A Review on Superhydrophobic Surface with Anti-Icing Properties in Overhead Transmission Lines
Abstract
1. Introduction
- Weather conditions: Extreme cold temperatures and heavy snowfall can make it difficult to access transmission lines and remove ice buildup. Additionally, high winds cause ice to form on transmission lines more quickly and in thicker layers.
- Line accessibility: Some transmission lines are located in remote or difficult-to-reach areas, making it challenging to perform regular maintenance and de-icing operations.
- Power outages: If ice buildup causes transmission lines to sag or come into contact with other lines or structures, it can lead to power outages. These outages can be difficult to repair, especially in remote or difficult-to-reach areas.
- Line damage: Ice buildup can cause damage to transmission lines and associated equipment, such as insulators and conductors. This damage can be costly to repair and can lead to further power outages.
- Safety concerns: De-icing transmission lines can be a dangerous task, as it often involves working at heights and in inclement weather conditions. Additionally, the use of de-icing chemicals and the operation of de-icing equipment can pose safety risks to workers.
- Environmental concerns: The use of de-icing chemicals can have negative impacts on the environment.
| Preparation Techniques | Modification Agents | Micro-Topography | CA (°) | Anti-Icing Effect | Ref. |
|---|---|---|---|---|---|
| Depositing | RTV SR modified with stearic acid | Micro nanoscale structured roughness surfaces | 150° at −10 °C | Few ice growth spots at a working temperature of −6 °C | [34] |
| Depositing | Silica nanoparticles into polyamide mesh | Controllable meshes with partially embedded nanoparticles | 153.1° | An ice adhesion strength of ∼1.9 kPa and a delayed freezing time of ∼1048 s | [35] |
| Depositing | MWCNTs mixed with FAS | Hierarchical structure and partially embedded structure | 162.5° | Completely melted with 120 s | [36] |
| Depositing | Doping PVC particles into a silicone matrix | A soft and rigid integrated (SRI) coating | 120°~150° | The ice adhesion of 34.6 kPa when the iced length was 20 cm | [37] |
| Acid etching | Hydrochloric acid-etched surface with FAS | Micro nanoscale holes | 165° | 0.58 kPa at −6 °C | [38] |
| Spray-coating technique | Al2O3 particles doped in SR solution | Cluster-like structure | 163.4 | 65.4 ± 18 kPa | [39] |
| Spin-coating | Carbon-black, titania or ceria nanopowders in RTV-SR | Grooves between rough asperities | ∼150° | Freezing was delayed to ∼12–13 min | [40] |
| Deposition | OD, PF modified with alkylsilane compounds | Micro nanoscale surface | ~160° | Shear stress of ice detachment (71.5 ± 15 kPa) | [41] |
| Anodization and deposition | HMDSO deposited on anodized aluminum | Coral-like nanostructure | ~156° | Keep icephobic properties to 15 icing/de-icing ARF = 2.7 | [42] |
| Deposition | Silicone rubber and alumina nanoparticles | Spongy-like and flower-like structure | 155° | ARF = 1.17 | [43] |
| Spray | Nano CaCo3, silica particles into fluorosilicic and E-51 | Ring-like pits structure | 166.4° | 20% of surface area covered by glaze ice at −5 °C | [44] |
| Chemical etching | Modified with PFPE | Micro/nanostructures | 160° | Delay the freezing time of water droplets to 5100 s | [45] |
| Salt etching | Modified with silane | Micro/nanostructure | 161.9 | 53% of the surface remained unfrozen in glaze ice after 50 min | [46] |
| RF magnetron sputtering | Sputtered by Zn target, modified with FAS-17 | Nanorods structure | 160° | Frost formation was delayed for 140 min at −10 °C, no degradation after 30 cycles of frosting/defrosting process | [47] |
| RF plasma-sputtering and anodization | PTFE or Teflon sputtered on anodized Al alloys | Nest-like micro nanostructure | 165° | low variation in ice adhesion strength after 15 icing/de-icing cycles | [48] |
| Laser ablation | Modified with methoxy silane | Micro-channel pattern | 169.9° | Superhydrophobicity withstands thermal aging, thermal cycling, UV exposure, long-term ambient outdoor environment exposure and corona exposure | [49] |
| Laser ablation | Modified with FAS-13 and PDMS | Micro pattern | ~145° | Ice adhesion of 60 kPa | [50] |
| Anodization and SLIPS | FAS and silicone oil | Nano-pores structure | 150.3° | Low ice adhesion withstands 150 icing/de-icing cycle | [51] |
2. Anti-Icing Mechanism of the Superhydrophobic Surface
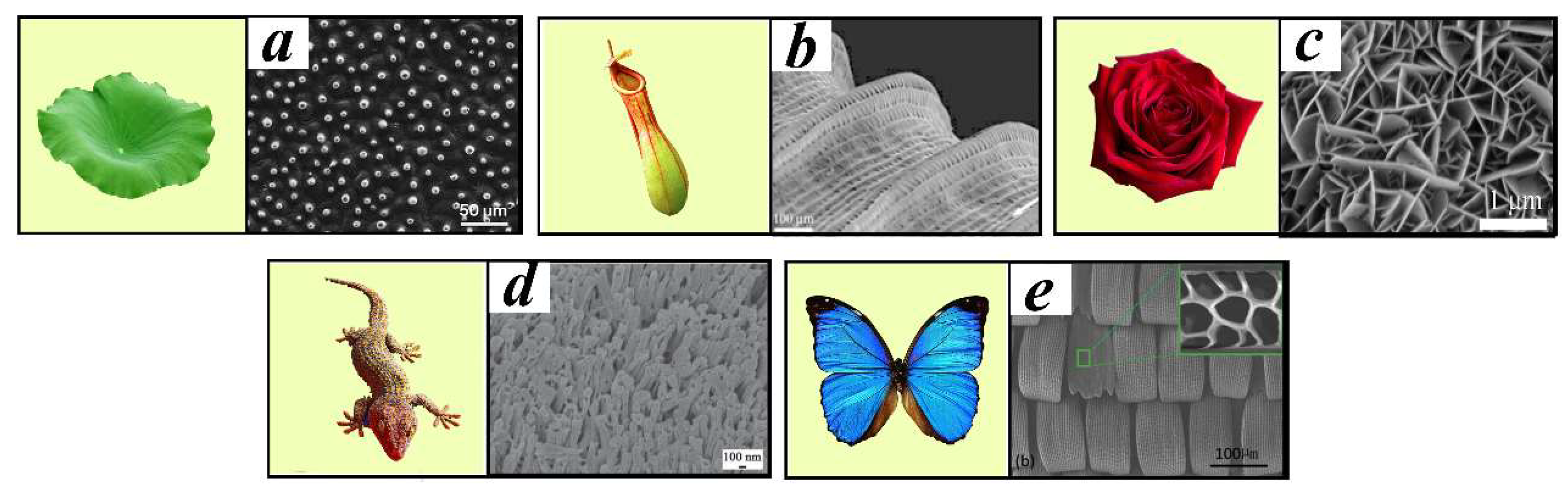
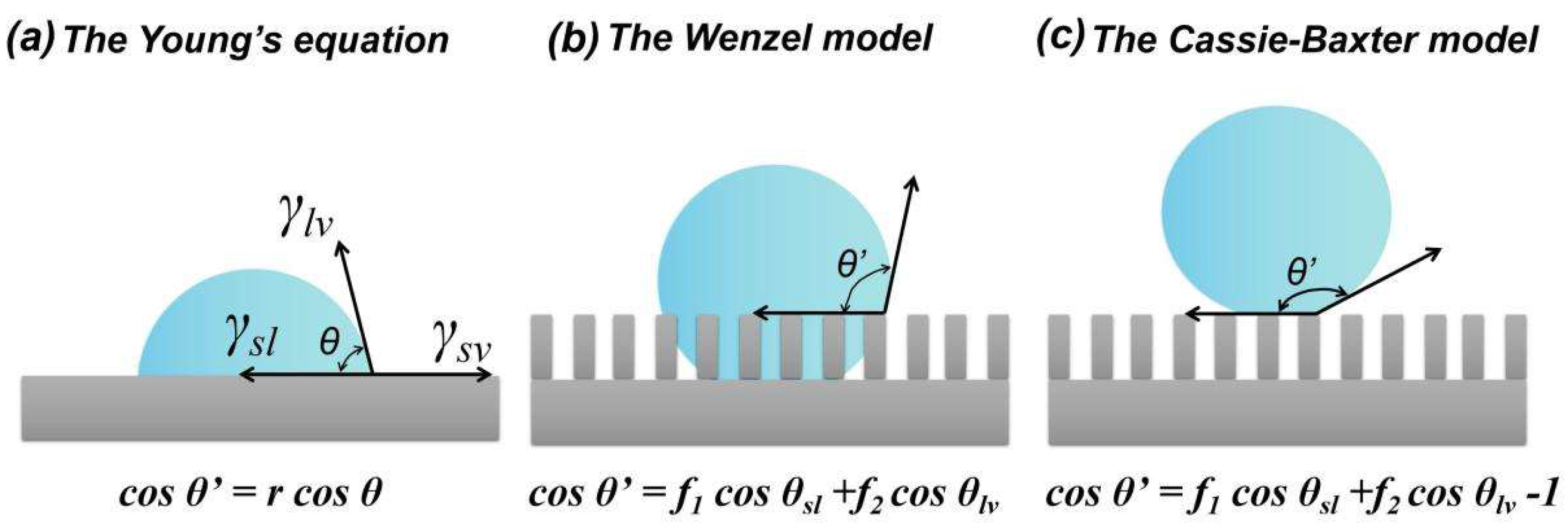
2.1. Wettability and Hydrophobicity
2.2. Icing and Icephobicity
3. Influencing Factors of Anti-Icing Properties
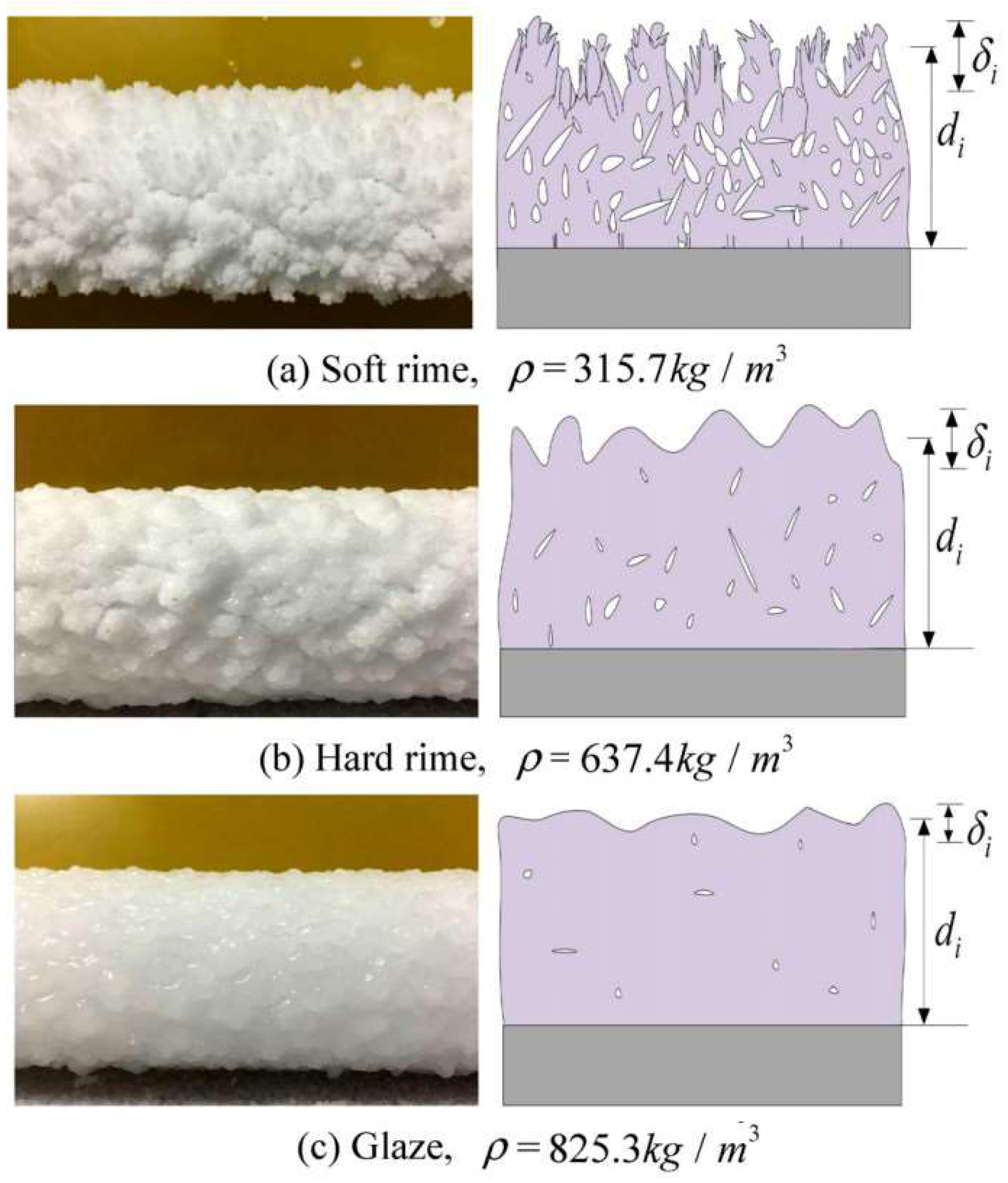
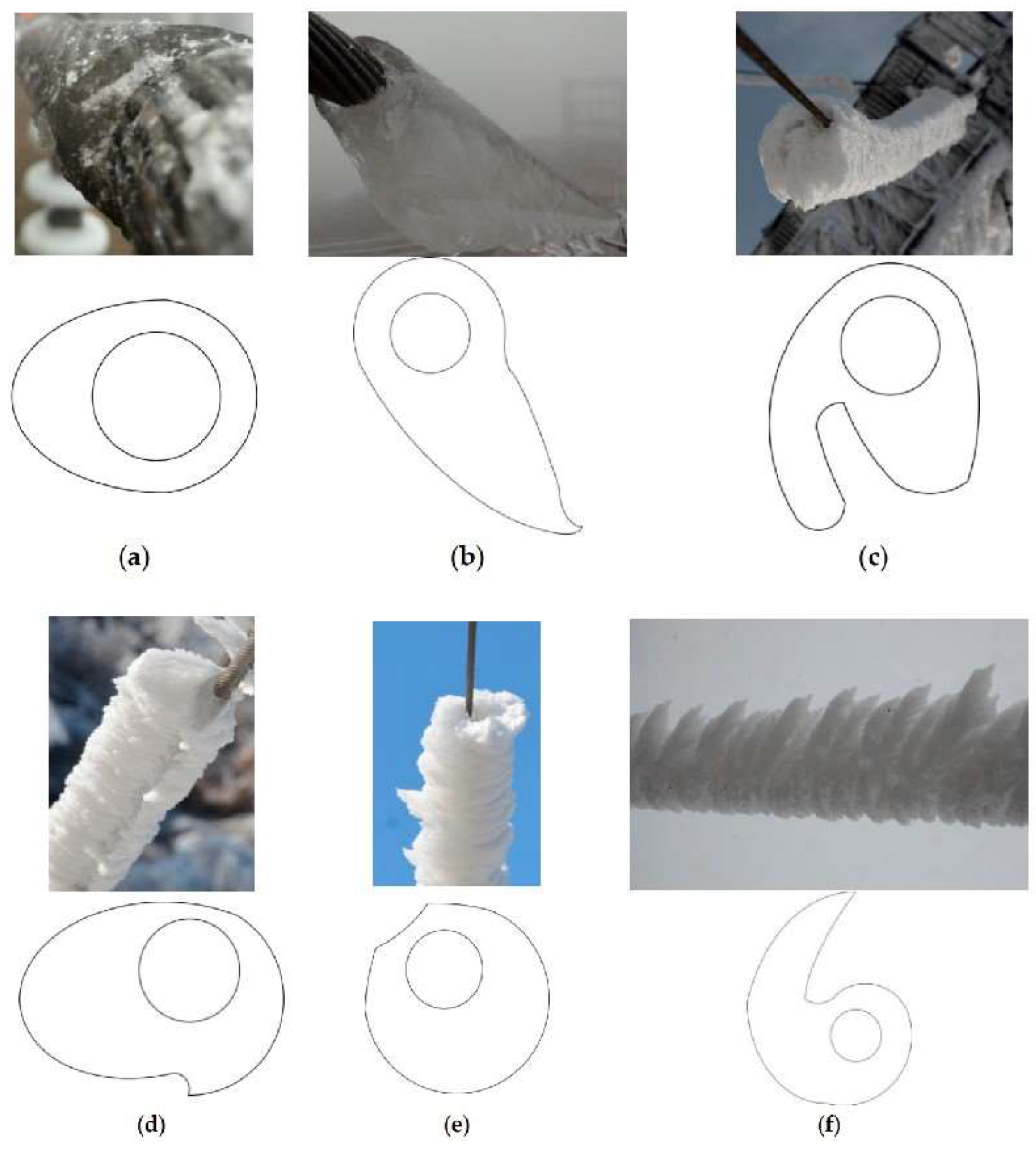
3.1. Icing Forms
3.2. External Factors
3.3. Internal Factors
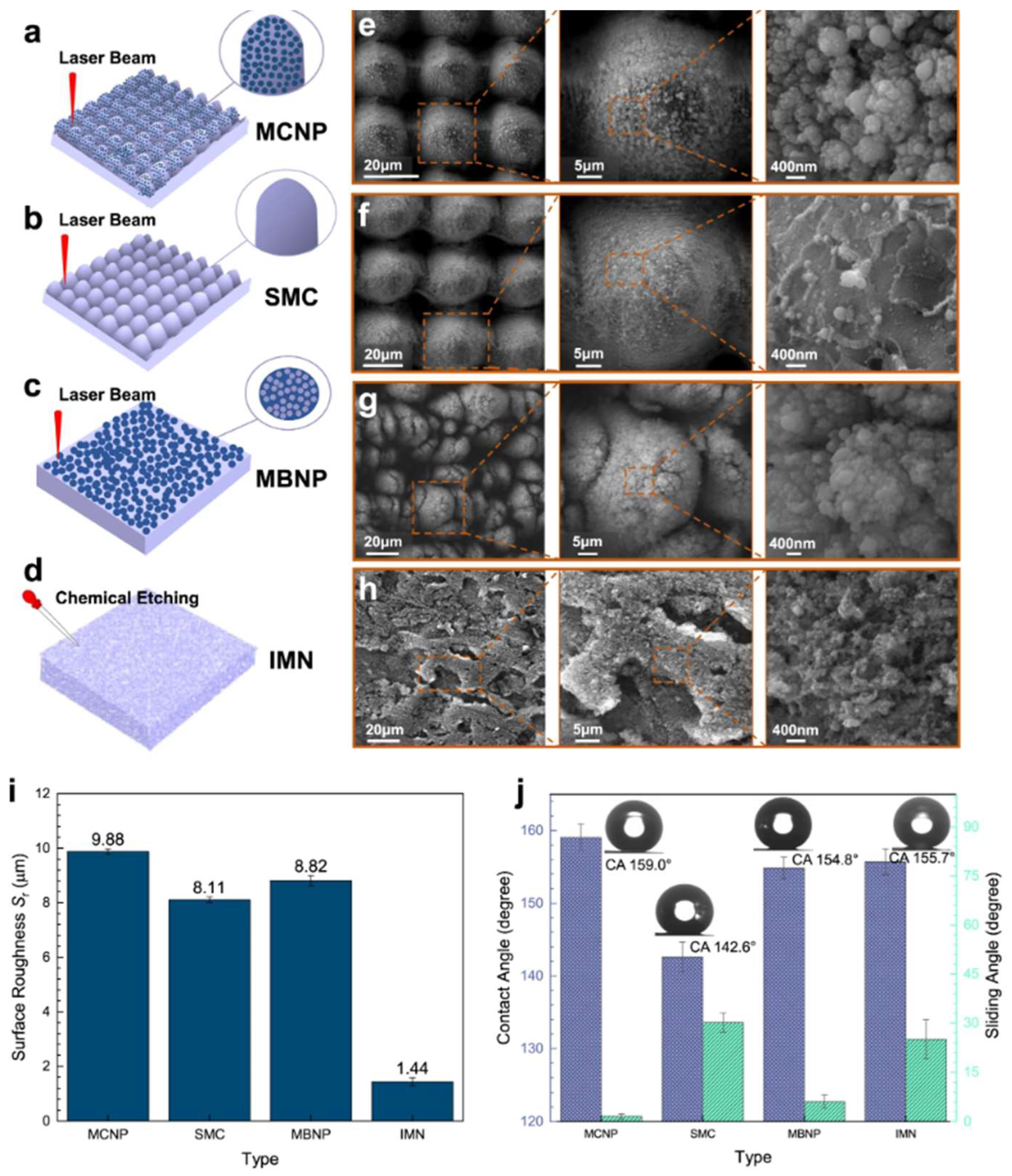
4. Preparation Techniques for Superhydrophobic Surfaces
4.1. Liquid-Phase Deposition
- Spraying: A superhydrophobic coating can be sprayed onto the surface of transmission lines using specialized equipment. This method is fast, efficient, and can provide a uniform coating.
- Painting: Superhydrophobic coatings can also be applied using a paint brush or roller. This method is more labor-intensive but can be useful for coating irregular or hard-to-reach surfaces.
- Dip coating: In this method, transmission lines are dipped into a solution containing the coating material and, after that, it is dried.
4.2. Chemical Etching
4.3. Anodization Technique
4.4. Laser Ablation Technique
4.5. New Progress in Preparation Techniques
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Designation Categories | Abbreviation |
| Room temperature vulcanized silicone rubber | RTV-SR |
| Silicone rubber | SR |
| Depositing | RF |
| Multiwall carbon nanotubes | MWCNTs |
| Polyvinyl chloride | PVC |
| Fluoroalkylsiane | FAS |
| Octadecyltrichlorosilane | OD |
| Perfluorooctyltrichlorosilane | PF |
| Fluoroalkylsioxane | FAS-17 |
| Hexamethyldimethyl ether | HMDSO |
| Aluminum | Al |
| polydimethylsiloxane | PDMS |
| Methoxypropyltrimethoxysilane | PFPE |
| Octadecyltrimethoxysilane | ODTMS |
| Hexadecyltrimethoxy silane | HDTMS |
| Octadecyl trichlorosilane | OTS |
| Polyurethane | PU |
| Graphene oxide | GO |
| Silane coupling agent | SCA |
| Contact angle | CA |
| Contact angle hysteresis | CAH |
| Median volume diameter of water droplets | MVD |
| Liquid water content in the air | LWC |
| Low-emissivity solar-assisted superhydrophobic | LE-SS |
| Adhesion reduction factor | ARF |
References
- Tao, B.; Cheng, L.; Wang, J.; Zhang, X.; Liao, R. A review on mechanism and application of functional coatings for overhead transmission lines. Front. Mater. 2022, 9, 1–21. [Google Scholar] [CrossRef]
- Villegas, M.; Zhang, Y.; Abu Jarad, N.; Soleymani, L.; Didar, T.F. Liquid-Infused Surfaces: A Review of Theory, Design, and Applications. ACS Nano 2019, 13, 8517–8536. [Google Scholar] [CrossRef]
- Celia, E.; Darmanin, T.; Taffin de Givenchy, E.; Amigoni, S.; Guittard, F. Recent advances in designing superhydrophobic surfaces. J. Colloid Interface Sci. 2013, 402, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, H.; Wang, G.; Liu, A. Recent Progress in Preparation and Anti-Icing Applications of Superhydrophobic Coatings. Coatings 2018, 8, 208. [Google Scholar] [CrossRef]
- Li, W.; Zhan, Y.; Yu, S. Applications of superhydrophobic coatings in anti-icing: Theory, mechanisms, impact factors, challenges and perspectives. Prog. Org. Coat. 2021, 152, 106117. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, S.; Shu, L.; Jiang, X.; Qiu, G.; Li, H. Influence of shed configuration on icing characteristics and flashover performance of 220 kV composite insulators. IEEE Trans. Dielect. Electr. Insul. 2016, 23, 319–330. [Google Scholar] [CrossRef]
- Savadjiev, K.; Farzaneh, M. Modeling of icing and ice shedding on overhead power lines based on statistical analysis of meteorological data. IEEE Trans. Power Deliv. 2004, 19, 715–721. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, R.; Zhang, Y.; Dong, X.; Huang, X. Review on flashover risk prediction method of iced insulator based on icing monitoring technology. Cold Reg. Sci. Technol. 2021, 185, 103252. [Google Scholar] [CrossRef]
- Wei, X.; Jia, Z.; Sun, Z.; Farzaneh, M.; Guan, Z. Effect of the Parameters of the Semiconductive Coating on the Anti-Icing Performance of the Insulators. IEEE Trans. Power Deliv. 2016, 31, 1413–1421. [Google Scholar] [CrossRef]
- Huang, W.; Hu, B.; Shahidehpour, M.; Sun, Y.; Sun, Q.; Yan, M.; Shao, C.; Xie, K. Preventive Scheduling for Reducing the Impact of Glaze Icing on Transmission Lines. IEEE Trans. Power Syst. 2022, 37, 1297–1310. [Google Scholar] [CrossRef]
- Lu, J.; Zeng, M.; Zeng, X.; Fang, Z.; Yuan, J. Analysis of Ice-Covering Characteristics of China Hunan Power Grid. IEEE Trans. Ind. Appl. 2015, 51, 1997–2002. [Google Scholar] [CrossRef]
- Zhou, F.; Zhu, J.; An, N.; Wang, C.; Liu, J.; Long, L. The anti-icing and deicing robot system for electricity transmission line based on external excitation resonant. IEEJ Trans. Electr. Electr. 2020, 15, 593–600. [Google Scholar] [CrossRef]
- Huang, Y.; Jiang, X.; Virk, M.S. Study of inverted T-shape insulator strings in icing conditions. Cold Reg. Sci. Technol. 2020, 173, 103021. [Google Scholar] [CrossRef]
- Jiang, X.; Fan, S.; Zhang, Z.; Sun, C.; Shu, L. Simulation and Experimental Investigation of DC Ice-Melting Process on an Iced Conductor. IEEE Trans. Power Deliv. 2010, 25, 919–929. [Google Scholar] [CrossRef]
- Jiang, X.; Meng, Z.; Zhang, Z.; Hu, J.; Lei, Y. DC ice-melting and temperature variation of optical fibre for ice-covered overhead ground wire. IET Gener. Transm. Dis. 2016, 10, 352–358. [Google Scholar] [CrossRef]
- Menini, R.; Farzaneh, M. Elaboration of Al2O3/PTFE icephobic coatings for protecting aluminum surfaces. Surf. Coat. Technol. 2009, 203, 1941–1946. [Google Scholar] [CrossRef]
- Nakayama, K.; Koyama, A.; Zhu, C.; Aoki, Y.; Habazaki, H. Rapid and Repeatable Self-Healing Superoleophobic Porous Aluminum Surface Using Infiltrated Liquid Healing Agent. Adv. Mater. Interfaces 2018, 5, 1800566. [Google Scholar] [CrossRef]
- Deng, L.; Wang, H.; Xun, Z.; Chen, R.; Ding, Y.; Liao, Q. Numerical Study of Droplet Impact Dynamics on the ACSR Cable for Studying Ice Adhesion and Accretion on Real Power Lines. J. Sci. Eng. Appl. 2020, 13, 21–27. [Google Scholar] [CrossRef]
- Fan, S.; Jiang, X.; Sun, C.; Zhang, Z.; Shu, L. Temperature characteristic of DC ice-melting conductor. Cold Reg. Sci. Technol. 2011, 65, 29–38. [Google Scholar] [CrossRef]
- Lu, J.; Guo, J.; Hu, J.; Yang, L.; Feng, T. Analysis of ice disasters on ultra-high-voltage direct-current transmission lines. Nat. Hazards 2017, 86, 203–217. [Google Scholar] [CrossRef]
- Wang, Z.; Zhu, Y.; Liu, X.; Zhao, Z.; Chen, J.; Jing, X.; Chen, H. Temperature self-regulating electrothermal pseudo-slippery surface for anti-icing. Chem. Eng. J. 2021, 422, 130110. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, Y.; Wang, D.; Liu, Z.; Liu, Y.; Pei, X.; Yu, B.; Zhou, F. Integration of Self-Lubrication and Near-Infrared Photothermogenesis for Excellent Anti-Icing/Deicing Performance. Adv. Funct. Mater. 2015, 25, 4237–4245. [Google Scholar] [CrossRef]
- Najibi, H.; Mohammadi, A.H.; Tohidi, B. Estimating the Hydrate Safety Margin in the Presence of Salt and/or Organic Inhibitor Using Freezing Point Depression Data of Aqueous Solutions. Ind. Eng. Chem. Res. 2006, 45, 4441–4446. [Google Scholar] [CrossRef]
- Golovin, K.; Dhyani, A.; Thouless, M.D.; Tuteja, A. Low–interfacial toughness materials for effective large-scale deicing. Science 2019, 364, 371–375. [Google Scholar] [CrossRef]
- Yan, X.; Li, J.; Li, L.; Huang, Z.; Hu, J.; Lu, M. An OH-PDMS-modified nano-silica/carbon hybrid coating for anti-icing of insulators part ii: Anti-icing performance. IEEE Trans. Dielect. Electr. Insul. 2016, 23, 2165–2173. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, Y.; Liu, Y.; Xu, R.; Liu, S.; Zhou, F. Robust Photothermal Coating Strategy for Efficient Ice Removal. ACS Appl. Mater. Interfaces 2020, 12, 46981–46990. [Google Scholar] [CrossRef]
- Jamil, M.I.; Zhan, X.; Chen, F.; Cheng, D.; Zhang, Q. Durable and Scalable Candle Soot Icephobic Coating with Nucleation and Fracture Mechanism. ACS Appl. Mater. Interfaces 2019, 11, 31532–31542. [Google Scholar] [CrossRef]
- Mohseni, M.; Recla, L.; Mora, J.; Gallego, P.G.; Agüero, A.; Golovin, K. Quasicrystalline Coatings Exhibit Durable Low Interfacial Toughness with Ice. ACS Appl. Mater. Interfaces 2021, 13, 36517–36526. [Google Scholar] [CrossRef]
- Shao, Y.; Zhao, J.; Fan, Y.; Wan, Z.; Lu, L.; Zhang, Z.; Ming, W.; Ren, L. Shape memory superhydrophobic surface with switchable transition between “Lotus Effect” to “Rose Petal Effect”. Chem. Eng. J. 2020, 382, 122989. [Google Scholar] [CrossRef]
- Sun, J.; Wang, C.; Song, J.; Huang, L.; Sun, Y.; Liu, Z.; Zhao, C.; Li, Y. Multi-functional application of oil-infused slippery Al surface: From anti-icing to corrosion resistance. J. Mater. Sci. 2018, 53, 16099–16109. [Google Scholar] [CrossRef]
- Liu, K.; Du, J.; Wu, J.; Jiang, L. Superhydrophobic gecko feet with high adhesive forces towards water and their bio-inspired materials. Nanoscale 2012, 4, 768–772. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Z.; Gong, G.; Wang, J.; Wu, J.; Yang, S.; Jiang, L. Icephobicity of Penguins Spheniscus Humboldti and an Artificial Replica of Penguin Feather with Air-Infused Hierarchical Rough Structures. J. Phys. Chem. C 2016, 120, 15923–15929. [Google Scholar] [CrossRef]
- Ijaola, A.O.; Bamidele, E.A.; Akisin, C.J.; Bello, I.T.; Oyatobo, A.T.; Abdulkareem, A.; Farayibi, P.K.; Asmatulu, E. Wettability Transition for Laser Textured Surfaces: A Comprehensive Review. Surf. Interfaces 2020, 21, 100802. [Google Scholar] [CrossRef]
- Wang, F.; Li, C.; Lv, Y.; Lv, F.; Du, Y. Ice accretion on superhydrophobic aluminum surfaces under low-temperature conditions. Cold Reg. Sci. Technol. 2010, 62, 29–33. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Xie, Q.; Duan, W.; Zhang, X.; Han, H. A Passive Anti-icing Strategy Based on a Superhydrophobic Mesh with Extremely Low Ice Adhesion Strength. J. Bionic Eng. 2021, 18, 55–64. [Google Scholar] [CrossRef]
- Wang, P.; Yang, M.; Zheng, B.; Guan, X.; Liao, Y.; Yue, Y.; Duan, W.; Zhang, Y. Soft and Rigid Integrated Durable Coating for Large-Scale Deicing. Langmuir 2023, 39, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, J.; Duan, W.; Li, C.; Han, H.; Xie, Q. A Superhydrophobic/Electrothermal/Photothermal Synergistically Anti-icing Strategy with Excellent Self-healable and Anti-abrasion Property. J. Bionic Eng. 2021, 18, 1147–1156. [Google Scholar] [CrossRef]
- Wang, F.; Lv, F.; Liu, Y.; Li, C.; Lv, Y. Ice adhesion on different microstructure superhydrophobic aluminum surfaces. J. Adhes. Sci. Technol. 2013, 27, 58–67. [Google Scholar] [CrossRef]
- Momen, G.; Farzaneh, M. Facile approach in the development of icephobic hierarchically textured coatings as corrosion barrier. Appl. Surf. Sci. 2014, 299, 41–46. [Google Scholar] [CrossRef]
- Arianpour, F.; Farzaneh, M.; Kulinich, S.A. Hydrophobic and ice-retarding properties of doped silicone rubber coatings. Appl. Surf. Sci. 2013, 265, 546–552. [Google Scholar] [CrossRef]
- Arianpour, F.; Farhadi, S.; Farzaneh, M. Effect of heterogeneity on hydro/ice-phobic properties of alkylsilane/fluoro-alkylsilane-based coatings on Al substrates. J. Coat. Technol. Res. 2017, 14, 267–275. [Google Scholar] [CrossRef]
- Foroughi Mobarakeh, L.; Jafari, R.; Farzaneh, M. Robust icephobic, and anticorrosive plasma polymer coating. Cold Reg. Sci. Technol. 2018, 151, 89–93. [Google Scholar] [CrossRef]
- Momen, G.; Jafari, R.; Farzaneh, M. Ice repellency behaviour of superhydrophobic surfaces: Effects of atmospheric icing conditions and surface roughness. Appl. Surf. Sci. 2015, 349, 211–218. [Google Scholar] [CrossRef]
- Guo, C.; Liao, R.; Yuan, Y.; Zuo, Z.; Zhuang, A. Glaze Icing on Superhydrophobic Coating Prepared by Nanoparticles Filling Combined with Etching Method for Insulators. J. Nanomater. 2015, 2015, 404071. [Google Scholar] [CrossRef]
- Fenero, M.; Knez, M.; Saric, I.; Petravic, M.; Grande, H.; Palenzuela, J. Omniphobic Etched Aluminum Surfaces with Anti-Icing Ability. Langmuir 2020, 36, 10916–10922. [Google Scholar] [CrossRef]
- Liao, R.; Zuo, Z.; Guo, C.; Yuan, Y.; Zhuang, A. Fabrication of superhydrophobic surface on aluminum by continuous chemical etching and its anti-icing property. Appl. Surf. Sci. 2014, 317, 701–709. [Google Scholar] [CrossRef]
- Zuo, Z.; Liao, R.; Zhao, X.; Song, X.; Qiao, Z.; Guo, C.; Zhuang, A.; Yuan, Y. Anti-frosting performance of superhydrophobic surface with ZnO nanorods. Appl. Therm. Eng. 2017, 110, 39–48. [Google Scholar] [CrossRef]
- Jafari, R.; Momen, G.; Farzaneh, M. Durability enhancement of icephobic fluoropolymer film. J. Coat. Technol. Res. 2016, 13, 405–412. [Google Scholar] [CrossRef]
- Lian, C.; Emersic, C.; Rajab, F.H.; Cotton, I.; Zhang, X.; Lowndes, R.; Li, L. Assessing the Superhydrophobic Performance of Laser Micropatterned Aluminium Overhead Line Conductor Material. IEEE Trans. Power Deliv. 2022, 37, 972–979. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Zhan, B.; Li, S.; Han, Z.; Liu, Y. A Novel Icephobic Strategy: The Fabrication of Biomimetic Coupling Micropatterns of Superwetting Surface. Adv. Mater. Interfaces 2019, 6, 1900864. [Google Scholar] [CrossRef]
- Yuan, Y.; Xiang, H.; Liu, G.; Wang, L.; Liu, H.; Liao, R. Self-Repairing Performance of Slippery Liquid Infused Porous Surfaces for Durable Anti-Icing. Adv. Mater. Interfaces 2022, 9, 2101968. [Google Scholar] [CrossRef]
- Barthlott, W.; Neinhuis, C. Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 1997, 202, 1–8. [Google Scholar] [CrossRef]
- Zhu, H.; Guo, Z.; Liu, W. Adhesion behaviors on superhydrophobic surfaces. Chem. Commun. 2014, 50, 3900–3913. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Gao, X.; Jiang, L. Directional adhesion of superhydrophobic butterfly wings. Soft Matter 2007, 3, 178–182. [Google Scholar] [CrossRef]
- Peng, Y.; Shang, J.; Liu, C.; Zhao, S.; Huang, C.; Bai, Y.; Li, Y. A universal replica molding strategy based on natural bio-templates for fabrication of robust superhydrophobic surfaces. Colloid Surf. A 2023, 660, 130879. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, Q.; Zheng, Y.; Xu, S. Composite structure and properties of the pitcher surface of the carnivorous plant Nepenthes and its influence on the insect attachment system. Prog. Nat. Sci. 2009, 19, 1657–1664. [Google Scholar] [CrossRef]
- Wu, L.; Wu, J.; Zhang, Z.; Zhang, C.; Zhang, Y.; Tang, A.; Li, L.; Zhang, G.; Zheng, Z.; Atrens, A.; et al. Corrosion resistance of fatty acid and fluoroalkylsilane-modified hydrophobic Mg-Al LDH films on anodized magnesium alloy. Appl. Surf. Sci. 2019, 487, 569–580. [Google Scholar] [CrossRef]
- Han, Z.; Fu, J.; Wang, Z.; Wang, Y.; Li, B.; Mu, Z.; Zhang, J.; Niu, S. Long-term durability of superhydrophobic properties of butterfly wing scales after continuous contact with water. Colloid Surf. A 2017, 518, 139–144. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, X.; Tao, J.; Zhu, C.; Lai, Y.; Chen, Z. Icephobic materials: Fundamentals, performance evaluation, and applications. Prog. Mater. Sci. 2019, 103, 509–557. [Google Scholar] [CrossRef]
- Raza Shaikh, A.; Qu, J.; Zhou, M.; Mulbah, C.K.Y. Recent progress on the surface finishing of metals and alloys to achieve superhydrophobic surfaces: A critical review. Trans. IMF 2021, 99, 61–72. [Google Scholar] [CrossRef]
- He, H.; Guo, Z. Superhydrophobic materials used for anti-icing Theory, application, and development. iScience 2021, 24, 103357. [Google Scholar] [CrossRef]
- Style, R.W.; Dufresne, E.R. Static wetting on deformable substrates, from liquids to soft solids. Soft Matter 2012, 8, 7177–7184. [Google Scholar] [CrossRef]
- Hazlett, R.D. Fractal applications: Wettability and contact angle. J. Colloid Interface Sci. 1990, 137, 527–533. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of Solid Surfaces to Wetting By Water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Mockenhaupt, B.; Ensikat, H.-J.; Spaeth, M.; Barthlott, W. Superhydrophobicity of Biological and Technical Surfaces under Moisture Condensation: Stability in Relation to Surface Structure. Langmuir 2008, 24, 13591–13597. [Google Scholar] [CrossRef] [PubMed]
- Orchard, M.J.; Kohonen, M.; Humphries, S. The influence of surface energy on the self-cleaning of insect adhesive devices. J. Exp. Biol. 2012, 215, 279–286. [Google Scholar] [CrossRef]
- Jiang, L.; Zhao, Y.; Zhai, J. A lotus-leaf-like superhydrophobic surface: A porous microsphere/ nanofiber composite film prepared by electrohydrodynamics. Angew. Chem. 2004, 116, 4438–4441. [Google Scholar] [CrossRef]
- Lai, Y.; Gao, X.; Zhuang, H.; Huang, J.; Lin, C.; Jiang, L. Designing superhydrophobic porous nanostructures with tunable water adhesion. Adv. Mater. 2009, 21, 3799–3803. [Google Scholar] [CrossRef]
- Shen, Y.; Xie, Y.; Tao, J.; Chen, H.; Zhu, C.; Jin, M.; Lu, Y. Rationally designed nanostructure features on superhydrophobic surfaces for enhancing self-propelling dynamics of condensed droplets. ACS Sustain. Chem. Eng. 2018, 7, 2702–2708. [Google Scholar] [CrossRef]
- Tian, J.; Zhang, Y.; Zhu, J.; Yang, Z.; Gao, X. Robust Nonsticky Superhydrophobicity by the Tapering of Aligned ZnO Nanorods. Chem. Phys. Chem. 2014, 15, 858–861. [Google Scholar] [CrossRef]
- Haikun Zheng, S.C.Y.Z. Anti-Icing & Icephobic Mechanism and Applications of Superhydrophobic/Ultra Slippery Surface. Prog. Chem. 2017, 29, 102–118. [Google Scholar]
- Wang, X.; Wang, S.; Xu, Q.; Mi, J. Thermodynamics of Ice Nucleation in Liquid Water. J. Phys. Chem. B 2015, 119, 1660–1668. [Google Scholar] [CrossRef]
- Matsumoto, M.; Saito, S.; Ohmine, I. Molecular dynamics simulation of the ice nucleation and growth process leading to water freezing. Nature 2002, 416, 409–413. [Google Scholar] [CrossRef]
- Chen, D.; Gelenter, M.D.; Hong, M.; Cohen, R.E.; McKinley, G.H. Icephobic Surfaces Induced by Interfacial Nonfrozen Water. ACS Appl. Mater. Interfaces 2017, 9, 4202–4214. [Google Scholar] [CrossRef]
- Schutzius, T.M.; Jung, S.; Maitra, T.; Eberle, P.; Antonini, C.; Stamatopoulos, C.; Poulikakos, D. Physics of Icing and Rational Design of Surfaces with Extraordinary Icephobicity. Langmuir 2015, 31, 4807–4821. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Tiwari, M.K.; Doan, N.V.; Poulikakos, D. Mechanism of supercooled droplet freezing on surfaces. Nat. Commun. 2012, 3, 615. [Google Scholar] [CrossRef]
- Eberle, P.; Tiwari, M.K.; Maitra, T.; Poulikakos, D. Rational nanostructuring of surfaces for extraordinary icephobicity. Nanoscale 2014, 6, 4874–4881. [Google Scholar] [CrossRef]
- Bai, G.; Gao, D.; Liu, Z.; Zhou, X.; Wang, J. Probing the critical nucleus size for ice formation with graphene oxide nanosheets. Nature 2019, 576, 437–441. [Google Scholar] [CrossRef]
- Shin, B.; Lee, K.-R.; Moon, M.-W.; Kim, H.-Y. Extreme water repellency of nanostructured low-surface-energy non-woven fabrics. Soft Matter 2012, 8, 1817–1823. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Z. Fundamentals of icing and common strategies for designing biomimetic anti-icing surfaces. J. Mater. Chem. A 2018, 6, 13549–13581. [Google Scholar] [CrossRef]
- Wang, H.; He, M.; Liu, H.; Guan, Y. One-Step Fabrication of Robust Superhydrophobic Steel Surfaces with Mechanical Durability, Thermal Stability, and Anti-icing Function. ACS Appl. Mater. Interfaces 2019, 11, 25586–25594. [Google Scholar] [CrossRef]
- Varanasi, K.K.; Deng, T.; Smith, J.D.; Hsu, M.; Bhate, N. Frost formation and ice adhesion on superhydrophobic surfaces. Appl. Phys. Lett. 2010, 97, 234102. [Google Scholar] [CrossRef]
- Liu, G.Y.; Yuan, Y.; Liao, R.J.; Xiang, H.Y.; Wang, L.; Yu, Q.; Zhang, C. Robust and self-healing superhydrophobic aluminum surface with excellent anti-icing performance. Surf. Interfaces 2022, 28, 101588. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, J. Ice Accretion Cause and Mechanism of Glaze on Wires of Power Transmission Lines. Adv. Mater. Res. 2011, 189, 3238–3242. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, X.; Jia, J.; Tian, Y.; Zhao, L.; Zhang, Y.; Cui, Y.; Li, X. Experimental study on the thermal conductivity for transmission line icing. Cold Reg. Sci. Technol. 2016, 129, 96–103. [Google Scholar] [CrossRef]
- Jiang, X.; Yi, H. Transmission line’s Icing and Protection; China Electric Power Press: Beijing, China, 2002. [Google Scholar]
- Fujimura, T.; Naito, K.; Hasegawa, Y.; Kawaguchi, T. Performance of insulators covered with snow or ice. IEEE Trans. Power Syst. 1979, 5, 1621–1631. [Google Scholar] [CrossRef]
- Farzaneh, M.; Baker, C.T.; Bernstorf, A.; Brown, K.; Chisholm, W.A.; de Tourreil, C.; Drapeau, J.F.; Fikke, S.; George, J.M.; Gnandt, E.; et al. Insulator icing test methods and procedures a position paper prepared by the ieee task force on insulator icing test methods. IEEE Trans. Power Deliv. 2003, 18, 1503–1515. [Google Scholar] [CrossRef]
- Mohammadian, B.; Abou Yassine, A.H.; Sarayloo, M.; Sojoudi, H. Prediction of wet snow shedding from surfaces under various heat transfer modes. Appl. Therm. Eng. 2022, 204, 117955. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, D.; Jiang, X.; Huang, H.; Gao, D.W. Study of the icing growth characteristic and its influencing factors for different types of insulators. Turk. J. Electr. Eng. 2016, 24, 1571–1585. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Zhang, X.; Zhou, P.; Sun, L.; Liu, L.; Zi, B.; Cui, G.; Ding, H. Atmospheric icing status and type of southwest China networks. MATEC Web Conf. 2016, 77, 06012. [Google Scholar]
- Korolev, A.; Isaac, G.A. Relative Humidity in Liquid, Mixed-Phase, and Ice Clouds. J. Atmos. Sci. 2006, 63, 2865–2880. [Google Scholar] [CrossRef]
- Parent, O.; Ilinca, A. Anti-icing and de-icing techniques for wind turbines: Critical review. Cold Reg. Sci. Technol. 2011, 65, 88–96. [Google Scholar] [CrossRef]
- Belaud, C.; Vercillo, V.; Kolb, M.; Bonaccurso, E. Development of nanostructured icephobic aluminium oxide surfaces for aeronautic applications. Surf. Coat. Technol. 2021, 405, 126652. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Bressler, A.H.; Castano, C.E.; Fergusson, C.P.; Mohammadi, R. Rational strategy for the atmospheric icing prevention based on chemically functionalized carbon soot coatings. Appl. Surf. Sci. 2016, 390, 452–460. [Google Scholar] [CrossRef]
- Sharifi, N.; Dolatabadi, A.; Pugh, M.; Moreau, C. Anti-icing performance and durability of suspension plasma sprayed TiO2 coatings. Cold Reg. Sci. Technol. 2019, 159, 1–12. [Google Scholar] [CrossRef]
- Stewart, R.E.; King, P. Freezing precipitation in winter storms. Mon. Weather Rev. 1987, 115, 1270–1280. [Google Scholar] [CrossRef]
- Chen, B.; Hu, W.; Pu, J. Characteristics of the raindrop size distribution for freezing precipitation observed in southern China. J. Geophys. Res. Atmos. 2011, 116, 1–10. [Google Scholar] [CrossRef]
- Moallem, E.; Cremaschi, L.; Fisher, D.E.; Padhmanabhan, S. Experimental measurements of the surface coating and water retention effects on frosting performance of microchannel heat exchangers for heat pump systems. Exp. Fluid Sci. 2012, 39, 176–188. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Castano, C.E.; Mohammadi, R.; Lazarov, Y.; Radeva, E.I. Delayed condensation and frost formation on superhydrophobic carbon soot coatings by controlling the presence of hydrophilic active sites. J. Phys. D Appl. Phys. 2018, 51, 055302. [Google Scholar] [CrossRef]
- Rønneberg, S.; Laforte, C.; Volat, C.; He, J.; Zhang, Z. The effect of ice type on ice adhesion. AIP Adv. 2019, 9, 055304. [Google Scholar] [CrossRef]
- Jiang, X.; Xiang, Z.; Zhang, Z.; Hu, J.; Hu, Q.; Shu, L. Predictive Model for Equivalent Ice Thickness Load on Overhead Transmission Lines Based on Measured Insulator String Deviations. IEEE Trans. Power Deliv. 2014, 29, 1659–1665. [Google Scholar] [CrossRef]
- Fan, C.; Jiang, X. Analysis of the Icing Accretion Performance of Conductors and Its Normalized Characterization Method of Icing Degree for Various Ice Types in Natural Environments. Energies 2018, 11, 2678. [Google Scholar] [CrossRef]
- Lina, H.; Shengsuo, N.; Yueyan, H.; Jingping, Z. Study on the Influence of Meteorological Conditions on Transmission Line Parameters. IOP Conf. Ser. Earth Environ. Sci. 2019, 218, 012103. [Google Scholar] [CrossRef]
- Xue, S.; Liu, Y.; Wang, Y.; Xiao, B.; Shi, X.; Yao, J.; Lv, X.; Yuan, W.; He, Y.; Tawfik, M. Variation in Anti-icing Power of Superhydrophobic Electrothermal Film under Different Temperatures and Wind Speeds. Int. J. Aerosp. Eng. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Kermani, M.; Farzaneh, M.; Kollar, L.E. The Effects of Wind Induced Conductor Motion on Accreted Atmospheric Ice. IEEE Trans. Power Deliv. 2013, 28, 540–548. [Google Scholar] [CrossRef]
- Ghassemi, M.; Farzaneh, M. Coupled Computational Fluid Dynamics and Heat Transfer Modeling of the Effects of Wind Speed and Direction on Temperature Increase of an Ice-Covered FRP Live-Line Tool. IEEE Trans. Power Deliv. 2015, 30, 2268–2275. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, L.; Wang, J.; Liu, G. Dynamic Characteristics Analysis of Ice-AdhesionTransmission Tower-Line System under Effect of Wind-Induced Ice Shedding. Comp. Model. Eng. 2020, 125, 647–670. [Google Scholar]
- Kim, P.; Wong, T.-S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-Infused Nanostructured Surfaces with Extreme Anti-Ice and Anti-Frost Performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef]
- Ren, C.; Li, M.; Huang, W.; Zhang, Y.; Huang, J. Superhydrophobic coating with excellent robustness and UV resistance fabricated using hydrothermal treated lignin nanoparticles by one-step spray. J. Mater. Sci. 2022, 57, 18356–18369. [Google Scholar] [CrossRef]
- Xu, P.; Ma, Y.; Zhu, J.; Zhao, L.; Shen, B.; Bian, X. Effect of TiO2 coating on the surface condition and corona characteristics of positive DC conductors with particle matters. High Volt 2022, 7, 147–157. [Google Scholar] [CrossRef]
- Hakansson, E.; Predecki, P.; Kumosa, M.S. Galvanic Corrosion of High Temperature Low Sag Aluminum Conductor Composite Core and Conventional Aluminum Conductor Steel Reinforced Overhead High Voltage Conductors. IEEE Trans. Reliab. 2015, 64, 928–934. [Google Scholar] [CrossRef]
- Carneiro, S.; Marti, J.R. Evaluation of corona and line models in electromagnetic transients simulations. IEEE Trans. Power Deliv. 1991, 6, 334–342. [Google Scholar] [CrossRef]
- Chang, J.-S.; Lawless, P.A.; Yamamoto, T. Corona discharge processes. IEEE Trans. Plasma Sci. 1991, 19, 1152–1166. [Google Scholar] [CrossRef]
- Stracqualursi, E.; Araneo, R.; Celozzi, S. The Corona Phenomenon in Overhead Lines: Critical Overview of Most Common and Reliable Available Models. Energies 2021, 14, 6612. [Google Scholar] [CrossRef]
- Phillips, A.; Childs, D.; Schneider, H. Aging of nonceramic insulators due to corona from water drops. IEEE Trans. Power Deliv. 1999, 14, 1081–1089. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Lei, Q.; Li, W.; Wu, Y. Influence of alternating current (AC) corona discharge on the superhydrophobicity of SiO2/fluorosilicon resin nano-composite coating. Appl. Surf. Sci. 2019, 478, 642–650. [Google Scholar] [CrossRef]
- Ouyang, X.; Yin, F.; Jia, Z.; Yang, S.; Wang, Y.; Bai, H.; Zhang, X.; Li, Y.; Chen, H. Research of biological contamination and its effect on the properties of RTV-coated insulators. Electr Pow. Syst. Res. 2019, 167, 138–149. [Google Scholar] [CrossRef]
- Cherney, E.; Gorur, R. RTV silicone rubber coatings for outdoor insulators. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 605–611. [Google Scholar] [CrossRef]
- Moreno, V.; Gorur, R. Effect of long-term corona on non-ceramic outdoor insulator housing materials. IEEE Trans. Dielectr. Electr. Insul. 2001, 8, 117–128. [Google Scholar] [CrossRef]
- Megala, V.; Sugumaran, C.P. Enhancement of Corona Onset Voltage Using PI/MWCNT Nanocomposite on HV Conductor. IEEE Trans. Plasma Sci. 2020, 48, 1122–1129. [Google Scholar] [CrossRef]
- Zhang, X.; Cotton, I.; Li, Q.; Rowland, S.M.; Emersic, C.; Lian, C.; Li, W. Experimental verification of the potential of superhydrophobic surfaces in reducing audible noise on HVAC overhead line conductors. High Volt. 2022, 7, 692–704. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, G.; Tao, J.; Zhu, C.; Liu, S.; Jin, M.; Xie, Y.; Chen, Z. Anti-Icing Performance of Superhydrophobic Texture Surfaces Depending on Reference Environments. Adv. Mater. Interfaces 2017, 4, 1700836. [Google Scholar] [CrossRef]
- Xu, Z.; Qi, H.; Cheng, Y.; He, X. Nanocoating: Anti-icing superamphiphobic surface on 1060 aluminum alloy mesh. Appl. Surf. Sci. 2019, 498, 143827. [Google Scholar] [CrossRef]
- Zhao, X.D.; Fan, H.M.; Liu, X.Y.; Pan, H.; Xu, H.Y. Pattern-Dependent Tunable Adhesion of Superhydrophobic MnO2 Nanostructured Film. Langmuir 2011, 27, 3224–3228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, D.; Zhang, Y.; Zhu, M. Lattice Boltzmann Modeling of Droplet Condensation on Superhydrophobic Nanoarrays. Langmuir 2014, 30, 12559–12569. [Google Scholar] [CrossRef]
- Wang, L.; Tian, Z.; Jiang, G.; Luo, X.; Chen, C.; Hu, X.; Zhang, H.; Zhong, M. Spontaneous dewetting transitions of droplets during icing & melting cycle. Nat. Commun. 2022, 13, 378. [Google Scholar]
- Zhao, W.; Wang, Y.; Han, M.; Xu, J.; Tam, K.C. Surface Modification, Topographic Design and Applications of Superhydrophobic Systems. Chem Eur. J. 2022, 28, e202202657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.; Wang, Z. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 18, 621–633. [Google Scholar] [CrossRef]
- Zhang, Z.; Xue, F.; Bai, W.; Shi, X.; Liu, Y.; Feng, L. Superhydrophobic surface on Al alloy with robust durability and excellent self-healing performance. Surf. Coat. Technol. 2021, 410, 126952. [Google Scholar] [CrossRef]
- Zhizhchenko, A.Y.; Shabalina, A.V.; Aljulaih, A.A.; Gurbatov, S.O.; Kuchmizhak, A.A.; Iwamori, S.; Kulinich, S.A. Stability of Octadecyltrimethoxysilane-Based Coatings on Aluminum Alloy Surface. Materials 2022, 15, 1804. [Google Scholar] [CrossRef]
- Xiang, H.; Yuan, Y.; Zhang, C.; Dai, X.; Zhu, T.; Song, L.; Gai, Y.; Liao, R. Key Factors Affecting Durable Anti-Icing of Slippery Surfaces: Pore Size and Porosity. ACS Appl. Mater. Interfaces 2023, 15, 3599–3612. [Google Scholar] [PubMed]
- Babich, A.; Pogosov, V. Effect of dielectric coating on the electron work function and the surface stress of a metal. Surf. Sci. 2009, 603, 2393–2397. [Google Scholar] [CrossRef]
- Pylarinos, D.; Siderakis, K.; Thalassinakis, E. Comparative investigation of silicone rubber composite and room temperature vulcanized coated glass insulators installed in coastal overhead transmission lines. IEEE Electr. Insul. Mag. 2015, 31, 23–29. [Google Scholar] [CrossRef]
- Nourai, A.; Kogan, V.; Schafer, C.M. Load leveling reduces T&D line losses. IEEE Trans. Power Deliv. 2008, 23, 2168–2173. [Google Scholar]
- Gao, N.; Li, W.-P.; Wang, W.-S.; Liu, D.-P.; Cui, Y.-M.; Guo, L.; Wang, G.-S. Balancing dielectric loss and magnetic loss in Fe–NiS2/NiS/PVDF composites toward strong microwave reflection loss. ACS Appl. Mater. Interfaces 2020, 12, 14416–14424. [Google Scholar] [CrossRef] [PubMed]
- Lazauskas, A.; Guobienė, A.; Prosyčevas, I.; Baltrušaitis, V.; Grigaliūnas, V.; Narmontas, P.; Baltrusaitis, J. Water droplet behavior on superhydrophobic SiO2 nanocomposite films during icing/deicing cycles. Mater. Charact. 2013, 82, 9–16. [Google Scholar] [CrossRef]
- Nakajima, A.; Hashimoto, K.; Watanabe, T.; Takai, K.; Yamauchi, G.; Fujishima, A. Transparent Superhydrophobic Thin Films with Self-Cleaning Properties. Langmuir 2000, 16, 7044–7047. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z.; Zhang, Y.; Xiang, B.; Tang, X.; She, H. Facile fabrication of superhydrophobic silica coatings with excellent corrosion resistance and liquid marbles. J. Sol. Gel Sci. Technol. 2016, 80, 208–214. [Google Scholar] [CrossRef]
- Jafari, R.; Farzaneh, M. Development a simple method to create the superhydrophobic composite coatings. J. Compos. Mater. 2012, 47, 3125–3129. [Google Scholar] [CrossRef]
- Li, J.; Wu, R.; Jing, Z.; Yan, L.; Zha, F.; Lei, Z. One-step spray-coating process for the fabrication of colorful superhydrophobic coatings with excellent corrosion resistance. Langmuir 2015, 31, 10702–10707. [Google Scholar] [CrossRef]
- Wang, G.; Li, A.; Li, K.; Zhao, Y.; Ma, Y.; He, Q. A fluorine-free superhydrophobic silicone rubber surface has excellent self-cleaning and bouncing properties. J. Colloid Interface Sci. 2021, 588, 175–183. [Google Scholar] [CrossRef]
- Han, B.; Wang, H.; Yuan, S.; Li, Y.; Zhang, X.; Lin, D.; Chen, L.; Zhu, Y. Durable and anti-corrosion superhydrophobic coating with bistratal structure prepared by ambient curing. Prog. Org. Coat. 2020, 149, 105922. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, A.G.; Sun, B.R.; Chen, K.S.; Yu, H.Z. Functional and versatile superhydrophobic coatings via stoichiometric silanization. Nat. Commun. 2021, 12, 982. [Google Scholar] [CrossRef]
- Zhou, Y.; Ma, Y.; Qi, C.; Shen, L.; Fu, Q.; Sun, Y.; Liu, Y. Superhydrophobic surface based on nano-engineering for enhancing the durability of anticorrosion. Surf. Eng. 2021, 37, 288–298. [Google Scholar] [CrossRef]
- Li, Y.; Ma, W.; Kwon, Y.S.; Li, W.; Yao, S.; Huang, B. Solar Deicing Nanocoatings Adaptive to Overhead Power Lines. Adv. Funct. Mater. 2022, 32, 2113297. [Google Scholar] [CrossRef]
- Xiao, X.; Xie, W.; Ye, Z. Preparation of corrosion-resisting superhydrophobic surface on aluminium substrate. Surf. Eng. 2019, 35, 411–417. [Google Scholar] [CrossRef]
- Wenxuan, Y.; Yuan, Y.; Guoyong, L.; Bing, Z.; Rongkai, Y. The anti-icing/frosting aluminum surface with hydrangea-like micro/nano structure prepared by chemical etching. Mater. Lett. 2018, 226, 4–7. [Google Scholar] [CrossRef]
- Jin, H.-y.; Nie, S.-c.; Li, Z.-w.; Tong, C.; Wang, K.-j. Investigation on Preparation and Anti-icing Performance of Super-hydrophobic Surface on Aluminum Conductor. Chin. J. Chem. Phys. 2018, 31, 216–222. [Google Scholar] [CrossRef]
- Zuo, Z.; Liao, R.; Guo, C.; Yuan, Y.; Zhao, X.; Zhuang, A.; Zhang, Y. Fabrication and anti-icing property of coral-like superhydrophobic aluminum surface. Appl. Surf. Sci. 2015, 331, 132–139. [Google Scholar] [CrossRef]
- Takahashi, H.; Chiba, M. Role of anodic oxide films in the corrosion of aluminum and its alloys. Corros. Rev. 2018, 36, 35–54. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Zhevnenko, S.N.; Emel’yanenko, A.M.; Gol’dshtein, R.V.; Epifanov, V.P. Adhesive strength of the contact of ice with a superhydrophobic coating. Dokl Chem. 2013, 448, 71–75. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, X.; Pang, G.; Zhang, F.; Han, Y.; Wang, X.; Liu, X.; Xue, L. Temperature-based adhesion tuning and superwettability switching on superhydrophobic aluminum surface for droplet manipulations. Surf. Coat. Technol. 2019, 375, 527–533. [Google Scholar] [CrossRef]
- Yong, J.; Yang, Q.; Guo, C.; Chen, F.; Hou, X. A review of femtosecond laser-structured superhydrophobic or underwater superoleophobic porous surfaces/materials for efficient oil/water separation. RSC Adv. 2019, 9, 12470–12495. [Google Scholar] [CrossRef] [PubMed]

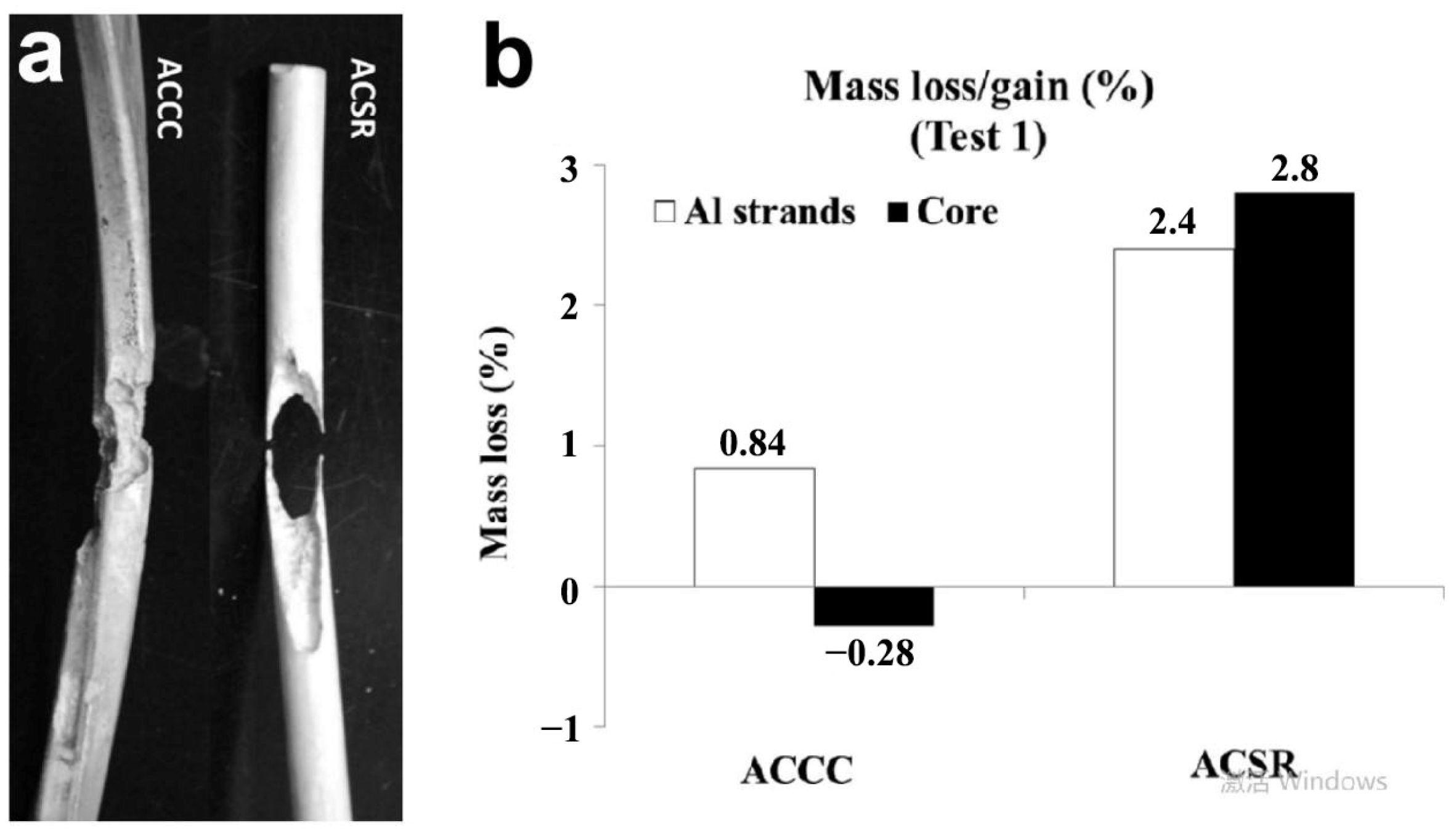

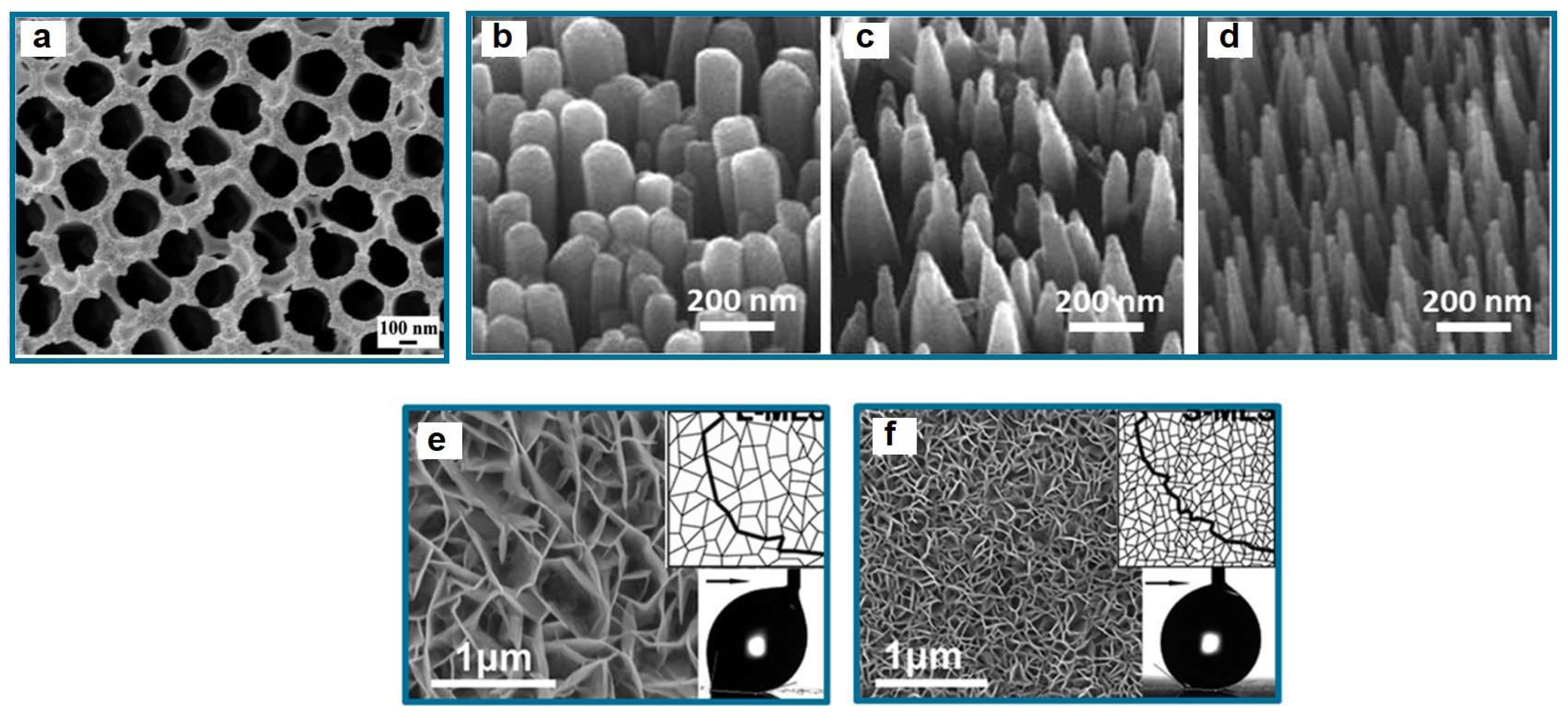


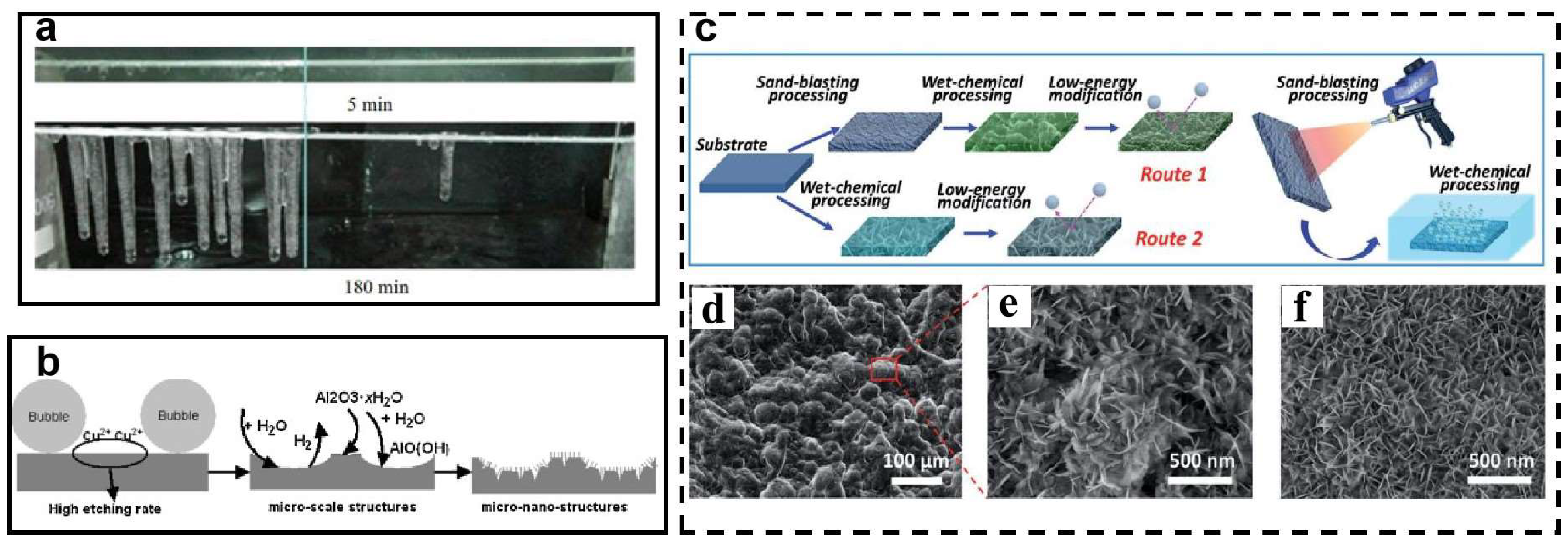
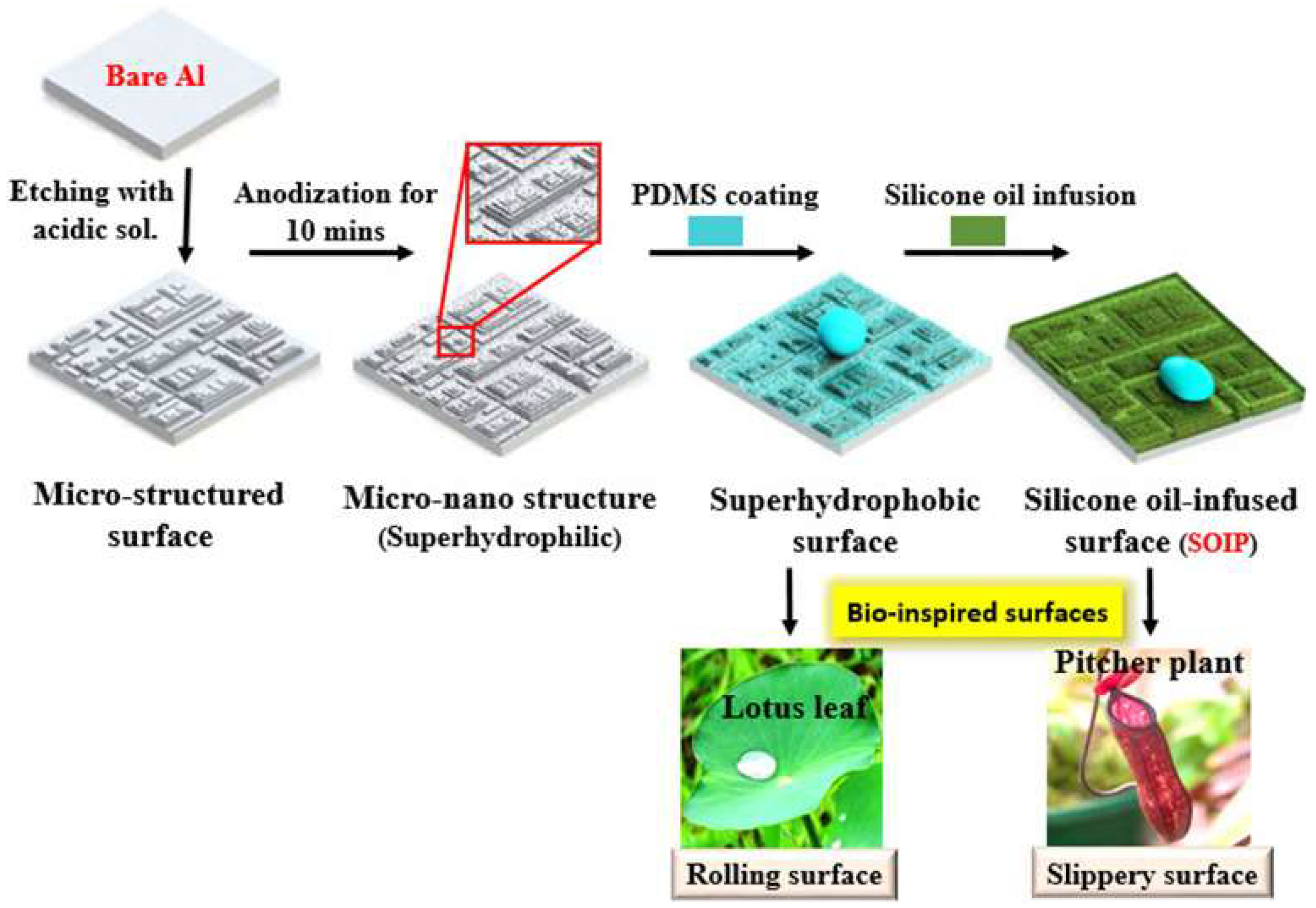
| Icing Types | Environment Temperatures (T/°C) | Wind Speed (v/m·s−1) | MVD (µm) | LWC (g/m3) | Density (g/m3) | Ice Adhesion | |
|---|---|---|---|---|---|---|---|
| Atmospheric icing (in-cloud icing) | Glaze | −5~0 | 1~10 | 1~20 | 0.05~0.6 | 0.1~0.3 | Strong |
| Hard rime | −15~−3 | 1~15 | 5~35 | 0.4~2.0 | 0.15~0.19 | Strong | |
| Soft rime | −25~−5 | 3~20 | 10~80 | 0.6~3.0 | <0.6 | Weak | |
| Precipitation icing | Freezing rain | −3~0 | 2~4 | 300~5000 | 0.11~2.5 | 0.2~0.6 | Strong |
| Wet snow | −2~3 | 0~9 | 100~300 | 0.02~0.3 | 0.4~0.6 | Initially weak, subsequently enhanced | |
| Frosting | Hoarfrost | −5~0 | low | / | / | <0.3 | Strong |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Bai, J.; He, J.; Ding, C.; Dai, X.; Ci, W.; Zhu, T.; Liao, R.; Yuan, Y. A Review on Superhydrophobic Surface with Anti-Icing Properties in Overhead Transmission Lines. Coatings 2023, 13, 301. https://doi.org/10.3390/coatings13020301
Li B, Bai J, He J, Ding C, Dai X, Ci W, Zhu T, Liao R, Yuan Y. A Review on Superhydrophobic Surface with Anti-Icing Properties in Overhead Transmission Lines. Coatings. 2023; 13(2):301. https://doi.org/10.3390/coatings13020301
Chicago/Turabian StyleLi, Bo, Jie Bai, Jinhang He, Chao Ding, Xu Dai, Wenjun Ci, Tao Zhu, Ruijin Liao, and Yuan Yuan. 2023. "A Review on Superhydrophobic Surface with Anti-Icing Properties in Overhead Transmission Lines" Coatings 13, no. 2: 301. https://doi.org/10.3390/coatings13020301
APA StyleLi, B., Bai, J., He, J., Ding, C., Dai, X., Ci, W., Zhu, T., Liao, R., & Yuan, Y. (2023). A Review on Superhydrophobic Surface with Anti-Icing Properties in Overhead Transmission Lines. Coatings, 13(2), 301. https://doi.org/10.3390/coatings13020301







