Hydrothermal Preparation of TiO2/Graphite Nanosheets Composites and Its Effect on Electrothermal Behavior
Abstract
1. Introduction
2. Experimental
2.1. Materials
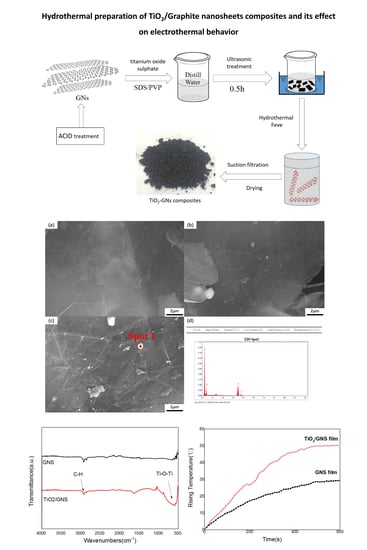
2.2. Preparation of TiO2/Graphite Nanosheets Composite
2.3. Characterization
3. Results and Discussion
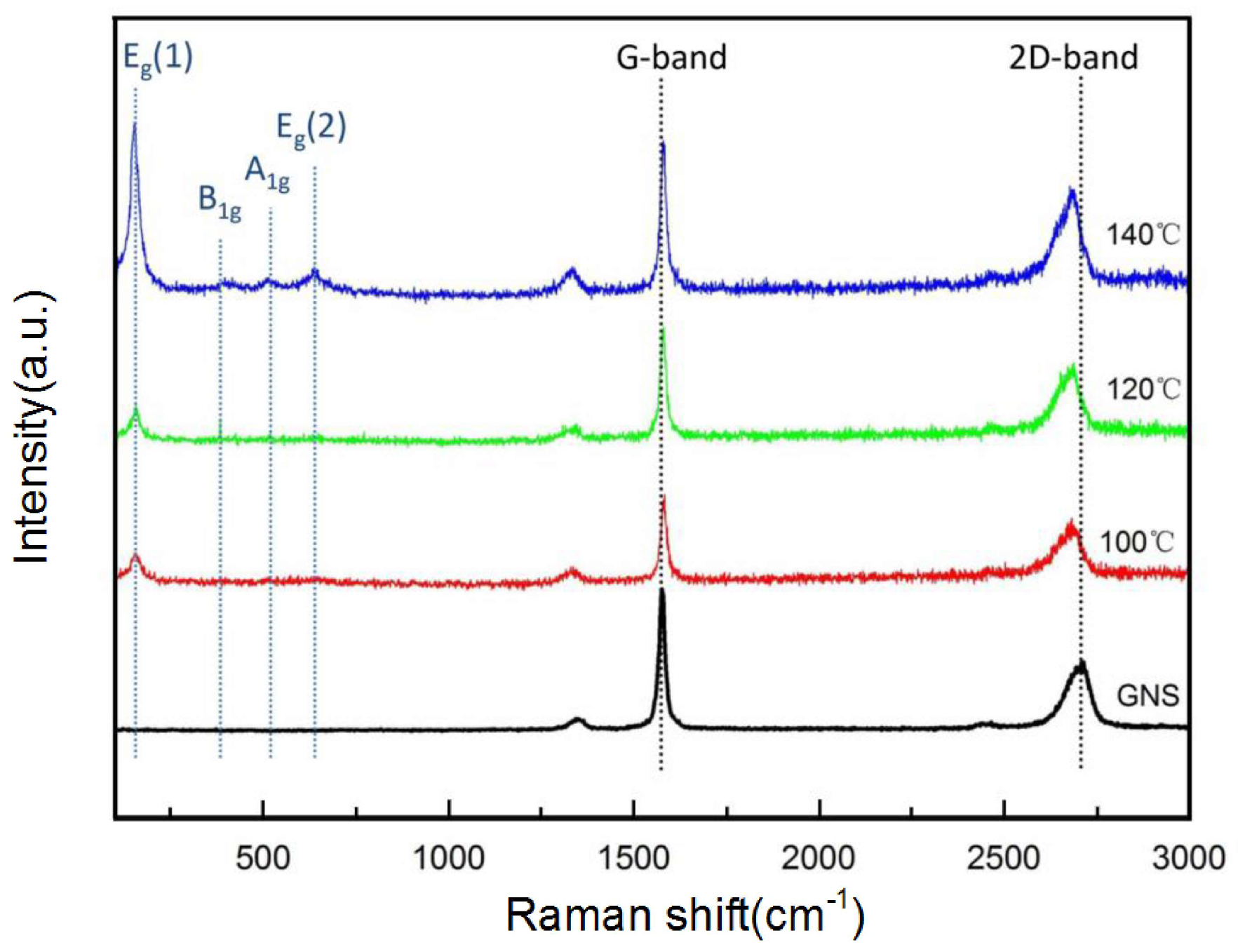
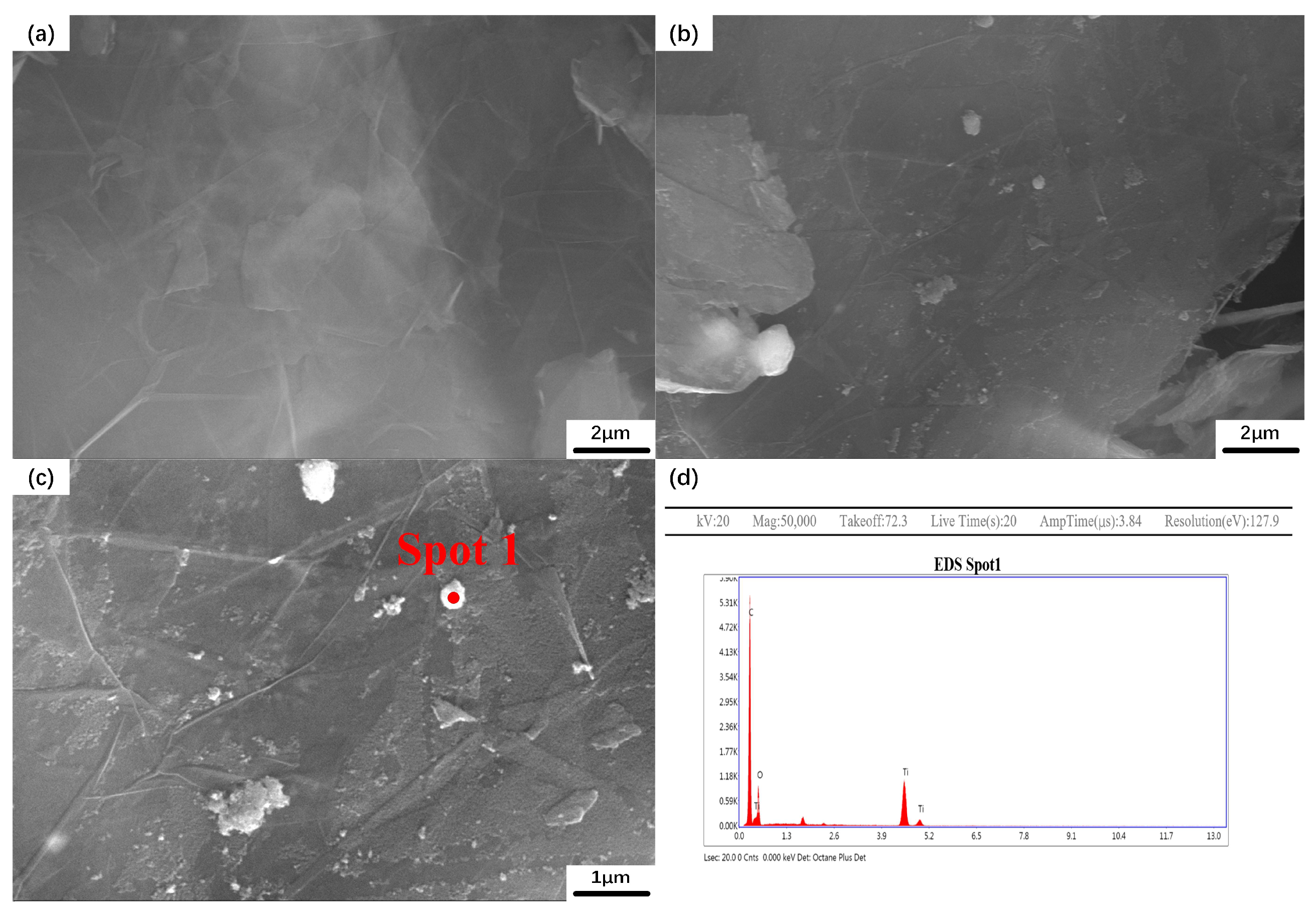
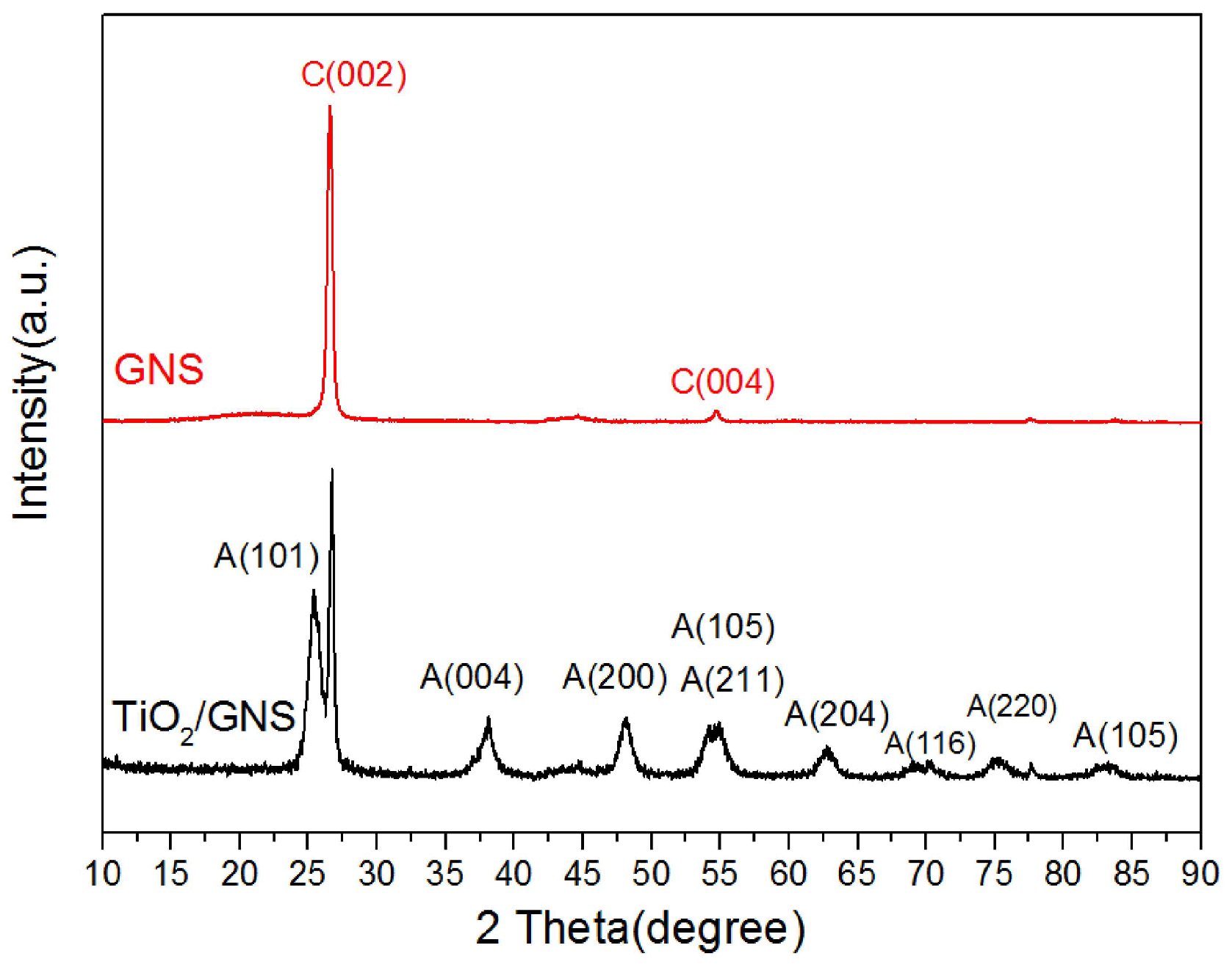
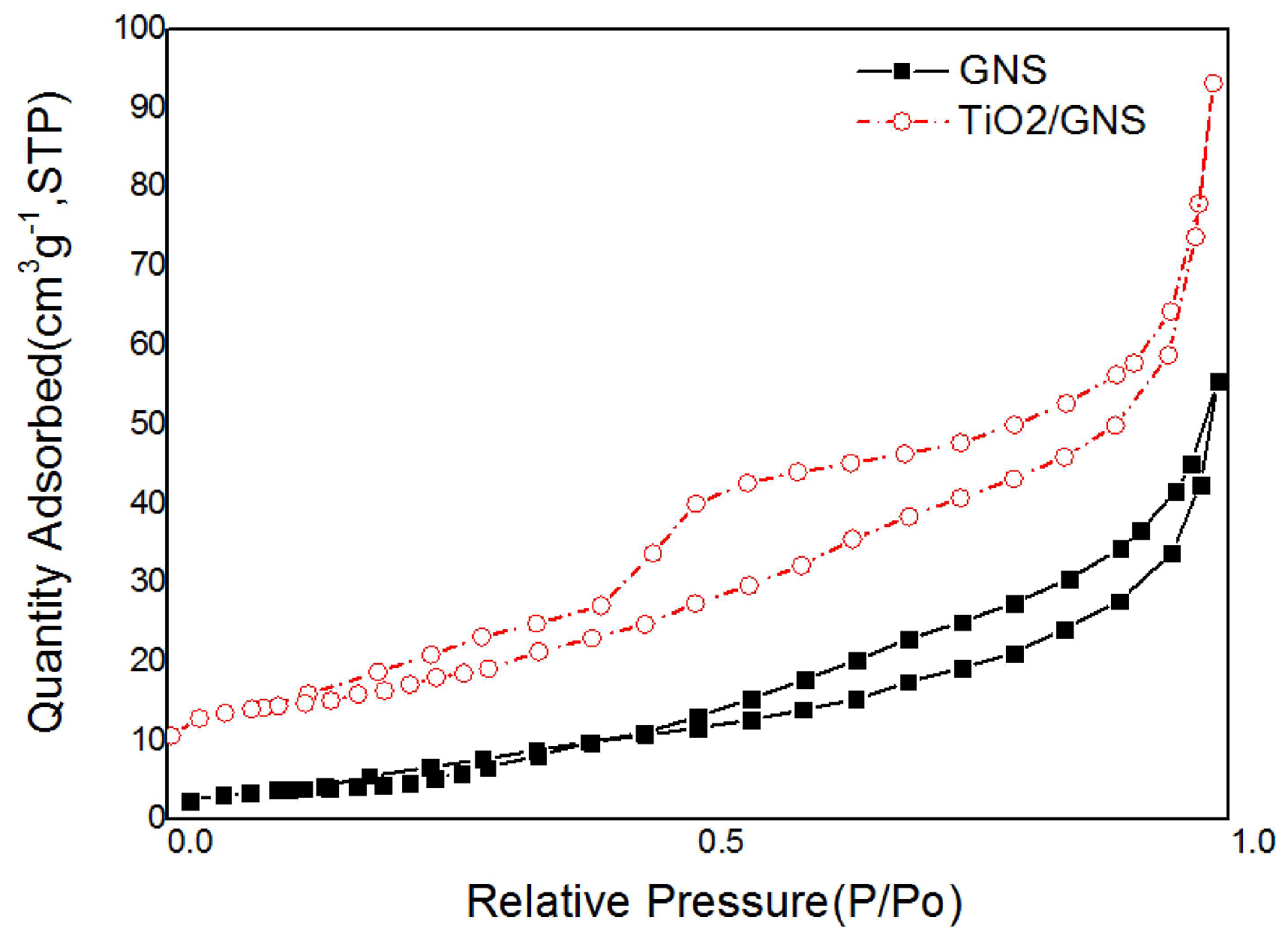
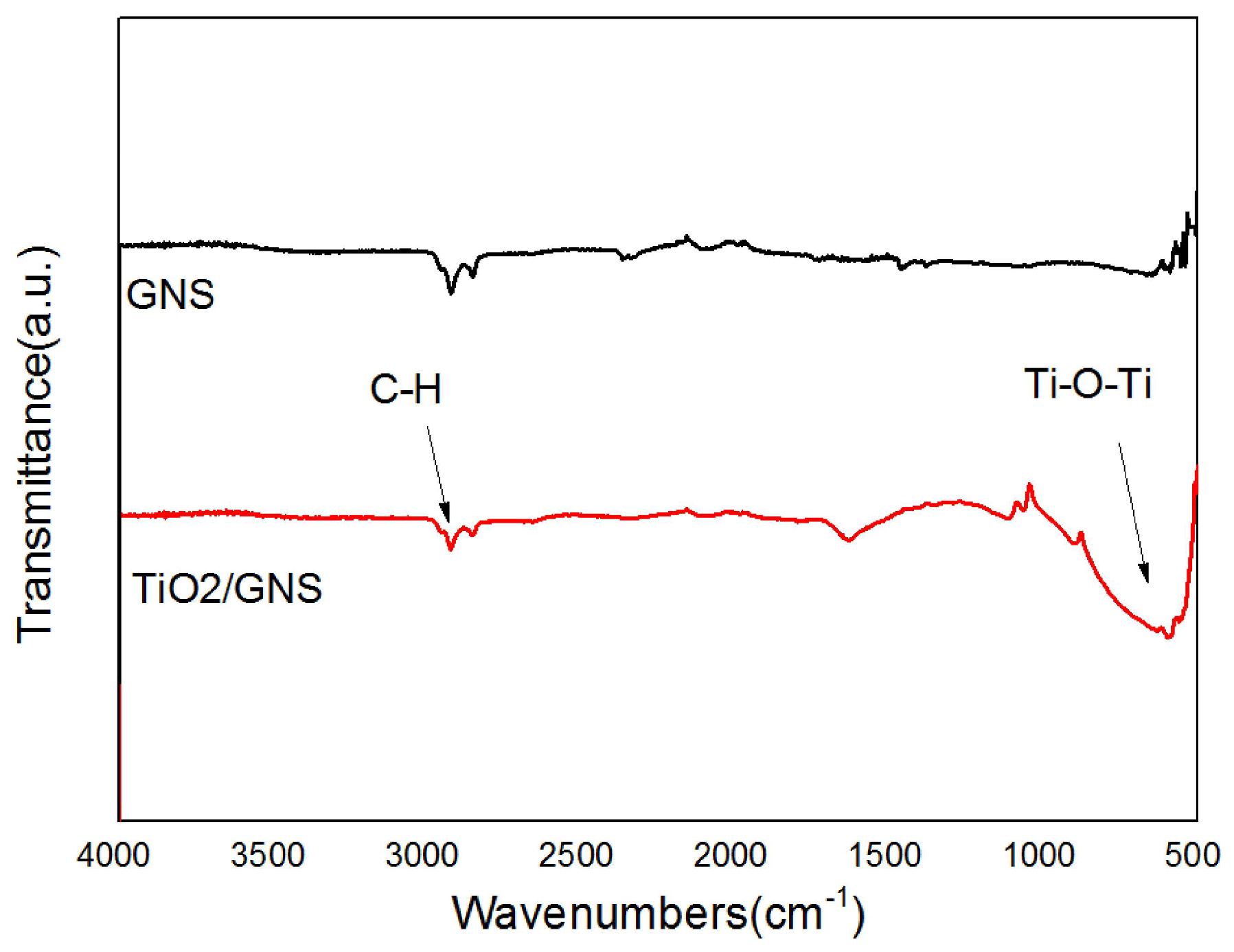
3.1. Characterization of the TiO2/GNs Composite
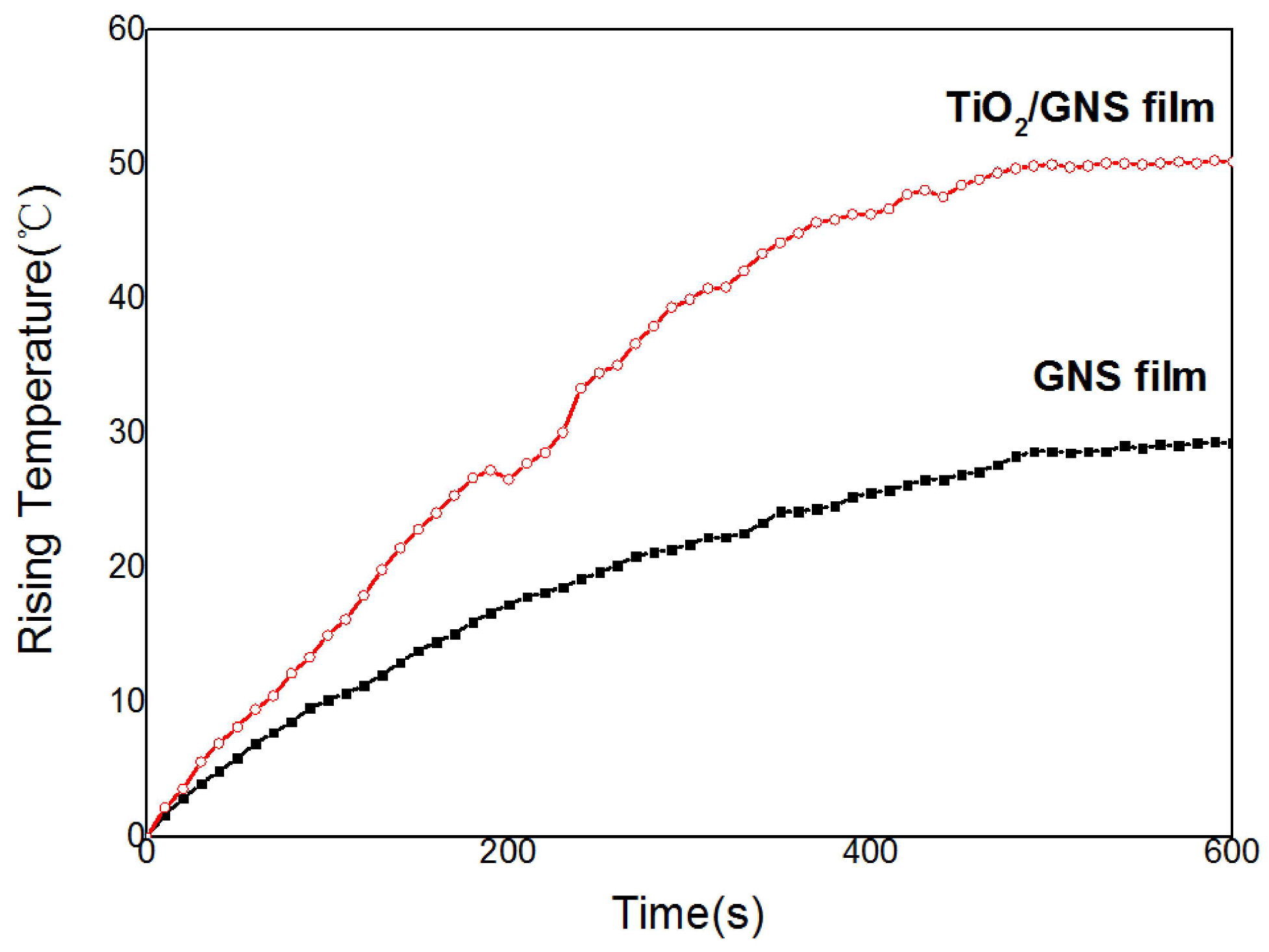
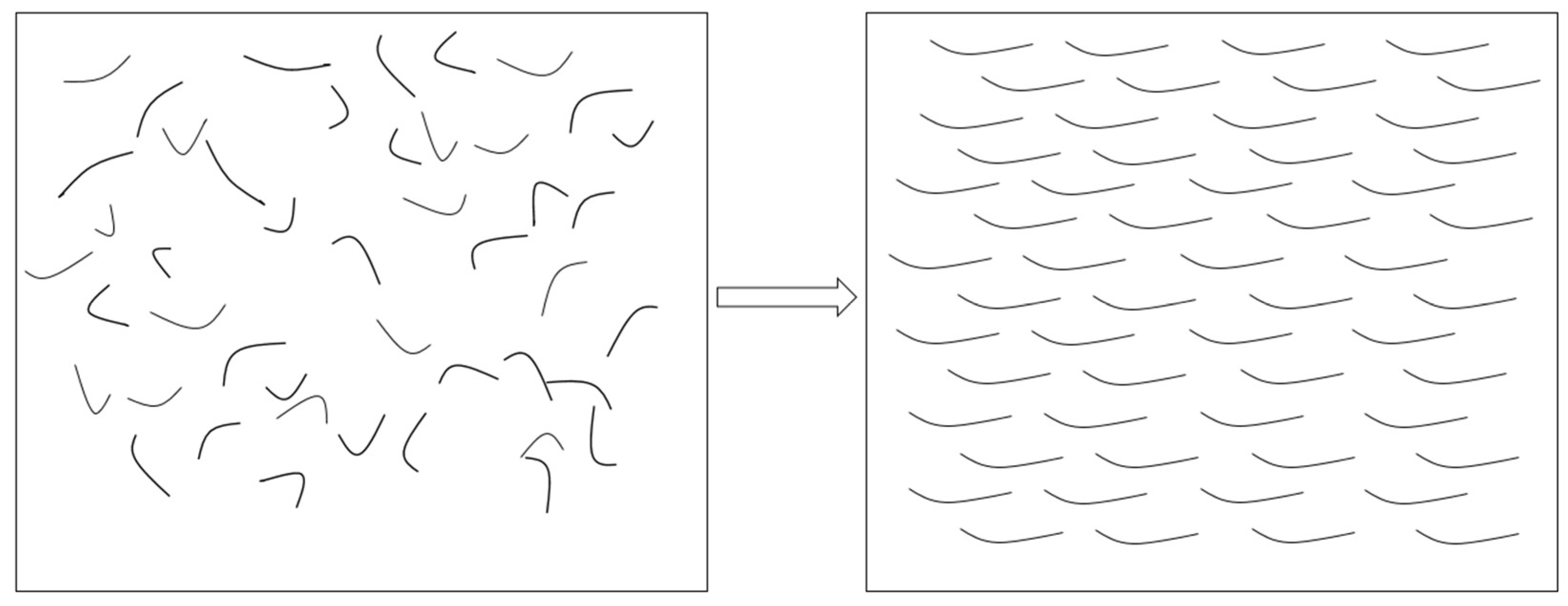
3.2. Electrothermal Application of TiO2/GNs Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abbas, Q.; Shinde, P.A.; Abdelkareem, M.A.; Alami, A.H.; Mirzaeian, M.; Yadav, A.; Olabi, A.G. Graphene Synthesis Techniques and Environmental Applications. Materials 2022, 15, 7804. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Tian, Y.; Ma, X.; Zhao, M.; Qian, J.; Wang, X. Screen-printed high-performance flexible electrothermal films based on three-dimensional intercalation graphene nanosheets/MWCNT/carbon black composite. ACS Appl. Mater. Interfaces 2020, 12, 48077–48083. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yu, C.; Zhang, L.; Zhang, Y.; Wei, B.; Liu, J.; Zhang, Y. High-Performance Electrothermal Film Based on Laser-Induced Graphene. Adv. Eng. Mater. 2022, 24, 2200368. [Google Scholar] [CrossRef]
- Shao, C.; Li, X.; Lin, S.; Zhuo, B.; Yang, S.; Yuan, Q. Characterization of nanocellulose–graphene electric heating membranes prepared via ultrasonic dispersion. J. Mater. Sci. 2020, 55, 421–437. [Google Scholar] [CrossRef]
- Li, X.; Shao, C.; Zhuo, B.; Yang, S.; Zhu, Z.; Su, C.; Yuan, Q. The use of nanofibrillated cellulose to fabricate a homogeneous and flexible graphene-based electric heating membrane. Int. J. Biol. Macromol. 2019, 139, 1103–1116. [Google Scholar] [CrossRef]
- Shao, C.; Zhu, Z.; Su, C.; Yang, S.; Yuan, Q. Thin Electric Heating Membrane Constructed with a Three-Dimensional Nanofibrillated Cellulose–Graphene–Graphene Oxide System. Materials 2018, 11, 1727. [Google Scholar] [CrossRef]
- Ren, K.; Liu, Z.; Wei, T.; Fan, Z. Recent developments of transition metal compounds-carbon hybrid electrodes for high energy/power supercapacitors. Nano-Micro Lett. 2021, 13, 237–268. [Google Scholar] [CrossRef]
- Wu, J.; Wang, H.; Qiu, J.; Shao, J.; Zhang, K.; Yan, L. Electrochemical exfoliation for few-layer graphene in molybdate aqueous solution and its application for fast electrothermal film. Prog. Nat. Sci. 2020, 30, 312–320. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, H.; Hu, S.; Song, J.; Zhang, X.; Hu, T.; Ye, H.; Xu, L. Ultraviolet light crosslinked graphene/multi-walled carbon nanotube hybrid films for highly robust, efficient and flexible electrothermal heaters. Compos. Sci. Technol. 2021, 221, 109183. [Google Scholar] [CrossRef]
- Chen, W.; Yang, S.; Wang, H.; Yang, K.; Wu, X.; Gao, F.; Zheng, B.; Qian, K.; Yao, W.; Zhang, T.; et al. Flexible, stretchable, waterproof (IPX7) electro-thermal films based on graphite nanoplatelets & polyurethane nanocomposites for wearable heaters. Chem. Eng. J. 2022, 431, 133990. [Google Scholar]
- Jayanthi, S.; Shenbagavalli, S.; Muthuvinayagam, M.; Sundaresan, B. Effect of nano TiO2 on the thransport, structural and thermal properties of PEMA-NaI solid polymer electrolytes for energy storage devices. Mater. Sci. Eng. B 2022, 285, 115942. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, M.J.; Min, Y.; Xu, M.; Mei, Z.; Liang, J.; Hu, J.; Yuan, S.; Xiao, S.; Duan, Y.; et al. Controllable formation of (004)-orientated Nb: TiO2 for high-performance transparent conductive oxide thin films with tunable near-infrared transmittance. ACS Appl. Mater. Interfaces 2017, 9, 29021–29029. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Hwang, J. rGO-wrapped Ag-doped TiO2 nanofibers for photocatalytic CO2 reduction under visible light. J. Clean. Prod. 2022, 374, 134022. [Google Scholar] [CrossRef]
- Niu, R.; Han, R.; Tang, S.; Zhu, J. Microwave selective heating ultrafast construction of coral-like TiO2-MXene/graphene hybrid architectures for high-performance lithium-ion battery. J. Power Sources 2022, 542, 231738. [Google Scholar] [CrossRef]
- Chen, X.; Zhan, Y.; Sun, A.; Feng, Q.; Yang, W.; Dong, H.; Chen, Y.; Zhang, Y. Anchoring the TiO2@ crumpled graphene oxide core–shell sphere onto electrospun polymer fibrous membrane for the fast separation of multi-component pollutant-oil–water emulsion. Sep. Purif. Technol. 2022, 298, 121605. [Google Scholar] [CrossRef]
- Boaz, P.T.; Livonia, M. Glass Sheets Carrying Water Based Paint 1. U.S. Patent No. 5582920, 10 December 1996. [Google Scholar]
- Ansari, A.R.; Ansari, S.A.; Parveen, N.; Ansari, M.O.; Osman, Z. Silver nanoparticles decorated on the surface of reduced graphene oxide coated titanium oxide nanocomposite for enhanced electrochemical supercapacitance performance. Ionics 2022, 28, 4793–4804. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, C.; Ma, Y.; Zhong, B.; Li, X.; Zhang, P. ZIF-67/GNs derived Co3O4/GNs multilayer flower and porous structure as an efficient electromagnetic wave absorbing material for excellent absorbing properties. Appl. Surf. Sci. 2022, 575, 151789. [Google Scholar] [CrossRef]
- Fu, H.; Cai, H.; Gray, K.A. Metal oxide encapsulated by 3D graphene oxide creates a nanocomposite with enhanced organic adsorption in aqueous solution. J. Hazard. Mater. 2023, 444, 130340. [Google Scholar] [CrossRef]
- Taheri, M.; Maaref, S.; Kantzas, A.; Bryant, S.; Trudel, S. Improving the colloidal stability of PEGylated BaTiO3 nanoparticles with surfactants. Chem. Phys. 2023, 564, 111701. [Google Scholar] [CrossRef]
- Gao, P.; Liu, W.; Yang, J. Using “intercalation bridging” to effectively improve the electrothermal properties of sheet graphite/ultrafine carbon powder conductive paste for screen printing. J. Mater. Sci. Mater. Electron. 2022, 33, 17599–17618. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, H.; Liu, G.; Su, Z.; Wu, J.; Liu, J.; Zhang, X.; Chen, Y.; Zhou, W. Light-weight, flexible, low-voltage electro-thermal film using graphite nanoplatelets for wearable/smart electronics and deicing devices. J. Alloys Compd. 2017, 699, 1049–1056. [Google Scholar] [CrossRef]
- Peng, T.; Si, Y.; Qian, J.; Zhang, Z.; Yan, X.; Zhu, C.; Hong, X. Reduced graphene oxide/MnFe2O4 nanocomposite papers for fast electrical heating and microwave absorption. Appl. Surf. Sci. 2023, 613, 156001. [Google Scholar] [CrossRef]
- Tembei, S.A.; Ali, M.K.; Hessein, A.; El-Bab, A.M.F.; Abd El-Moneim, A. High-performance flexible electrothermal Joule heaters from laser reduced FN Co-doped graphene oxide with extended Sp2 networks. FlatChem 2022, 36, 100437. [Google Scholar] [CrossRef]
- Owji, E.; Ostovari, F.; Keshavarz, A. Electro-thermal properties and characterization of the flexible polyurethane-graphene nanocomposite films. Phys. Scr. 2022, 97, 105704. [Google Scholar] [CrossRef]


| Composition | Sodium Silicate | Water | Silicone Oil | MgO | NaOH | Sb2O3 |
|---|---|---|---|---|---|---|
| Contains | 65 g | 60 g | 0.3 g | 0.3 g | 0.1 g | 0.2 g |
| Element | Weight % | Atomic % | Net Int. | Error % | K-Ratio |
|---|---|---|---|---|---|
| C | 63.30 | 74.44 | 3109.42 | 4.74 | 0.4307 |
| O | 25.06 | 22.13 | 526.64 | 10.90 | 0.0316 |
| Ti | 11.63 | 3.43 | 1275.51 | 2.46 | 0.0955 |
| Sample | Voltage/Power | Max Temperature (°C) | Speed | Reference |
|---|---|---|---|---|
| Graphene (GE)/UC film | 30 V | 199 | - | [21] |
| GNSs/MWCNTs/CB | 3 V | 175 | 17.5 °C/s | [2] |
| laser-induced graphene/PI flim | - | 270 | 27 °C/s | [3] |
| water-dispersible graphene films | 10 V | 147 | 11.8 °C/s | [8] |
| GNP/PU film | 5–24 V | - | 25 °C/min | [10] |
| GNP films | 3–5 V | - | 25–65 °C/min | [22] |
| graphene sheets/NFC membrane | 2000 W·m−2 | 60 | 20 °C/min | [5] |
| RGO/MnFe2O4 paper | 0.35 A | 100.2 | 6.68 °C/s | [23] |
| F-N Co-doped laser reduced GO | 9 V | 365 | 385.33 °C/s | [24] |
| polyurethane/graphene | 22 V | 75 | - | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Tian, W.; Kang, S.; Zhong, B.; Qin, C.; Wang, H. Hydrothermal Preparation of TiO2/Graphite Nanosheets Composites and Its Effect on Electrothermal Behavior. Coatings 2023, 13, 226. https://doi.org/10.3390/coatings13020226
Wang C, Tian W, Kang S, Zhong B, Qin C, Wang H. Hydrothermal Preparation of TiO2/Graphite Nanosheets Composites and Its Effect on Electrothermal Behavior. Coatings. 2023; 13(2):226. https://doi.org/10.3390/coatings13020226
Chicago/Turabian StyleWang, Chunyu, Weiyao Tian, Sibo Kang, Bo Zhong, Chunlin Qin, and Hongyang Wang. 2023. "Hydrothermal Preparation of TiO2/Graphite Nanosheets Composites and Its Effect on Electrothermal Behavior" Coatings 13, no. 2: 226. https://doi.org/10.3390/coatings13020226
APA StyleWang, C., Tian, W., Kang, S., Zhong, B., Qin, C., & Wang, H. (2023). Hydrothermal Preparation of TiO2/Graphite Nanosheets Composites and Its Effect on Electrothermal Behavior. Coatings, 13(2), 226. https://doi.org/10.3390/coatings13020226