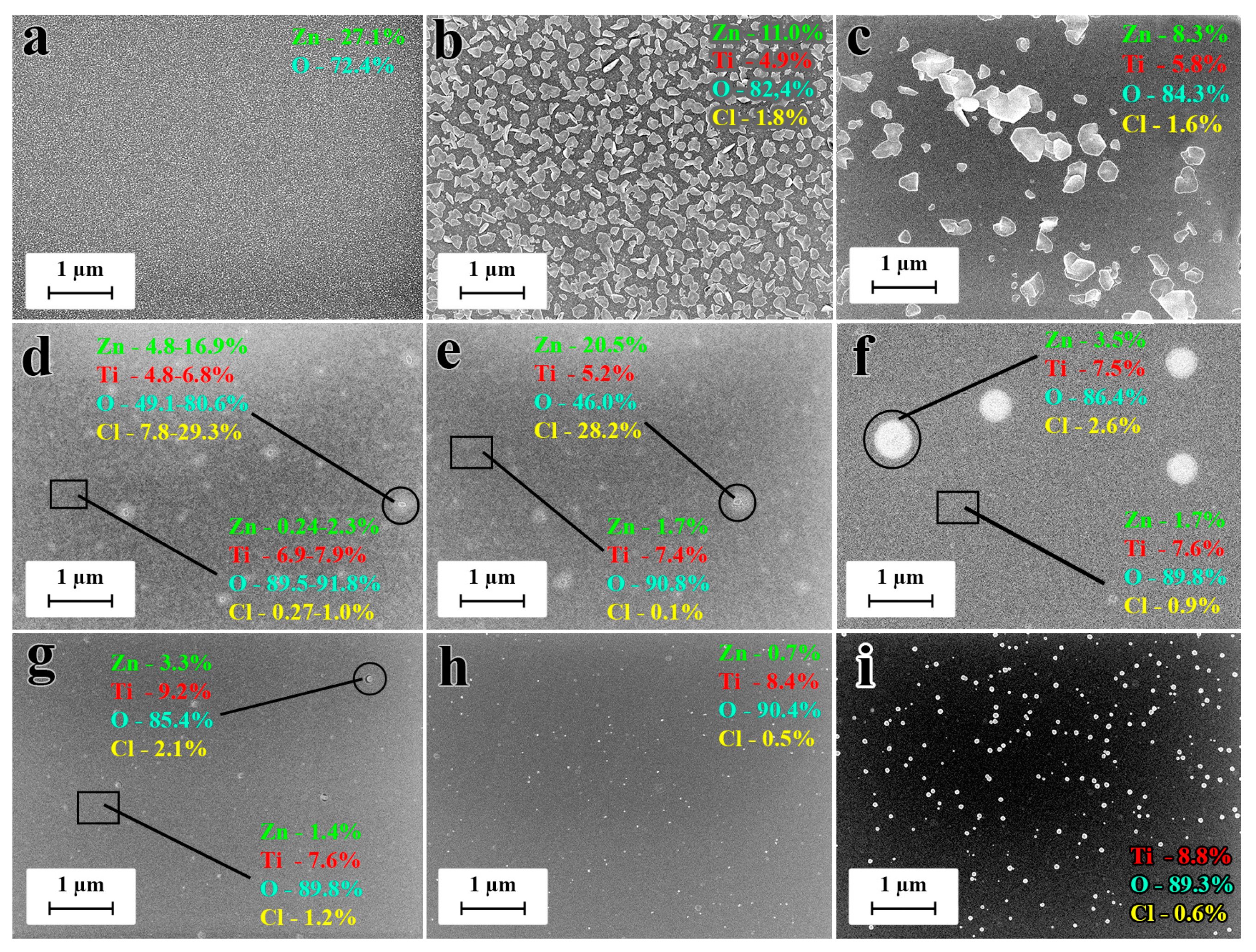
3.2. Structure and Morphology of the Films
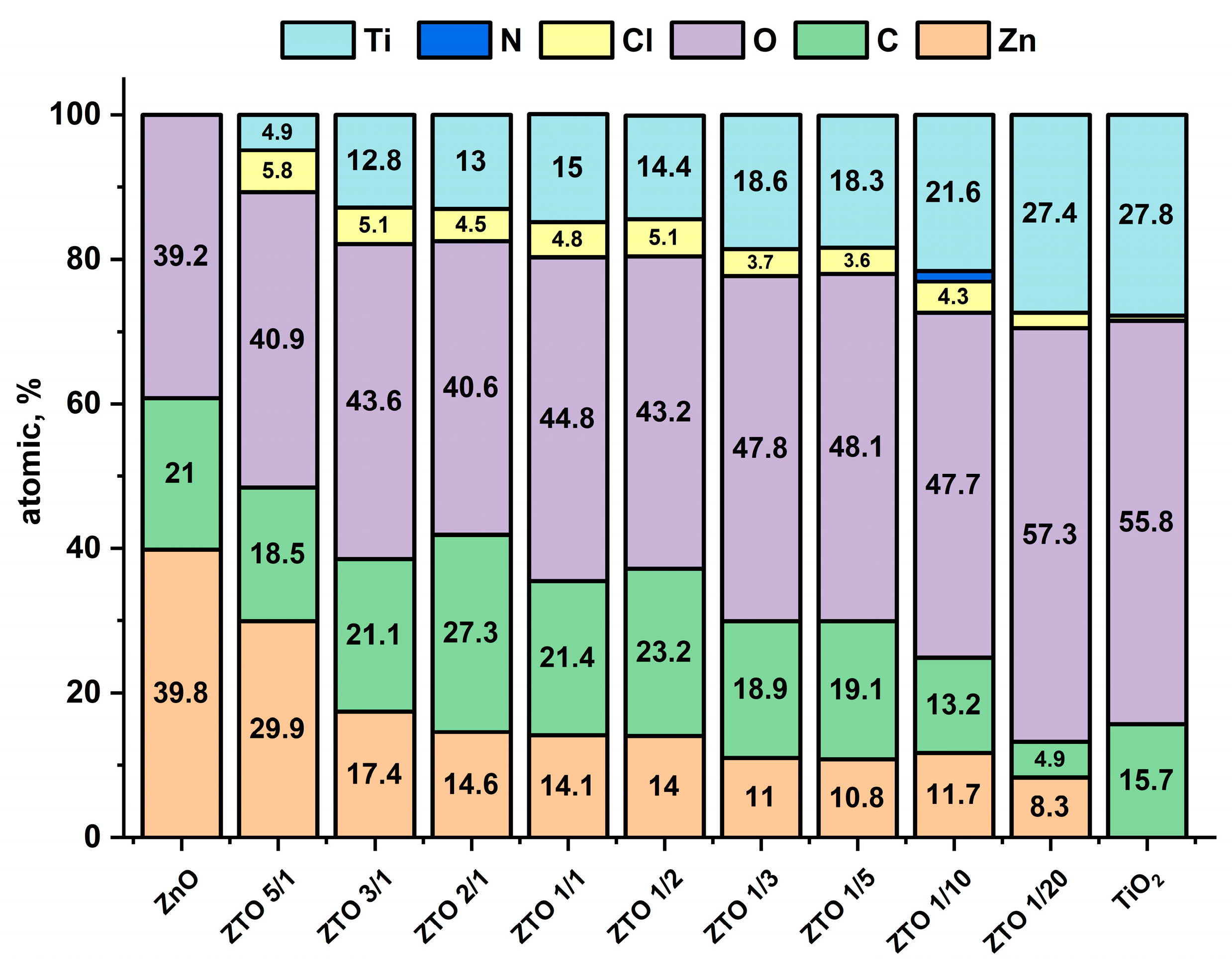
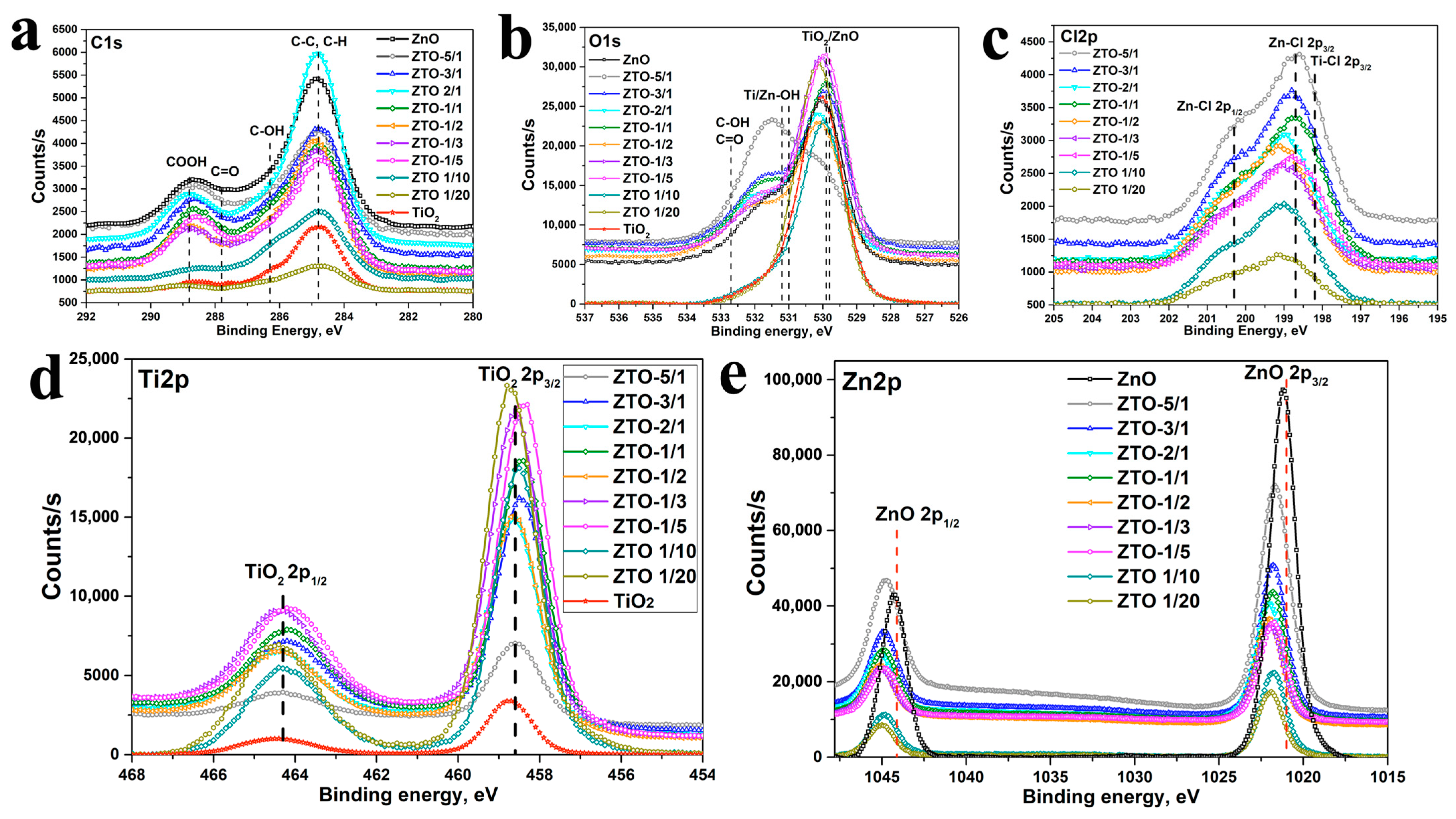
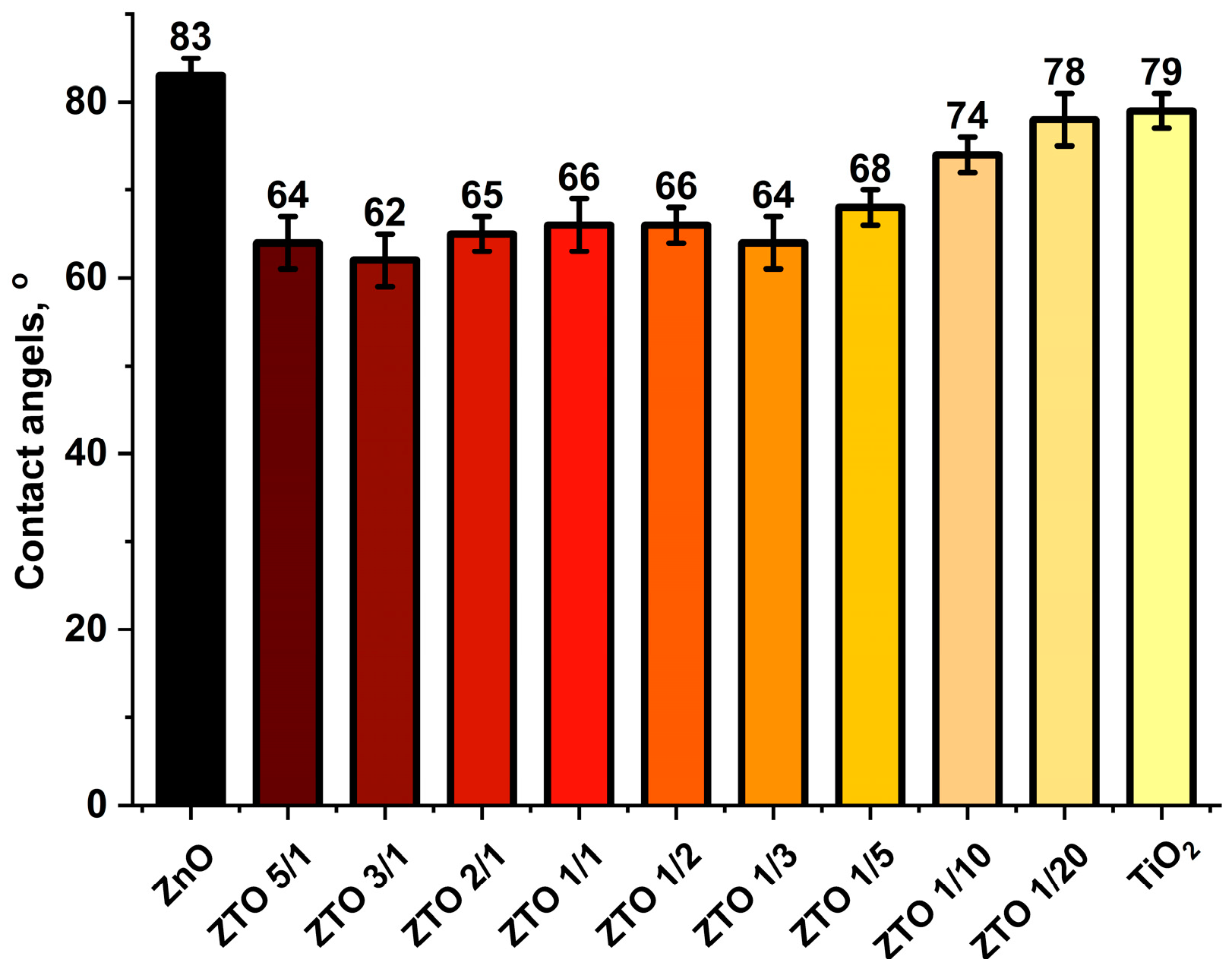
3.3. Surface Composition and Wettability of the Nanocoatings
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ding, S.-J.; Wu, X. Superior Atomic Layer Deposition Technology for Amorphous Oxide Semiconductor Thin-Film Transistor Memory Devices. Chem. Mater. 2020, 32, 1343–1357. [Google Scholar] [CrossRef]
- Tsai, Y.; Li, Z.; Hu, S. Recent Progress of Atomic Layer Technology in Spintronics: Mechanism, Materials and Prospects. Nanomaterials 2022, 12, 661. [Google Scholar] [CrossRef]
- Biyikli, N.; Haider, A. Atomic layer deposition: An enabling technology for the growth of functional nanoscale semiconductors. Semicond. Sci. Technol. 2017, 32, 093002. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, Y. Interface Tailoring of Heterogeneous Catalysts by Atomic Layer Deposition. ACS Catal. 2018, 8, 10064–10081. [Google Scholar] [CrossRef]
- Eswar, N.K.R.; Singh, S.A.; Heo, J. Atomic layer deposited photocatalysts: Comprehensive review on viable fabrication routes and reactor design approaches for photo-mediated redox reactions. J. Mater. Chem. A 2019, 7, 17703–17734. [Google Scholar] [CrossRef]
- Brinkmann, K.O.; Gahlmann, T.; Riedl, T. Atomic Layer Deposition of Functional Layers in Planar Perovskite Solar Cells. Sol. RRL 2019, 4, 1900332. [Google Scholar] [CrossRef]
- Niu, W.; Li, X.; Karuturi, S.K.; Fam, D.W.; Fan, H.; Shrestha, S.; Wong, L.H.; Tok, A.I. Applications of atomic layer deposition in solar cells. Nanotechnology 2015, 26, 064001. [Google Scholar] [CrossRef]
- Xu, H.; Akbari, M.K.; Kumar, S.; Verpoort, F.; Zhuiykov, S. Atomic layer deposition—state-of-the-art approach to nanoscale hetero-interfacial engineering of chemical sensors electrodes: A review. Sens. Actuators B Chem. 2021, 331, 129403. [Google Scholar] [CrossRef]
- Graniel, O.; Weber, M.; Balme, S.; Miele, P.; Bechelany, M. Atomic layer deposition for biosensing applications. Biosens. Bioelectron. 2018, 122, 147–159. [Google Scholar] [CrossRef]
- Koshtyal, Y.; Olkhovskii, D.; Rumyantsev, A.; Maximov, M. Applications and Advantages of Atomic Layer Deposition for Lithium-Ion Batteries Cathodes: Review. Batteries 2022, 8, 184. [Google Scholar] [CrossRef]
- Tiurin, O.; Ein-Eli, Y. A Critical Review: The Impact of the Battery Electrode Material Substrate on the Composition and Properties of Atomic Layer Deposition (ALD) Coatings. Adv. Mater. Interfaces 2019, 6, 1901455. [Google Scholar] [CrossRef]
- Ahmed, B.; Xia, C.; Alshareef, H.N. Electrode surface engineering by atomic layer deposition: A promising pathway toward better energy storage. Nano Today 2016, 11, 250–271. [Google Scholar] [CrossRef]
- Hashemi Astaneh, S.; Faverani, L.P.; Sukotjo, C.; Takoudis, C.G. Atomic layer deposition on dental materials: Processing conditions and surface functionalization to improve physical, chemical, and clinical properties—A review. Acta Biomater. 2021, 121, 103–118. [Google Scholar] [CrossRef]
- Perrotta, A.; Werzer, O.; Coclite, A.M. Strategies for Drug Encapsulation and Controlled Delivery Based on Vapor-Phase Deposited Thin Films. Adv. Eng. Mater. 2018, 20, 1700639. [Google Scholar] [CrossRef]
- Mackus, A.J.M.; Schneider, J.R.; MacIsaac, C.; Baker, J.G.; Bent, S.F. Synthesis of Doped, Ternary, and Quaternary Materials by Atomic Layer Deposition: A Review. Chem. Mater. 2018, 31, 1142–1183. [Google Scholar] [CrossRef]
- Coll, M.; Napari, M. Atomic layer deposition of functional multicomponent oxides. APL Mater. 2019, 7, 110901. [Google Scholar] [CrossRef]
- Bergum, K.; Hansen, P.-A.; Fjellvåg, H.; Nilsen, O. Structural, electrical and optical characterization of Ti-doped ZnO films grown by atomic layer deposition. J. Alloys Compd. 2014, 616, 618–624. [Google Scholar] [CrossRef]
- Bergum, K.; Fjellvåg, H.; Nilsen, O. Thickness dependent structural, optical and electrical properties of Ti-doped ZnO films prepared by atomic layer deposition. Appl. Surf. Sci. 2015, 332, 494–499. [Google Scholar] [CrossRef]
- Coutancier, D.; Zhang, S.T.; Bernardini, S.; Fournier, O.; Mathieu-Pennober, T.; Donsanti, F.; Tchernycheva, M.; Foldyna, M.; Schneider, N. ALD of ZnO: Ti: Growth Mechanism and Application as an Efficient Transparent Conductive Oxide in Silicon Nanowire Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 21036–21044. [Google Scholar] [CrossRef]
- Bakos, L.P.; Justh, N.; Moura da Silva Bezerra da Costa, U.C.; Laszlo, K.; Labar, J.L.; Igricz, T.; Varga-Josepovits, K.; Pasierb, P.; Farm, E.; Ritala, M.; et al. Photocatalytic and Gas Sensitive Multiwalled Carbon Nanotube/TiO(2)-ZnO and ZnO-TiO(2) Composites Prepared by Atomic Layer Deposition. Nanomaterials 2020, 10, 252. [Google Scholar] [CrossRef]
- Wang, C.-C.; Lin, J.-W.; Yu, Y.-H.; Lai, K.-H.; Lee, S.-M.; Chiu, K.-F.; Kei, C.-C. Nanolaminated ZnO–TiO2 coated lithium-rich layered oxide cathodes by atomic layer deposition for enhanced electrochemical performances. J. Alloys Compd. 2020, 842, 155845. [Google Scholar] [CrossRef]
- Kim, M.-w.; Yoon, H.; Ohm, T.Y.; Jo, H.S.; An, S.; Choi, S.K.; Park, H.; Al-Deyab, S.S.; Min, B.K.; Swihart, M.T.; et al. Nanotextured cupric oxide nanofibers coated with atomic layer deposited ZnO-TiO2 as highly efficient photocathodes. Appl. Catal. B Environ. 2017, 201, 479–485. [Google Scholar] [CrossRef]
- Kim, M.-W.; Kim, K.; Ohm, T.Y.; Yoon, H.; Joshi, B.; Samuel, E.; Swihart, M.T.; Choi, S.K.; Park, H.; Yoon, S.S. Electrosprayed BiVO4 nanopillars coated with atomic-layer-deposited ZnO/TiO2 as highly efficient photoanodes for solar water splitting. Chem. Eng. J. 2018, 333, 721–729. [Google Scholar] [CrossRef]
- Kim, T.-G.; Joshi, B.; Park, C.-W.; Samuel, E.; Kim, M.-W.; Swihart, M.T.; Yoon, S.S. Supersonically sprayed iron oxide nanoparticles with atomic-layer-deposited ZnO/TiO2 layers for solar water splitting. J. Alloys Compd. 2019, 798, 35–44. [Google Scholar] [CrossRef]
- Ageh, V.; Rajamure, R.; Ho, Y.H.; Scharf, T.W. Nanocrystalline zinc titanate coatings for corrosion protection. Nanomater. Energy 2014, 3, 47–52. [Google Scholar] [CrossRef]
- Ageh, V.; Mohseni, H.; Scharf, T.W. Processing–structure–tribological property interrelationships of zinc titanate coatings grown by atomic layer deposition. Surf. Coat. Technol. 2014, 241, 112–117. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, X.; Xiong, Z.; Huang, Q.; Yang, X.; Yan, H.; Ma, J.; Feng, Q.; Shen, Z. Novel micro/nanostructured TiO2/ZnO coating with antibacterial capacity and cytocompatibility. Ceram. Int. 2018, 44, 9711–9719. [Google Scholar] [CrossRef]
- Felizco, J.; Juntunen, T.; Uenuma, M.; Etula, J.; Tossi, C.; Ishikawa, Y.; Tittonen, I.; Uraoka, Y. Enhanced Thermoelectric Transport and Stability in Atomic Layer Deposited-HfO(2)/ZnO and TiO(2)/ZnO-Sandwiched Multilayer Thin Films. ACS Appl. Mater. Interfaces 2020, 12, 49210–49218. [Google Scholar] [CrossRef]
- Su, C.Y.; Wang, C.C.; Hsueh, Y.C.; Gurylev, V.; Kei, C.C.; Perng, T.P. Enabling high solubility of ZnO in TiO(2) by nanolamination of atomic layer deposition. Nanoscale 2015, 7, 19222–19230. [Google Scholar] [CrossRef]
- Torrisi, G.; Di Mauro, A.; Scuderi, M.; Nicotra, G.; Impellizzeri, G. Atomic layer deposition of ZnO/TiO2 multilayers: Towards the understanding of Ti-doping in ZnO thin films. RSC Adv. 2016, 6, 88886–88895. [Google Scholar] [CrossRef]
- Wan, Z.; Kwack, W.-S.; Lee, W.-J.; Jang, S., II; Kim, H.-R.; Kim, J.-W.; Jung, K.-W.; Min, W.-J.; Yu, K.-S.; Park, S.-H.; et al. Electrical and optical properties of Ti doped ZnO films grown on glass substrate by atomic layer deposition. Mater. Res. Bull. 2014, 57, 23–28. [Google Scholar] [CrossRef]
- Qian, X.; Gao, M.-Y.; Cao, Y.-Q.; Guo, B.-L.; Li, A.-D. Preparation and phase structures of Zn–Ti–O ternary compounds by atomic layer deposition. J. Vac. Sci. Technol. A Vac. Surf. Film. 2013, 31, 01A133. [Google Scholar] [CrossRef]
- Ye, Z.Y.; Lu, H.L.; Geng, Y.; Gu, Y.Z.; Xie, Z.Y.; Zhang, Y.; Zhang, D.W. Structural, electrical, and optical properties of Ti-doped ZnO films fabricated by atomic layer deposition. Nanoscale Res. Lett. 2013, 8, 1–6. [Google Scholar] [CrossRef]
- Lee, D.-J.; Kim, K.-J.; Kim, S.-H.; Kwon, J.-Y.; Xu, J.; Kim, K.-B. Atomic layer deposition of Ti-doped ZnO films with enhanced electron mobility. J. Mater. Chem. C 2013, 1, 4761–4769. [Google Scholar] [CrossRef]
- Borgese, L.; Bontempi, E.; Depero, L.E.; Colombi, P.; Alessandri, I. Tailoring phase and composition at the nanoscale: Atomic layer deposition of Zn–Ti–O thin films. CrystEngComm 2011, 13, 6621–6624. [Google Scholar] [CrossRef]
- Hazra, S.K.; Borgese, L.; Federici, S.; Bontempi, E.; Ferrari, M.; Ferrari, V.; Plaisier, J.R.; Santarelli, X.; Zerauschek, G.; Lausi, A.; et al. Electrical resistivity of Ti–Zn mixed oxide thin films deposited by atomic layer deposition. Thin Solid Film. 2012, 520, 5151–5154. [Google Scholar] [CrossRef]
- Doyle, S.; Ryan, L.; McCarthy, M.M.; Modreanu, M.; Schmidt, M.; Laffir, F.; Povey, I.M.; Pemble, M.E. Combinatorial ALD for the growth of ZnO/TiO2nanolaminates and mixed ZnO/TiO2nanostructured films. Mater. Adv. 2022, 3, 2896–2907. [Google Scholar] [CrossRef]
- Ageh, V.; Mohseni, H.; Scharf, T.W. Lubricious zinc titanate coatings for high temperature applications. Surf. Coat. Technol. 2013, 237, 241–247. [Google Scholar] [CrossRef]
- Niemelä, J.-P.; Marin, G.; Karppinen, M. Titanium dioxide thin films by atomic layer deposition: A review. Semicond. Sci. Technol. 2017, 32, 093005. [Google Scholar] [CrossRef]
- Nazarov, D.; Ezhov, I.; Yudintceva, N.; Mitrofanov, I.; Shevtsov, M.; Rudakova, A.; Maximov, M. MG-63 and FetMSC Cell Response on Atomic Layer Deposited TiO2 Nanolayers Prepared Using Titanium Tetrachloride and Tetraisopropoxide. Coatings 2022, 12, 668. [Google Scholar] [CrossRef]
- Drozd, V.E.; Titov, V.V.; Kasatkin, I.A.; Basov, L.L.; Lisachenko, A.A.; Stroyuk, O.L.; Kuchmiy, S.Y. Structure, optical properties and visible-light-induced photochemical activity of nanocrystalline ZnO films deposited by atomic layer deposition onto Si(100). Thin Solid Film. 2014, 573, 128–133. [Google Scholar] [CrossRef]
- Nazarov, D.; Ezhov, I.; Yudintceva, N.; Shevtsov, M.; Rudakova, A.; Kalganov, V.; Tolmachev, V.; Zharova, Y.; Lutakov, O.; Kraeva, L.; et al. Antibacterial and Osteogenic Properties of Ag Nanoparticles and Ag/TiO2 Nanostructures Prepared by Atomic Layer Deposition. J. Funct. Biomater. 2022, 13, 62. [Google Scholar] [CrossRef]
- Lu, X.; Wang, Y.; Yang, X.; Zhang, Q.; Zhao, Z.; Weng, L.T.; Leng, Y. Spectroscopic analysis of titanium surface functional groups under various surface modification and their behaviors in vitro and in vivo. J. Biomed. Mater. Res. Part A 2008, 84, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.-H.; Bernard, C.; Variola, F.; Zalzal, S.F.; Wuest, J.D.; Rosei, F.; Nanci, A. Characterization of a bioactive nanotextured surface created by controlled chemical oxidation of titanium. Surf. Sci. 2006, 600, 4613–4621. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Bankar, D.B.; Hawaldar, R.R.; Arbuj, S.S.; Moulavi, M.H.; Shinde, S.T.; Takle, S.P.; Shinde, M.D.; Amalnerkar, D.P.; Kanade, K.G. ZnCl2 loaded TiO2 nanomaterial: An efficient green catalyst to one-pot solvent-free synthesis of propargylamines. RSC Adv. 2019, 9, 32735–32743. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-Y.; Wang, L.-C.; Liu, W.-S.; Wang, C.-C.; Perng, T.-P. Photocatalysis and Hydrogen Evolution of Al- and Zn-Doped TiO2 Nanotubes Fabricated by Atomic Layer Deposition. ACS Appl. Mater. Interfaces 2018, 10, 33287–33295. [Google Scholar] [CrossRef]
- Rudakova, A.V.; Emeline, A.V.; Romanychev, A.I.; Bahnemann, D.W. Photoinduced hydrophilic behavior of TiO2 thin film on Si substrate. J. Alloys Compd. 2021, 872, 159746. [Google Scholar] [CrossRef]
- Nazarov, D.V.; Smirnov, V.M.; Zemtsova, E.G.; Yudintceva, N.M.; Shevtsov, M.A.; Valiev, R.Z. Enhanced Osseointegrative Properties of Ultra-Fine-Grained Titanium Implants Modified by Chemical Etching and Atomic Layer Deposition. ACS Biomater. Sci. Eng. 2018, 4, 3268–3281. [Google Scholar] [CrossRef]
- Motola, M.; Capek, J.; Zazpe, R.; Bacova, J.; Hromadko, L.; Bruckova, L.; Ng, S.; Handl, J.; Spotz, Z.; Knotek, P.; et al. Thin TiO2 Coatings by ALD Enhance the Cell Growth on TiO2 Nanotubular and Flat Substrates. ACS Appl. Bio Mater. 2020, 3, 6447–6456. [Google Scholar] [CrossRef]
- Blendinger, F.; Seitz, D.; Ottenschlager, A.; Fleischer, M.; Bucher, V. Atomic Layer Deposition of Bioactive TiO2 Thin Films on Polyetheretherketone for Orthopedic Implants. ACS Appl. Mater. Interfaces 2021, 13, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- De Dicastillo, C.L.; Vidal, C.P.; Falcó, I.; Sánchez, G.; Márquez, P.; Escrig, J. Antimicrobial Bilayer Nanocomposites Based on the Incorporation of As-Synthetized Hollow Zinc Oxide Nanotubes. Nanomaterials 2020, 10, 503. [Google Scholar] [CrossRef]
- Li, L.; Yu, P.; Li, Y.; Wu, X.; Li, W.; Cheng, X. A Facile Approach to Fabricating Antibacterial Textile with High Efficiency and Compact Process. Adv. Mater. Interfaces 2021, 8, 2101197. [Google Scholar] [CrossRef]
- Yao, L.; Wu, X.; Wu, S.; Pan, X.; Tu, J.; Chen, M.; Al-Bishari, A.M.; Al-Baadani, M.A.; Yao, L.; Shen, X.; et al. Atomic layer deposition of zinc oxide on microrough zirconia to enhance osteogenesis and antibiosis. Ceram. Int. 2019, 45, 24757–24767. [Google Scholar] [CrossRef]


| Nominal ZnO/TiO2 Ratio | Temperature/ Substrate | Thickness/Growth Rate | Composition | Ref. |
|---|---|---|---|---|
| DEZ-TiCl4-H2O precursors | ||||
| 200/2, 200/4, 200/6, 200/8 | 170 °C/glass and Si (100) | 112–117 nm/not discussed | Close to theoretical | [28] |
| 1/200, 1/100, 1/50, 1/25 | 100 °C/Si, polycarbonate membrane | 28 nm/slight increase in growth rate by Zn doping | Zn content increase on the surface Presence of Cl | [29] |
| 13/1, 14/1, 15/1 | 120, 160, 200, 240 °C/quartz and silicon | 53–82 nm depending on deposition temperature (theoretical thickness—100 nm) | The real Ti concentration (4%) is more than the nominal one (2%) | [30] |
| DEZ-TTIP-H2O precursors | ||||
| 5/1, 10/1, 15/1, 20/1, 25/1, 30/1 | 200 °C/single-crystalline Si (100), soda-lime glass | 200 nm/the growth rates were slightly below the expected values | The linear correlation between pulses ration and Ti content up to 9.1% of pulse TTIP. A slight deviation at 16.7% pulse TTIP. | [17] |
| 1/1, 5/1, 10/1, 20/1, 25/1 | 200 °C/Corning glass | 50 nm/- | Nonlinear correlation Ti pulses and Ti content Ti doping is homogeneous | [31] |
| 1/1, 2/1, 5/1, 10/1, 20/1 | 200 °C/borosilicate glass, n-doped Si (100) | 9–148 nm/close to theoretical at low doping ratio and lower at high doping ratio (samples—ZTO 1/1, 2/1) | Order of the precursors’ pulse affects the doping mechanism and film composition | [19] |
| 1/2 2/5 1/3 | 200 °C/p-type (100) Si | 160–185 nm/GPC of TiO2 deposited on ZnO-terminated surface is faster than that of TiO2, the GPC of ZnO on TiO2-terminated surface is slower | Zn/Ti cycle ratio effect on the film composition is weak | [32] |
| 20/1, 10/1, 5/1, 2/1, 1/1 | 200 °C/ Si, thermally grown SiO2, quartz | 80–106 nm/thicknesses were thinner than expected for samples with cycle ratio of ZnO/TiO2 less than 10 | - | [33] |
| From 99/1 to 4/1 (9 types) | 200 °C/Thermally oxidized SiO2 (100 nm) on Si | 50–58 nm/growth rates were higher than the estimated films exhibited a layer-by-layer structure | Ti concentration is more than expected. Adsorption of TTIP on the ZnO surface was enhanced relative to the TiO2 | [34] |
| DEZ-TDMAT-H2O precursors | ||||
| 1/1, 1/2, 1/3, 1/4 | 90 °C/p-type (100) Si | 8.3–43.8 nm/thickness is less than expected and the difference increases with loop cycles number | Experimental and theoretical composition are close | [35,36] |
| 1/1 | 200 °C/Si(100) wafers and glass substrates | A nucleation delay for ZnO deposited on the Si and TiO2. TiO2 growth on ZnO is greater than that of pure TiO2 | Lower concentration of Ti3+ comparing to pure TiO2 and multilayered TiO2/ZnO | [37] |
| 2/1 | 200 °C/Si(100) | 170, 1100 nm | Zn/Ti = 1/1 (Ti concentration is more than expected) The coating is oxygen deficient | [38] |
| Composition of Supercycle (ZnO/TiO2 Pulse Ratio) | Number of Supercycles | Number of Cycles | Estimated Thickness *, nm |
|---|---|---|---|
| 1/0 | 222 | 222 | 40.0 |
| 5/1 | 42 | 252 | 40.1 |
| 3/1 | 67 | 268 | 39.9 |
| 2/1 | 96 | 288 | 39.8 |
| 1/1 | 170 | 340 | 40.0 |
| 1/2 | 138 | 414 | 40.0 |
| 1/3 | 116 | 464 | 40.0 |
| 1/5 | 88 | 525 | 40.0 |
| 1/10 | 55 | 605 | 40.2 |
| 1/20 | 31 | 651 | 39.7 |
| 0/1 | 720 | 720 | 39.6 |
| Sample | Thickness (Ellipsometry), nm | Thickness (XRR), nm | Roughness, nm | Density, g/cm3 |
|---|---|---|---|---|
| ZnO | 42 ± 0.5 | 43.2 | 2.43 | 5.88 |
| ZTO–5/1 | 43.1 ± 0.4 | 45.2 | 6.28 | 3.76 |
| ZTO–3/1 | 43.6 ± 0.6 | 37.9 | 2.03 | 5.15 |
| ZTO–1/1 | 38.2 ± 0.3 | 34.7 | 0.26 | 3.96 |
| ZTO–1/3 | 41.5 ± 0.4 | 39.1 | 0.89 | 4.1 |
| ZTO–1/5 | 40.4 ± 0.5 | 36.5 | 1.08 | 3.88 |
| ZTO–1/20 | 40.2 ± 0.3 | 37.0 | 0.95 | 3.87 |
| TiO2 | 43.7 ± 0.7 | 37.7 | 0.67 | 4.11 |
| Strain | ZnO | ZTO–1/1 | TiO2 | Control |
|---|---|---|---|---|
| S. aureus | No growth | No growth | ~10 | 1 × 107 |
| A. baumannii | No growth | No growth | 1 × 106 | 1 × 106 |
| P. aeruginosa | ~100 | ~1000 | 1 × 107 | 1 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazarov, D.; Kozlova, L.; Rudakova, A.; Zemtsova, E.; Yudintceva, N.; Ovcharenko, E.; Koroleva, A.; Kasatkin, I.; Kraeva, L.; Rogacheva, E.; et al. Atomic Layer Deposition of Chlorine Containing Titanium–Zinc Oxide Nanofilms Using the Supercycle Approach. Coatings 2023, 13, 960. https://doi.org/10.3390/coatings13050960
Nazarov D, Kozlova L, Rudakova A, Zemtsova E, Yudintceva N, Ovcharenko E, Koroleva A, Kasatkin I, Kraeva L, Rogacheva E, et al. Atomic Layer Deposition of Chlorine Containing Titanium–Zinc Oxide Nanofilms Using the Supercycle Approach. Coatings. 2023; 13(5):960. https://doi.org/10.3390/coatings13050960
Chicago/Turabian StyleNazarov, Denis, Lada Kozlova, Aida Rudakova, Elena Zemtsova, Natalia Yudintceva, Elizaveta Ovcharenko, Alexandra Koroleva, Igor Kasatkin, Ludmila Kraeva, Elizaveta Rogacheva, and et al. 2023. "Atomic Layer Deposition of Chlorine Containing Titanium–Zinc Oxide Nanofilms Using the Supercycle Approach" Coatings 13, no. 5: 960. https://doi.org/10.3390/coatings13050960
APA StyleNazarov, D., Kozlova, L., Rudakova, A., Zemtsova, E., Yudintceva, N., Ovcharenko, E., Koroleva, A., Kasatkin, I., Kraeva, L., Rogacheva, E., & Maximov, M. (2023). Atomic Layer Deposition of Chlorine Containing Titanium–Zinc Oxide Nanofilms Using the Supercycle Approach. Coatings, 13(5), 960. https://doi.org/10.3390/coatings13050960









