Adjustable Underwater Gas Transportation Using Bioinspired Superhydrophobic Elastic String
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Treatment
2.3. Stability Test and Regeneration
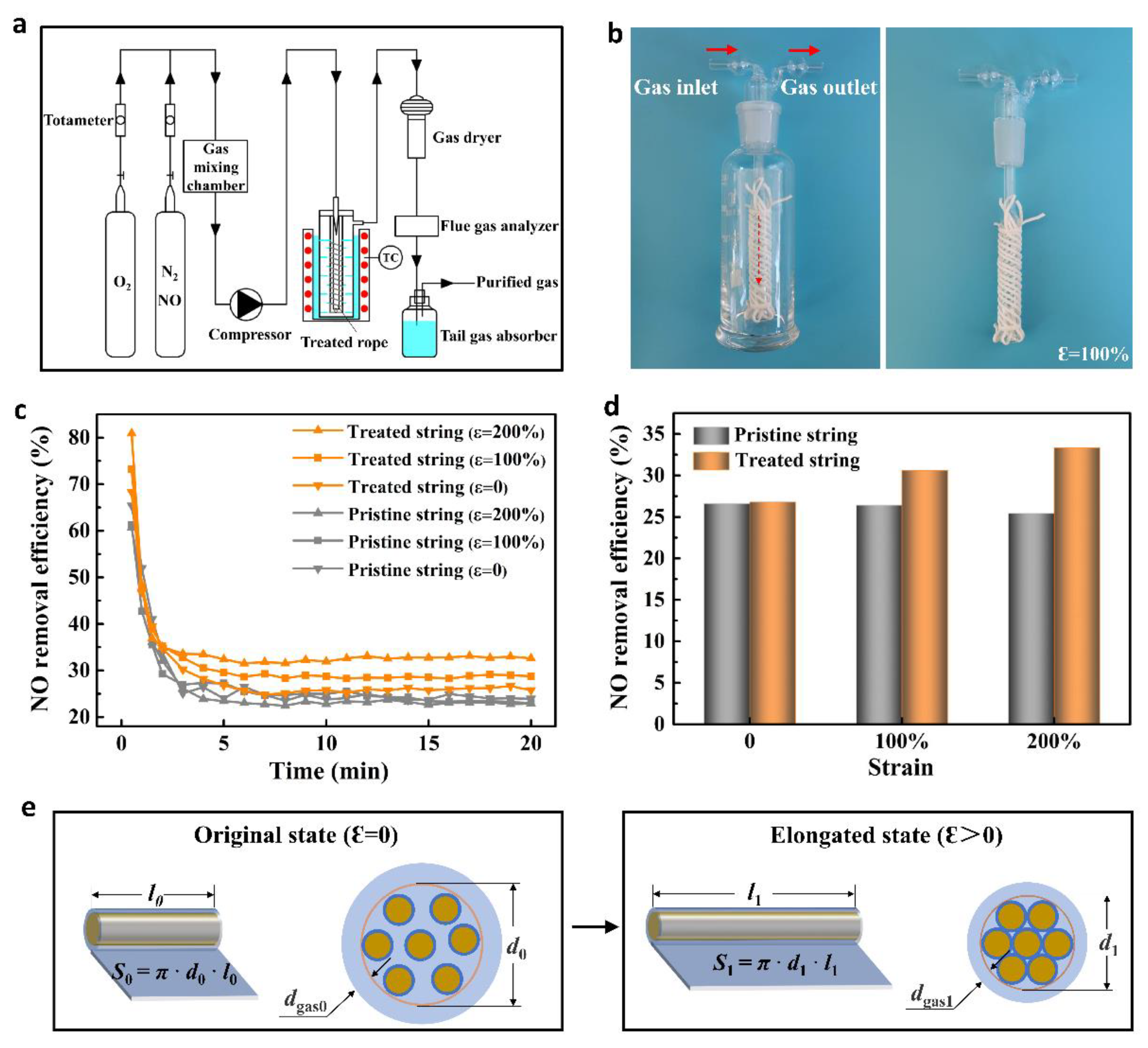
2.4. Gas Transportation
2.5. Removal of NO
2.6. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, H.; Scott, K. An empirical model approach to gas evolution reactions in a centrifugal field. J. Electroanal. Chem. 2003, 544, 75–85. [Google Scholar] [CrossRef]
- Dubois, M.R.; Dubois, D.L. Development of molecular electrocatalysts for CO2 reduction and H2 production/oxidation. Acc. Chem. Res. 2009, 42, 1974–1982. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Fan, S.; Wang, Y.; Rossouw, D.; Wang, Y.; Botton, G.A.; Mi, Z. Tunable syngas production from CO2 and H2O in an aqueous photoelectrochemical cell. Angew. Chem. Int. Ed. Engl. 2016, 55, 14262–14266. [Google Scholar] [CrossRef] [PubMed]
- Mukumoto, M.; Ohshima, T.; Ozaki, M.; Konishi, H.; Maeda, N.; Nakamura, Y. Effect of microbubbled water on the removal of a biofilm attached to orthodontic appliances—An in vitro study. Dent. Mater. J. 2012, 31, 821–827. [Google Scholar] [CrossRef][Green Version]
- Qi, Y.; Hu, X.; Liu, Y.; Sun, D.; Li, R.; Zhu, H. Highly efficient and reversible absorption of SO2 by hydroxyl ammonium ionic liquids at low partial pressure. J. Chem. Technol. Biotechnol. 2019, 94, 3325–3332. [Google Scholar] [CrossRef]
- Wang, P.; Yang, L.; Li, J.; Sadeh, B. Zn/Zno heterostructure for the application of mo degradation and no removal. Catal. Lett. 2020, 150, 1985–1992. [Google Scholar] [CrossRef]
- Wang, R.; Wang, M.; Wang, C.; Yang, Q.; Wang, J.; Jiang, L. Thermally driven interfacial switch between adhesion and antiadhesion on gas bubbles in aqueous media. ACS Appl. Mater. Interfaces 2019, 11, 37365–37370. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, B.; Ma, H.; Li, Z.; Xiao, X.; Zhang, Y.; Cui, X.; Yu, C.; Cao, M.; Jiang, L. Bioinspired pressure-tolerant asymmetric slippery surface for continuous self-transport of gas bubbles in aqueous environment. ACS Nano 2018, 12, 2048–2055. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; He, Z.; Gong, H.; Xu, X.; Zhao, M.; Qin, H. Efficient gas transportation using bioinspired superhydrophobic yarn as the gas-siphon underwater. ACS Appl. Mater. Interfaces 2020, 12, 18174–18181. [Google Scholar] [CrossRef]
- Li, W.; Zhang, J.; Xue, Z.; Wang, J.; Jiang, L. Spontaneous and directional bubble transport on porous copper wires with complex shapes in aqueous media. ACS Appl. Mater. Interfaces 2018, 10, 3076–3081. [Google Scholar] [CrossRef]
- Zhu, S.; Li, J.; Cai, S.; Bian, Y.; Chen, C.; Xu, B.; Su, Y.; Hu, Y.; Wu, D.; Chu, J. Unidirectional transport and effective collection of underwater CO2 bubbles utilizing ultrafast-laser-ablated janus foam. ACS Appl. Mater. Interfaces 2020, 12, 18110–18115. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhu, X.; Li, K.; Cao, M.; Jiang, L. Manipulating bubbles in aqueous environment via a lubricant-infused slippery surface. Adv. Funct. Mater. 2017, 27, 1701605. [Google Scholar] [CrossRef]
- Shi, C.; Cui, X.; Zhang, X.; Tchoukov, P.; Liu, Q.; Encinas, N.; Paven, M.; Geyer, F.; Vollmer, D.; Xu, Z.; et al. Interaction between air bubbles and superhydrophobic surfaces in aqueous solutions. Langmuir 2015, 31, 7317–7327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, P.; Yi, B.; Wang, Z.; Huang, X.; Jiang, L.; Yao, X. Bio-inspired elastic liquid-infused material for on-demand underwater manipulation of air bubbles. ACS Nano 2019, 13, 10596–10602. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, H.R.S.; Zhang, G.; Tang, Y.; Ruan, W.J.; Li, J.B.; Xie, D.T.; Ni, J.P.; Ni, C.S. A highly efficient dual-phase GaN(O)/Nb2O5(N) photocatalyst prepared through nitridation and reoxidation process for NO removal. Chem. Eng. J. 2020, 402, 126199. [Google Scholar] [CrossRef]
- Lv, F.; Yao, D.; Wang, Y.; Wang, C.; Zhu, P.; Hong, Y. Recycling of waste nylon 6/spandex blended fabrics by melt processing. Compos. B Eng. 2015, 77, 232–237. [Google Scholar] [CrossRef]
- Kiumarsi, A.; Parvinzadeh, M. Enzymatic hydrolysis of nylon 6 fiber using lipolytic enzyme. J. Appl. Polym. Sci. 2010, 116, 3140–3147. [Google Scholar] [CrossRef]
- Qu, R.; Gao, J.; Tang, B.; Ma, Q.; Qu, B.; Sun, C. Preparation and property of polyurethane/nanosilver complex fibers. Appl. Surf. Sci. 2014, 294, 81–88. [Google Scholar] [CrossRef]
- Meng, X.; Tian, X.; Xia, Y.; Xiong, Z. Multifunctional alginate-based carbon aerogels for oil–water mixture and emulsion separation. J. Dispers. Sci. Technol. 2021, 1–8. [Google Scholar] [CrossRef]
- Tian, X.; Zhu, H.; Meng, X.; Wang, J.; Zheng, C.; Xia, Y.; Xiong, Z. Amphiphilic calcium alginate carbon aerogels: Broad-spectrum adsorbents for ionic and solvent dyes with multiple functions for decolorized oil–water separation. ACS Appl. Mater. Interfaces 2020, 8, 12755–12767. [Google Scholar] [CrossRef]
- Parvinzadeh, M.; Assefipour, R.; Kiumarsi, A. Biohydrolysis of nylon 6,6 fiber with different proteolytic enzymes. Polym. Degrad. Stab. 2009, 94, 1197–1205. [Google Scholar] [CrossRef]
- Zheng, C.; Sun, Y.; Cui, Y.; Yang, W.; Lu, Z.; Shen, S.; Xia, Y.; Xiong, Z. Superhydrophobic and flame-retardant alginate fabrics prepared through a one-step dip-coating surface-treatment. Cellulose 2021, 28, 5973–5984. [Google Scholar] [CrossRef]
- Zheng, C.; Jin, F.; Zhao, Y.; Zheng, M.; Liu, J.; Dong, X.; Xiong, Z.; Xia, Y.; Duan, X. Light-driven micron-scale 3D hydrogel actuator produced by two-photon polymerization microfabrication. Sens. Actuator B-Chem. 2020, 304, 127345. [Google Scholar] [CrossRef]
- Xiong, Z.; Zheng, C.; Xia, Y. Polymer self-etching for superhydrophobicity through a green hot-pressing-exfoliation process: Low and high adhesion. Macromol. Mater. Eng. 2016, 301, 653–658. [Google Scholar] [CrossRef]
- Ying, T.; Su, J.; Jiang, Y.; Ni, L.; Jiang, X.; Ke, Q.; Xu, H. Superhydrophobic MOFs decorated on hierarchically micro/nanofibrous membranes for high-performance emulsified oily wastewater separation and cationic dyes adsorption. J. Mater. Chem. A 2022, 10, 829–845. [Google Scholar] [CrossRef]
- Du, X.; He, J. Structurally colored surfaces with antireflective, self-cleaning, and antifogging properties. J. Colloid Interface Sci. 2012, 381, 189–197. [Google Scholar] [CrossRef]
- Pugno, M.C.; Misseroni, D.; Pugno, N.M. Air-encapsulating elastic mechanism of submerged Taraxacum blowballs. Mater. Today Bio 2021, 9, 100095. [Google Scholar] [CrossRef]
- Xue, X.; Wang, R.; Lan, L.; Wang, J.; Xue, Z.; Jiang, L. Reliable manipulation of gas bubble size on superaerophilic cones in aqueous media. ACS Appl. Mater. Interfaces 2018, 10, 5099–5106. [Google Scholar] [CrossRef]
- Zhizhchenko, A.Y.; Shabalina, A.V.; Aljulaih, A.A.; Gurbatov, S.O.; Kuchmizhak, A.A.; Iwamori, S.; Kulinich, S.A. Stability of octadecyltrimethoxysilane-based coatings on aluminum alloy surface. Materials 2022, 15, 1804. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Han, K.; Lu, C. Release of sulfur dioxide and nitric oxide and characteristic of coal combustion under the effect of calcium based organic compounds. Chem. Eng. J. 2011, 168, 255–261. [Google Scholar] [CrossRef]
- Wei, Z.S.; Niu, H.J.; Ji, Y.F. Simultaneous removal of SO2 and NOx by microwave with potassium permanganate over zeolite. Fuel Process. Technol. 2009, 90, 324–329. [Google Scholar] [CrossRef]
- Hutson, N.D.; Krzyzynska, R.; Srivastava, R.K. Simultaneous removal of SO2, NOX, and Hg from coal flue gas using a NaClO2-enhanced wet scrubber. Ind. Eng. Chem. Res. 2008, 47, 5825–5831. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Sheng, C.; Zhang, Y.; Zhao, L. Simultaneous removal of NO and SO2 from coal-fired flue gas by UV/H2O2 advanced oxidation process. Chem. Eng. J. 2010, 162, 1006–1011. [Google Scholar] [CrossRef]
- Su, C.; Ran, X.; Hu, J.; Shao, C. Photocatalytic process of simultaneous desulfurization and denitrification of flue gas by TiO2-polyacrylonitrile nanofibers. Environ. Sci. Technol. 2013, 47, 11562–11568. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Zhang, H.; Huang, W.; Mao, L.; Ke, Y.; Gao, M.; Yu, B. Highly responsive flexible strain sensor using polystyrene nanoparticle doped reduced graphene oxide for human health monitoring. Carbon 2018, 140, 286–295. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Pan, J.; Tang, A. Investigation on the removal of NO from SO2-containing simulated flue gas by an ultraviolet/fenton-like reaction. Energy Fuels 2012, 26, 5430–5436. [Google Scholar] [CrossRef]
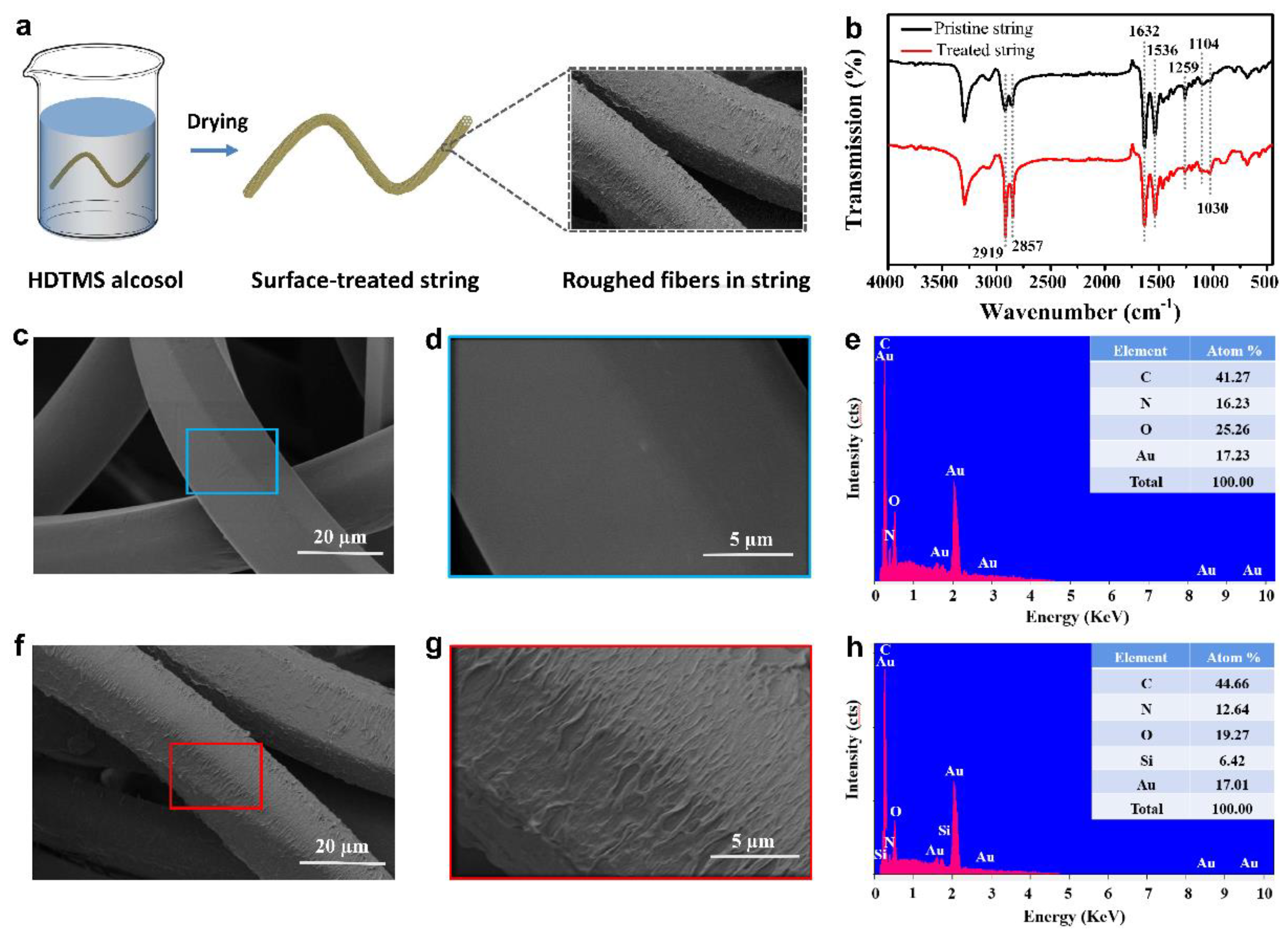
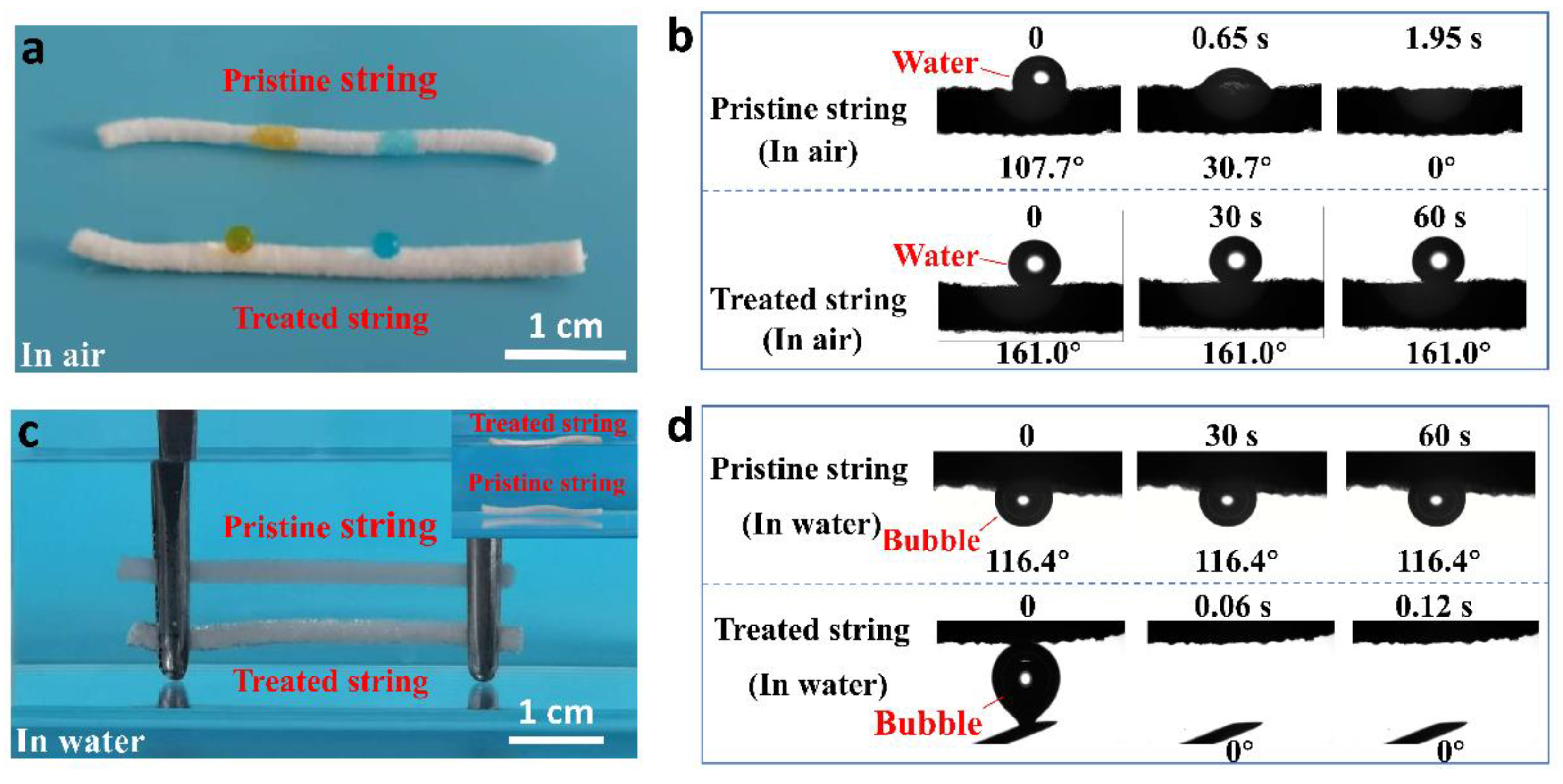
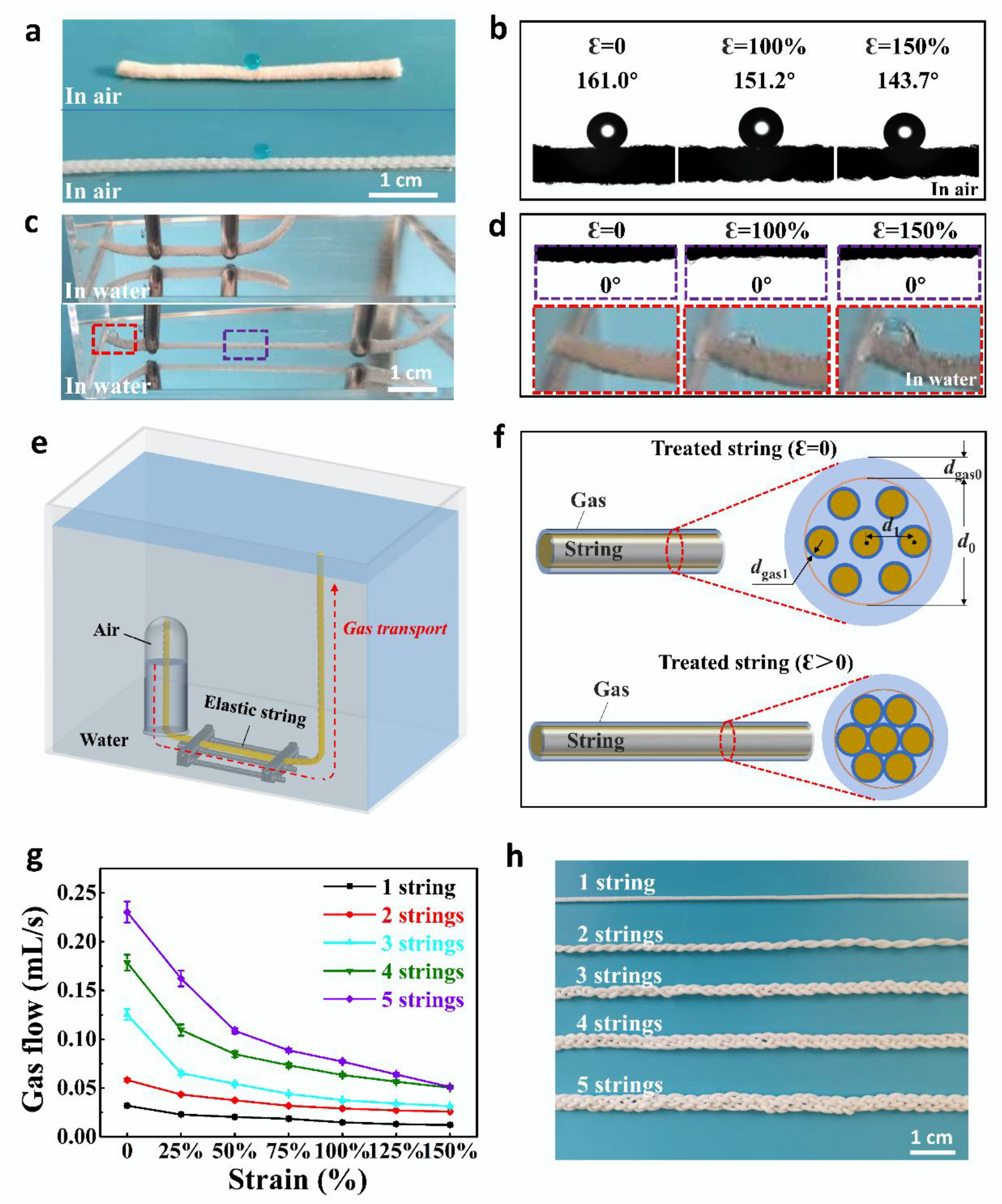
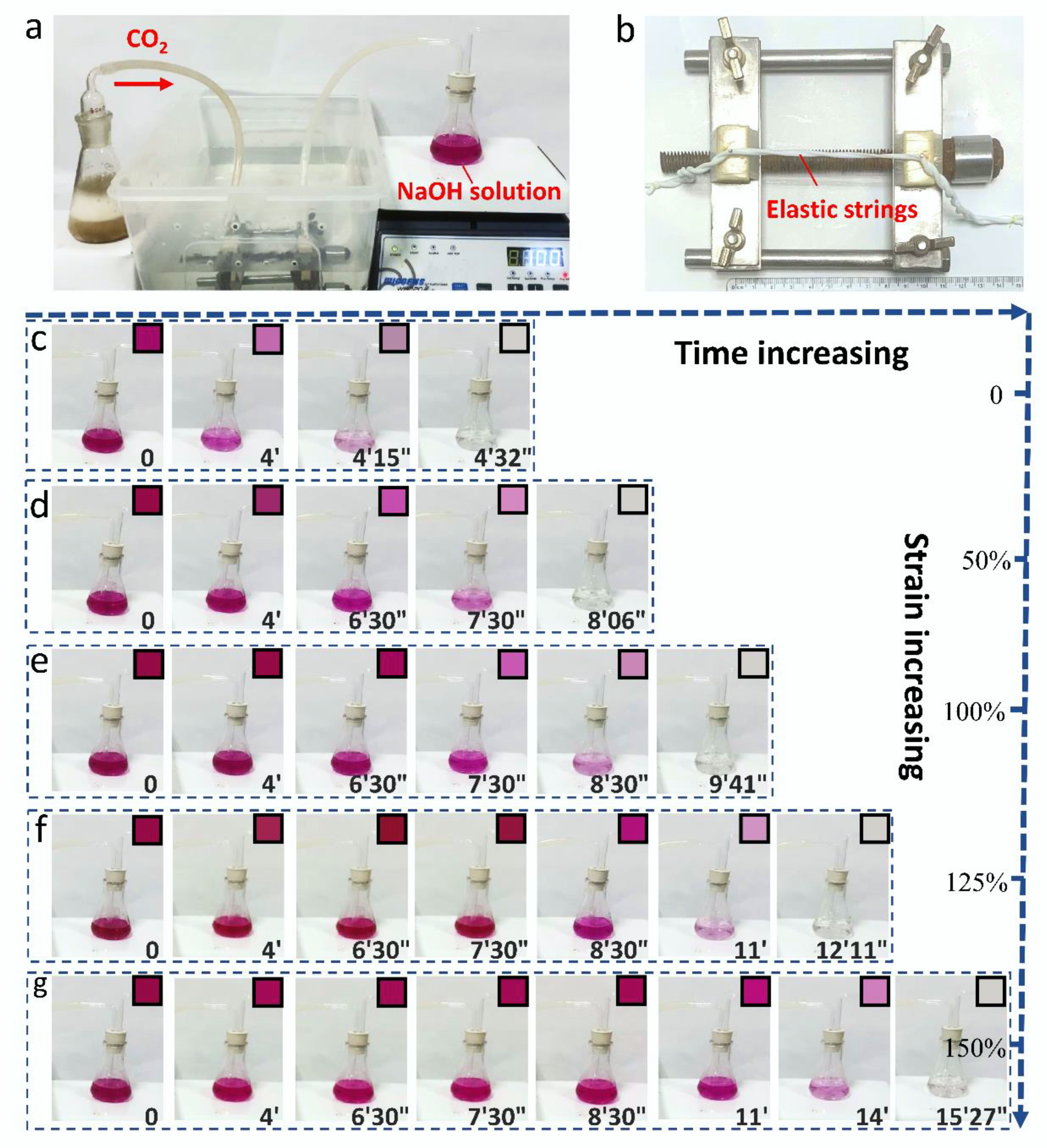
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Liu, M.; Li, X.; Sun, D.; Xia, Y.; Xiong, Z. Adjustable Underwater Gas Transportation Using Bioinspired Superhydrophobic Elastic String. Coatings 2022, 12, 638. https://doi.org/10.3390/coatings12050638
Sun Y, Liu M, Li X, Sun D, Xia Y, Xiong Z. Adjustable Underwater Gas Transportation Using Bioinspired Superhydrophobic Elastic String. Coatings. 2022; 12(5):638. https://doi.org/10.3390/coatings12050638
Chicago/Turabian StyleSun, Yaping, Meichen Liu, Xinlei Li, Deshuai Sun, Yanzhi Xia, and Zhong Xiong. 2022. "Adjustable Underwater Gas Transportation Using Bioinspired Superhydrophobic Elastic String" Coatings 12, no. 5: 638. https://doi.org/10.3390/coatings12050638
APA StyleSun, Y., Liu, M., Li, X., Sun, D., Xia, Y., & Xiong, Z. (2022). Adjustable Underwater Gas Transportation Using Bioinspired Superhydrophobic Elastic String. Coatings, 12(5), 638. https://doi.org/10.3390/coatings12050638







