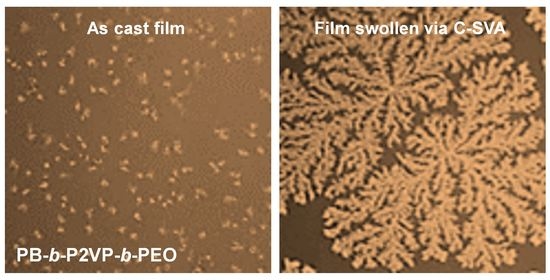
Crystallization of Poly(ethylene oxide)-Based Triblock Copolymers in Films Swollen-Rich in Solvent Vapors
Abstract
1. Introduction
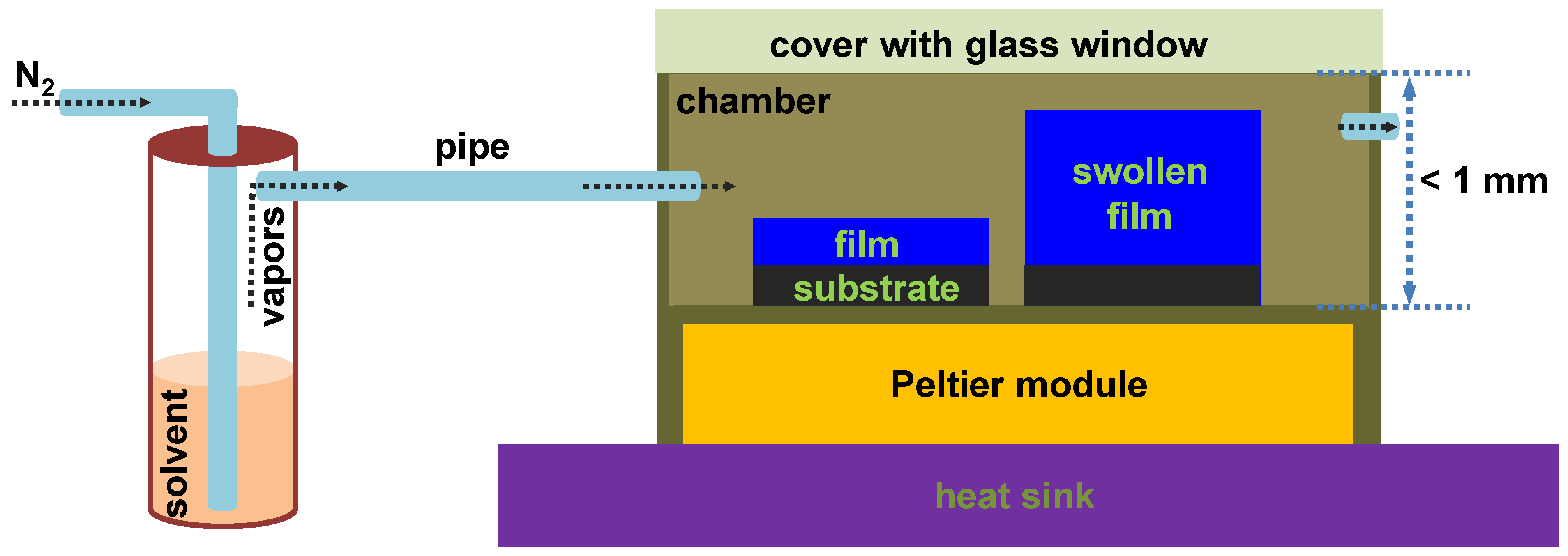
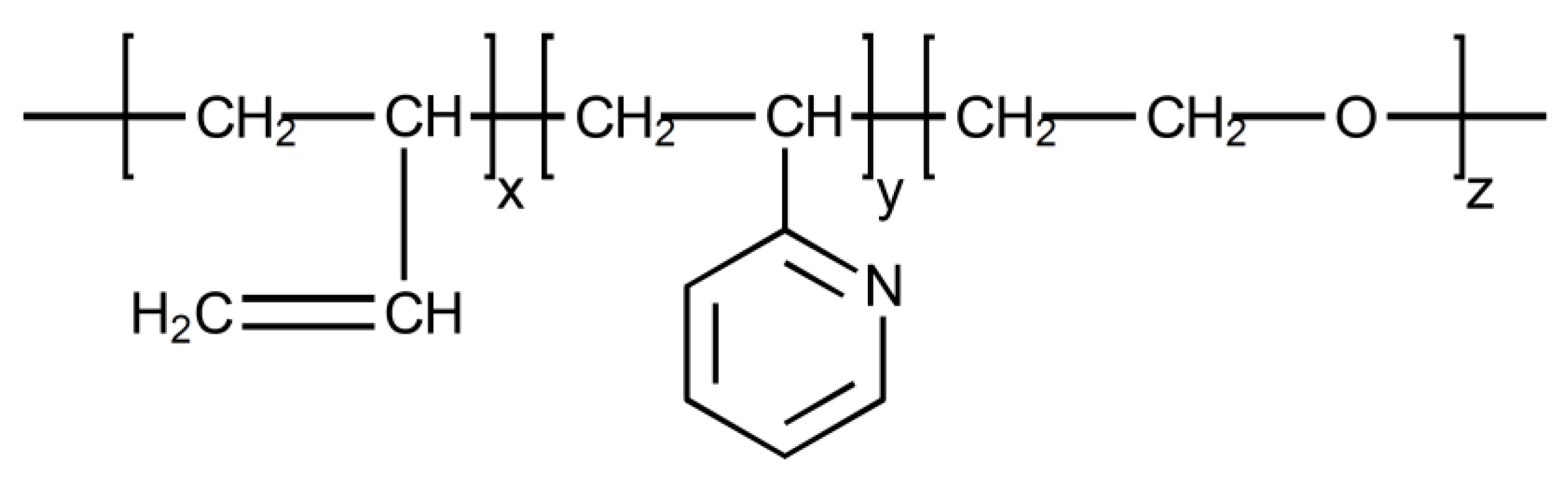
2. Materials and Methods
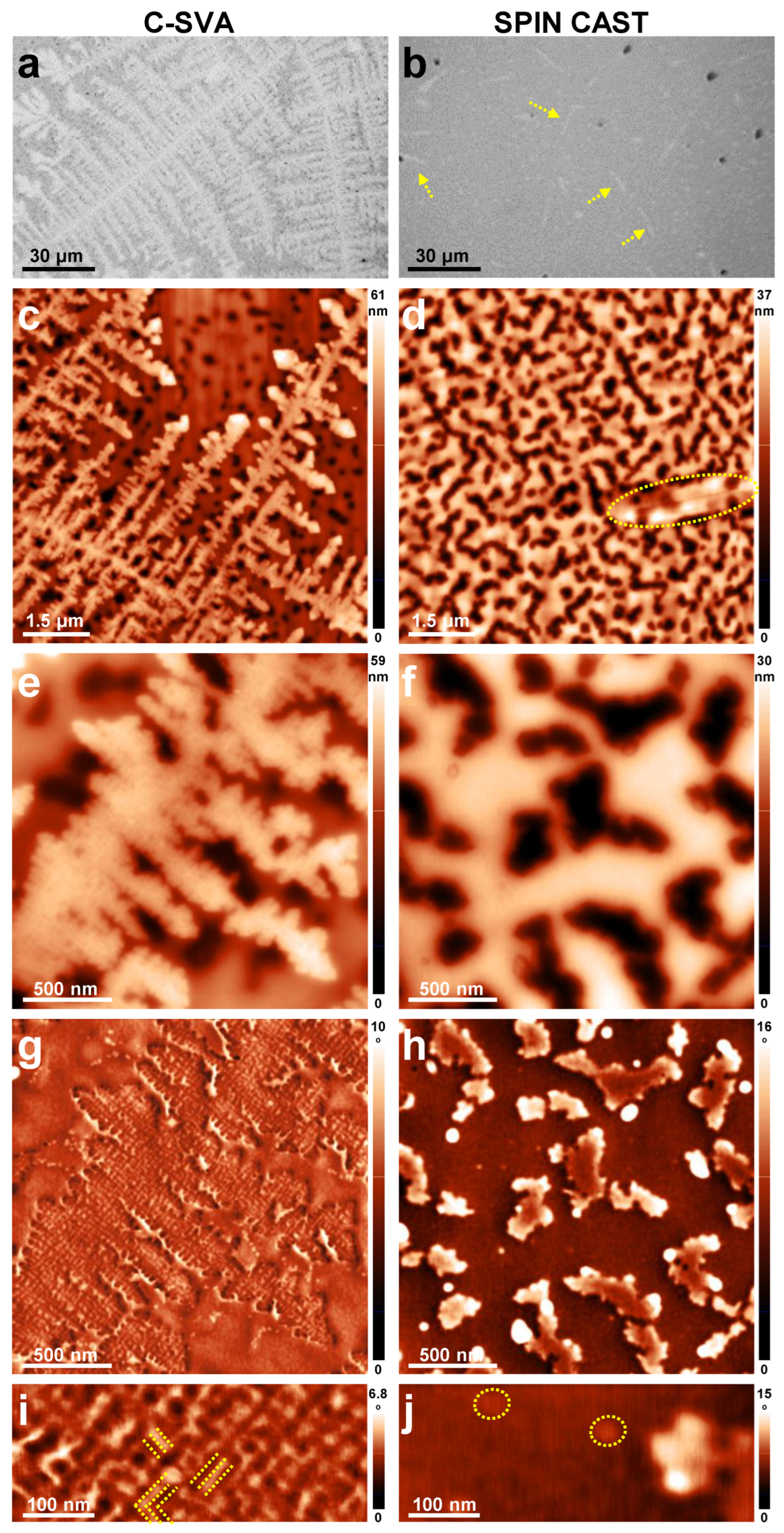
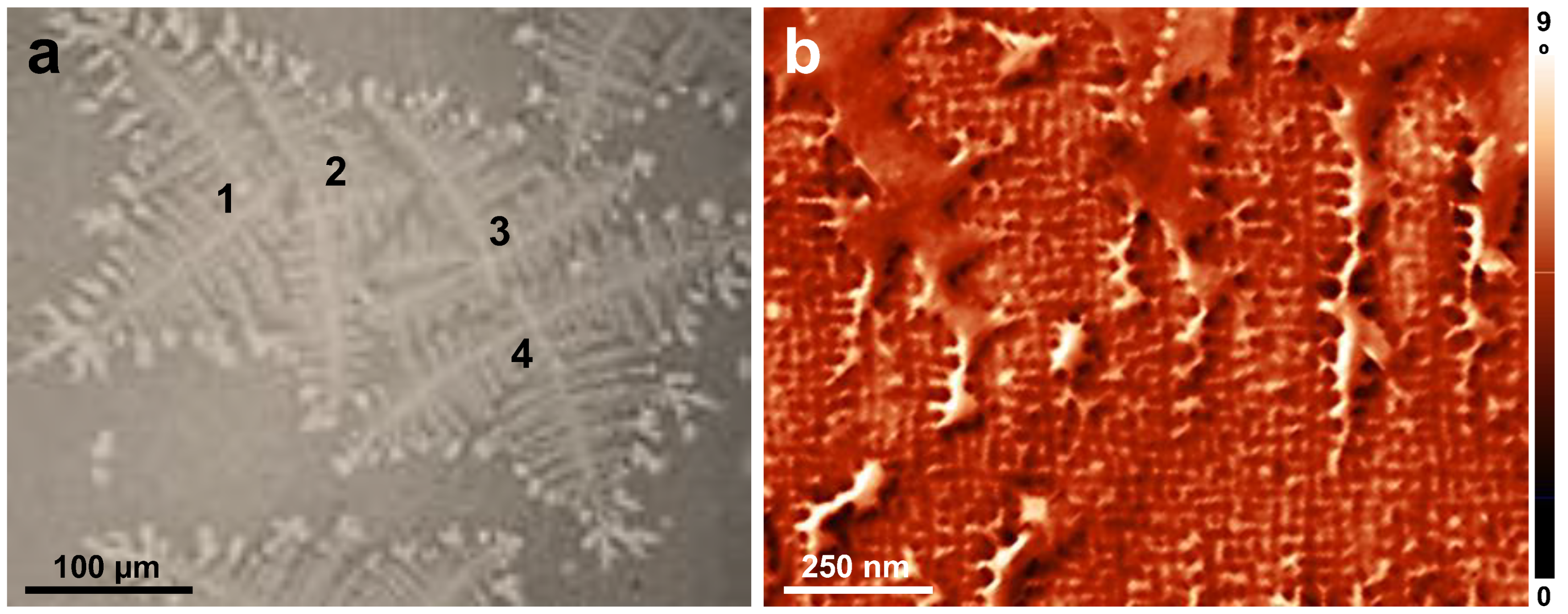
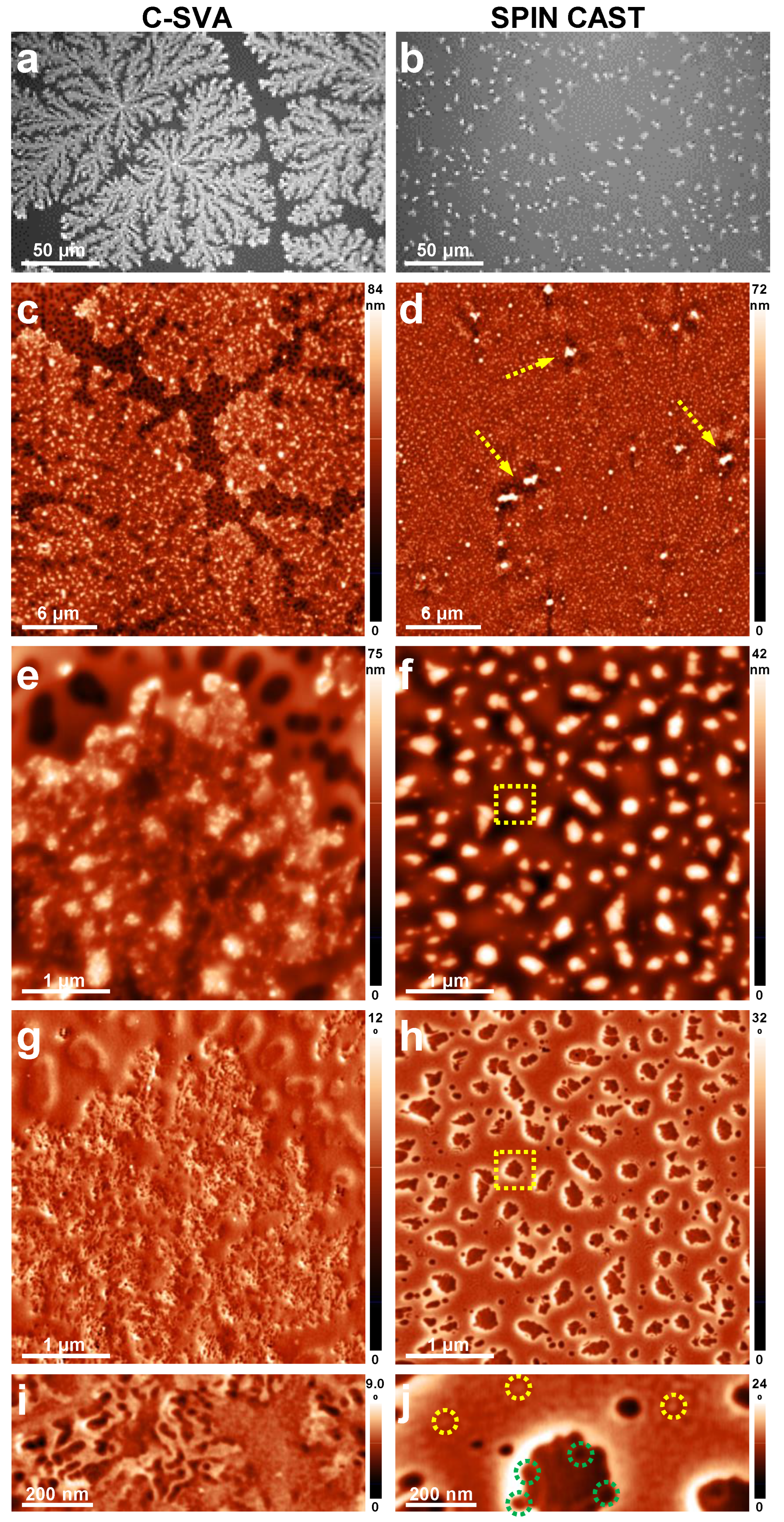
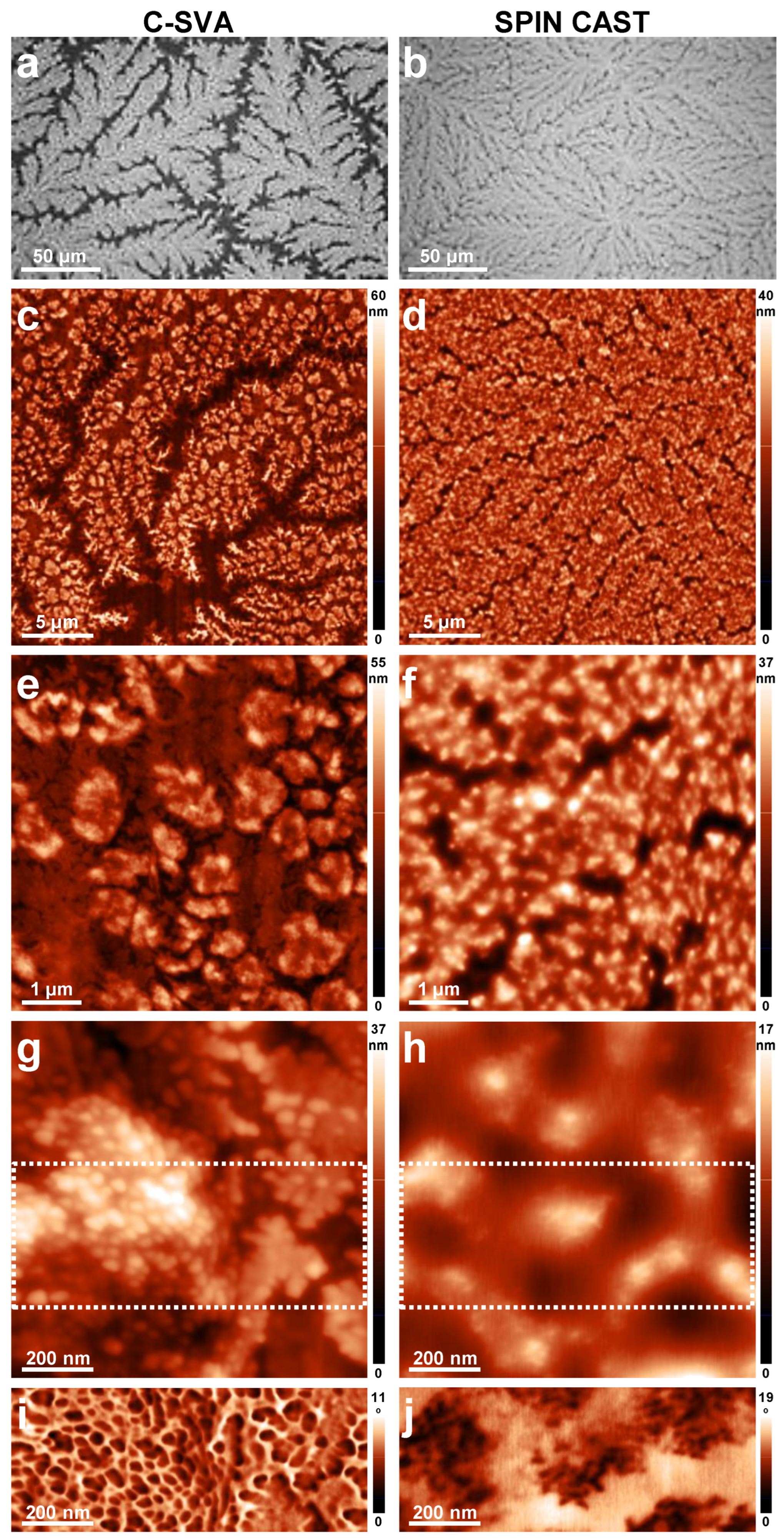
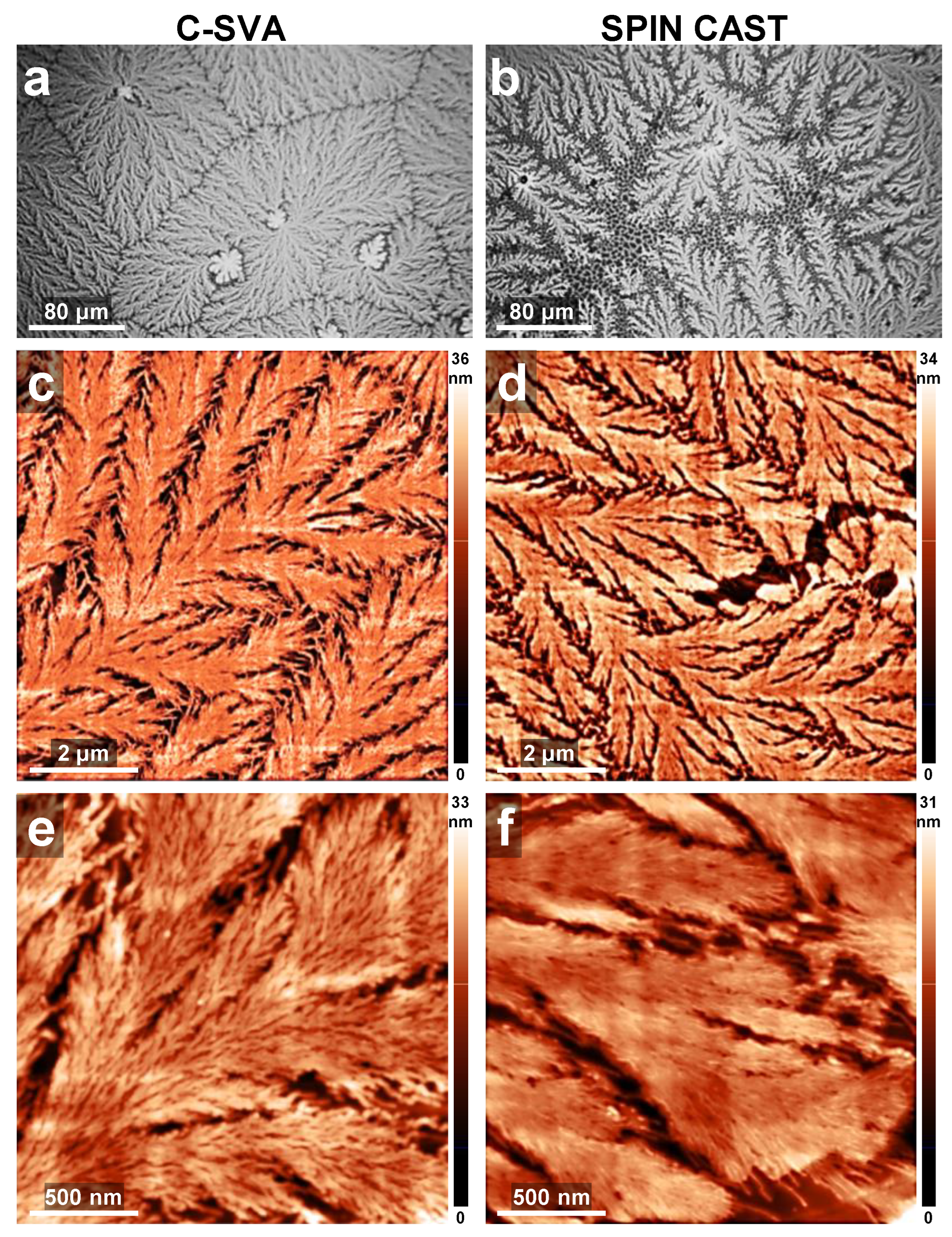
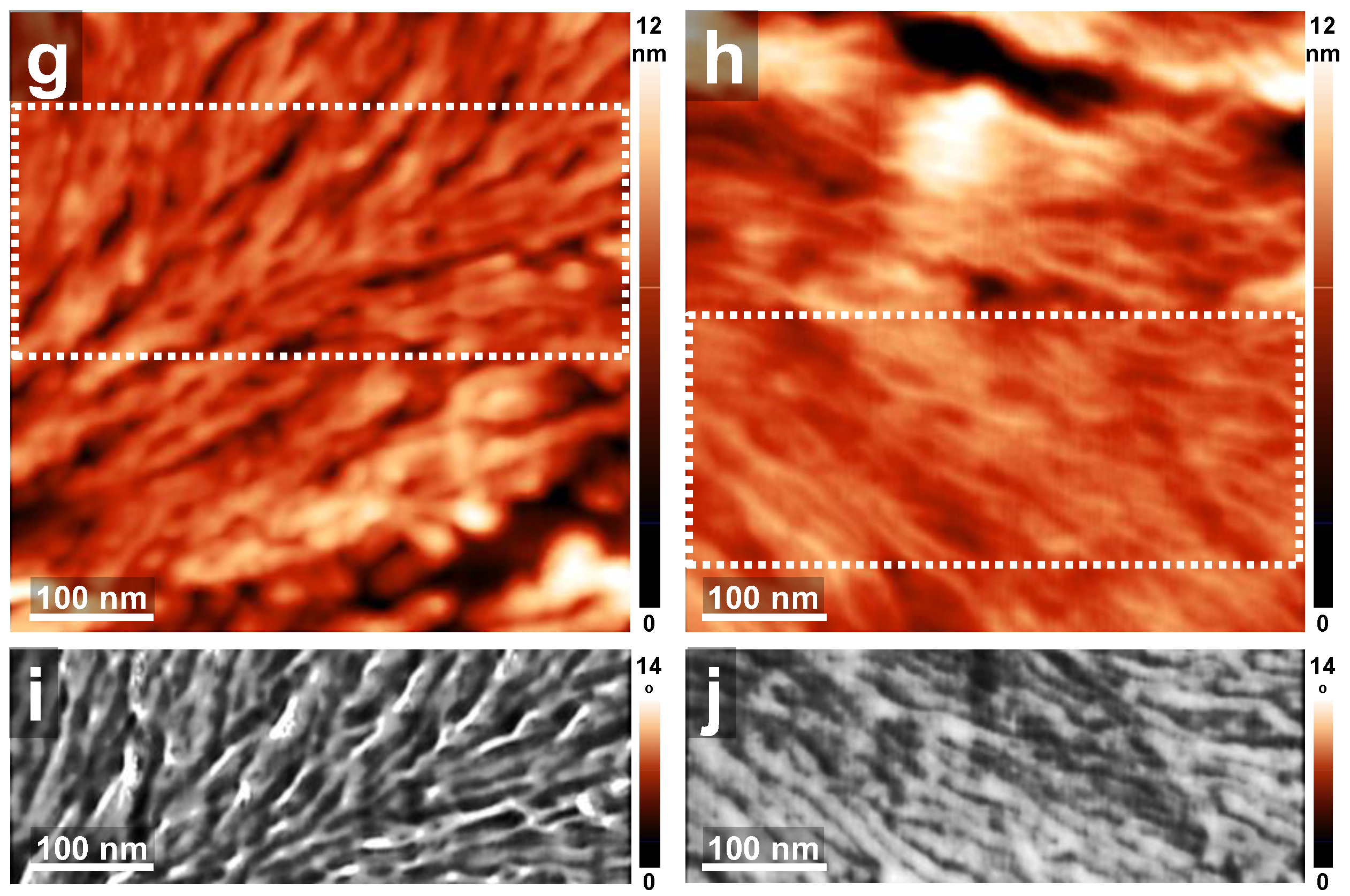
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ronca, S. Chapter 10—Polyethylene. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Oxford, UK; pp. 247–278. ISBN 978-0-323-35824-8.
- Babutan, I.; Lucaci, A.-D.; Botiz, I. Antimicrobial Polymeric Structures Assembled on Surfaces. Polymers 2021, 13, 1552. [Google Scholar] [CrossRef] [PubMed]
- Moohan, J.; Stewart, S.A.; Espinosa, E.; Rosal, A.; Rodríguez, A.; Larrañeta, E.; Donnelly, R.F.; Domínguez-Robles, J. Cellulose Nanofibers and Other Biopolymers for Biomedical Applications. A Review. Appl. Sci. 2020, 10, 65. [Google Scholar] [CrossRef]
- Pattanashetti, N.A.; Heggannavar, G.B.; Kariduraganavar, M.Y. Smart Biopolymers and Their Biomedical Applications. Procedia Manuf. 2017, 12, 263–279. [Google Scholar] [CrossRef]
- Smith, M.; Kar-Narayan, S. Piezoelectric Polymers: Theory, Challenges and Opportunities. Int. Mater. Rev. 2022, 67, 65–88. [Google Scholar] [CrossRef]
- Yao, H.; Fan, Z.; Cheng, H.; Guan, X.; Wang, C.; Sun, K.; Ouyang, J. Recent Development of Thermoelectric Polymers and Composites. Macromol. Rapid Commun. 2018, 39, 1700727. [Google Scholar] [CrossRef]
- Tarcan, R.; Handrea-Dragan, M.; Leordean, C.-I.; Cioban, R.C.; Kiss, G.-Z.; Zaharie-Butucel, D.; Farcau, C.; Vulpoi, A.; Simon, S.; Botiz, I. Development of Polymethylmethacrylate/Reduced Graphene Oxide Composite Films as Thermal Interface Materials. J. Appl. Polym. Sci. 2022, 139, e53238. [Google Scholar] [CrossRef]
- Foster, D.P.; Majumdar, D. Critical Behavior of Magnetic Polymers in Two and Three Dimensions. Phys. Rev. E 2021, 104, 024122. [Google Scholar] [CrossRef]
- Botiz, I.; Durbin, M.M.; Stingelin, N. Providing a Window into the Phase Behavior of Semiconducting Polymers. Macromolecules 2021, 54, 5304–5320. [Google Scholar] [CrossRef]
- Botiz, I.; Astilean, S.; Stingelin, N. Altering the Emission Properties of Conjugated Polymers. Polym. Int. 2016, 65, 157–163. [Google Scholar] [CrossRef]
- Nguyen, H.V.-T.; Jiang, Y.; Mohapatra, S.; Wang, W.; Barnes, J.C.; Oldenhuis, N.J.; Chen, K.K.; Axelrod, S.; Huang, Z.; Chen, Q.; et al. Bottlebrush Polymers with Flexible Enantiomeric Side Chains Display Differential Biological Properties. Nat. Chem. 2022, 14, 85–93. [Google Scholar] [CrossRef]
- Song, X.; Gan, B.; Qi, S.; Guo, H.; Tang, C.Y.; Zhou, Y.; Gao, C. Intrinsic Nanoscale Structure of Thin Film Composite Polyamide Membranes: Connectivity, Defects, and Structure–Property Correlation. Environ. Sci. Technol. 2020, 54, 3559–3569. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Guo, H.; Qin, M.; Wang, Y.; Zhao, J.; Sun, H.; Wang, H.; Wang, Y.; Zhou, X.; Facchetti, A.; et al. Imide-Functionalized Thiazole-Based Polymer Semiconductors: Synthesis, Structure–Property Correlations, Charge Carrier Polarity, and Thin-Film Transistor Performance. Chem. Mater. 2018, 30, 7988–8001. [Google Scholar] [CrossRef]
- Botiz, I. Prominent Processing Techniques to Manipulate Semiconducting Polymer Microstructure. J. Mater. Chem. C 2023, 11, 364–405. [Google Scholar] [CrossRef]
- Botiz, I.; Freyberg, P.; Leordean, C.; Gabudean, A.-M.; Astilean, S.; Yang, A.C.-M.; Stingelin, N. Emission Properties of MEH-PPV in Thin Films Simultaneously Illuminated and Annealed at Different Temperatures. Synth. Met. 2015, 199, 33–36. [Google Scholar] [CrossRef]
- Peng, Z.; Stingelin, N.; Ade, H.; Michels, J.J. A Materials Physics Perspective on Structure-Processing-Function Relations in Blends of Organic Semiconductors. Nat. Rev. Mater. 2023, 1–17. [Google Scholar] [CrossRef]
- Dimov, I.B.; Moser, M.; Malliaras, G.G.; McCulloch, I. Semiconducting Polymers for Neural Applications. Chem. Rev. 2022, 122, 4356–4396. [Google Scholar] [CrossRef] [PubMed]
- de Leon, A.C.C.; da Silva, Í.G.M.; Pangilinan, K.D.; Chen, Q.; Caldona, E.B.; Advincula, R.C. High Performance Polymers for Oil and Gas Applications. React. Funct. Polym. 2021, 162, 104878. [Google Scholar] [CrossRef]
- Pham, Q.-T.; Chern, C.-S. Applications of Polymers in Lithium-Ion Batteries with Enhanced Safety and Cycle Life. J. Polym. Res. 2022, 29, 124. [Google Scholar] [CrossRef]
- Yarali, E.; Baniasadi, M.; Zolfagharian, A.; Chavoshi, M.; Arefi, F.; Hossain, M.; Bastola, A.; Ansari, M.; Foyouzat, A.; Dabbagh, A.; et al. Magneto-/ Electro-responsive Polymers toward Manufacturing, Characterization, and Biomedical/Soft Robotic Applications. Appl. Mater. Today 2022, 26, 101306. [Google Scholar] [CrossRef]
- Angel, N.; Li, S.; Yan, F.; Kong, L. Recent Advances in Electrospinning of Nanofibers from Bio-Based Carbohydrate Polymers and Their Applications. Trends Food Sci. Technol. 2022, 120, 308–324. [Google Scholar] [CrossRef]
- He, Y.; Kukhta, N.A.; Marks, A.; Luscombe, C.K. The Effect of Side Chain Engineering on Conjugated Polymers in Organic Electrochemical Transistors for Bioelectronic Applications. J. Mater. Chem. C 2022, 10, 2314–2332. [Google Scholar] [CrossRef] [PubMed]
- Handrea-Dragan, M.; Botiz, I. Multifunctional Structured Platforms: From Patterning of Polymer-Based Films to Their Subsequent Filling with Various Nanomaterials. Polymers 2021, 13, 445. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, A.K.; Wu, F.; Mincheva, R.; Hakkarainen, M.; Raquez, J.-M.; Mielewski, D.F.; Narayan, R.; Netravali, A.N.; Misra, M. Sustainable Polymers. Nat. Rev. Methods Primers 2022, 2, 1–27. [Google Scholar] [CrossRef]
- Chohan, J.S.; Boparai, K.S.; Singh, R.; Hashmi, M.S.J. Manufacturing Techniques and Applications of Polymer Matrix Composites: A Brief Review. Adv. Mater. Process. Technol. 2022, 8, 884–894. [Google Scholar] [CrossRef]
- Sun, C.; Zhu, C.; Meng, L.; Li, Y. Quinoxaline-Based D–A Copolymers for the Applications as Polymer Donor and Hole Transport Material in Polymer/Perovskite Solar Cells. Adv. Mater. 2022, 34, 2104161. [Google Scholar] [CrossRef]
- Ritsema van Eck, G.C.; Chiappisi, L.; de Beer, S. Fundamentals and Applications of Polymer Brushes in Air. ACS Appl. Polym. Mater. 2022, 4, 3062–3087. [Google Scholar] [CrossRef]
- Kirillova, A.; Yeazel, T.R.; Asheghali, D.; Petersen, S.R.; Dort, S.; Gall, K.; Becker, M.L. Fabrication of Biomedical Scaffolds Using Biodegradable Polymers. Chem. Rev. 2021, 121, 11238–11304. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F.S.U.; Tremel, K.; Saur, A.-K.; Link, S.; Kayunkid, N.; Brinkmann, M.; Herrero-Carvajal, D.; Navarrete, J.T.L.; Delgado, M.C.R.; Ludwigs, S. Influence of Processing Solvents on Optical Properties and Morphology of a Semicrystalline Low Bandgap Polymer in the Neutral and Charged States. Macromolecules 2013, 46, 4924–4931. [Google Scholar] [CrossRef]
- Baklar, M.A.; Koch, F.; Kumar, A.; Buchaca Domingo, E.; Campoy-Quiles, M.; Feldman, K.; Yu, L.; Wöbkenberg, P.; Ball, J.; Wilson, R.M.; et al. Solid-State Processing of Organic Semiconductors. Adv. Mater. 2010, 22, 3942–3947. [Google Scholar] [CrossRef]
- Botiz, I.; Codescu, M.-A.; Farcau, C.; Leordean, C.; Astilean, S.; Silva, C.; Stingelin, N. Convective Self-Assembly of π-Conjugated Oligomers and Polymers. J. Mater. Chem. C 2017, 5, 2513–2518. [Google Scholar] [CrossRef]
- Peterson, G.W.; Lee, D.T.; Barton, H.F.; Epps, T.H.; Parsons, G.N. Fibre-Based Composites from the Integration of Metal–Organic Frameworks and Polymers. Nat. Rev. Mater. 2021, 6, 605–621. [Google Scholar] [CrossRef]
- Chowdhury, M.; Sajjad, M.T.; Savikhin, V.; Hergué, N.; Sutija, K.B.; Oosterhout, S.D.; Toney, M.F.; Dubois, P.; Ruseckas, A.; Samuel, I.D.W. Tuning Crystalline Ordering by Annealing and Additives to Study Its Effect on Exciton Diffusion in a Polyalkylthiophene Copolymer. Phys. Chem. Chem. Phys. 2017, 19, 12441–12451. [Google Scholar] [CrossRef] [PubMed]
- Todor-Boer, O.; Petrovai, I.; Tarcan, R.; Vulpoi, A.; David, L.; Astilean, S.; Botiz, I. Enhancing Photoluminescence Quenching in Donor–Acceptor PCE11:PPCBMB Films through the Optimization of Film Microstructure. Nanomaterials 2019, 9, 1757. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Y.; Yao, Z.-F.; Lu, Y.; Zhang, S.; Ahmad, Z.; Wang, J.-Y.; Gu, X.; Pei, J. Achieving High Alignment of Conjugated Polymers by Controlled Dip-Coating. Adv. Electron. Mater. 2020, 6, 2000080. [Google Scholar] [CrossRef]
- Xiao, M.; Kang, B.; Lee, S.B.; Perdigão, L.M.A.; Luci, A.; Warr, D.A.; Senanayak, S.P.; Nikolka, M.; Statz, M.; Wu, Y.; et al. Anisotropy of Charge Transport in a Uniaxially Aligned Fused Electron-Deficient Polymer Processed by Solution Shear Coating. Adv. Mater. 2020, 32, 2000063. [Google Scholar] [CrossRef]
- Basu, A.; Niazi, M.R.; Scaccabarozzi, A.D.; Faber, H.; Fei, Z.; Anjum, D.H.; Paterson, A.F.; Boltalina, O.; Heeney, M.; Anthopoulos, T.D. Impact of P-Type Doping on Charge Transport in Blade-Coated Small-Molecule:Polymer Blend Transistors. J. Mater. Chem. C 2020, 8, 15368–15376. [Google Scholar] [CrossRef]
- Grozev, N.; Botiz, I.; Reiter, G. Morphological Instabilities of Polymer Crystals. Eur. Phys. J. E 2008, 27, 63–71. [Google Scholar] [CrossRef]
- Botiz, I.; Grozev, N.; Schlaad, H.; Reiter, G. The Influence of Protic Non-Solvents Present in the Environment on Structure Formation of Poly(γ-Benzyl-L-Glutamate in Organic Solvents. Soft Matter 2008, 4, 993–1002. [Google Scholar] [CrossRef]
- Darko, C.; Botiz, I.; Reiter, G.; Breiby, D.W.; Andreasen, J.W.; Roth, S.V.; Smilgies, D.M.; Metwalli, E.; Papadakis, C.M. Crystallization in Diblock Copolymer Thin Films at Different Degrees of Supercooling. Phys. Rev. E 2009, 79, 041802. [Google Scholar] [CrossRef]
- Jahanshahi, K.; Botiz, I.; Reiter, R.; Thomann, R.; Heck, B.; Shokri, R.; Stille, W.; Reiter, G. Crystallization of Poly(γ-Benzyl L-Glutamate) in Thin Film Solutions: Structure and Pattern Formation. Macromolecules 2013, 46, 1470–1476. [Google Scholar] [CrossRef]
- Jahanshahi, K.; Botiz, I.; Reiter, R.; Scherer, H.; Reiter, G. Reversible Nucleation, Growth, and Dissolution of Poly(γ-Benzyl l-Glutamate) Hexagonal Columnar Liquid Crystals by Addition and Removal of a Nonsolvent. Cryst. Growth Des. 2013, 13, 4490–4494. [Google Scholar] [CrossRef]
- Singh, M.; Agrawal, A.; Wu, W.; Masud, A.; Armijo, E.; Gonzalez, D.; Zhou, S.; Terlier, T.; Zhu, C.; Strzalka, J.; et al. Soft-Shear-Aligned Vertically Oriented Lamellar Block Copolymers for Template-Free Sub-10 Nm Patterning and Hybrid Nanostructures. ACS Appl. Mater. Interfaces 2022, 14, 12824–12835. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Weber, C.; Schubert, U.S.; Hoogenboom, R. Thermoresponsive Polymers with Lower Critical Solution Temperature: From Fundamental Aspects and Measuring Techniques to Recommended Turbidimetry Conditions. Mater. Horiz. 2017, 4, 109–116. [Google Scholar] [CrossRef]
- Macedo, A.S.; Carvalho, E.O.; Cardoso, V.F.; Correia, D.M.; Tubio, C.R.; Fidalgo-Marijuan, A.; Botelho, G.; Lanceros-Méndez, S. Tailoring Electroactive Poly(Vinylidene Fluoride-co-Trifluoroethylene) Microspheres by a Nanoprecipitation Method. Mater. Lett. 2020, 261, 127018. [Google Scholar] [CrossRef]
- Lin, X.; Liu, R.; Ding, C.; Deng, J.; Guo, Y.; Long, S.; Li, L.; Li, M. Modulation of Microstructure and Charge Transport in Polymer Monolayer Transistors by Solution Aging. Chin. J. Chem. 2021, 39, 3079–3084. [Google Scholar] [CrossRef]
- Khalil, Y.; Hopkinson, N.; Kowalski, A.; Fairclough, J.P.A. Characterisation of UHMWPE Polymer Powder for Laser Sintering. Materials 2019, 12, 3496. [Google Scholar] [CrossRef]
- Jana, A.; Selvaraj, S.; Subramani, K. A Novel Technique for the Development of Acetabular Cup by Cold Isostatic Compaction and Sintering of UHMWPE Powder with Optimized Processing Parameters. Polym. Eng. Sci. 2021, 61, 2536–2556. [Google Scholar] [CrossRef]
- Khan, A.L.T.; Sreearunothai, P.; Herz, L.M.; Banach, M.J.; Köhler, A. Morphology-Dependent Energy Transfer within Polyfluorene Thin Films. Phys. Rev. B 2004, 69, 085201. [Google Scholar] [CrossRef]
- Danesh, C.D.; Starkweather, N.S.; Zhang, S. In Situ Study of Dynamic Conformational Transitions of a Water-Soluble Poly (3-Hexylthiophene) Derivative by Surfactant Complexation. J. Phys. Chem. B 2012, 116, 12887–12894. [Google Scholar] [CrossRef]
- Adachi, T.; Tong, L.; Kuwabara, J.; Kanbara, T.; Saeki, A.; Seki, S.; Yamamoto, Y. Spherical Assemblies from π-Conjugated Alternating Copolymers: Toward Optoelectronic Colloidal Crystals. J. Am. Chem. Soc. 2013, 135, 870–876. [Google Scholar] [CrossRef]
- Guha, S.; Chandrasekhar, M.; Scherf, U.; Knaapila, M. Tuning Structural and Optical Properties of Blue-Emitting Polymeric Semiconductors. Phys. Status Solidi B 2011, 248, 1083–1090. [Google Scholar] [CrossRef]
- Tung, K.-P.; Chen, C.-C.; Lee, P.; Liu, Y.-W.; Hong, T.-M.; Hwang, K.C.; Hsu, J.H.; White, J.D.; Yang, A.C.-M. Large Enhancements in Optoelectronic Efficiencies of Nano-Plastically Stressed Conjugated Polymer Strands. ACS Nano 2011, 5, 7296–7302. [Google Scholar] [CrossRef] [PubMed]
- Loo, Y.L.; Register, R.A.; Ryan, A.J. Modes of Crystallization in Block Copolymer Microdomains: Breakout, Templated, and Confined. Macromolecules 2002, 35, 2365–2374. [Google Scholar] [CrossRef]
- Bao, J.; Dong, X.; Chen, S.; Lu, W.; Zhang, X.; Chen, W. Confined Crystallization, Melting Behavior and Morphology in PEG-b-PLA Diblock Copolymers: Amorphous versus Crystalline PLA. J. Polym. Sci. 2020, 58, 455–465. [Google Scholar] [CrossRef]
- MacFarlane, L.R.; Shaikh, H.; Garcia-Hernandez, J.D.; Vespa, M.; Fukui, T.; Manners, I. Functional Nanoparticles through π-Conjugated Polymer Self-Assembly. Nat. Rev. Mater. 2021, 6, 7–26. [Google Scholar] [CrossRef]
- Stevens, C.A.; Kaur, K.; Klok, H.-A. Self-Assembly of Protein-Polymer Conjugates for Drug Delivery. Adv. Drug Deliv. Rev. 2021, 174, 447–460. [Google Scholar] [CrossRef]
- Scanga, R.A.; Reuther, J.F. Helical Polymer Self-Assembly and Chiral Nanostructure Formation. Polym. Chem. 2021, 12, 1857–1897. [Google Scholar] [CrossRef]
- Kos, P.I.; Ivanov, V.A.; Chertovich, A.V. Crystallization of Semiflexible Polymers in Melts and Solutions. Soft Matter 2021, 17, 2392–2403. [Google Scholar] [CrossRef]
- Jin, F.; Yuan, S.; Wang, S.; Zhang, Y.; Zheng, Y.; Hong, Y.; Miyoshi, T. Polymer Chains Fold Prior to Crystallization. ACS Macro Lett. 2022, 11, 284–288. [Google Scholar] [CrossRef]
- Sheng, J.; Chen, W.; Cui, K.; Li, L. Polymer Crystallization under External Flow. Rep. Prog. Phys. 2022, 85, 036601. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Zheng, Y.; Yuan, W.; Zhou, J.; Yu, C.; Bao, Y.; Shan, G.; Pan, P. Hierarchical Ordering and Multilayer Structure of Poly(ε-Caprolactone) End-Functionalized by a Liquid Crystalline Unit: Role of Polymer Crystallization. Polym. Chem. 2021, 12, 4175–4183. [Google Scholar] [CrossRef]
- Hamley, I.W. Crystallization in Block Copolymers. In Interfaces Crystallization Viscoelasticity; Springer: Berlin/Heidelberg, Germany, 1999; pp. 113–137. ISBN 978-3-540-48836-1. [Google Scholar]
- Castillo, R.V.; Müller, A.J. Crystallization and Morphology of Biodegradable or Biostable Single and Double Crystalline Block Copolymers. Prog. Polym. Sci. 2009, 34, 516–560. [Google Scholar] [CrossRef]
- Le, T.P.; Smith, B.H.; Lee, Y.; Litofsky, J.H.; Aplan, M.P.; Kuei, B.; Zhu, C.; Wang, C.; Hexemer, A.; Gomez, E.D. Enhancing Optoelectronic Properties of Conjugated Block Copolymers through Crystallization of Both Blocks. Macromolecules 2020, 53, 1967–1976. [Google Scholar] [CrossRef]
- He, Y.; Eloi, J.-C.; Harniman, R.L.; Richardson, R.M.; Whittell, G.R.; Mathers, R.T.; Dove, A.P.; O’Reilly, R.K.; Manners, I. Uniform Biodegradable Fiber-Like Micelles and Block Comicelles via “Living” Crystallization-Driven Self-Assembly of Poly(l-Lactide) Block Copolymers: The Importance of Reducing Unimer Self-Nucleation via Hydrogen Bond Disruption. J. Am. Chem. Soc. 2019, 141, 19088–19098. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. Block Copolymers. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2002; Volume 1, pp. 457–482. [Google Scholar]
- Xiong, S.; Li, D.; Hur, S.-M.; Craig, G.S.W.; Arges, C.G.; Qu, X.-P.; Nealey, P.F. The Solvent Distribution Effect on the Self-Assembly of Symmetric Triblock Copolymers during Solvent Vapor Annealing. Macromolecules 2018, 51, 7145–7151. [Google Scholar] [CrossRef]
- Shi, L.-Y.; Yin, C.; Zhou, B.; Xia, W.; Weng, L.; Ross, C.A. Annealing Process Dependence of the Self-Assembly of Rod–Coil Block Copolymer Thin Films. Macromolecules 2021, 54, 1657–1664. [Google Scholar] [CrossRef]
- Ginige, G.; Song, Y.; Olsen, B.C.; Luber, E.J.; Yavuz, C.T.; Buriak, J.M. Solvent Vapor Annealing, Defect Analysis, and Optimization of Self-Assembly of Block Copolymers Using Machine Learning Approaches. ACS Appl. Mater. Interfaces 2021, 13, 28639–28649. [Google Scholar] [CrossRef] [PubMed]
- Atanase, L.I.; Lerch, J.-P.; Caprarescu, S.; Iurciuc, C.E.; Riess, G. Micellization of PH-Sensitive Poly(Butadiene)-Block-Poly(2 Vinylpyridine)-Block-Poly(Ethylene Oxide) Triblock Copolymers: Complex Formation with Anionic Surfactants. J. Appl. Polym. Sci. 2017, 134, 45313. [Google Scholar] [CrossRef]
- Zhao, W.; Su, Y.; Müller, A.J.; Gao, X.; Wang, D. Direct Relationship Between Interfacial Microstructure and Confined Crystallization in Poly(Ethylene Oxide)/Silica Composites: The Study of Polymer Molecular Weight Effects. J. Polym. Sci. B Polym. Phys. 2017, 55, 1608–1616. [Google Scholar] [CrossRef]
- Babutan, I.; Todor-Boer, O.; Atanase, L.I.; Vulpoi, A.; Simon, S.; Botiz, I. Self-Assembly of Block Copolymers on Surfaces Exposed to Space-Confined Solvent Vapor Annealing. Polymer 2023, 273, 125881. [Google Scholar] [CrossRef]
- Babutan, I.; Todor-Boer, O.; Atanase, L.I.; Vulpoi, A.; Botiz, I. Self-Assembly of Block Copolymers in Thin Films Swollen-Rich in Solvent Vapors. Polymers 2023, 15, 1900. [Google Scholar] [CrossRef] [PubMed]
- Reiter, G.; Botiz, I.; Graveleau, L.; Grozev, N.; Albrecht, K.; Mourran, A.; Möller, M. Morphologies of Polymer Crystals in Thin Films. In Lecture Notes in Physics: Progress in Understanding of Polymer Crystallization; Reiter, G., Strobl, G.R., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; Volume 714, pp. 179–200. [Google Scholar] [CrossRef]
- Soum, A.; Fontanille, M.; Sigwalt, P. Anionic Polymerization of 2-Vinylpyridine Initiated by Symmetrical Organomagnesium Compounds in Tetrahydrofuran. J. Polym. Sci. Polym. Chem. Ed. 1977, 15, 659–673. [Google Scholar] [CrossRef]
- Atanase, L.I.; Riess, G. Stabilization of Non-Aqueous Emulsions by Poly(2-Vinylpyridine)-b-Poly(Butadiene) Block Copolymers. Colloids Surf. A Physicochem. Eng. Asp. 2014, 458, 19–24. [Google Scholar] [CrossRef]
- Botiz, I.; Schlaad, H.; Reiter, G. Processes of Ordered Structure Formation in Polypeptide Thin Film Solutions. In Self Organized Nanostructures of Amphiphilic Block Copolymers II; Springer: Berlin/Heidelberg, Germany, 2011; Volume 242, pp. 117–149. [Google Scholar]
- Kovacs, A.J.; Straupe, C.; Gonthier, A. Isothermal Growth, Thickening, and Melting of Polyethylene Oxide) Single Crystals in the Bulk. II. J. Polym. Sci. Polym. Symp. 1977, 59, 31–54. [Google Scholar] [CrossRef]
- Lysenko, E.A.; Bronich, T.K.; Slonkina, E.V.; Eisenberg, A.; Kabanov, V.A.; Kabanov, A.V. Block Ionomer Complexes with Polystyrene Core-Forming Block in Selective Solvents of Various Polarities. 2. Solution Behavior and Self-Assembly in Nonpolar Solvents. Macromolecules 2002, 35, 6344–6350. [Google Scholar] [CrossRef]
- Changez, M.; Kang, N.-G.; Koh, H.-D.; Lee, J.-S. Effect of Solvent Composition on Transformation of Micelles to Vesicles of Rod−Coil Poly(n-Hexyl Isocyanate-Block-2-Vinylpyridine) Diblock Copolymers. Langmuir 2010, 26, 9981–9985. [Google Scholar] [CrossRef]
- Ferreiro, V.; Douglas, J.F.; Warren, J.; Karim, A. Growth Pulsations in Symmetric Dendritic Crystallization in Thin Polymer Blend Films. Phys. Rev. E 2002, 65, 051606. [Google Scholar] [CrossRef]
- Ferreiro, V.; Douglas, J.F.; Warren, J.A.; Karim, A. Nonequilibrium Pattern Formation in the Crystallization of Polymer Blend Films. Phys. Rev. E 2002, 65, 042802. [Google Scholar] [CrossRef]
- Riess, G. Micellization of Block Copolymers. Prog. Polym. Sci. 2003, 28, 1107–1170. [Google Scholar] [CrossRef]
- Lerch, J.-P. Synthesis and Characterization of Amphiphilic Block- and Graft Terpolymers. Study of Inorganic Dispersions in Aqueous and Non-Aqueous Media. Ph.D. Thesis, University Haute Alsace, Mulhouse, France, 1996. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babutan, I.; Todor-Boer, O.; Atanase, L.I.; Vulpoi, A.; Botiz, I. Crystallization of Poly(ethylene oxide)-Based Triblock Copolymers in Films Swollen-Rich in Solvent Vapors. Coatings 2023, 13, 918. https://doi.org/10.3390/coatings13050918
Babutan I, Todor-Boer O, Atanase LI, Vulpoi A, Botiz I. Crystallization of Poly(ethylene oxide)-Based Triblock Copolymers in Films Swollen-Rich in Solvent Vapors. Coatings. 2023; 13(5):918. https://doi.org/10.3390/coatings13050918
Chicago/Turabian StyleBabutan, Iulia, Otto Todor-Boer, Leonard Ionut Atanase, Adriana Vulpoi, and Ioan Botiz. 2023. "Crystallization of Poly(ethylene oxide)-Based Triblock Copolymers in Films Swollen-Rich in Solvent Vapors" Coatings 13, no. 5: 918. https://doi.org/10.3390/coatings13050918
APA StyleBabutan, I., Todor-Boer, O., Atanase, L. I., Vulpoi, A., & Botiz, I. (2023). Crystallization of Poly(ethylene oxide)-Based Triblock Copolymers in Films Swollen-Rich in Solvent Vapors. Coatings, 13(5), 918. https://doi.org/10.3390/coatings13050918