Cissus antractica-ZnO NPs Induce Apoptosis in A549 Cells through ROS-Generated p53/Bcl-2/Bax Signaling Pathways and Inhibition of Inflammatory Cytokines
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
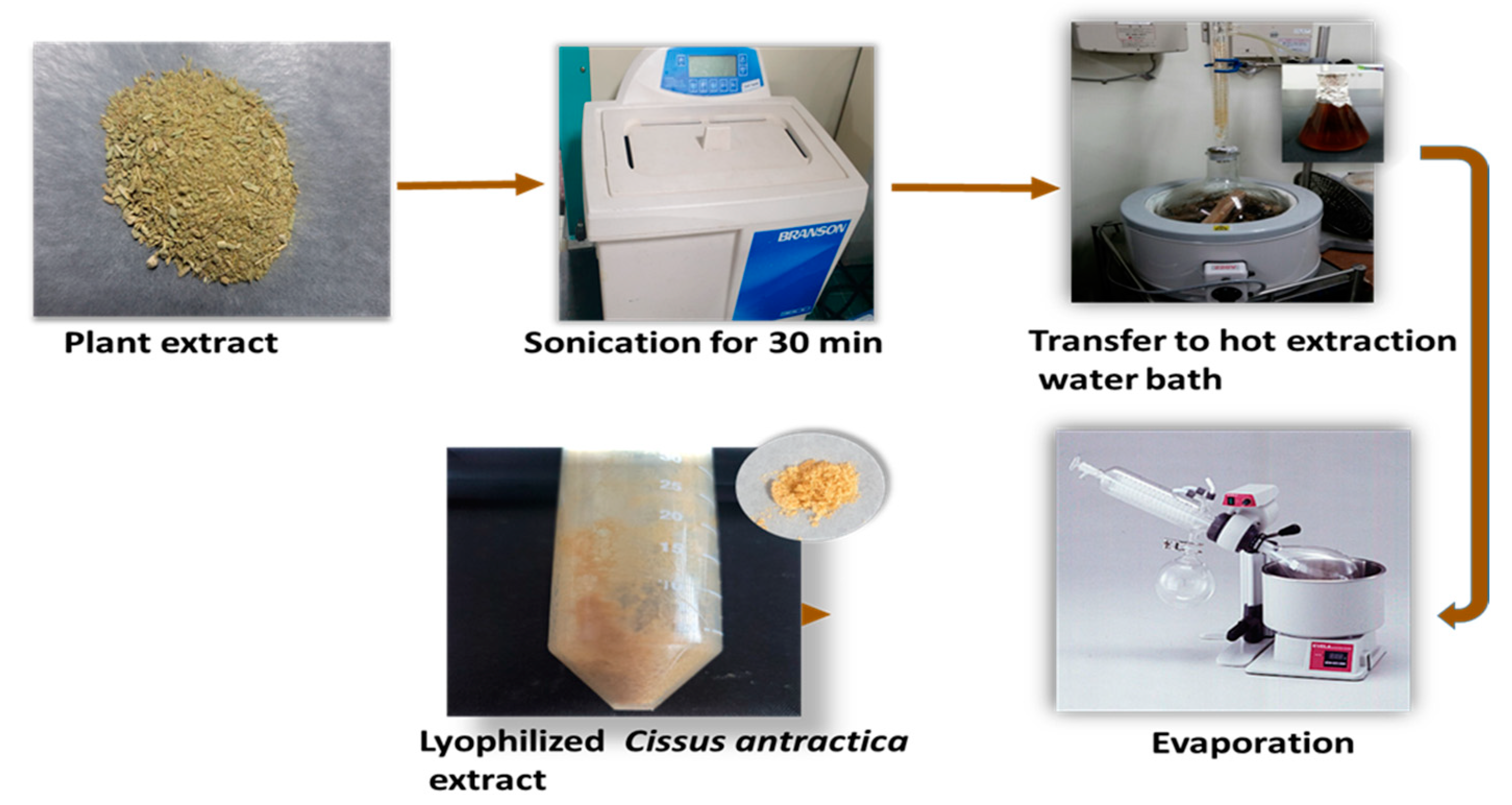
2.2. Preparation of Cissus antractica Water Extract
2.3. ZnO NPs Using Co-Precipitation Method
2.4. Characterization of ZnO Nanoparticles
2.4.1. UV-Vis Spectrophotometry
2.4.2. FT-IR Spectroscopy
2.4.3. XRD Analysis
2.4.4. FE-TEM Analysis
2.5. Cell Culture
2.6. Cytotoxicity Assay
2.7. Reactive Oxygen Species (ROS) Assay
2.8. Colony Formation Assay
2.9. Wound-Healing Assay
2.10. Hoechst Staining
2.11. PI Staining
2.12. Quantitative Reverse Transcription PCR (qRT-PCR)
3. Results and Discussion
3.1. Synthesis of CA-ZnO NPs
3.2. Characterization of CA-ZnO NPs
3.3. UV-Vis Spectral Analysis
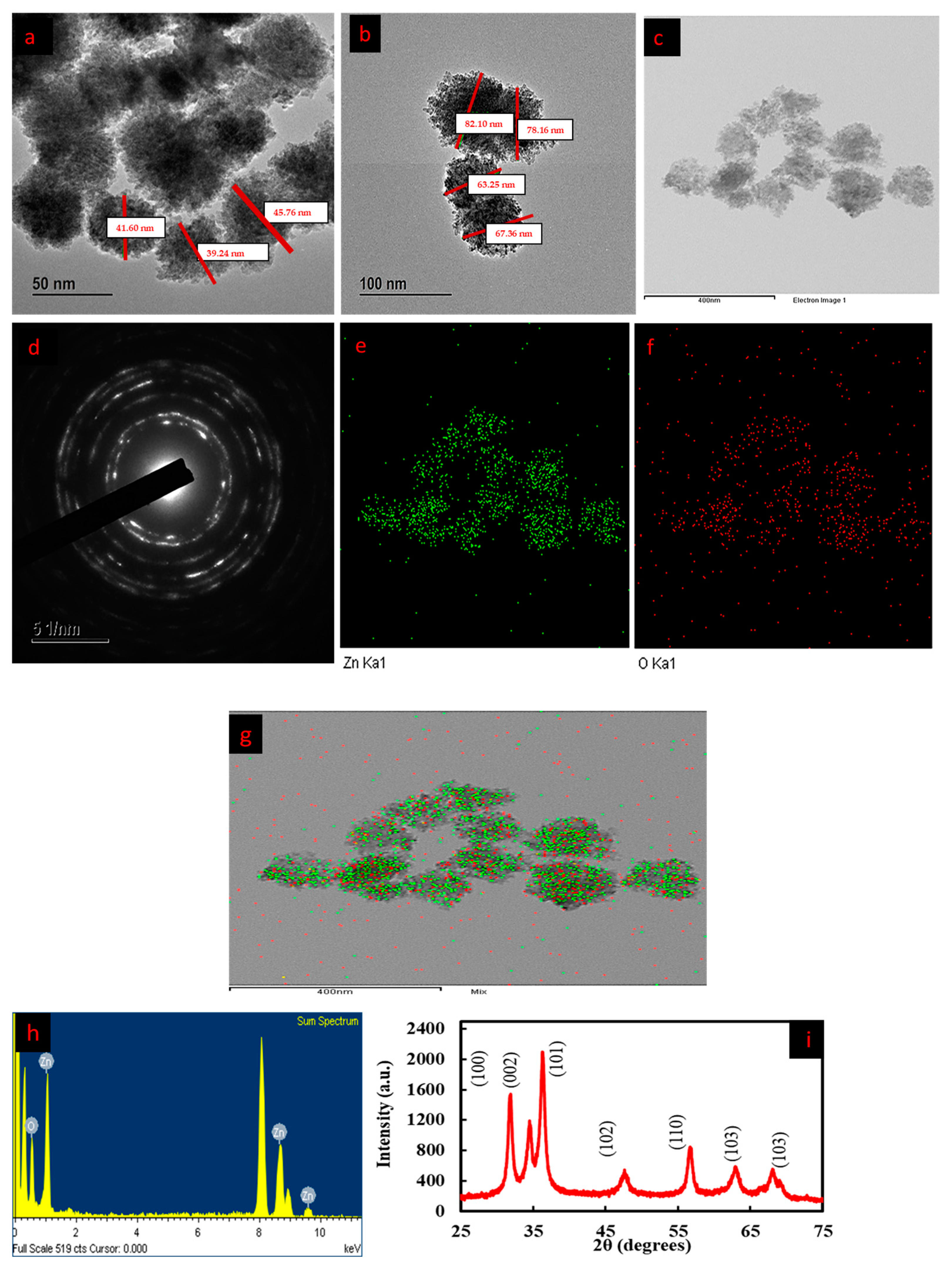
3.4. FE-TEM and EDX Analysis
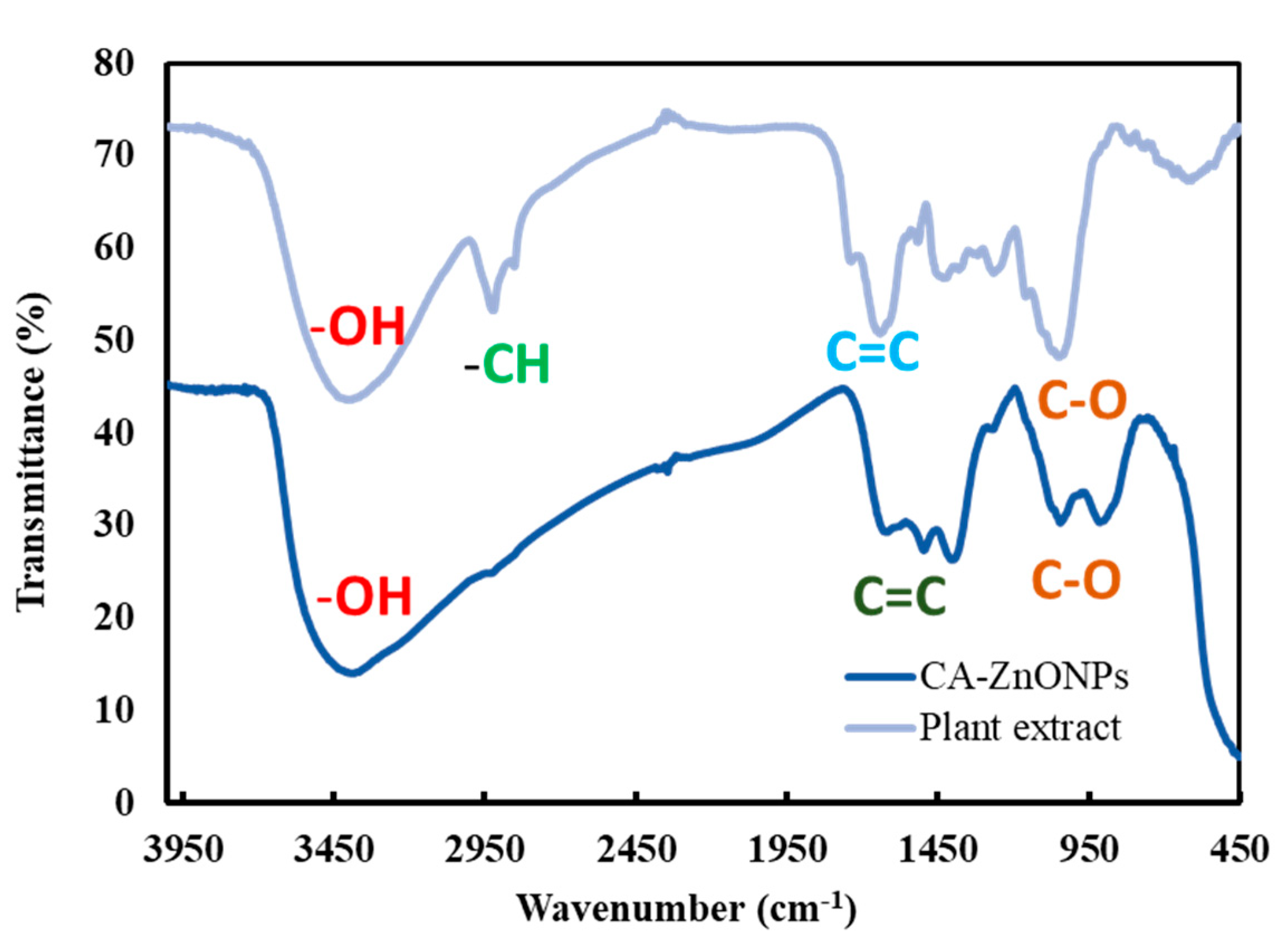
3.5. Fourier-Transform Infrared (FT-IR) Spectroscopy Analysis
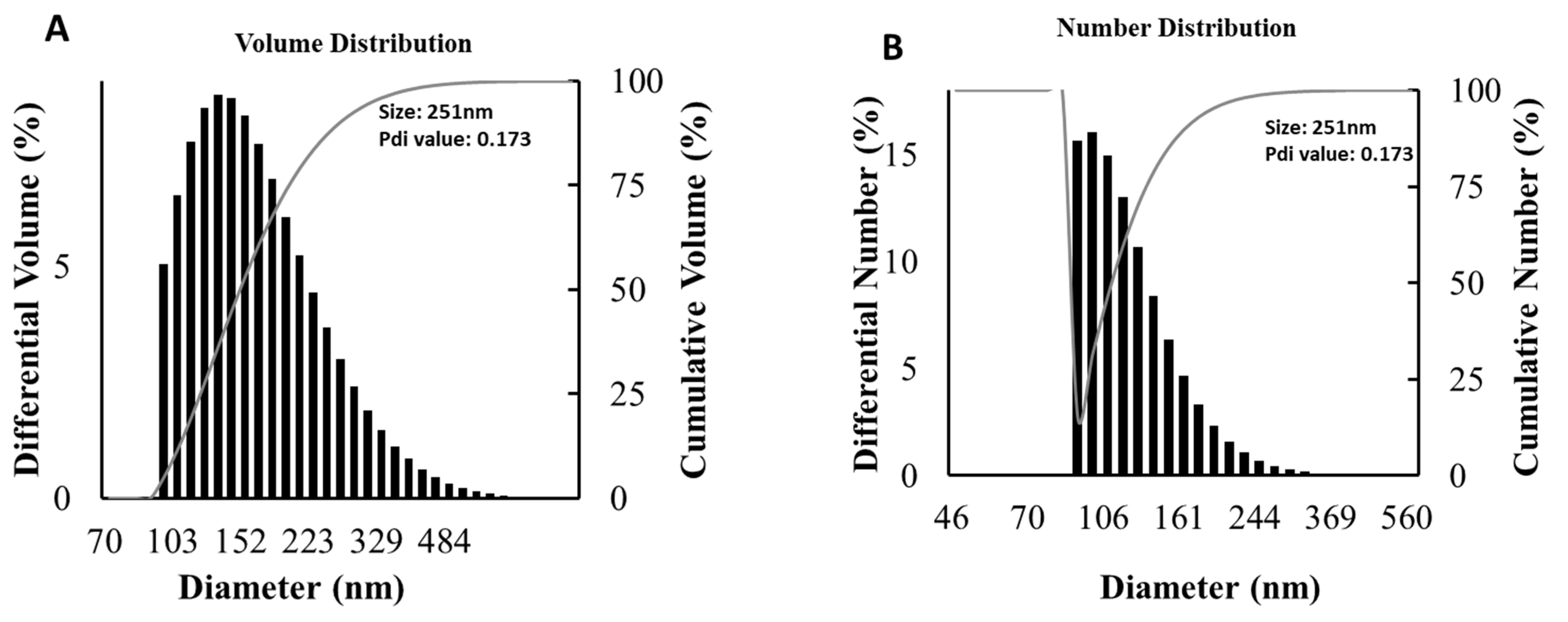
3.6. Particle Size Distribution Analysis
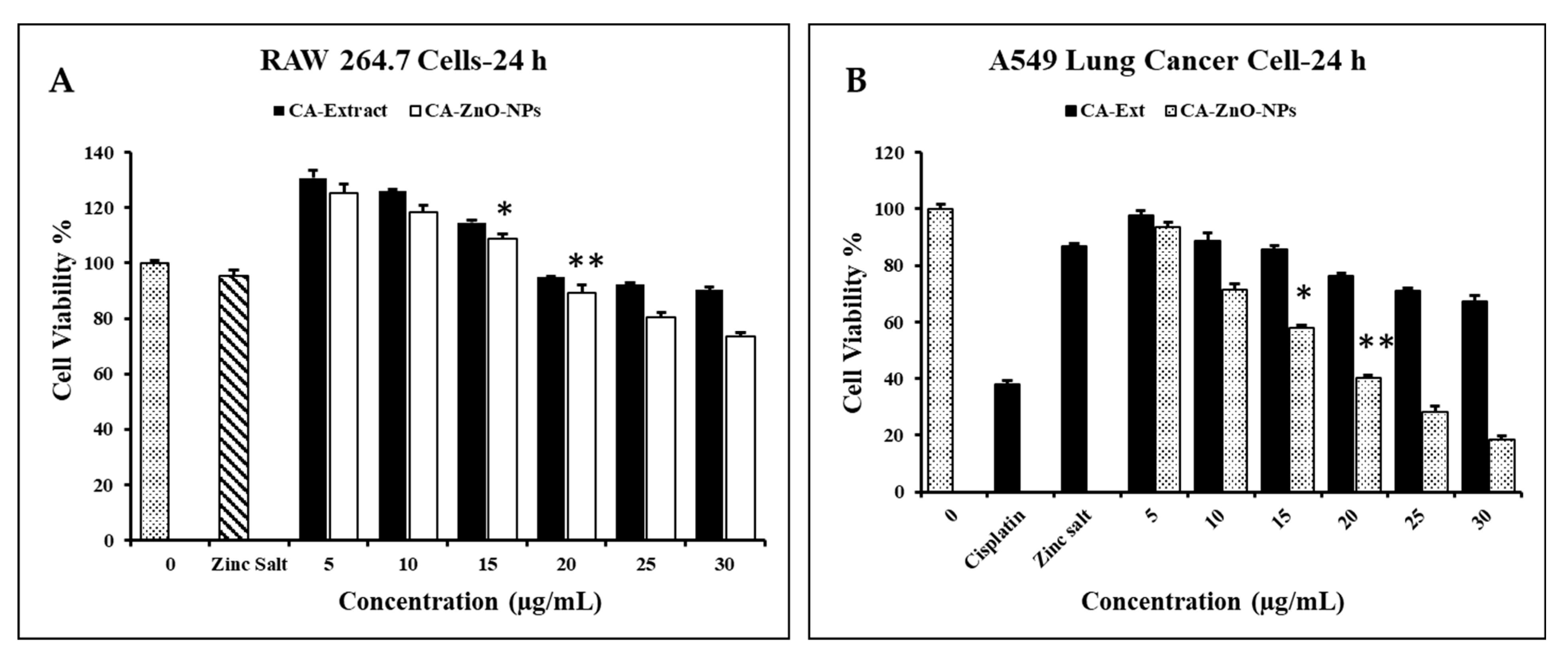
3.7. Evaluation of Cell Cytotoxicity
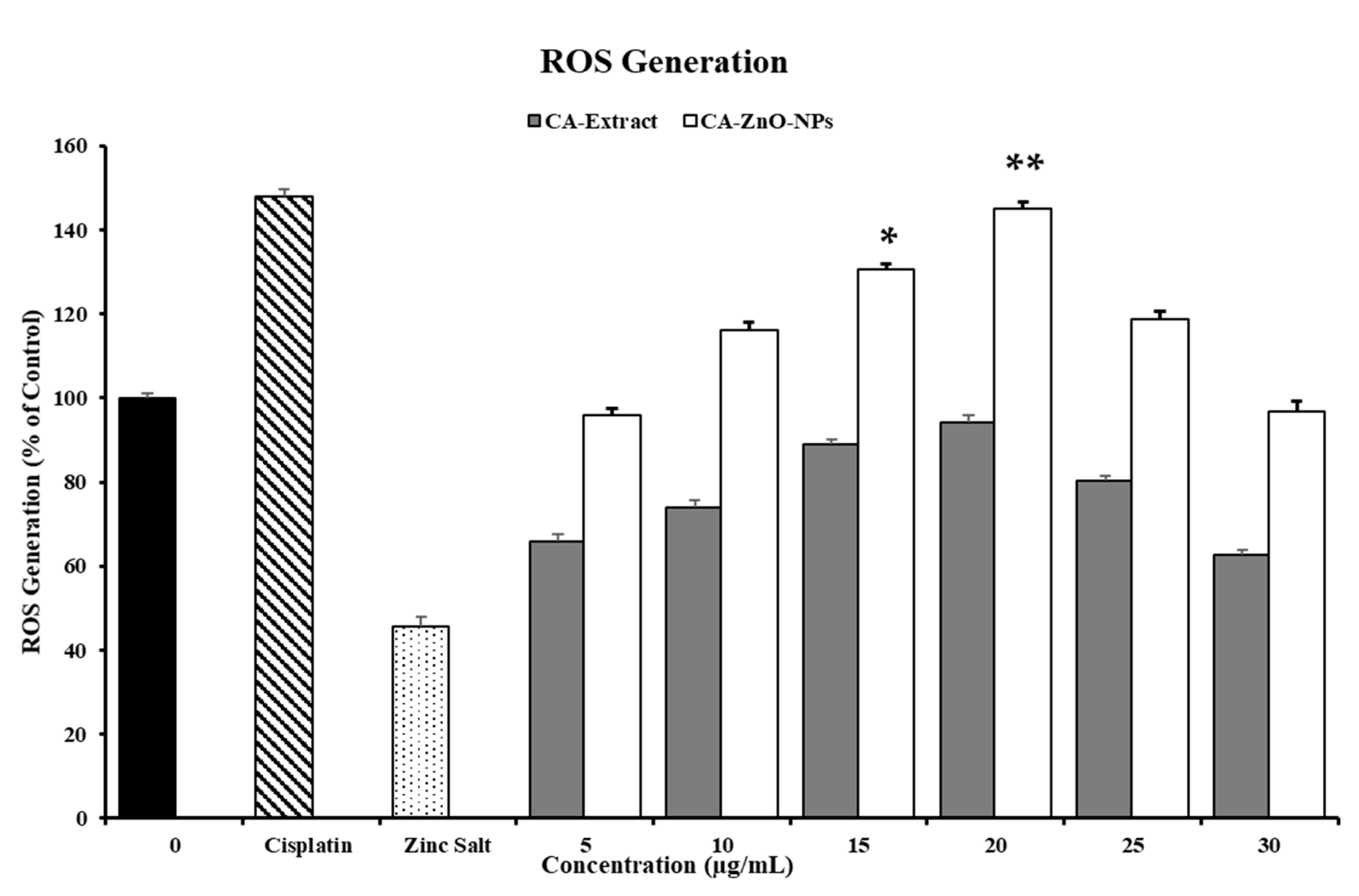
3.8. In Vitro ROS Induced by CA-ZnO NPs in Cancer Cells
3.9. Inhibition of Colony Formation of Cancer Cells
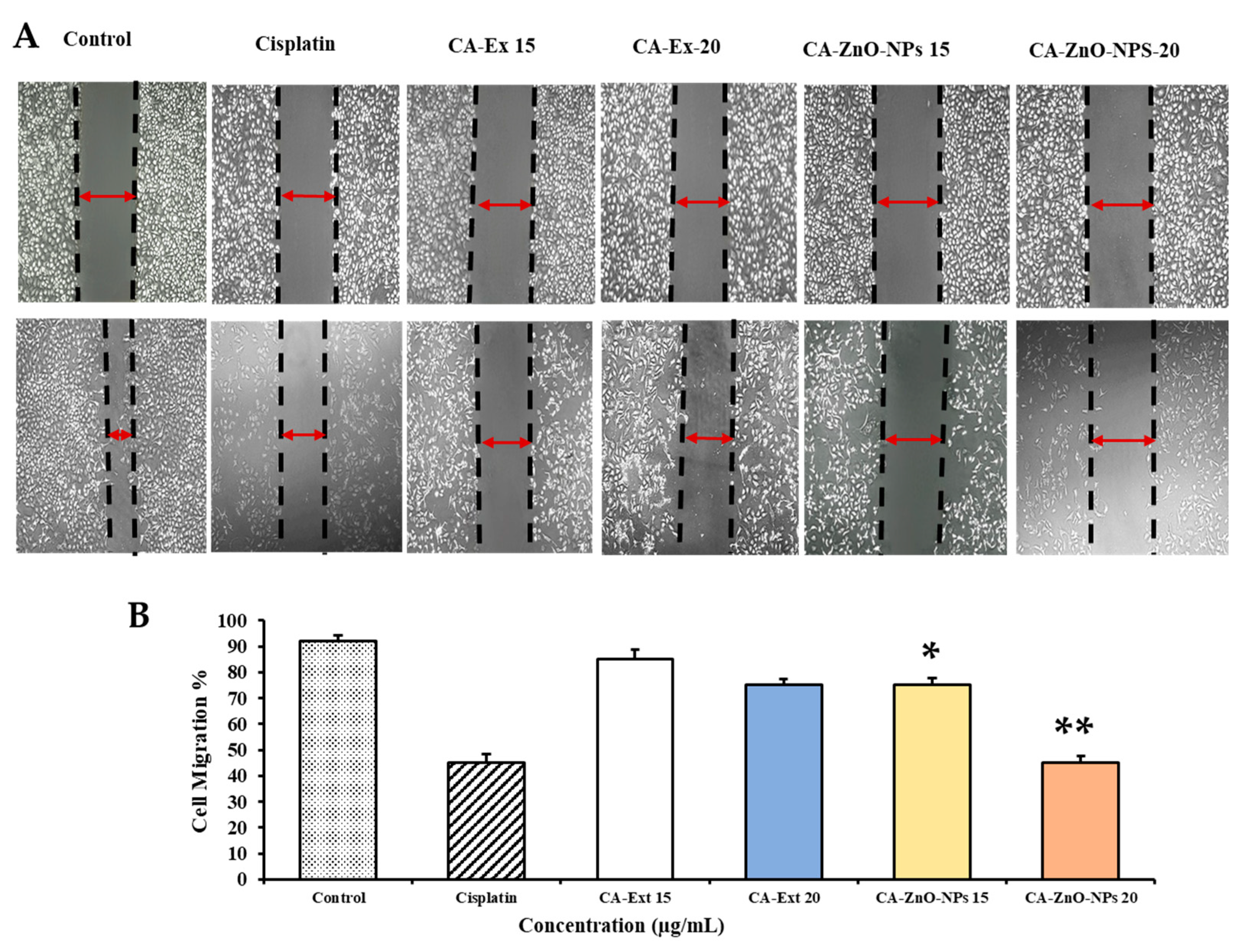
3.10. CA-ZnO NPs Inhibit Migration of Cancer Cells
3.11. Investigation of CA-ZnO-NP-Induced Apoptosis by Hoechst-33342/PI Dye Staining
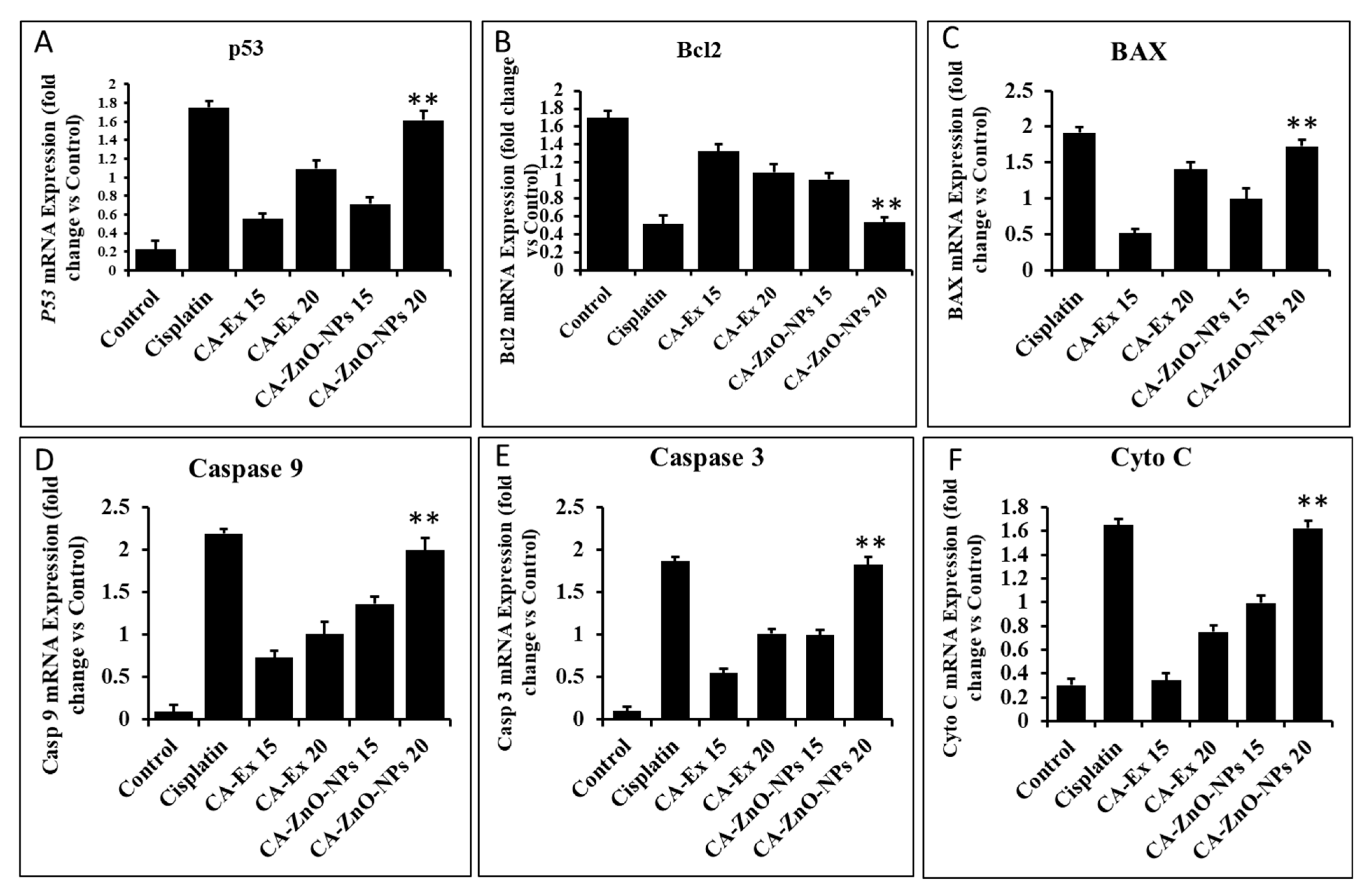
3.12. By Controlling Apoptotic Gene Expression, CA-ZnO NPs Induced Apoptosis
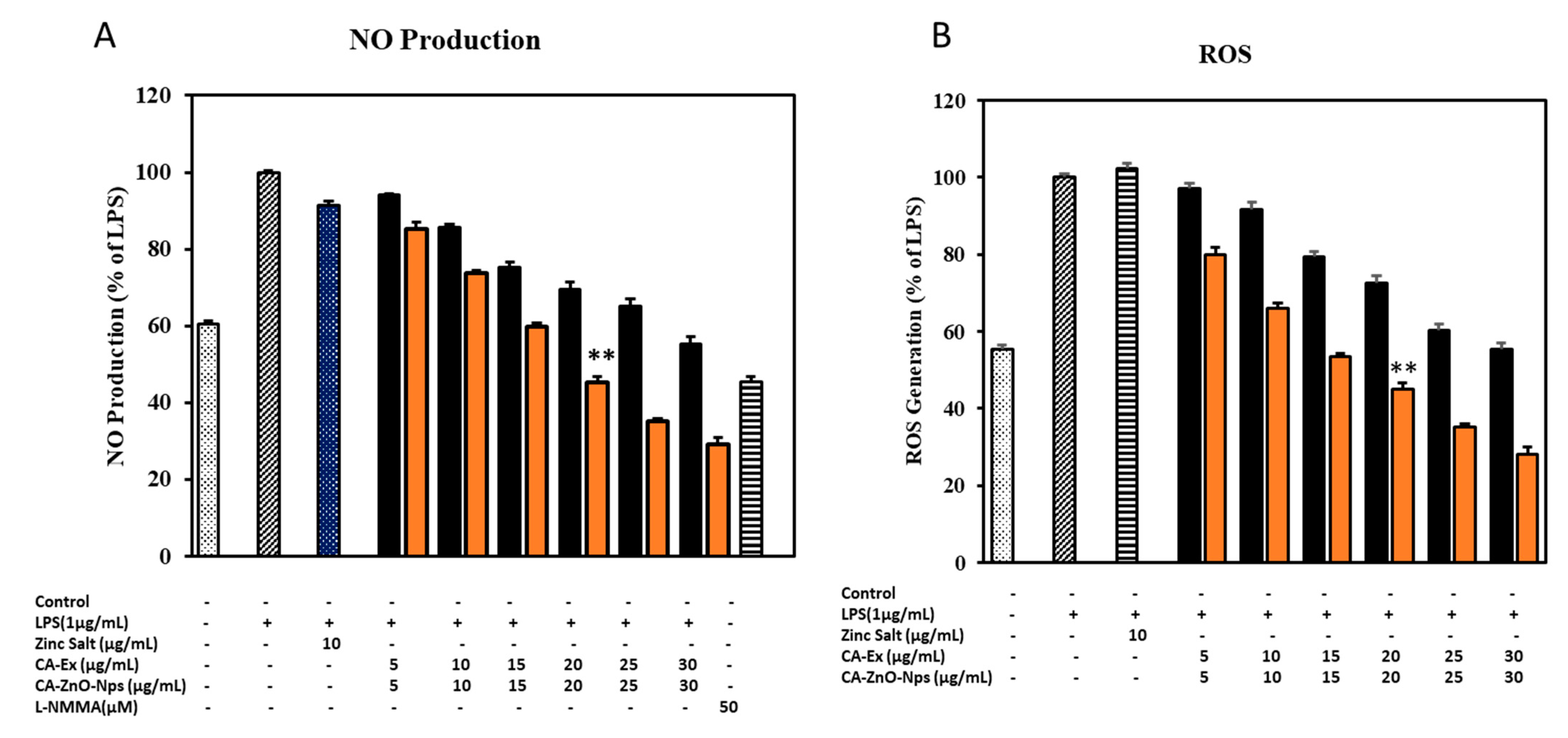
3.13. CA-ZnO NPs Increased NO Production while Inhibiting ROS Generation Caused by LPS
3.14. The Effect of CA-ZnO NPs on Inflammatory Cytokines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Medzhitov, R. Inflammation 2010: New adventures of an old flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Ding, A. Nonresolving Inflammation. Cell 2010, 140, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Kumar, D.; Bhat, Z.A.; Singh, P.; Bhujbal, S.S.; Mudgade, S.C.; Deoda, R.S. Antiallergic and anti-inflammatory properties of methanolic extract of stem bark of Ailanthus excelsa Roxb. Lat. Am. J. Pharm. 2010, 29, 1289–1295. [Google Scholar]
- Kumar, V.; Bhat, Z.A.; Kumar, D.; Khan, N.; Chashoo, I. Evaluation of anti-inflammatory potential of leaf extracts of Skimmia anquetilia. Asian Pac. J. Trop. Biomed. 2012, 2, 627–630. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, R.; Maheshwari, S.U.; Harish, G.; Raj, J.B.; Kamali, S.; Hemamalani, D.; Varma, J.B.; Reddy, C.U. Investigation of in-vitro anti-inflammatory, anti-platelet and anti-arthritic activities in the leaves of Anisomeles malabarica Linn. Biol. Chem. Sci. 2010, 1, 745–752. [Google Scholar]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I. GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- US Preventive Services Task Force. Screening for lung cancer: US Preventive Services Task Force recommendation statement. JAMA 2021, 325, 962–970. [Google Scholar] [CrossRef]
- Lee, S.-B.; Lee, J.-S.; Moon, S.-O.; Lee, H.-D.; Yoon, Y.-S.; Son, C.-G. A standardized herbal combination of Astragalus membranaceus and Paeonia japonica, protects against muscle atrophy in a C26 colon cancer cachexia mouse model. J. Ethnopharmacol. 2021, 267, 113470. [Google Scholar] [CrossRef] [PubMed]
- Alsharairi, N.A. The effects of dietary supplements on asthma and lung cancer risk in smokers and non-smokers: A review of the literature. Nutrients 2019, 11, 725. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Zhang, D.; Ma, X.-L.; Gu, Y.; Huang, H.; Zhang, G.-W. Green synthesis of metallic nanoparticles and their potential applications to treat cancer. Front. Chem. 2020, 8, 799. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Yang, J.; Yang, Q.; Yuan, W. Enantioselective reduction of fluorenones in surfactant-aqueous solution by fruits and vegetables. J. Mol. Catal. B Enzym. 2009, 61, 284–288. [Google Scholar] [CrossRef]
- Hameed, S.; Iqbal, J.; Ali, M.; Khalil, A.T.; Abbasi, B.A.; Numan, M.; Shinwari, Z.K. Green synthesis of zinc nanoparticles through plant extracts: Establishing a novel era in cancer theranostics. Mater. Res. Express 2019, 6, 102005. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Anusuya, S.; Anbazhagan, V. Anticancer, anti-diabetic, antimicrobial activity of zinc oxide nanoparticles: A comparative analysis. J. Mol. Struct. 2022, 1263, 133139. [Google Scholar] [CrossRef]
- Velsankar, K.; Venkatesan, A.; Muthumari, P.; Suganya, S.; Mohandoss, S.; Sudhahar, S. Green inspired synthesis of ZnO nanoparticles and its characterizations with biofilm, antioxidant, anti-inflammatory, and anti-diabetic activities. J. Mol. Struct. 2022, 1255, 132420. [Google Scholar] [CrossRef]
- Prashanth, G.; Prashanth, P.; Nagabhushana, B.; Ananda, S.; Krishnaiah, G.; Nagendra, H.; Sathyananda, H.; Rajendra Singh, C.; Yogisha, S.; Anand, S.; et al. Comparison of anticancer activity of biocompatible ZnO nanoparticles prepared by solution combustion synthesis using aqueous leaf extracts of Abutilon indicum, Melia azedarach and Indigofera tinctoria as biofuels. Artif. Cells Nanomed. Biotechnol. 2018, 46, 968–979. [Google Scholar] [CrossRef]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Balandrin, M.F.; Kinghorn, A.D.; Farnsworth, N.R. Plant-derived natural products in drug discovery and development: An overview. ACS Symp. Ser. 1993, 534, 2–12. [Google Scholar]
- Yang, D.-C.; Kang, S.C.; Jung, D.-H.; Nahar, J.; Mathiyalagan, R.; Rupa, E.J.; Ramadhania, Z.M.; Han, Y. A Focused Review on Molecular Signalling Mechanisms of Ginsenosides Anti-Lung Cancer and Anti-inflammatory Activities. Anti-Cancer Agents Med. Chem. 2022, 23, 3–14. [Google Scholar] [CrossRef]
- Liu, X.-Q.; Ickert-Bond, S.M.; Chen, L.-Q.; Wen, J. Molecular phylogeny of Cissus L. of Vitaceae (the grape family) and evolution of its pantropical intercontinental disjunctions. Mol. Phylogenetics Evol. 2013, 66, 43–53. [Google Scholar] [CrossRef]
- Gerrath, J.M.; Posluszny, U. Morphological and anatomical development in the Vitaceae. VI. Cissus antarctica. Can. J. Bot. 1994, 72, 635–643. [Google Scholar] [CrossRef]
- Balasubramanian, P.; Rajasekaran, A.; Prasad, S.N. Folk medicine of the irulas of Coimbatore forests. Anc. Sci. Life 1997, 16, 222–226. [Google Scholar] [PubMed]
- Manokari, M.; Shekhawat, M.S. An updated review on Cissus vitiginea L.(Family: Vitaceae)-An important medicinal climber. World News Nat. Sci. 2019, 22, 75–83. [Google Scholar]
- Payani, S.; Bhaskar, M.; Kumar, G.S.; Pradeepkiran, J.A. A study on antimicrobial and anticancer properties of Cissus quadrangulris using lung cancer cell line. Cancer Treat. Res. Commun. 2023, 36, 100732. [Google Scholar] [CrossRef]
- Chauhan, A.; Kumar, A. In-silico Molecular Interaction Studies of Biologically Active Secondary Metabolites of Cissus quadrangularis L. as a Potential Anti-cancer Drug. Int. J. Plant Environ. 2023, 9, 168–171. [Google Scholar] [CrossRef]
- Méndez López, L.F. Metabolomic Profile and Bioassay-Guided Phytochemical Analysis of the Stems from Cissus trifoliata, Evaluation of Their Antibacterial and Cytotoxic Activity, and Determination of the Mechanism of Action of One Active Compound. Doctoral Dissertation, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, Mexico, 2020. [Google Scholar]
- Purohit, S.; Pancholi, D.; Kunwar, N.; Choudhary, R.; Jain, R. Characterization of AgNPs biosynthesized from stem and leaf extracts of Cissus quadrangularis and C. rotundifolia. AIP Conf. Proc. 2023, 2752, 030003. [Google Scholar]
- Gupta, A.; Mehta, S.K.; Qayoom, I.; Gupta, S.; Singh, S.; Kumar, A. Biofunctionalization with Cissus quadrangularis Phytobioactives Accentuates Nano-Hydroxyapatite Based Ceramic Nano-Cement for Neo-Bone Formation in Critical Sized Bone Defect. Int. J. Pharm. 2023, 642, 123110. [Google Scholar] [CrossRef]
- Jin, Y.; Huynh, D.T.N.; Myung, C.-S.; Heo, K.-S. Ginsenoside Rh1 Prevents Migration and Invasion through Mitochondrial ROS-Mediated Inhibition of STAT3/NF-κB Signaling in MDA-MB-231 Cells. Int. J. Mol. Sci. 2021, 22, 10458. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-P.; Hsieh, C.-H.; Liu, C.-Y.; Lin, K.-H.; Wu, P.-T.; Chen, K.-M.; Fang, K. Reactive oxygen species-driven mitochondrial injury induces apoptosis by teroxirone in human non-small cell lung cancer cells. Oncol. Lett. 2017, 14, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Fowsiya, J.; Madhumitha, G.; Al-Dhabi, N.A.; Arasu, M.V. Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 162, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Goutam, S.P.; Yadav, A.K.; Das, A.J. Coriander extract mediated green synthesis of zinc oxide nanoparticles and their structural, optical and antibacterial properties. J. Nanosci. Technol. 2017, 3, 249–252. [Google Scholar]
- Rupa, E.J.; Kaliraj, L.; Abid, S.; Yang, D.-C.; Jung, S.-K. Synthesis of a zinc oxide nanoflower photocatalyst from sea buckthorn fruit for degradation of industrial dyes in wastewater treatment. Nanomaterials 2019, 9, 1692. [Google Scholar] [CrossRef]
- Kim, W.J.; Soshnikova, V.; Markus, J.; Oh, K.H.; Anandapadmanaban, G.; Mathiyalagan, R.; Perez, Z.E.J.; Kim, Y.J.; Yang, D.C. Room temperature synthesis of germanium dioxide nanorods and their in vitro photocatalytic application. Optik 2019, 178, 664–668. [Google Scholar] [CrossRef]
- Bisht, G.; Rayamajhi, S. ZnO nanoparticles: A promising anticancer agent. Nanobiomedicine 2016, 3, 9. [Google Scholar] [CrossRef]
- Shen, C.; James, S.A.; de Jonge, M.D.; Turney, T.W.; Wright, P.F.; Feltis, B.N. Relating cytotoxicity, zinc ions, and reactive oxygen in ZnO nanoparticle–exposed human immune cells. Toxicol. Sci. 2013, 136, 120–130. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc oxide nanoparticles: A promising nanomaterial for biomedical applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Truong-Tran, A.Q.; Carter, J.; Ruffin, R.; Zalewski, P.D. New insights into the role of zinc in the respiratory epithelium. Immunol. Cell Biol. 2001, 79, 170–177. [Google Scholar] [CrossRef]
- Sharma, S.; Pujari, P.; Sudarshan, K.; Dutta, D.; Mahapatra, M.; Godbole, S.; Jayakumar, O.; Tyagi, A. Positron annihilation studies in ZnO nanoparticles. Solid State Commun. 2009, 149, 550–554. [Google Scholar] [CrossRef]
- Rasmussen, J.W.; Martinez, E.; Louka, P.; Wingett, D.G. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin. Drug Deliv. 2010, 7, 1063–1077. [Google Scholar] [CrossRef] [PubMed]
- Sarmiento-Salinas, F.L.; Perez-Gonzalez, A.; Acosta-Casique, A.; Ix-Ballote, A.; Diaz, A.; Treviño, S.; Rosas-Murrieta, N.H.; Millán-Perez-Peña, L.; Maycotte, P. Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci. 2021, 284, 119942. [Google Scholar] [CrossRef]
- Dröse, S.; Brandt, U. Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 748, pp. 145–169. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging biological principles of metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, S.; Lallemand, B.; Dubois, J. Induction of phase II enzymes glutathione-s-transferase and NADPH: Quinone oxydoreductase 1 with novel sulforaphane derivatives in human keratinocytes: Evaluation of the intracellular GSH level. Pharmacol. Pharm. 2014, 5, 937–943. [Google Scholar] [CrossRef][Green Version]
- Rani, N.; Saini, K. Biogenic metal and metal oxides nanoparticles as anticancer agent: A review. Proc. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1225, 012043. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis, N.H.; Naidu, R. Mechanism of apoptosis induced by curcumin in colorectal cancer. Int. J. Mol. Sci. 2019, 20, 2454. [Google Scholar] [CrossRef]
- George, B.P.; Abrahamse, H. Increased oxidative stress induced by Rubus bioactive compounds induce apoptotic cell death in human breast cancer cells. Oxidative Med. Cell. Longev. 2019, 2019, 6797921. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y.; St. Clair, D.K. ROS and p53: A versatile partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535. [Google Scholar] [CrossRef]
- Omoyeni, O.A.; Hussein, A.; Meyer, M.; Green, I.; Iwuoha, E. Pleiocarpa pycnantha leaves and its triterpenes induce apoptotic cell death in Caco-2 cells in vitro. BMC Complement. Altern. Med. 2015, 15, 224. [Google Scholar] [CrossRef][Green Version]
- Kang, M.H.; Reynolds, C.P. Bcl-2 inhibitors: Targeting mitochondrial apoptotic pathways in cancer therapy. Clin. Cancer Res. 2009, 15, 1126–1132. [Google Scholar] [CrossRef]
- Zheng, J.H.; Viacava Follis, A.; Kriwacki, R.W.; Moldoveanu, T. Discoveries and controversies in BCL-2 protein-mediated apoptosis. FEBS J. 2016, 283, 2690–2700. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Yu, X.; Yu, T.; Zheng, X.; Chu, Q. Radix tetrastigma inhibits the non-small cell lung cancer via Bax/Bcl-2/Caspase-9/Caspase-3 pathway. Nutr. Cancer 2022, 74, 320–332. [Google Scholar] [CrossRef]
- Agarwal, H.; Shanmugam, V. A review on anti-inflammatory activity of green synthesized zinc oxide nanoparticle: Mechanism-based approach. Bioorganic Chem. 2020, 94, 103423. [Google Scholar] [CrossRef]
- Rajakumar, G.; Thiruvengadam, M.; Mydhili, G.; Gomathi, T.; Chung, I.-M. Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess Biosyst. Eng. 2018, 41, 21–30. [Google Scholar] [CrossRef]
- Clancy, R.M.; Amin, A.R.; Abramson, S.B. The role of nitric oxide in inflammation and immunity. Arthritis Rheum. 1998, 41, 1141–1151. [Google Scholar] [CrossRef]
- Salvemini, D. Regulation of cyclooxygenase enzymes by nitric oxide. Cell. Mol. Life Sci. 1997, 53, 576–582. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Bao, Y.; Wang, X.; Zhai, J.; Zhan, X.; Zhang, H. Characterization and anti-inflammation of a polysaccharide produced by Chaetomium globosum CGMCC 6882 on LPS-induced RAW 264.7 cells. Carbohydr. Polym. 2021, 251, 117129. [Google Scholar] [CrossRef] [PubMed]
- Šoltés, L.; Kogan, G. Hyaluronan: A Harbinger of the Status and Functionality of the Joint; Apple Academic Press/Taylor & Francis Group: Burlington, ON, Canada; Palm Bay, FL, USA, 2014; pp. 259–286. [Google Scholar]
- Cao, J.; Naeem, M.; Noh, J.-K.; Lee, E.H.; Yoo, J.-W. Dexamethasone phosphate-loaded folate-conjugated polymeric nanoparticles for selective delivery to activated macrophages and suppression of inflammatory responses. Macromol. Res. 2015, 23, 485–492. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Patel, G.; Xue, Q.; Njateng, G.S.S.; Cai, S.; Cheng, G.; Kai, G. In vitro and in vivo anti-inflammatory effects of different extracts from Epigynum auritum through down-regulation of NF-κB and MAPK signaling pathways. J. Ethnopharmacol. 2020, 261, 113105. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, S.-M.; eun Lee, J.; Kim, J.-H.J. Anti-inflammatory activity of mulberry leaf extract through inhibition of NF-κB. J. Funct. Foods 2013, 5, 178–186. [Google Scholar] [CrossRef]





| Gene | Primer Sequences (5′-3′) |
|---|---|
| p53 | F: TCT TGGGCC TGT GTT ATC TCC R: CGC CCA TGC AGG AAC TGT TA |
| Bcl2 | F: GAA GGG CAG CCG TTA GGAAA R: GCG CCC AAT ACG ACC AAA TC |
| BAX | F: GGT TGC CCT CTT CTA CTT T R: AGC CAC CCT GGT CTT G |
| CASPASE 3 | F: GAA GGA ACA CGC CAG GAA AC R: GCA AAG TGA AAT GTA GCA CCA A |
| CASPASE 9 | F: GCC CGA GTT TGA GAG GAA AA R: CAC AGC CAG ACC AGG AC |
| Cyto-c | F: CAGAAGGAAGTTAGGCC R: CGTCGCAGTGGATGATGTG |
| COX-2 | F: CCT GAG CAT CTA CGG TTT GC R: ACT GCT CAT CAC CCC ATT CA |
| TNF-α | F: GCCAGAATGCTGCAGGACTT R: GGCCTAAGGTCCACTTGTGTCA |
| iNOS | F: CCT GAG CAT CTA CGG TTT GC R: ACT GCT CAT CAC CCC ATT CA |
| IL-6 | F: AGGGTTGCCAGATGCAATAC R: AAACCAAGGCACAGTGGAAC |
| IL-8 | F: CCGGAGAGGAGACTTCACAG R: GGAAATTGGGGTAGGAAGGA |
| GAPDH | F: CAA GGT CAT CCA TGA CAA CTT TG R: GTC CAC CAC CCT GTT GCT GTA G |
| Element | Weight % | Atomic % |
|---|---|---|
| O K | 35.36 | 69.08 |
| Zn K | 64.64 | 30.92 |
| Totals | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rupa, E.J.; Nahar, J.; Al-Amin, M.; Park, J.-K.; Murugesan, M.; Awais, M.; Lee, S.-J.; Kim, I.M.; Ling, L.; Yang, D.-C.; et al. Cissus antractica-ZnO NPs Induce Apoptosis in A549 Cells through ROS-Generated p53/Bcl-2/Bax Signaling Pathways and Inhibition of Inflammatory Cytokines. Coatings 2023, 13, 2077. https://doi.org/10.3390/coatings13122077
Rupa EJ, Nahar J, Al-Amin M, Park J-K, Murugesan M, Awais M, Lee S-J, Kim IM, Ling L, Yang D-C, et al. Cissus antractica-ZnO NPs Induce Apoptosis in A549 Cells through ROS-Generated p53/Bcl-2/Bax Signaling Pathways and Inhibition of Inflammatory Cytokines. Coatings. 2023; 13(12):2077. https://doi.org/10.3390/coatings13122077
Chicago/Turabian StyleRupa, Esrat Jahan, Jinnatun Nahar, Md. Al-Amin, Jin-Kyu Park, Mohanapriya Murugesan, Muhammad Awais, Seung-Jin Lee, Il Mun Kim, Li Ling, Deok-Chun Yang, and et al. 2023. "Cissus antractica-ZnO NPs Induce Apoptosis in A549 Cells through ROS-Generated p53/Bcl-2/Bax Signaling Pathways and Inhibition of Inflammatory Cytokines" Coatings 13, no. 12: 2077. https://doi.org/10.3390/coatings13122077
APA StyleRupa, E. J., Nahar, J., Al-Amin, M., Park, J.-K., Murugesan, M., Awais, M., Lee, S.-J., Kim, I. M., Ling, L., Yang, D.-C., Yang, D.-U., Jung, D.-H., & Jung, S.-K. (2023). Cissus antractica-ZnO NPs Induce Apoptosis in A549 Cells through ROS-Generated p53/Bcl-2/Bax Signaling Pathways and Inhibition of Inflammatory Cytokines. Coatings, 13(12), 2077. https://doi.org/10.3390/coatings13122077








