Abstract
Cr-doped Co3O4 thin films were prepared by spray pyrolysis on soda-lima glass. The structure and morphology of the prepared samples were characterized by grazing incidence X-ray diffraction (GIXRD), Fourier-transform infrared spectroscopy (FTIR) and Scanning electron Microscopy (SEM). The results indicated the formation of a single cubic spinel phase with a crystallite size of about 6 nm. Different electronic transitions and estimations of the band gap structure were determined from the optical absorption spectra. The dependence of electrical resistivity on Cr content was investigated. It was found that the resistivity increased by increasing the Cr content. In addition, the effect of photon excitation on the electrical properties showed that the electrical resistivity decreased under visible illumination (λvis. = 532 nm) and increased under infrared illumination (λIR = 780 nm). The relative change in resistivity (sensitivity) under both light illuminations was enhanced by increasing the Cr content. Therefore, this work introduces a new application for Co3O4 material as a light detector.
1. Introduction
Thin film materials introduce unique physical and chemical properties in comparison to bulk ones. These properties arise mainly from quantum confinement effects and the increase of the surface atoms decreasing the particle size [1,2,3]. Transition metal oxide thin films have attracted physical properties, such as their chemical stability, tunning their electric and optical properties [4,5,6,7]. Cobalt oxide is known as a promising material due to its applications in many fields, such as CO gas sensors [8], solar absorbers at high temperatures [9], photocatalysts for dye degradation in water purification, and magnetic materials [10]. In the form of thin films, cobalt oxide is used as an electrode in super capacitor design and in the fabrication of electrochromic devices [11] as a result of changing its optical properties under an external electrical stimulus [12]. Enhancing the different properties of Co3O4 could be obtained by changing the method of preparation to control particle size and morphology [13] or by making substitutional doping with other transition elements [14,15]. The effect of doping on the different properties of Co3O4 arises from the ionic radius, electronic structure and the cation distribution of the dopant ions. Therefore, monitoring of the physical properties could be obtained by changing the type of dopant in addition to its concentration. Furthermore, the method of preparation for Co3O4 thin film has great effects on the magnetic, electric and optical properties of the prepared samples. These effects are attributed to the change in the crystallite size, morphology and texture of the prepared samples [10,11,12]. In particular, many techniques have been reported for the synthesis of Co3O4 thin films, such as chemical vapor deposition [13,14] and chemical spray pyrolysis [16]. Owing to their simplicity and inexpensiveness, chemical spray pyrolysis techniques have been used to prepare thin films.
Cr-doping has considerable effects on the different properties of Co3O4, especially on their optical absorption in the visible region [9]. In addition, the optical properties of Co3O4 could be changed under the effect of an external electrical signal [14].
In the present work, the change in the electrical properties under the effect of photon irradiation in both visible and near infrared regions at different concentrations of Cr doping will be investigated.
2. Materials and Methods
Chemical spray pyrolysis was used to deposit cobalt oxide and chromium-doped cobalt oxide thin films (CrxCo3−xO4) on properly cleaned soda lime glass substrates. High-purity Cr-nitrates (Cr(NO3)3·9H2O) (99.9%, LOBAChemie) and Co-nitrates (Co(NO3)2·6H2O) (98%, Merk, India) were dissolved in a solution of distilled water and ethanol at a ratio of 1:3, creating the precursor solution. A two-directional motorized micro nozzle with air as a carrier gas was used. The growth time was kept constant for all sprayed films (20 s) and the optimal substrate temperature for synthesizing CrxCo3−xO4 thin films of good quality was found to be 400 ± 10 °C. Furthermore, grazing incidence X-ray diffraction (GIXRD) (Philips X’Pert PRO MRD triple axis diffractometer (angular resolution 0.01°, precision 0.001°)) was used to obtain the cubic phase structure of the samples. The crystal structure of the CrxCo3−xO4 was refined using the Rietveld profile method, using MAUD software [17]. Fourier transform infrared spectroscopy (FTIR) was performed at room temperature in transmission mode (Shimadzu, Qatr-S single reflection ATR, Kyoto, Japan) in the range of 400–4000 cm−1. A scanning electron microscope (SEM) (Zeiss Field-emission SEM (FE-SEM), Jena, Germany) was used to determine the surface morphology. The optical properties of the films were measured using a UV-Vis optical spectroscopy (Jasco Ltd. V-670 UV-Vis-NIR, Heckmondwike, UK) spectrophotometer with a working range of 200–800 nm. In order to study the photo-resistance behavior of the investigated films, a two probe method with a KEITHLEY 6514 system electrometer was used in dark and under illumination of both infra-red and visible light sources.
3. Results
3.1. X-ray Analysis
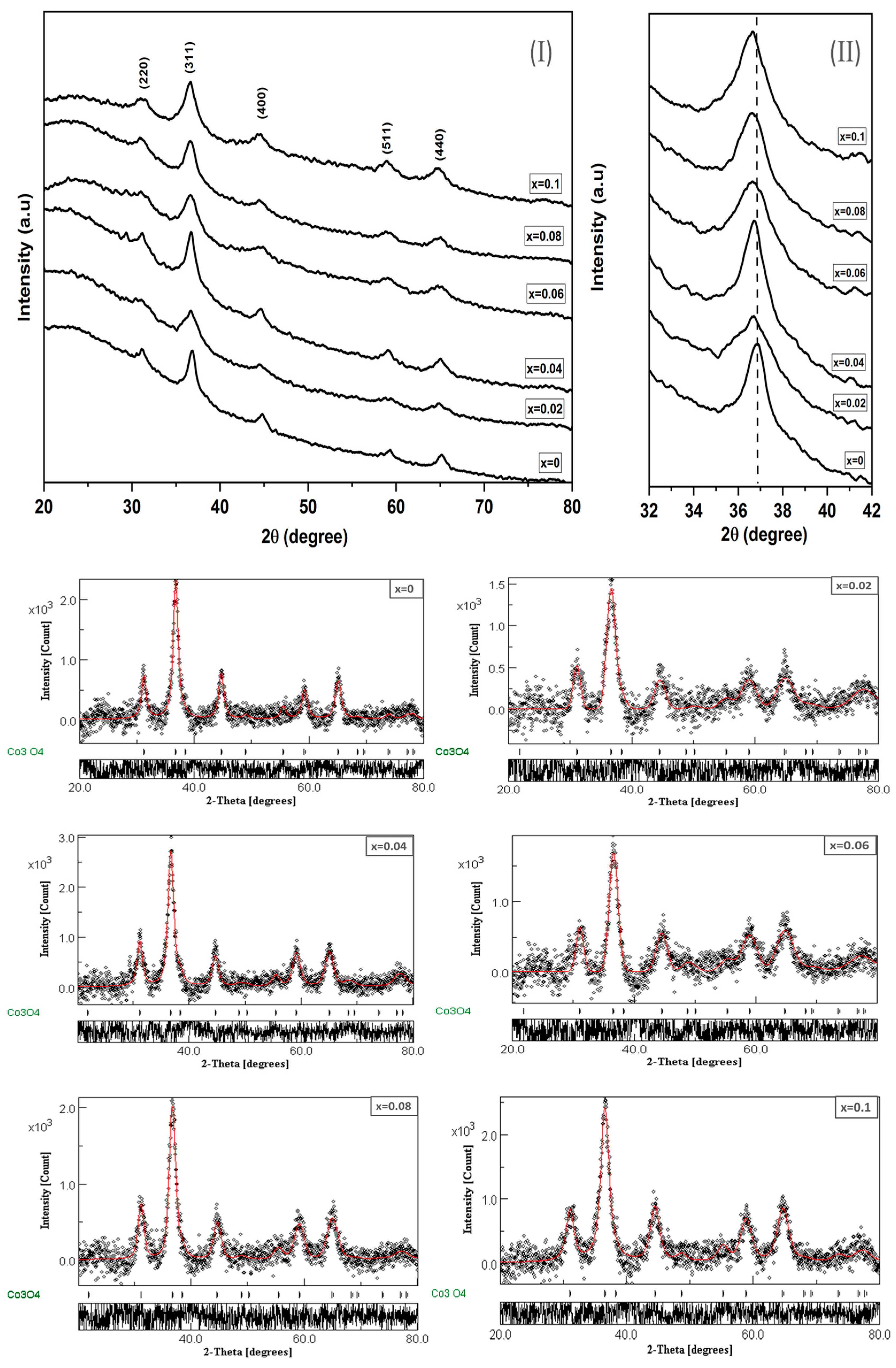
Figure 1I depicts the grazing incident X-ray diffractograms (GIXRD) of Co3O4 and Cr3+ doped Co3O4 thin film samples with varying concentrations (x = 0.02, 0.04, 0.06, 0.08 and 0.1). Consistent with ICSD card 28058 [18] and JCPDS No. 24-0326 [19], a single cubic structure phase of Co3O4 and CrxCo3−xO4 thin films, respectively, was obtained from diffraction peaks at (220), (311), (400), (511), and (440). GIXRD measurements show that the undoped and Cr3+ doped Co3O4 thin films form in a spinel structure system with a cubic lattice structure (space group: ) [20]. However, Figure 1II reveals that effective incorporation of Cr3+ dopant in the Co3O4 host matrix causes a shift of the prominent peak of the crystal planes (311) toward the lower diffraction angles, which in turn means a decrease in inter planar distances. This could be explained by the larger ionic radius of Cr3+ (0.63 Å) [10] compared to that of Co3+ (0.53 Å) [19]. This proves that the Cr3+ ion substitutes for the positions of Co3+ ions in the structure.
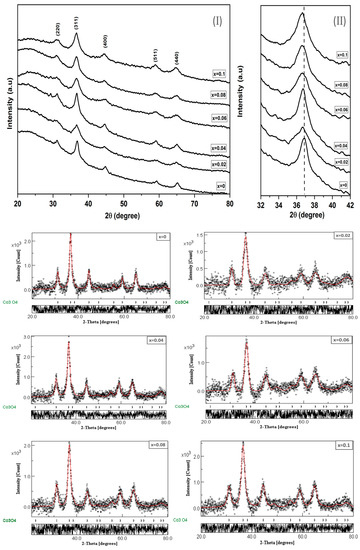
Figure 1.
(I) XRD patterns of Cr doped Co3O4 (II) the shifting of (311) peaks positions to lower 2θ values and Rietveld refinement profile for CrxCo3−xO4 thin films using MAUD program.
Furthermore, to examine the crystal structural phase and to obtain the lattice constants, all investigated samples were examined for Rietveld analysis using the MAUD software program [21], and the fitted profile results for all investigated thin films are shown in Figure 1. In addition, the refinement cycle was repeated until convergence with a goodness factor near one was attained [10].
The lattice constant (a) of all prepared CrxCo3−xO4 thin films was evaluated from the fitted data and is listed in Table 1. The theoretical density Dx-ray is calculated from:
where Z is the number of molecules per unit cell (Z = 8), M is the molecular weight of the undoped and doped spinel cobalt, V is the volume of the unit cell and NA is Avogadro’s number. Table 1 shows that the Dx-ray decreased with the addition of Cr3+ content. This can be attributed to the substitution of Cr3+ ions (51.996 amu) by heavier Co2+ ions (58.933 amu) [10]. Moreover, the micro strain and the average crystallite size are determined from the Williamson–Hall (W-H) equation [22]. Table 1 depicts the change in strain (ε) and average crystallite size (R) with the Cr3+ content. It is obvious that the crystallite size decreases with increasing the Cr3+ content while the strain has inverse behavior. This could be understood in terms of the difference in the ionic radii of Co3+ and Cr3+, where the presence of Cr3+, which has a larger ionic radius, causes more stress on the unit cell. This reflects the increase of the macrostrain with increasing the Cr content. Moreover, the increase in the macrostrain increases the lattice imperfection, which prevents crystal growth and thus decreases the crystallite size. For more confirmation about the defects in the prepared films, the dislocation density (δ), the length of dislocation lines per crystal lattice unit volume, is calculated from equation [23] δ = , where R is the crystallite size.

Table 1.
Lattice parameter (a), cell volume (V), crystallite size (R), strain (ε) and dislocation density (δ), for CrxCo3−xO4 thin film samples.
It is clear from Table 1 that the obtained dislocation density is significantly increased with increasing Cr3+ content which infers that dopant atoms occupy substitutional positions in the matrix. The variation in δ and strain in the investigated films confirms the presence of defects in the lattice structure due to the contribution of the Cr3+ ions that cause changes in different physical properties.
3.2. FTIR Analysis
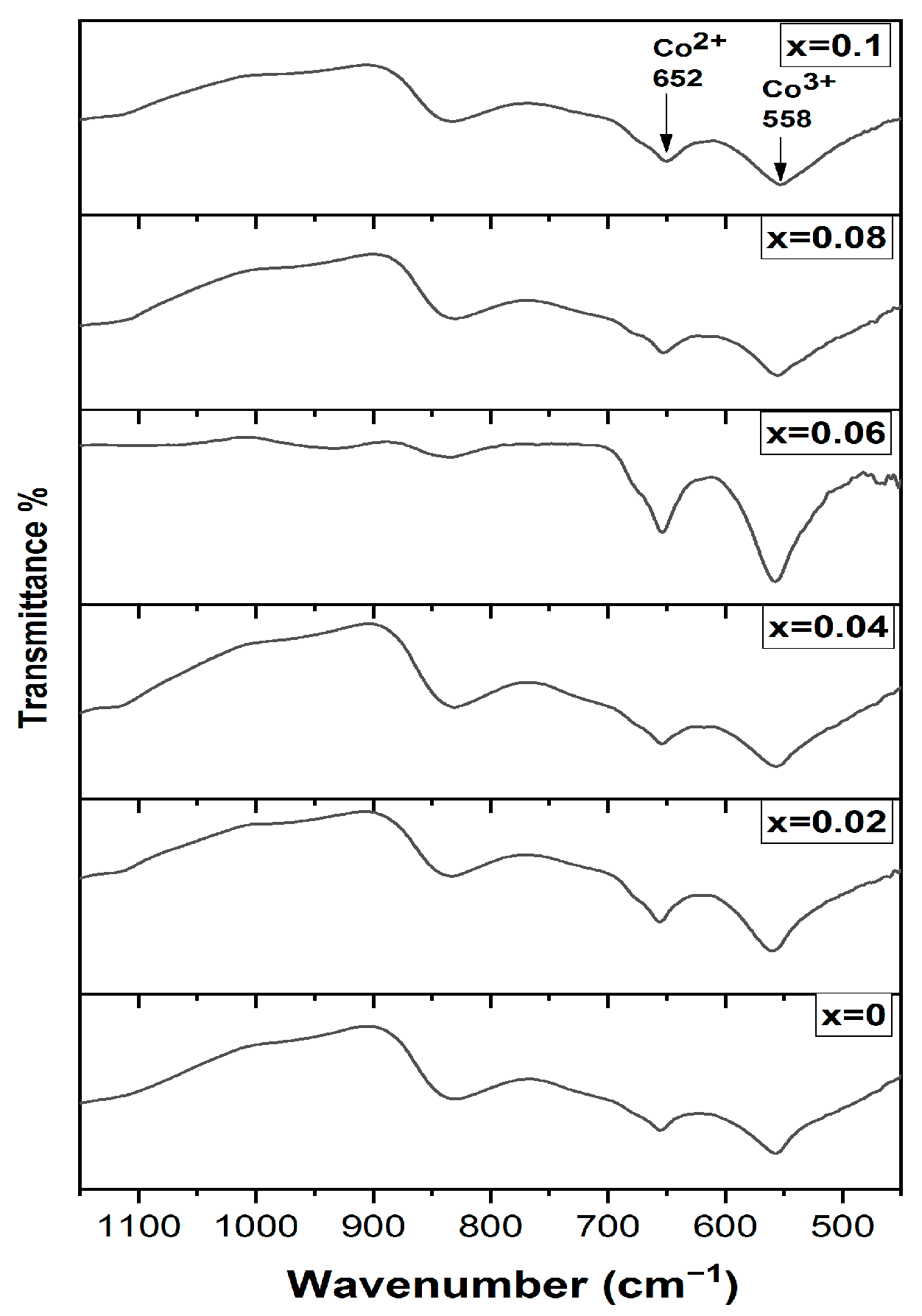
To study the cation distribution, the FTIR spectra for all investigated samples are shown in Figure 2. It is obvious that there are two main bands around 558 cm−1 and 650 cm−1. The first one is assigned to the LO vibrational mode of Co2+ ions in the tetrahedral environment of oxygen ions (Co2+–O) while the band above 650 cm−1 corresponds to the longitudinal optical vibrations (LO) of Co3+ in the octahedral site of the spinel, which is related to (Co3+–O) bond [24,25]. Furthermore, the dopant samples exhibit a small shift in the octahedral site band to a higher value, which confirms the presence of the Cr3+ ions in the octahedral site. The appearance of the band around 834 cm−1 is attributed to one of the vibrational modes of the nitrate group of the precursors [26]. The study of the FTIR spectrum confirms that Co2+ ions occupy the tetrahedral sites (A site) while, both Co3+ and Cr3+ ions occupy the octahedral sites (B site), i.e., the proposed cation distribution is [Co2+]A.O.(Co3+(1−x)Cr3+x)B.
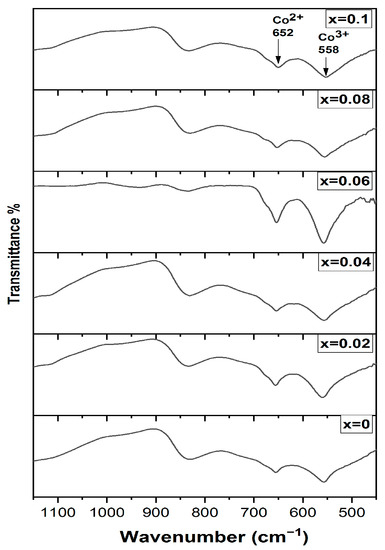
Figure 2.
FTIR spectra of Cr doped Co3O4 thin films.
3.3. Scanning Electron Microscope (SEM)
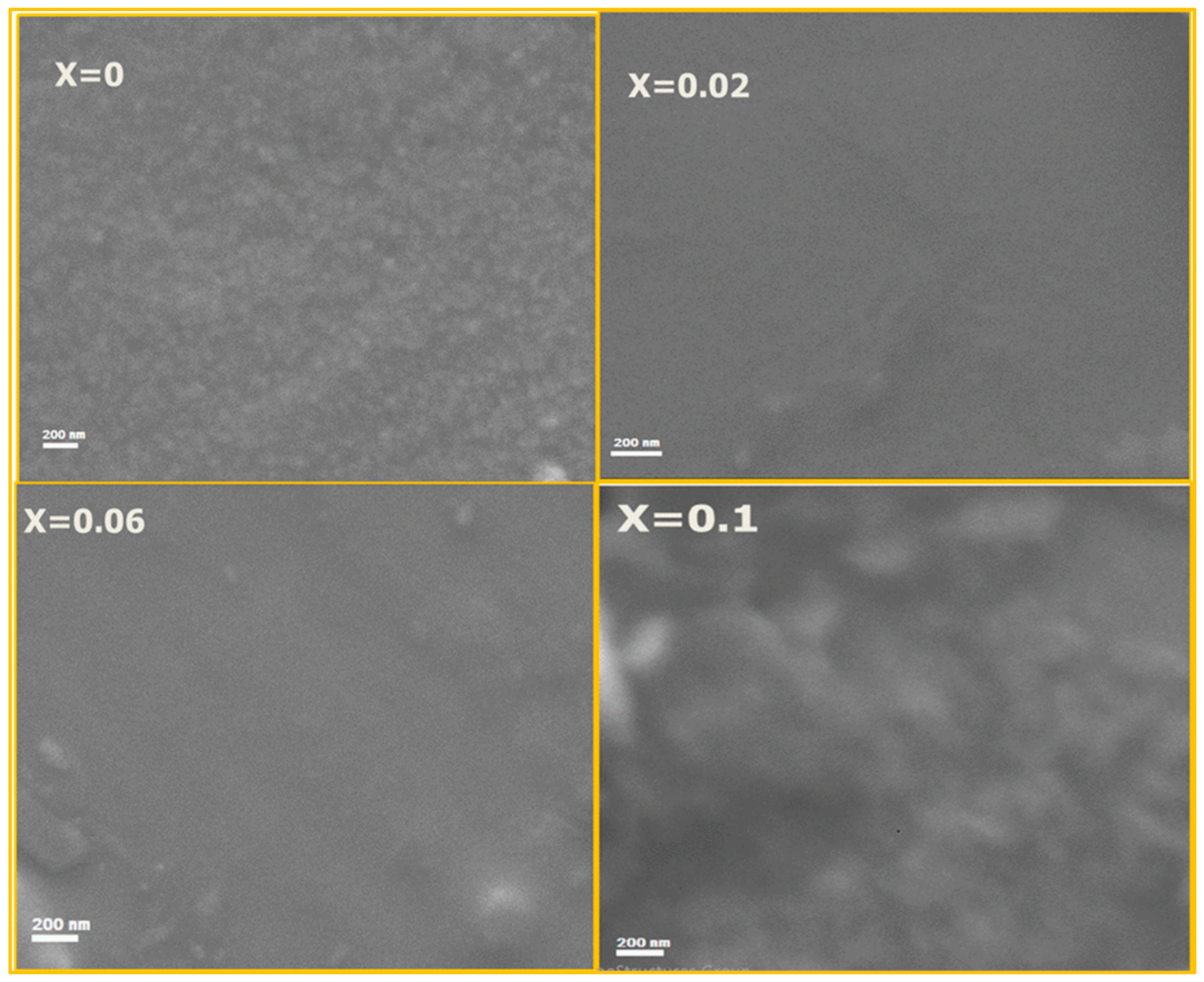
Figure 3 depicts the scanning electron microscope images for x = 0.0, 0.02, 0.06 and 0.1 of CrxCo3−xO4 thin films. One can see that the surface image of the samples is homogeneous and smooth. It is also observed that the un-doped cobalt spinel film shows a granular surface, which disappeared in Cr3+ doped films due to the decrease of the crystallite size, as mentioned in the X-ray study.
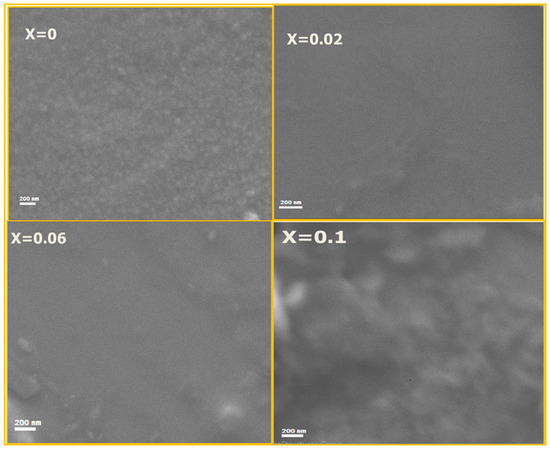
Figure 3.
SEM images of x = 0.00, 0.02, 0.04 and 0.1 of Cr doped Co3O4 thin films.
3.4. Optical Properties and Band Gap
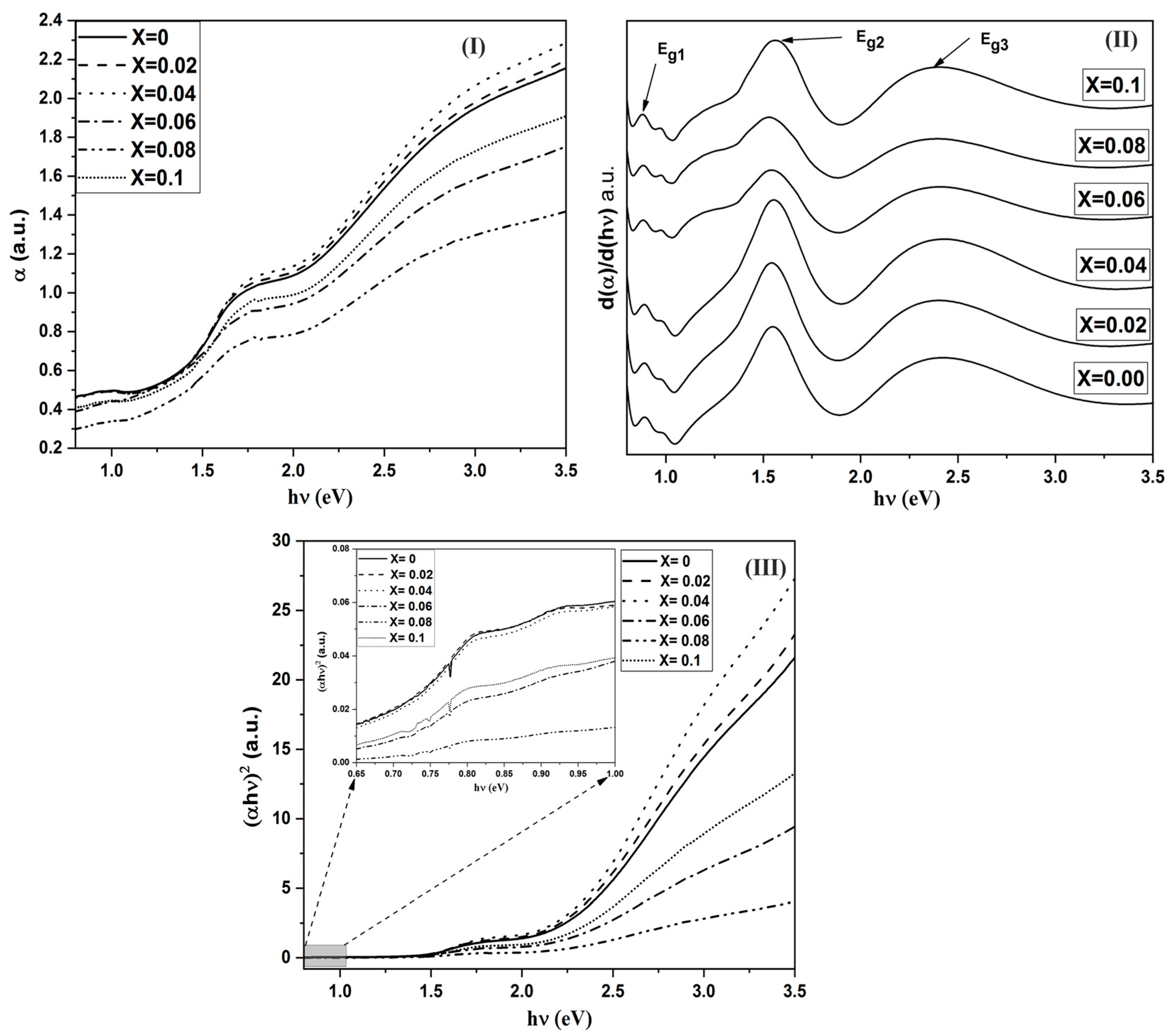
It is known that optical properties strongly depend on the electronic structure, which in turn is affected by the dopant elements. The absorption spectra for the investigated samples, in the wavelength range from 350 to 2700 nm, are given in Figure 4I. It is obvious that for pure Co3O4 (x = 0.0), there are three main absorption bands. To obtain the precise locations of these bands, a relation between the derivative of the absorption coefficient with respect to the photon energy is shown in Figure 4II. The first sharp transition is at 0.77 eV. This band is attributed to the charge transfer transition between d(2g) Co3+ in the octahedral site to d(t2) Co2+ in the tetrahedral site. This mechanism is called the internal oxidation process. The 2nd band is located at 1.56 eV. This band originates from the charge transfer transition between P(O−2) and d(eg Co3+). Finally, the 3rd transition at 2.38 eV is obtained because of the charge transfer P(O−2) to d(t2 Co2+) [27,28,29,30]. Furthermore, there is a small transition beside Eg1 band (around 0.9 eV) and the intensity of this band decreases by increasing Cr content. This transition is attributed to the excitation of the small hopping polaron formed between Co3+ and Co2+ [31]. Moreover, the width of the 2nd band (Eg2) increases from the red side of the band by increasing Cr3+ content. The effects of Cr doping on the positions of the absorption bands are listed in Table 2. One can observe that the positions of the three bands have no considerable variations with increasing Cr content (x). The only observed change is the effect of Cr3+ on the width of the 2nd band transition. From the above observations, one may conclude that the presence of Cr3+ ions in the B-site causes the presence of new states in the d(eg) level of Co3+. As the presence of Cr3+ ions in the octahedral site impedes the internal oxidation between Co3+ and Co2+, the intensity of the absorption of the corresponding band relative to the undoped sample decreases.
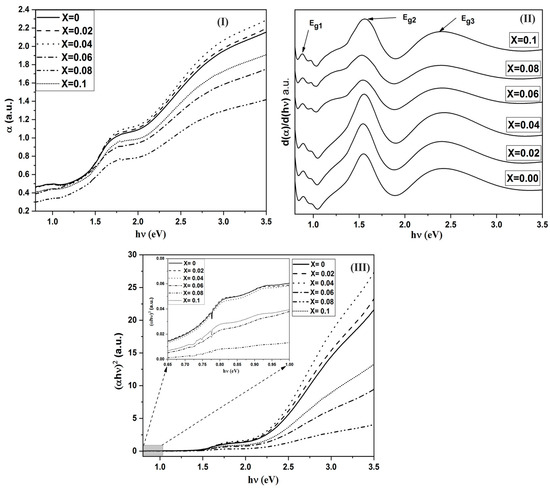
Figure 4.
(I) the absorption coefficient against the photon energy for all films of CrxCo3−xO, (II) the derivative of α against the photon energy for CrxCo3−xO4 samples and (III) the variation of (αhν)2 against the photon energy for all films of CrxCo3−xO4.

Table 2.
The energy band gaps (Eg1, Eg2 and Eg3) obtained from Tauc and derivative relations, hopping lengths and sub-sites radii for CrxCo3−xO4 thin film samples.
To obtain the type of the three band transitions, the Tauc & Davis–Mott relation is used, where according to this relation [32,33]:
αhν = A(hν − Eg)n
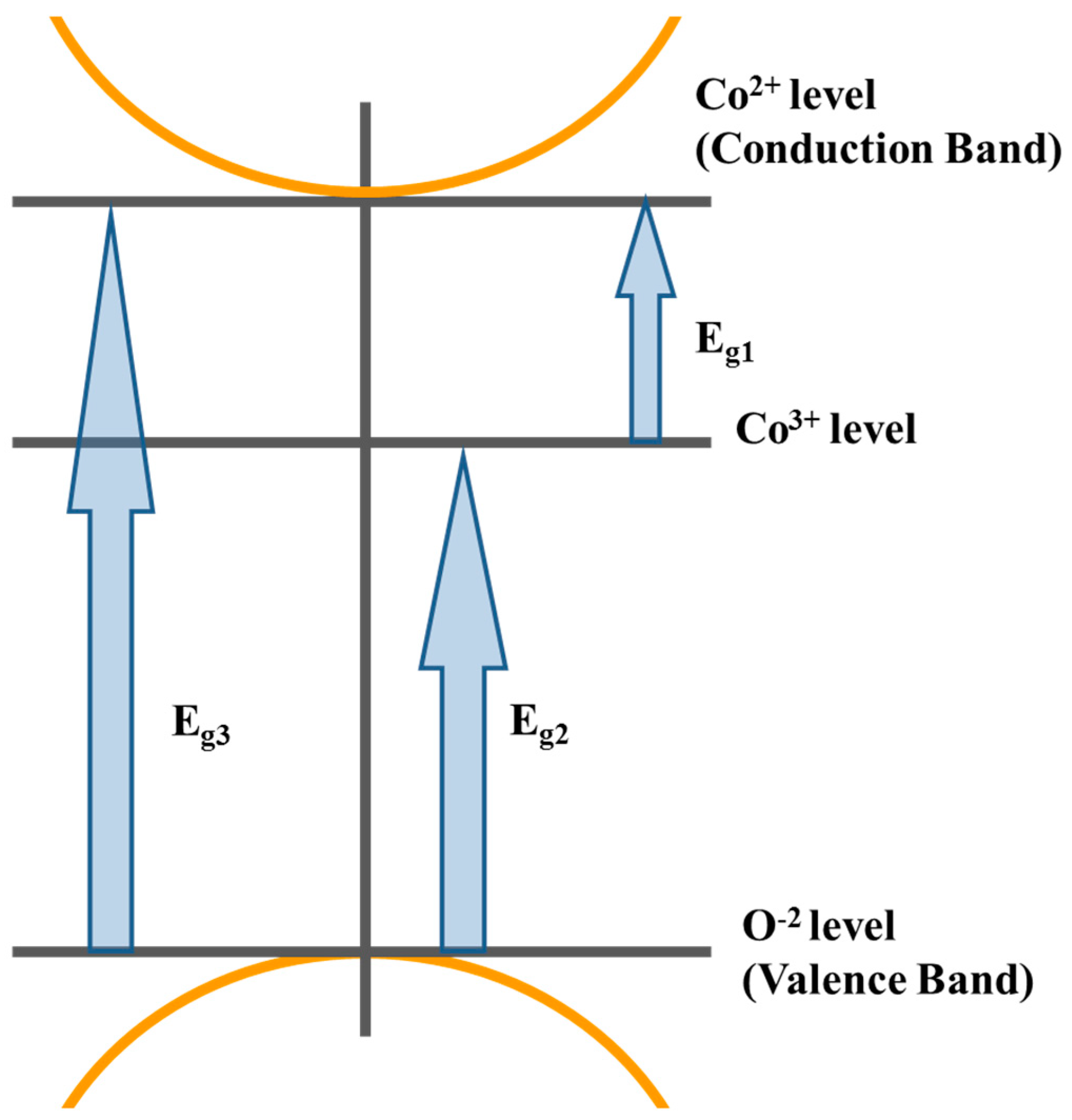
The value of n determines the type of electronic transition or optical transition. If n = 1/2 then the transition is a direct allowed transition, n = 2 indirect allowed transition, n = 3/2 direct forbidden transition, and n = 3 is the indirect forbidden energy [34]. The plot of (αhν)2 vs. the photon energy (hν), as shown in Figure 4III, exhibiting acute absorption boundary and Eg was calculated by fitting the linear part of each region. By comparing the values of the energy gaps obtained from Tauc’s relation and the differentiation method, there is a good match between the two methods. The difference arises from the linear fitting of Tauc’s method. Therefore, we may assume that the derivative method is more accurate. Hence, we can conclude that the electronic transitions belong to the direct allowed type. Furthermore, the sum of Eg1 and Eg2 is almost equal to Eg3. This means that the d(eg) level of Co3+ lies in the energy gap between the valance band of P(O−2) and the conduction band of d(t2) of Co2+ in tetrahedral sites. The proposed energy band diagram is shown in Figure 5.
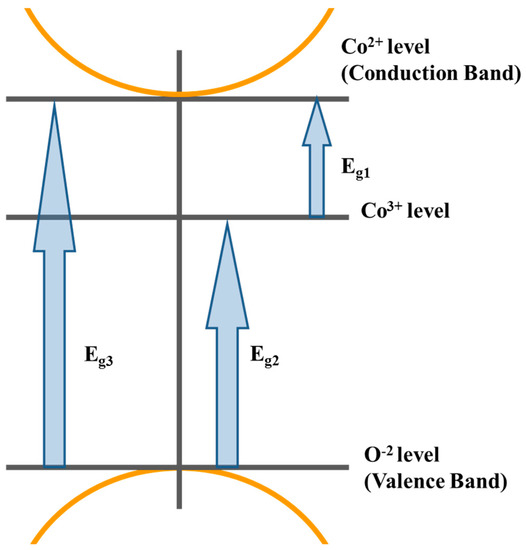
Figure 5.
The proposed schematic diagram for the optical energy bands of spinel cobalt oxide.
3.5. Photo-Resistance Measurements
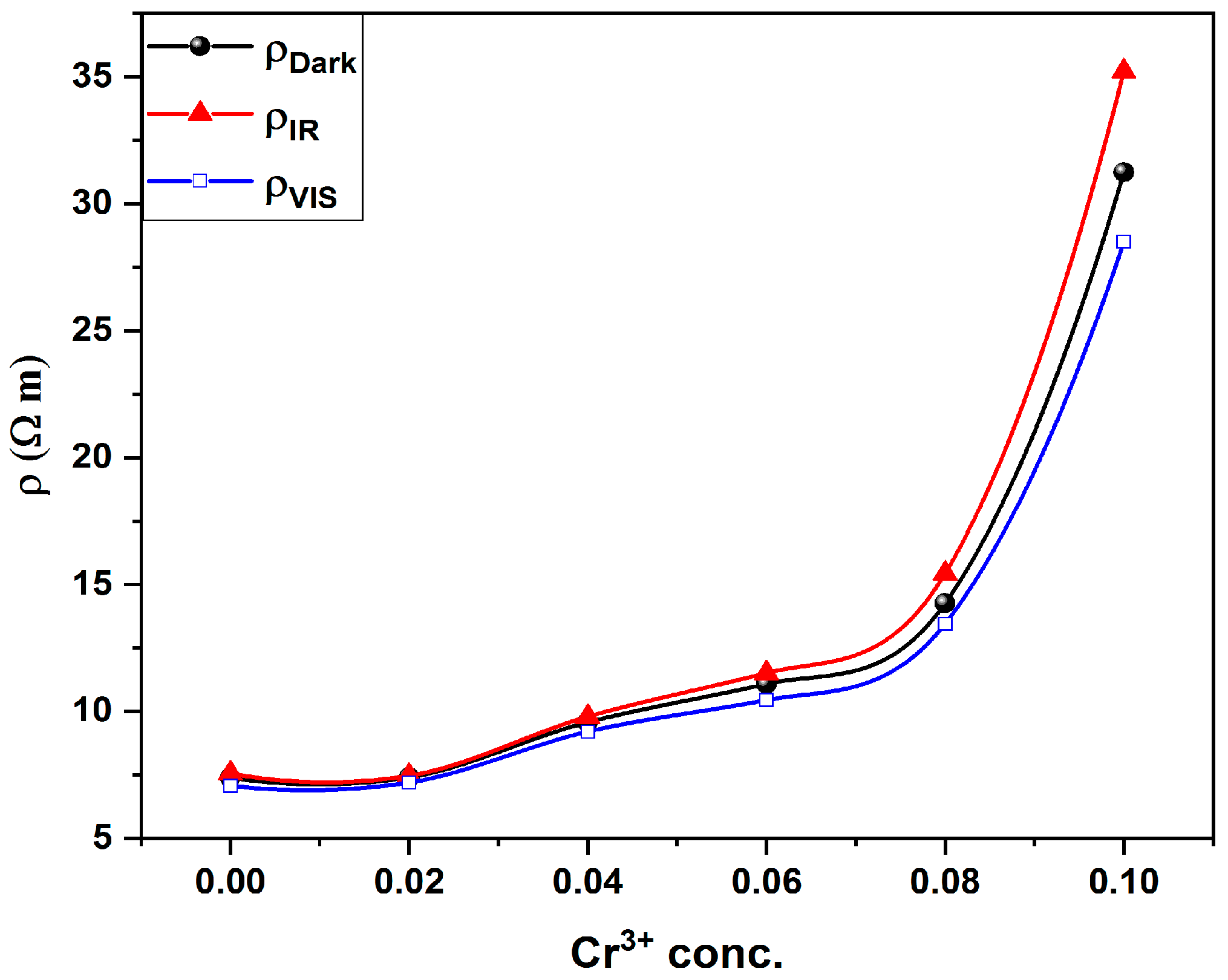
The dependence of room temperature resistivity (dark measurement) on Cr content is shown in Figure 6. It is obvious that, as the Cr content increases, the resistivity increases. This could be explained in light of two factors:
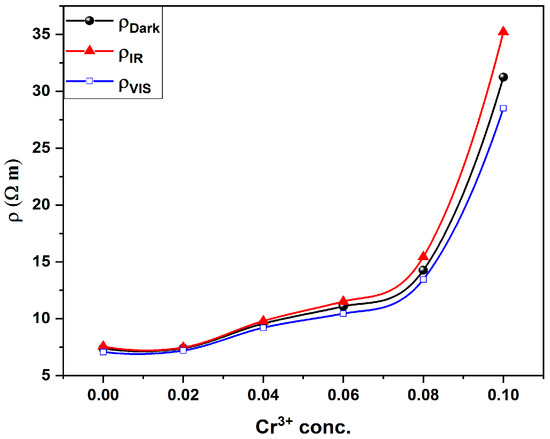
Figure 6.
Resistivity of (dark, IR and Vis) against Cr3+ concentration for all investigated thin films.
- The conduction mechanism in Co3O4 is due to the formation of a small polaron hopping between Co3+ and Co2+. As the Co3+ in the octahedral site is substituted by Cr3+ then the amount of Co3+ decreases and so the hopping between Co3+ and Co2+ decreases and subsequently, the resistivity increases.
- As the ionic radius of Cr3+ is greater than the radius Co3+ then the rate of hopping will be lower because of the large distance between the ions and this in turn will increase the resistivity.
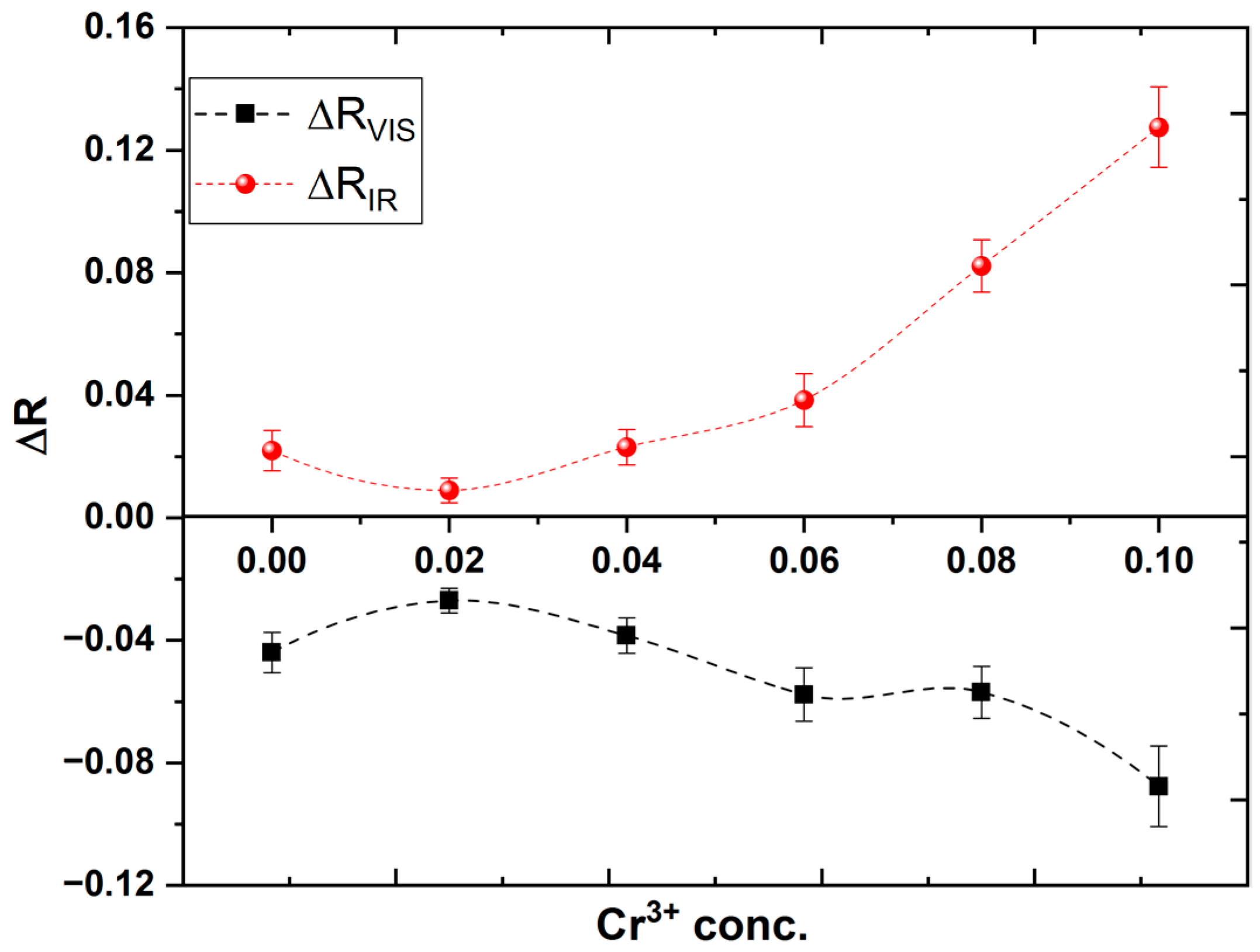
To study the effect of the electronic transition on the electrical resistivity of the investigated samples, two excitation wavelengths are used. One of them matches the Eg2 transition (1.56 eV or λir = 780 nm) and the other for Eg3 transition (2.38 eV or λvis = 532 nm). LED’s at the proper wavelengths are used as a source of excitation. Figure 6 shows the variation in electric resistivity under the illumination of both excitation wavelengths. It is valuable to mention that the resistivity of the samples increases under illumination by λir while it decreases under illumination by λvis. Additionally, as shown in Figure 7, the relative change in resistivity increases with increasing Cr content (x) for both wavelengths.
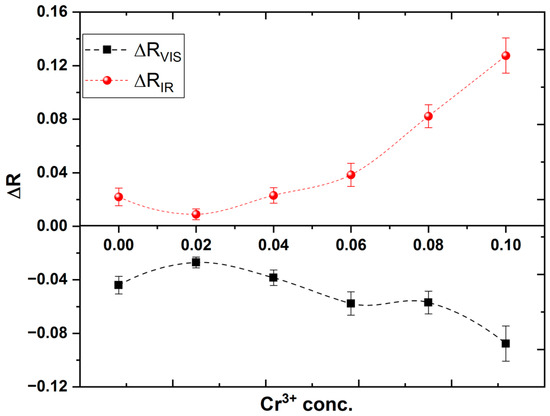
Figure 7.
Relative change in resistivity against Cr3+ concentration for all investigated thin films.
The decrease in resistivity under the illumination of λvis. could be understood in terms of the generation of electron-hole pairs in the valance and conduction bands, as mentioned in the 3rd band transition from the absorption spectrum. While λir could transfer the electrons from the valance band to the level of d(eg) of Co3+ in the octahedral site, which lies in the energy gap. The trapping of the exciting electrons in the d(eg) level will change the valance state of some Co3+ ions into Co2+ which in turn decreases the hopping between Co3+ in octahedral sites and Co2+ in the tetrahedral sites, which results in the decrease of the resistivity under the illumination of this wavelength.
4. Conclusions
A single cubic spinel phase of Cr-doped Co3O4 thin films has been prepared by the spray pyrolysis technique. From the optical absorption spectrum, the main three electronic transitions are attributed to the transition between Co3+ to Co2+, the transition from the valance band to Co3+ and the 3rd one from the transition from the valance to the conduction band. The electrical resistivity increases by increasing the Cr content, which is explained in terms of the increase of the lattice parameter and the decrease of Co content in the samples. The electrical resistivity was found to decrease under visible illumination (λvis. = 532 nm), while it increased under infrared illumination (λIR = 780 nm). Sensitivity under light illumination was enhanced by increasing the Cr content. This study introduces the samples as photocatalyst materials under visible illumination and as photodetectors for the near IR region.
Author Contributions
Conceptualization, A.M.F. and H.M.E.-S.; Data curation, A.M.F., H.E., M.S. and H.M.E.-S.; Formal analysis, A.M.F. and H.M.E.-S.; Funding acquisition, M.S. and H.M.E.-S.; Investigation, A.M.F. and H.M.E.-S.; Methodology, A.M.F. and H.M.E.-S.; Resources, M.S.; Validation, A.M.F., H.E., M.S. and H.M.E.-S.; Visualization, A.M.F., H.E., M.S. and H.M.E.-S.; Writing—original draft, A.M.F., M.S. and H.M.E.-S.; Writing—review & editing, A.M.F., H.E., M.S. and H.M.E.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Deanship Scientific Research at Umm Al-Qura University, grant number (22UQU4320621DSR01).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at Umm Al-Qura University for supporting this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siegel, R.W. Cluster-Assembled Nanophase Materials. Annu. Rev. Mater. Sci. 1991, 21, 559–578. [Google Scholar] [CrossRef]
- Kodama, R.H. Magnetic Nanoparticles. J. Magn. Magn. Mater. 1999, 200, 359–372. [Google Scholar] [CrossRef]
- Gleiter, H.H. Gleiter (1989). Prog. Mater. Sci 1989, 33, 323. [Google Scholar]
- Cheng, C.-S.; Serizawa, M.; Sakata, H.; Hirayama, T. Electrical Conductivity of Co3O4 Films Prepared by Chemical Vapour Deposition. Mater. Chem. Phys. 1998, 53, 225–230. [Google Scholar] [CrossRef]
- Raveau, B. Transition Metal Oxides: Promising Functional Materials. J. Eur. Ceram. Soc. 2005, 25, 1965–1969. [Google Scholar] [CrossRef]
- Makhlouf, S.A.; Bakr, Z.H.; Al-Attar, H.; Moustafa, M.S. Structural, Morphological and Electrical Properties of Cr2O3 Nanoparticles. Mater. Sci. Eng. B 2013, 178, 337–343. [Google Scholar] [CrossRef]
- Fouad, O.A.; El-Shall, M.S. Microwave Irradiation Assisted Growth of Cu, Ni, Co Metals And/or Oxides Nanoclusters and Their Catalytic Performance. Nano Brief. Rep. Rev. 2012, 7, 1250034. [Google Scholar] [CrossRef]
- Yamaura, H.; Tamaki, J.; Moriya, K.; Miura, N.; Yamazoe, N. Highly Selective CO Sensor Using Indium Oxide Doubly Promoted by Cobalt Oxide and Gold. J. Electrochem. Soc. 1997, 144, L158. [Google Scholar] [CrossRef]
- Chidambaram, K.; Malhotra, L.K.; Chopra, K.L. Spray-Pyrolysed Cobalt Black as a High Temperature Selective Absorber. Thin Solid Films 1982, 87, 365–371. [Google Scholar] [CrossRef]
- Faramawy, A.M.; Mattei, G.; Scian, C.; Elsayed, H.; Ismail, M.I.M. Cr3+substituted Aluminum Cobalt Ferrite Nanoparticles: Influence of Cation Distribution on Structural and Magnetic Properties. Phys. Scr. 2021, 96, 125849. [Google Scholar] [CrossRef]
- Maruyama, T.; Arai, S. Electrochromic Properties of Cobalt Oxide Thin Films Prepared by Chemical Vapor Deposition. J. Electrochem. Soc. 1996, 143, 1383. [Google Scholar] [CrossRef]
- Granqvist, C.G. Handbook of Inorganic Electrochromic Materials; Elsevier: Amsterdam, The Netherlands, 1995; ISBN 008053290X. [Google Scholar]
- Makhlouf, S.A.; Bakr, Z.H.; Aly, K.I.; Moustafa, M.S. Structural, Electrical and Optical Properties of Co3O4 Nanoparticles. Superlattices Microstruct. 2013, 64, 107–117. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Wang, T.; Zhou, J.; Zhao, Y. Synthesis and Magnetic Properties of Nanoporous Co3O4 Nanoflowers. Microporous Mesoporous Mater. 2008, 114, 257–261. [Google Scholar] [CrossRef]
- Reena, R.S.; Aslinjensipriya, A.; Jose, M.; Das, S.J. Investigation on Structural, Optical and Electrical Nature of Pure and Cr-Incorporated Cobalt Oxide Nanoparticles Prepared via Co-Precipitation Method for Photocatalytic Activity of Methylene Blue Dye. J. Mater. Sci. Mater. Electron. 2020, 31, 22057–22074. [Google Scholar] [CrossRef]
- Singh, R.N.; Koenig, J.; Poillerat, G.; Chartier, P. Electrochemical Studies on Protective Thin Co3O4 and NiCo2O4 Films Prepared on Titanium by Spray Pyrolysis for Oxygen Evolution. J. Electrochem. Soc. 1990, 137, 1408. [Google Scholar] [CrossRef]
- Matthies, S.; Lutterotti, L.; Wenk, H.R. Advances in Texture Analysis from Diffraction Spectra. J. Appl. Crystallogr. 1997, 30, 31–42. [Google Scholar] [CrossRef]
- Kalinic, B.; Girardi, L.; Ragonese, P.; Faramawy, A.; Mattei, G.; Frasconi, M.; Baretta, R.; Bogialli, S.; Roverso, M.; Rizzi, G.A.; et al. Diffusion-Driven Formation of Co3O4 Nanopetals Layers for Photoelectrochemical Degradation of Organophosphate Pesticides. Appl. Surf. Sci. 2022, 596, 153552. [Google Scholar] [CrossRef]
- Liao, W.M.; Zhao, P.P.; Cen, B.H.; Jia, A.P.; Lu, J.Q.; Luo, M.F. Co–Cr–O Mixed Oxides for Low–temperature Total Oxidation of Propane: Structural Effects, Kinetics, and Spectroscopic Investigation. Chin. J. Catal. 2020, 41, 442–453. [Google Scholar] [CrossRef]
- Roth, W.L. The Magnetic Structure of Co3O4. J. Phys. Chem. Solids 1964, 25, 1–10. [Google Scholar] [CrossRef]
- Lutterotti, L.; Gualtieri, A.; Aldrighetti, S. Rietveld Refinement Using Debye-Scherrer Film Techniques. Mater. Sci. Forum 1996, 228–231, 29–34. [Google Scholar] [CrossRef]
- Williamson, G.; Hall, W. X-ray Line Broadening from Filed Aluminium and Wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Amer, M.A.; Meaz, T.M.; Attalah, S.S.; Ghoneim, A.I. Annealing Effect on Structural Phase Transition of as-Synthesized Mg0.1Sr0.1Mn0.8Fe2O4 Nanoparticles. J. Alloys Compd. 2016, 654, 45–55. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Raja, A.; Devarajan, P.A. Structural Elucidation and Spectral Characterizations of Co3O4 Nanoflakes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 114, 586–591. [Google Scholar] [CrossRef]
- Tang, C.W.; Wang, C.B.; Chien, S.H. Characterization of Cobalt Oxides Studied by FT-IR, Raman, TPR and TG-MS. Thermochim. Acta 2008, 473, 68–73. [Google Scholar] [CrossRef]
- Roy, M.; Ghosh, S.; Naskar, M.K. Solvothermal Synthesis of Cr2O3 Nanocubes via Template-Free Route. Mater. Chem. Phys. 2015, 159, 101–106. [Google Scholar] [CrossRef]
- Hitkari, G.; Sandhya, S.; Gajanan, P.; Shrivash, M.K.; Deepak, K. Synthesis of Chromium Doped Cobalt Oxide (Cr: Co3O4) Nanoparticles by Co-Precipitation Method and Enhanced Photocatalytic Properties in the Visible Region. J. Mater. Sci. Eng 2018, 7, 2169–2222. [Google Scholar]
- Pal, J.; Chauhan, P. Study of Physical Properties of Cobalt Oxide (Co3O4) Nanocrystals. Mater. Charact. 2010, 61, 575–579. [Google Scholar] [CrossRef]
- Jacobs, J.-P.; Maltha, A.; Reintjes, J.G.H.; Drimal, J.; Ponec, V.; Brongersma, H.H. The Surface of Catalytically Active Spinels. J. Catal. 1994, 147, 294–300. [Google Scholar] [CrossRef]
- Shelef, M.; Wheeler, M.A.Z.; Yao, H.C. Ion Scattering Spectra from Spinel Surfaces. Surf. Sci. 1975, 47, 697–703. [Google Scholar] [CrossRef]
- Smart, T.; Pham, T.A.; Ping, Y.; Ogitsu, T. The Nature of Band Gap of Co3O4-a Revisit from First-Principles. APS March Meet. Abstr. 2019, 2019, C11.006. [Google Scholar]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi (b) 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Mott, N.F.; Davis, E.A. Conduction in Non-Crystalline Systems V. Conductivity, Optical Absorption and Photoconductivity in Amorphous Semiconductors. Philos. Mag. 1970, 22, 903–922. [Google Scholar]
- Louardi, A.; Rmili, A.; Ouachtari, F.; Bouaoud, A.; Elidrissi, B.; Erguig, H. Characterization of Cobalt Oxide Thin Films Prepared by a Facile Spray Pyrolysis Technique Using Perfume Atomizer. J. Alloys Compd. 2011, 509, 9183–9189. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).