Hot-Corrosion Behavior of Gd2O3–Yb2O3 Co-Doped YSZ Thermal Barrier Coatings in the Presence of V2O5 Molten Salt
Abstract
:1. Introduction
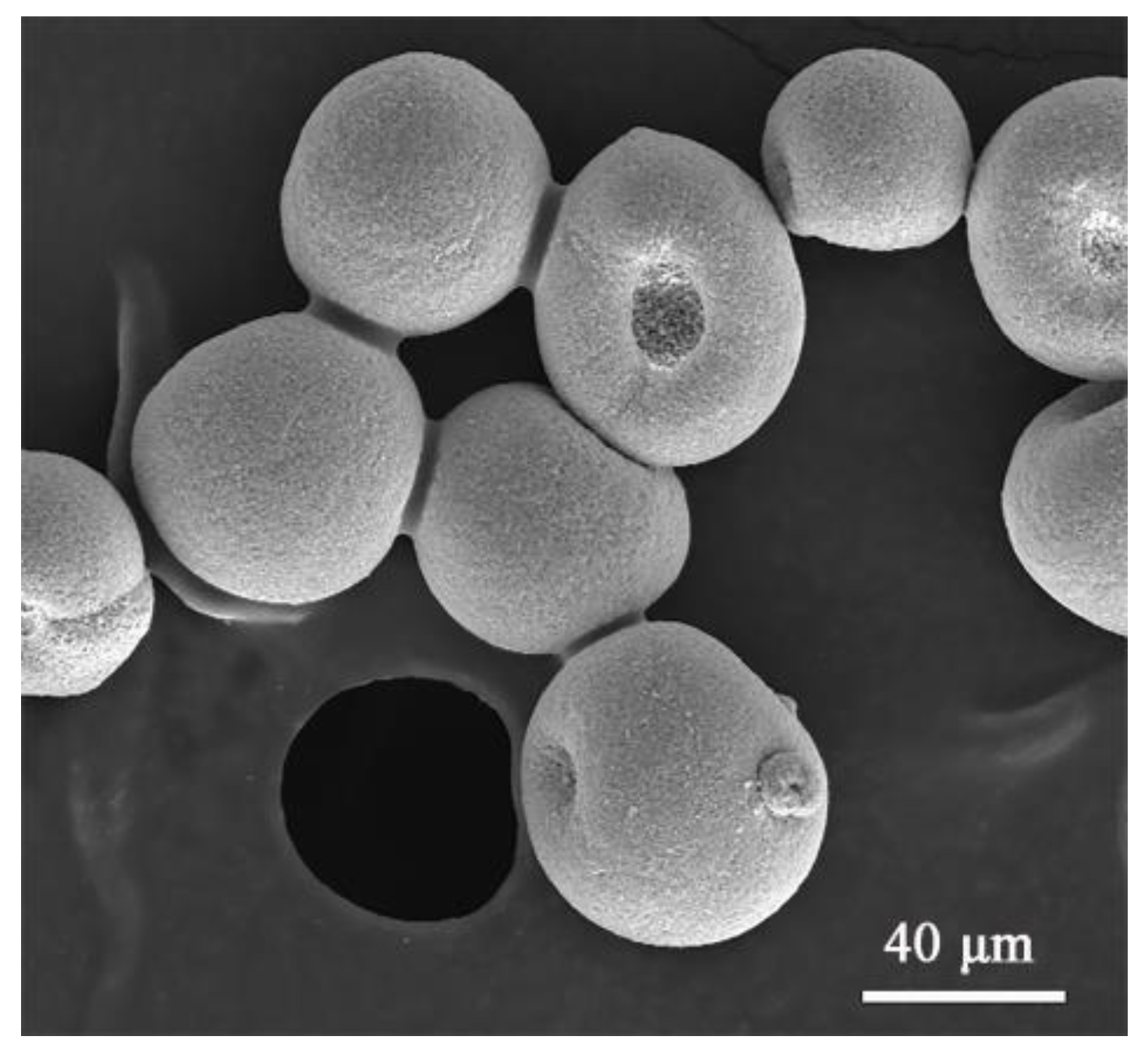
2. Experimental Procedures
3. Results and Discussion
4. Conclusions
- (1)
- The GdYb-YSZ TBCs experienced some degree of attack by V2O5, but they showed better corrosion resistance compared to YSZ TBCs. This improved resistance can be attributed to the higher rare-earth content and lower basicity of Yb2O3.
- (2)
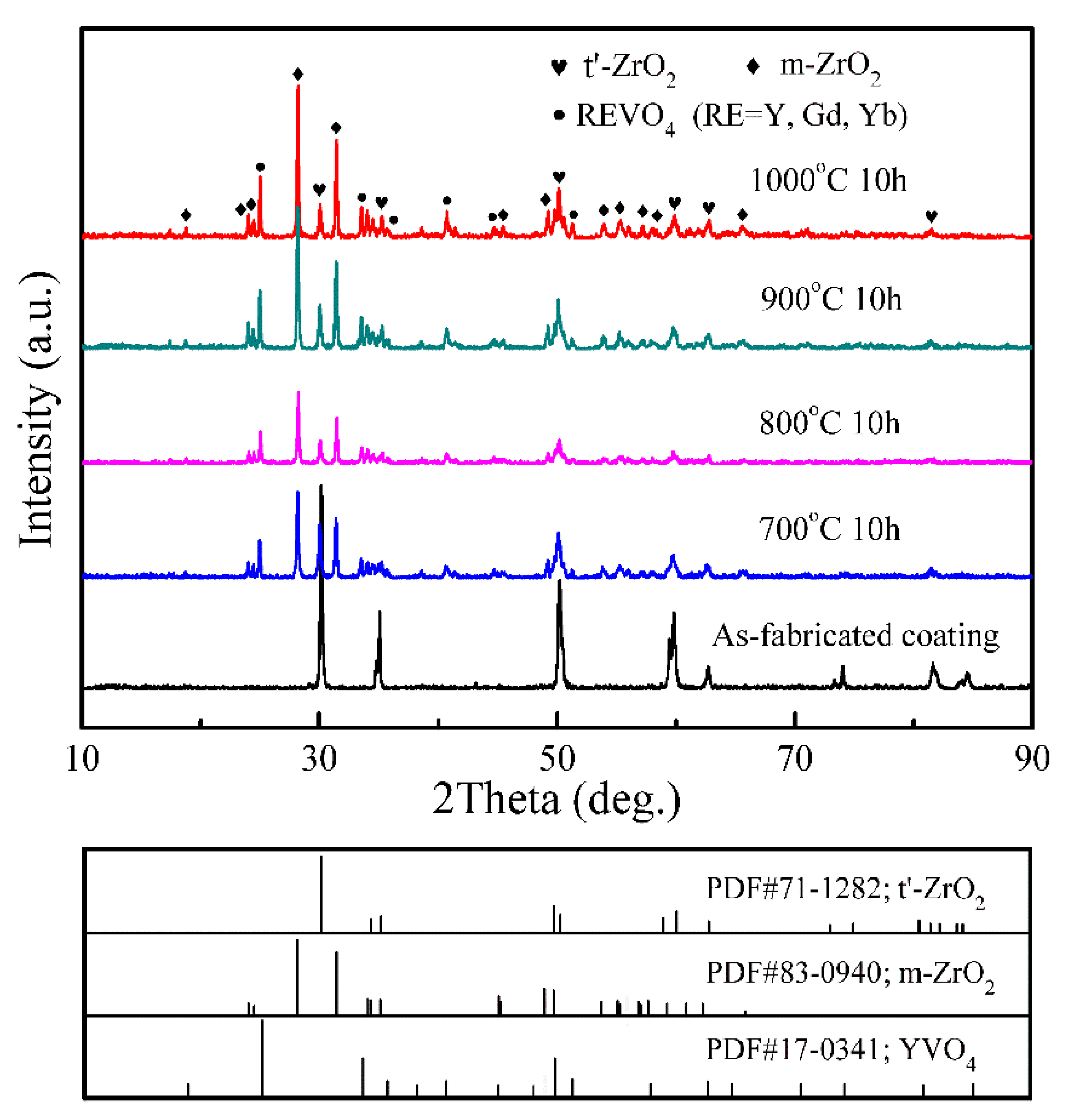
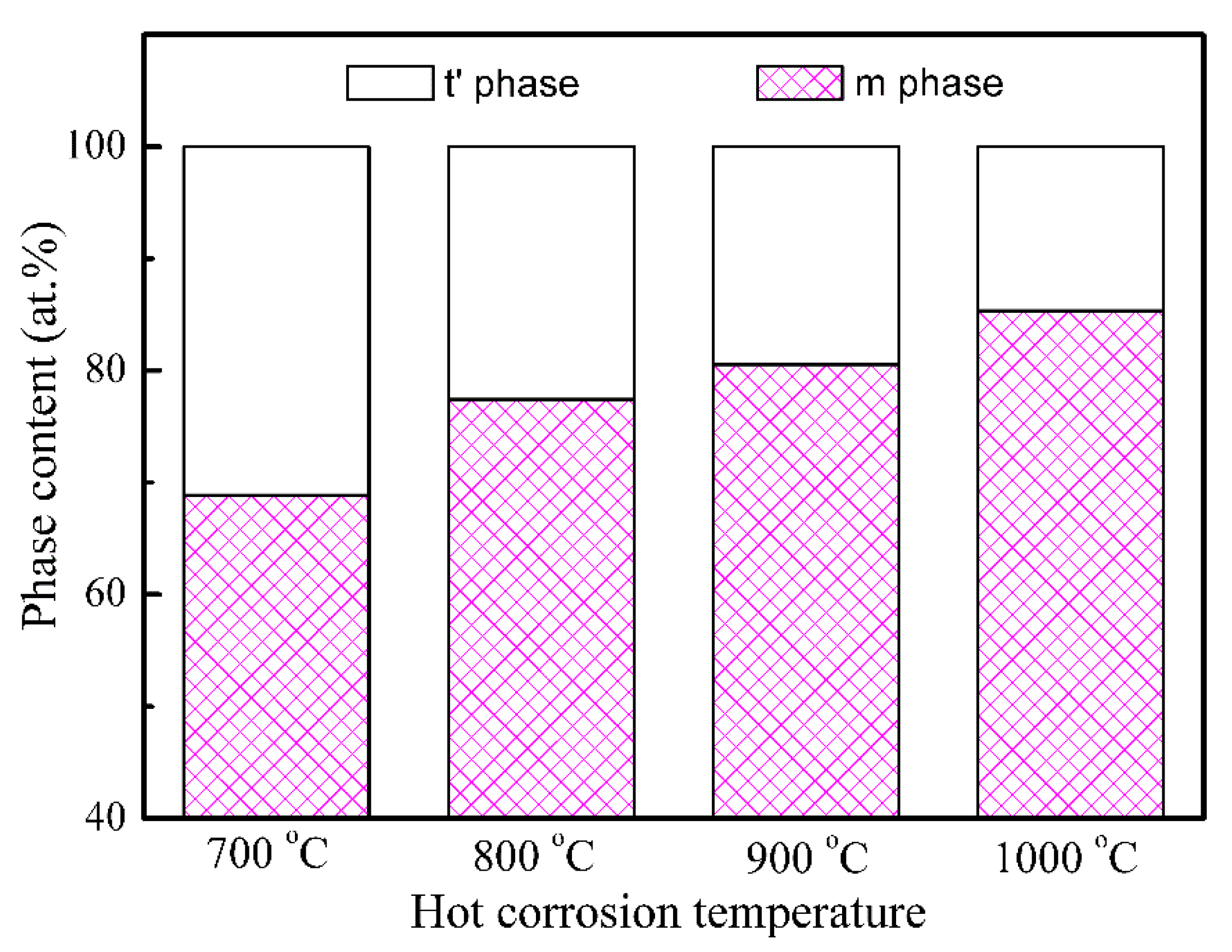
- The as-fabricated GdYb-YSZ TBCs contained t′ phase, and even after hot corrosion, the t′ phase was still detectable on the corroded surfaces, indicating that the coatings had a certain level of resistance to phase decomposition. As the temperature of corrosion increased, the quantity of t′ phase decreased, while the amount of m phase increased.
- (3)
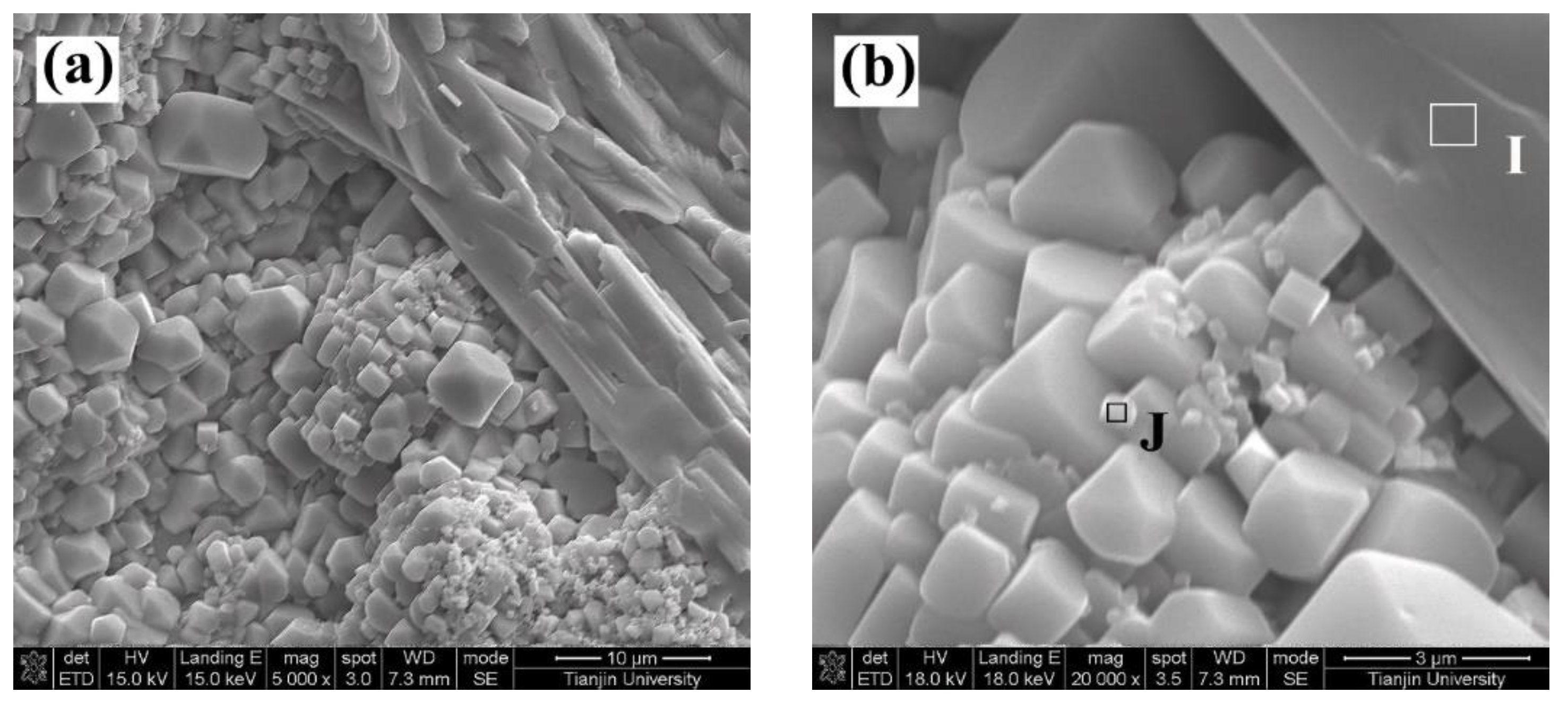
- In addition to the m phase, Yb- and Gd-doped YVO4 were generated as the corrosion products of the GdYb-YSZ TBCs in V2O5. Higher temperatures had no effect on the type of corrosion products but changed the morphologies of Yb- and Gd-doped YVO4 crystals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaßen, R.; Jarligo, M.O.; Steinke, T.; Mack, D.E.; Stöver, D. Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Yang, S.J.; Song, W.J.; Dingwell, D.B.; He, J.; Guo, H.B. Surface roughness affects metastable non-wetting behavior of silicate melts on thermal barrier coatings. Rare Met. 2022, 41, 469–481. [Google Scholar] [CrossRef]
- Guo, L.; Gao, Y.; Ye, F.X.; Zhang, X.M. CMAS corrosion behavior and protection method of thermal barrier coatings for aeroengine. Acta Metall. Sin. 2021, 57, 1184–1198. [Google Scholar]
- Yang, J.; Bai, S.; Sun, J.; Wu, H.; Sun, S.; Wang, S.; Li, Y.; Ma, W.; Tang, X.; Xu, D. Microstructural understanding of the oxidation and inter-diffusion behavior of Cr-coated Alloy 800H in supercritical water. Corros. Sci. 2023, 211, 110910. [Google Scholar] [CrossRef]
- Yang, J.; Shang, L.; Sun, J.; Bai, S.; Wang, S.; Liu, J.; Yun, D.; Ma, D. Restraining the Cr-Zr interdiffusion of Cr-coated Zr alloys in high temperature environment: A Cr/CrN/Cr coating approach. Corros. Sci. 2023, 214, 111015. [Google Scholar] [CrossRef]
- Xue, Z.L.; Guo, H.B.; Gong, S.K.; Xu, H.B. Novel ceramic materials for thermal barrier coatings. J. Aeronaut. Mater. 2018, 38, 10–20. [Google Scholar]
- Xu, Z.H.; Zhou, X.; Wang, K.; Dai, J.W.; He, L.M. Thermal barrier coatings of new rare-earth composite oxide by EB-PVD. J. Alloys Compd. 2014, 587, 126–132. [Google Scholar] [CrossRef]
- Liu, Y.; Ravichandran, R.; Chen, K.Y.; Patnaik, P. Application of machine learning to solid particle erosion of APS-TBC and EB-PVD TBC at elevated temperatures. Coatings 2021, 11, 11070845. [Google Scholar] [CrossRef]
- Xie, Z.H.; Liu, Q.; Lee, K.I.; Zhu, W.; Wu, L.T.; Wu, R.T. The effect of bond coat roughness on the cmas hot corrosion resistance of EB-PVD thermal barrier coatings. Coatings 2022, 12, 12050596. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Wei, L.L.; He, Q.; Deng, Y.P.; He, W.T.; Guo, H.B. PS−PVD alumina overlayer on thermal barrier coatings against CMAS attack. J. Therm. Spray Techn. 2021, 30, 864–872. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Song, W.J.; Guo, L.; Li, X.X.; He, W.T.; Yan, X.D.; Dingwell, D.B.; Guo, H.B. Molten-volcanic-ash-phobic thermal barrier coating based on biomimetic structure. Adv. Sci. 2023, 10, 2205156. [Google Scholar] [CrossRef]
- Guo, Y.Q.; Guo, L.; Li, X.X.; Jiang, C.Y.; Wei, L.L.; Zhu, X.Y.; Liu, D.R.; Song, W.J.; Dingwell, D.B.; Guo, H.B. Ultrafast laser reconstructed PS-PVD thermal barrier coatings with superior silicophobic triple-scale micro/nano structure. Mater. Des. 2023, 228, 111846. [Google Scholar] [CrossRef]
- Loghman-Estarki, M.R.; Shoja Razavi, R.S.; Edris, H.; Pourbafrany, M.; Jamali, H.; Ghasemi, R. Life time of new SYSZ thermal barrier coatings produced by plasma spraying method under thermal shock test and high temperature treatment. Ceram. Int. 2014, 40, 1405–1414. [Google Scholar] [CrossRef]
- Tsipas, S.A. Effect of dopants on the phase stability of zirconia-based plasma sprayed thermal barrier coatings. J. Eur. Ceram. Soc. 2010, 30, 61–72. [Google Scholar] [CrossRef]
- Zhang, X.M.; Xin, H.; Guo, L. Crystallization behavior of calcium–magnesium–alumina–silicate coupled with NaCl/Na2SO4. Corros. Commun. 2023, 10, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhou, C.G. Microstructure and thermal properties of nanostructured gadolinia doped yttria-stabilized zirconia thermal barrier coatings produced by air plasma spraying. Ceram. Int. 2016, 42, 13047–13052. [Google Scholar] [CrossRef]
- Wei, X.D.; Hou, G.L.; An, Y.L.; Yang, P.; Zhao, X.Q.; Zhou, H.D.; Chen, J.M. Effect of doping CeO2 and Sc2O3 on structure, thermal properties and sintering resistance of YSZ. Ceram. Int. 2021, 47, 6875–6883. [Google Scholar] [CrossRef]
- Liu, Z.G.; Zhang, W.H.; Ouyang, J.H.; Zhou, Y. Novel thermal barrier coatings based on rare-earth zirconates/YSZ double-ceramic-layer system deposited by plasma spraying. J. Alloys Compd. 2015, 647, 438–444. [Google Scholar] [CrossRef]
- Wang, Y.H.; Ma, Z.; Liu, L.; Liu, Y.B. Reaction products of Sm2Zr2O7 with calcium-magnesium-aluminum-silicate (CMAS) and their evolution. J. Adv. Ceram. 2021, 10, 1389–1397. [Google Scholar] [CrossRef]
- Guo, L.; Li, B.W.; Cheng, Y.X.; Wang, L. Composition optimization, high-temperature stability, and thermal cycling performance of Sc-doped Gd2Zr2O7 thermal barrier coatings: Theoretical and experimental studies. J. Adv. Ceram. 2022, 11, 454–469. [Google Scholar] [CrossRef]
- Guo, L.; Yan, Z.; Li, Z.H.; Yu, J.X.; Wang, Q.; Li, M.Z.; Ye, F.X. GdPO4 as a novel candidate for thermal barrier coating applications at elevated temperatures. Surf. Coat. Technol. 2018, 349, 400–406. [Google Scholar] [CrossRef]
- She, Y.J.; Guo, Y.W.; Tan, Z.X.; Liao, K. Na2SO4 + V2O5 Corrosion behavior of BaNd2Ti3O10 for thermal barrier coating applications. Coatings 2020, 10, 901. [Google Scholar] [CrossRef]
- Huang, H.; Liu, C.; Ni, L.Y.; Zhou, C.G. Evaluation of microstructural evolution of thermal barrier coatings exposed to Na2SO4 using impedance spectroscopy. Corros. Sci. 2011, 53, 1369–1374. [Google Scholar] [CrossRef]
- Liu, Z.G.; Ouyang, J.H.; Zhou, Y.; Zhu, R.X. Hot corrosion of V2O5-coated NdMgAl11O19 ceramic in air at 950 °C. J. Eur. Ceram. Soc. 2013, 33, 1975–1979. [Google Scholar] [CrossRef]
- Yin, Y.C.; Ma, W.; Jin, X.L.; Li, X.Y.; Bai, Y.; Jia, R.L.; Dong, H.Y. Hot corrosion behavior of the La2(Zr0.7Ce0.3)2O7 ceramic in molten V2O5 and a Na2SO4+V2O5 salt mixture. J. Alloys Compd. 2016, 689, 123–129. [Google Scholar] [CrossRef]
- Chen, X.L.; Sun, Y.W.; Chen, D.X.; Li, J.; Li, W.; Zeng, D.H.; Wu, D.L.; Zou, B.L.; Cao, X.Q. A comparative investigation on the corrosion degradation of plasma sprayed YSZ and LnMgAl11O19 (Ln = Nd, Sm, Gd) coatings exposed to the molten V2O5 + Na2SO4 salt mixture at 1100 °C. J. Eur. Ceram. Soc. 2019, 39, 3778–3787. [Google Scholar] [CrossRef]
- Rahmatnezhad, K.; Zarastvand, M.R.; Talebitooti, R. Mechanism study and power transmission feature of acoustically stimulated and thermally loaded composite shell structures with double curvature. Compos. Struct. 2021, 276, 114557. [Google Scholar] [CrossRef]
- Talebitooti, R.; Gohari, H.D.; Zarastvand, M.R. Multi objective optimization of sound transmission across laminated composite cylindrical shell lined with porous core investigating Non-dominated Sorting Genetic Algorithm. Aerosp. Sci. Technol. 2017, 69, 269–280. [Google Scholar] [CrossRef]
- Bajpai, P.; Das, A.; Bhattacharya, P.; Madayi, S.; Kulkarni, K.; Omar, S. Hot corrosion of stabilized zirconia thermal barrier coatings and the role of Mg inhibitor. J. Am. Ceram. Soc. 2015, 98, 2655–2661. [Google Scholar] [CrossRef]
- Loghman-Estarki, M.R.; Nejati, M.; Edris, H.; Shoja Razavi, R.S.; Jamali, H.; Pakseresht, A.H. Evaluation of hot corrosion behavior of plasma sprayed Scandia and yttria co-stabilized nanostructured thermal barrier coatings in the presence of molten sulfate and vanadate salt. J. Eur. Ceram. Soc. 2015, 35, 693–702. [Google Scholar] [CrossRef]
- Liu, H.F.; Xiong, X.; Li, X.B.; Wang, Y.L. Hot corrosion behavior of Sc2O3-Y2O3-ZrO2 thermal barrier coatings in presence of Na2SO4 + V2O5 molten salt. Corros. Sci. 2014, 85, 87–93. [Google Scholar] [CrossRef]
- Loghman-Estarki, M.R.; Shoja Razavi, R.S.; Edris, H.; Bakhshi, S.R.; Nejati, M.; Jamali, H. Comparison of hot corrosion behavior of nanostructured ScYSZ and YSZ thermal barrier coatings. Ceram. Int. 2016, 42, 7432–7439. [Google Scholar] [CrossRef]
- Habibi, M.H.; Guo, S.M. The hot corrosion behavior of plasma sprayed zirconia coatings stabilized with yttria, ceria, and titania in sodium sulfate and vanadium oxide. Mater. Corros. 2015, 66, 270–277. [Google Scholar] [CrossRef]
- Hajizadeh-Oghaz, M.; Razavi, R.S.; Ghasemi, A.; Valefi, Z. Na2SO4 and V2O5 molten salts corrosion resistance of plasma-sprayed nanostructured ceria and yttria co-stabilized zirconia thermal barrier coatings. Ceram. Int. 2016, 42, 5433–5446. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, C.L.; He, Q.; Li, Z.H.; Yu, J.X.; Liu, X.C.; Ye, F.X. Corrosion products evolution and hot corrosion mechanisms of REPO4 (RE= Gd, Nd, La) in the presence of V2O5 + Na2SO4 molten salt. J. Eur. Ceram. Soc. 2019, 39, 1496–1506. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, C.L.; Li, M.Z.; Sun, W.; Zhang, Z.Y.; Ye, F.X. Hot corrosion evaluation of Gd2O3-Yb2O3 co-doped Y2O3 stabilized ZrO2 thermal barrier oxides exposed to Na2SO4+V2O5 molten salt. Ceram. Int. 2017, 43, 2780–2785. [Google Scholar] [CrossRef]
- Guo, L.; Li, M.Z.; Ye, F.X. Phase stability and thermal conductivity of RE2O3 (RE=La, Nd, Gd, Yb) and Yb2O3 co-doped Y2O3 stabilized ZrO2 ceramics. Ceram. Int. 2016, 42, 7360–7365. [Google Scholar] [CrossRef]
- Hui, Y.; Zhao, S.M.; Xu, J.Y.; Zou, B.L.; Wang, Y.; Cai, X.L.; Zhu, L.; Cao, X.Q. High temperature corrosion behavior of zirconia ceramic in molten Na2SO4+NaVO3 salt mixture. Ceram. Int. 2016, 42, 341–350. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Q.; Yu, Z.; Wang, H.; Zhang, T. Influence of Y2O3 addition on the microstructure of TiC reinforced Ti-based composite coating prepared by laser cladding. Mater. Charact. 2022, 189, 111962. [Google Scholar] [CrossRef]
- Johns, R.L. Oxide acid-base reactions in ceramic corrosion. High Temp. Sci. 1989, 27, 369–380. [Google Scholar]
- Jones, R.L. Scandia-stabilized zirconia for resistance to molten vanadate-sulfate corrosion. Surf. Coat. Technol. 1989, 39–40, 89–96. [Google Scholar] [CrossRef]
- Xu, Z.H.; He, L.M.; Mu, R.D.; He, S.M.; Huang, G.H.; Cao, X.Q. Hot corrosion behavior of La2Zr2O7 with the addition of Y2O3 thermal barrier coatings in contacts with vanadate-sulfate salts. J. Alloys Compd. 2010, 504, 382–385. [Google Scholar] [CrossRef]



| Element | Ni | Co | Cr | Al | Y |
|---|---|---|---|---|---|
| Content | Bal. | 20–22 | 24 | 20 | 1.5 |
| Parameter | NiCoCrAlY | GYb–YSZ |
|---|---|---|
| Current (A) | 530 | 600 |
| Voltage (V) | 50 | 56 |
| Primary gas, Ar (L/min) | 40 | 40 |
| Secondary gas, H2 (L/min) | 10 | 10 |
| Feedstock giving rate (g/min) | 30 | 30 |
| Spray distance (mm) | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; She, Y.; Liao, K. Hot-Corrosion Behavior of Gd2O3–Yb2O3 Co-Doped YSZ Thermal Barrier Coatings in the Presence of V2O5 Molten Salt. Coatings 2023, 13, 886. https://doi.org/10.3390/coatings13050886
Li Y, She Y, Liao K. Hot-Corrosion Behavior of Gd2O3–Yb2O3 Co-Doped YSZ Thermal Barrier Coatings in the Presence of V2O5 Molten Salt. Coatings. 2023; 13(5):886. https://doi.org/10.3390/coatings13050886
Chicago/Turabian StyleLi, Yang, Yajuan She, and Kai Liao. 2023. "Hot-Corrosion Behavior of Gd2O3–Yb2O3 Co-Doped YSZ Thermal Barrier Coatings in the Presence of V2O5 Molten Salt" Coatings 13, no. 5: 886. https://doi.org/10.3390/coatings13050886
APA StyleLi, Y., She, Y., & Liao, K. (2023). Hot-Corrosion Behavior of Gd2O3–Yb2O3 Co-Doped YSZ Thermal Barrier Coatings in the Presence of V2O5 Molten Salt. Coatings, 13(5), 886. https://doi.org/10.3390/coatings13050886





