Kinetic Analysis of Oxygen Evolution on Spin-Coated Thin-Film Electrodes via Electrochemical Impedance Spectroscopy
Abstract
:1. Introduction
2. Experimental Details
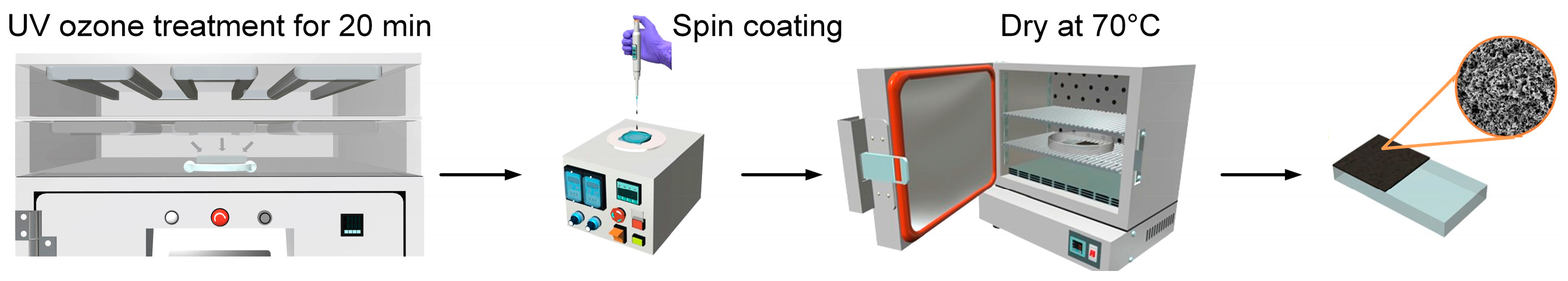
2.1. Electrocatalyst Synthesis and Fabrication of Thin-Film Electrodes
2.2. Materials’ Characterization
3. Results and Discussion
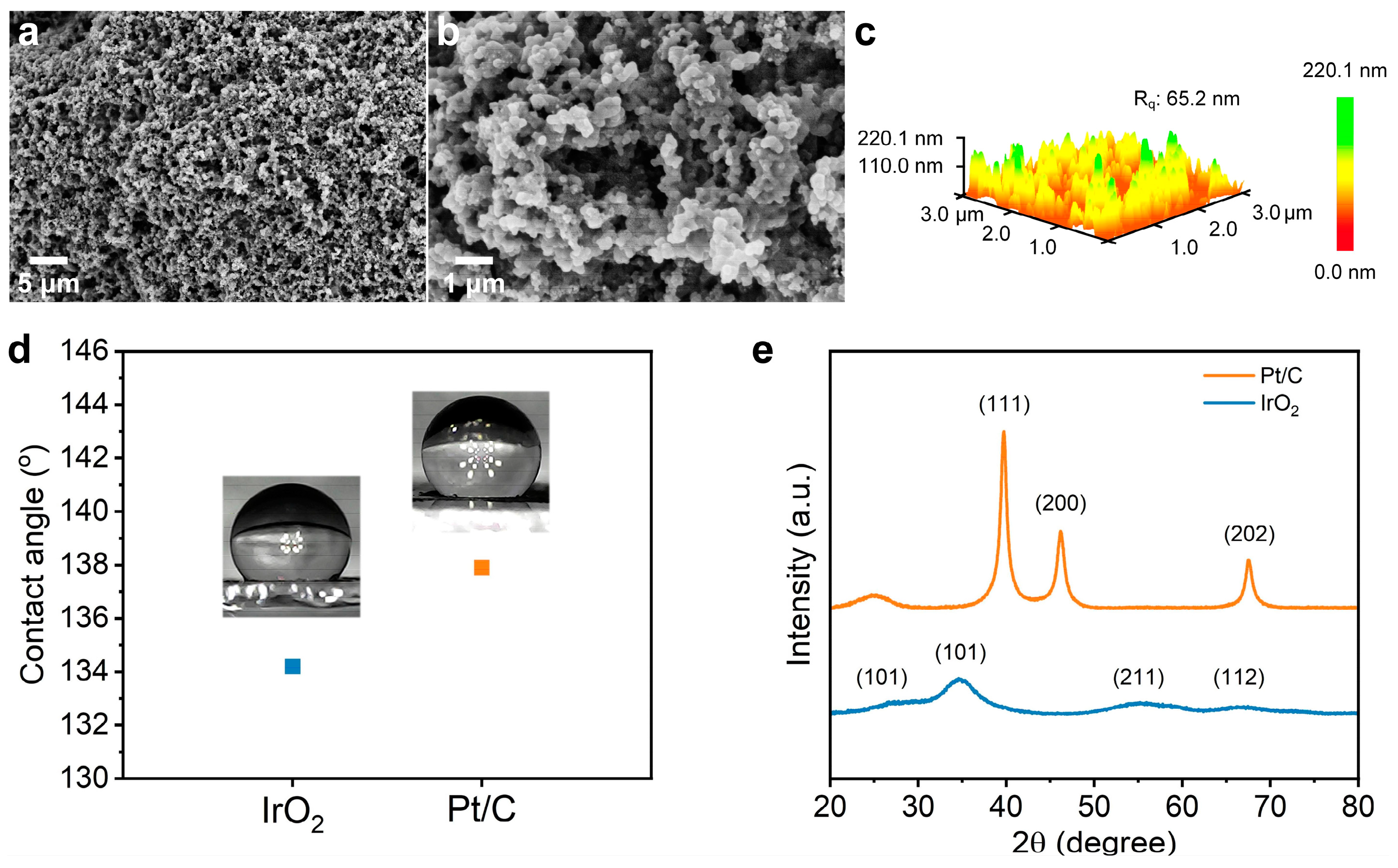
3.1. Characterization of Thin-Film Electrodes
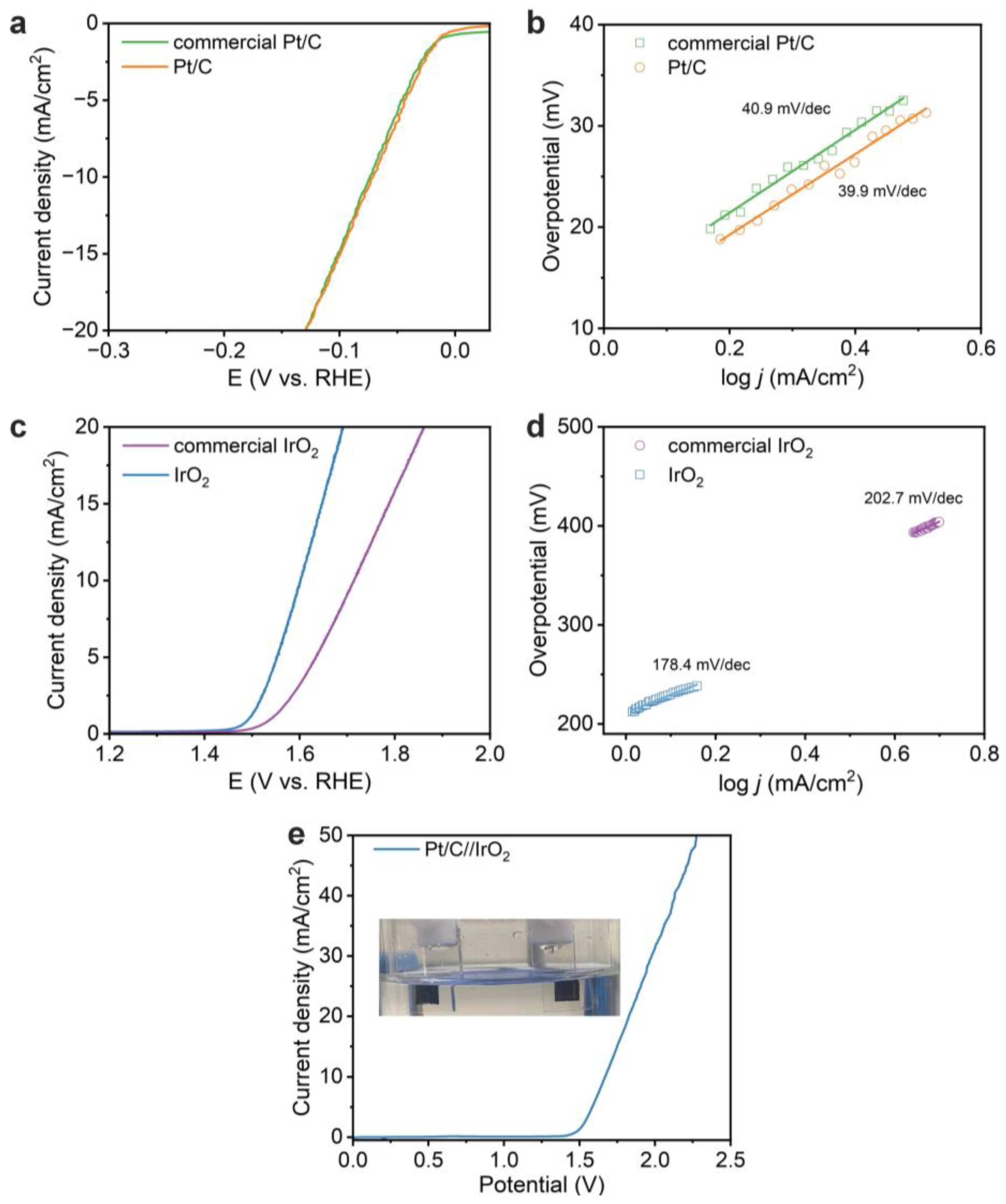
3.2. Electrochemical Properties of Thin-Film Electrodes
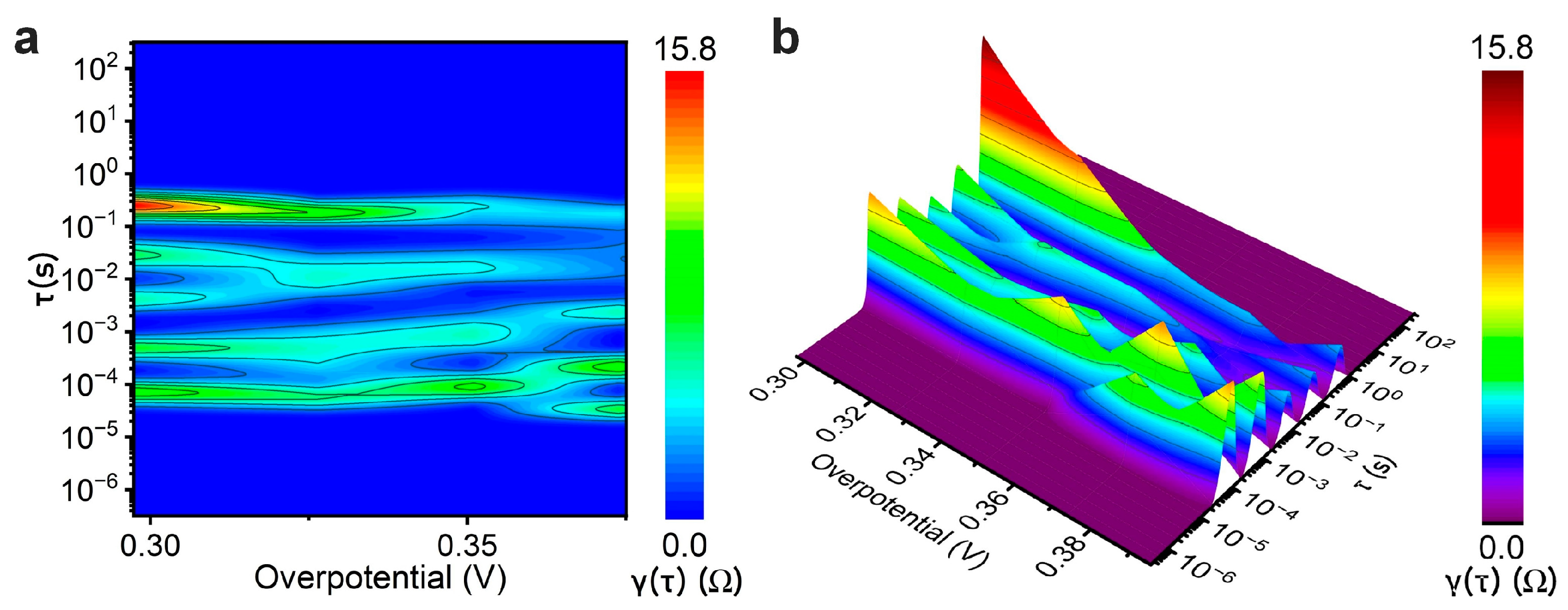
3.3. Kinetic Analysis of Oxygen Evolution on IrO2 Using Electrochemical Impedance Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kakoulaki, G.; Kougias, I.; Taylor, N.; Dolci, F.; Moya, J.; Jäger-Waldau, A. Green hydrogen in Europe-A regional assessment: Substituting existing production with electrolysis powered by renewables. Energy Convers. Manag. 2021, 228, 113649. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Stern, L.-A.; Hu, X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014, 43, 6555–6569. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.-C.; Yeh, Y.-X.; Ting, Y.-C.; Lin, S.-H.; Sasaki, K.; Choi, Y.; Lu, S.-Y. Modulation of the coordination environment enhances the electrocatalytic efficiency of Mo single atoms toward water splitting. J. Mater. Chem. A 2022, 10, 8784–8797. [Google Scholar] [CrossRef]
- Vos, J.G.; Wezendonk, T.A.; Jeremiasse, A.W.; Koper, M.T. MnOx/IrOx as selective oxygen evolution electrocatalyst in acidic chloride solution. J. Am. Chem. Soc. 2018, 140, 10270–10281. [Google Scholar] [CrossRef] [PubMed]
- Tee, S.Y.; Win, K.Y.; Teo, W.S.; Koh, L.D.; Liu, S.; Teng, C.P.; Han, M.Y. Recent progress in energy-driven water splitting. Adv. Sci. 2017, 4, 1600337. [Google Scholar] [CrossRef]
- Mehrpooya, M.; Ghorbani, B.; Ekrataleshian, A.; Mousavi, S.A. Investigation of hydrogen production by sulfur-iodine thermochemical water splitting cycle using renewable energy source. Int. J. Energy Res. 2021, 45, 14845–14869. [Google Scholar] [CrossRef]
- Huang, C.L.; Sasaki, K.; Senthil Raja, D.; Hsieh, C.T.; Wu, Y.J.; Su, J.T.; Cheng, C.C.; Cheng, P.Y.; Lin, S.H.; Choi, Y. Twinning enhances efficiencies of metallic catalysts toward electrolytic water splitting. Adv. Energy Mater. 2021, 11, 2101827. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.; Wu, H.; Cheng, J. Kinetics and oxidation pathways of Fe3+-catalyzed carbon-assisted water electrolysis for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 20432–20447. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, D.; Deng, T.; He, G.; Chen, A.; Sun, X.; Yang, Y.; Miao, P. Research progress of oxygen evolution reaction catalysts for electrochemical water splitting. ChemSusChem 2021, 14, 5359–5383. [Google Scholar] [CrossRef]
- Singh, M.R.; Clark, E.L.; Bell, A.T. Effects of electrolyte, catalyst, and membrane composition and operating conditions on the performance of solar-driven electrochemical reduction of carbon dioxide. Phys. Chem. Chem. Phys. 2015, 17, 18924–18936. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative strategies for electrocatalytic water splitting. Accounts Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Raja, D.S.; Huang, C.-L.; Chen, Y.-A.; Choi, Y.; Lu, S.-Y. Composition-balanced trimetallic MOFs as ultra-efficient electrocatalysts for oxygen evolution reaction at high current densities. Appl. Catal. B 2020, 279, 119375. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Chen, F.-Y.; Li, B.; Yu, S.-W.; Finfrock, Y.Z.; Meira, D.M.; Yan, Q.-Q.; Zhu, P.; Chen, M.-X.; Song, T.-W.; et al. Non-iridium-based electrocatalyst for durable acidic oxygen evolution reaction in proton exchange membrane water electrolysis. Nat. Mater. 2023, 22, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-W.; Liang, H.-W. Ir-based bifunctional electrocatalysts for overall water splitting. Catal. Sci. Technol. 2021, 11, 4673–4689. [Google Scholar] [CrossRef]
- Fu, L.; Zeng, X.; Cheng, G.; Luo, W. IrCo nanodendrite as an efficient bifunctional electrocatalyst for overall water splitting under acidic conditions. ACS Appl. Mater. Interfaces 2018, 10, 24993–24998. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Hu, X.; Li, Y.; Cheng, G.; Luo, W. IrW nanobranches as an advanced electrocatalyst for pH-universal overall water splitting. Nanoscale 2019, 11, 8898–8905. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, S.; Mao, Q.; Yin, S.; Wang, Z.; Xu, Y.; Li, X.; Wang, L.; Wang, H. Ultrafine ruthenium-iridium-tellurium nanotubes for boosting overall water splitting in acidic media. J. Mater. Chem. A 2022, 10, 2021–2026. [Google Scholar] [CrossRef]
- Yang, N.; Tian, S.; Feng, Y.; Hu, Z.; Liu, H.; Tian, X.; Xu, L.; Hu, C.; Yang, J. Introducing High-Valence Iridium Single Atoms into Bimetal Phosphides toward High-Efficiency Oxygen Evolution and Overall Water Splitting. Small 2023, 19, 2207253. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, X.; An, Q.; Liu, M.; Zhou, W.; Li, Y.; Hu, F.; Liu, Q.; Su, H. Dynamically-evolved surface heterojunction in iridium nanocrystals boosting acidic oxygen evolution and overall water splitting. J. Energy Chem. 2023, 78, 374–380. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, C.-Q.; Liu, W.; Hung, S.-F.; Bin Yang, H.; Gao, J.; Cai, W.; Chen, H.M.; Li, J.; Liu, B. Coordination engineering of iridium nanocluster bifunctional electrocatalyst for highly efficient and pH-universal overall water splitting. Nat. Commun. 2020, 11, 4246. [Google Scholar] [CrossRef]
- Wan, C.; Duan, X.; Huang, Y. Molecular Design of Single-Atom Catalysts for Oxygen Reduction Reaction. Adv. Energy Mater. 2020, 10, 1903815. [Google Scholar] [CrossRef]
- Liu, B.-J.; Yin, T.-H.; Lin, Y.-W.; Chang, C.-W.; Yu, H.-C.; Lim, Y.; Lee, H.; Choi, C.; Tsai, M.-K.; Choi, Y. A Cost-Effective, Nanoporous, High-Entropy Oxide Electrode for Electrocatalytic Water Splitting. Coatings 2023, 13, 1461. [Google Scholar] [CrossRef]
- Yu, S.; Li, K.; Wang, W.; Xie, Z.; Ding, L.; Kang, Z.; Wrubel, J.; Ma, Z.; Bender, G.; Yu, H.; et al. Tuning Catalyst Activation and Utilization Via Controlled Electrode Patterning for Low-Loading and High-Efficiency Water Electrolyzers. Small 2022, 18, 2107745. [Google Scholar] [CrossRef]
- Chen, S.; Li, X.; Kao, C.-W.; Luo, T.; Chen, K.; Fu, J.; Ma, C.; Li, H.; Li, M.; Chan, T.-S.; et al. Unveiling the Proton-Feeding Effect in Sulfur-Doped Fe-N-C Single-Atom Catalyst for Enhanced CO2 Electroreduction. Angew. Chem.-Int. Edit. 2022, 61, e202206233. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Wang, C.; Zhao, Y.; Zhang, Y.; Gao, J.; Sun, L.; Hou, J. Regulating electronic states of nitride/hydroxide to accelerate kinetics for oxygen evolution at large current density. Nat. Commun. 2023, 14, 1873. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Wang, W.; Xie, F. Investigation of oxygen evolution reaction kinetic process and kinetic parameters on iridium electrode by electrochemistry impedance spectroscopy analysis. J. Electroanal. Chem. 2020, 871, 114281. [Google Scholar] [CrossRef]
- Schröder, J.; Mints, V.A.; Bornet, A.; Berner, E.; Fathi Tovini, M.; Quinson, J.; Wiberg, G.K.H.; Bizzotto, F.; El-Sayed, H.A.; Arenz, M. The Gas Diffusion Electrode Setup as Straightforward Testing Device for Proton Exchange Membrane Water Electrolyzer Catalysts. JACS Au 2021, 1, 247–251. [Google Scholar] [CrossRef]
- Costa, F.R.; Franco, D.V.; Da Silva, L.M. Electrochemical impedance spectroscopy study of the oxygen evolution reaction on a gas-evolving anode composed of lead dioxide microfibers. Electrochim. Acta 2013, 90, 332–343. [Google Scholar] [CrossRef]
- Benck, J.D.; Pinaud, B.A.; Gorlin, Y.; Jaramillo, T.F. Substrate selection for fundamental studies of electrocatalysts and photoelectrodes: Inert potential windows in acidic, neutral, and basic electrolyte. PLoS ONE 2014, 9, e107942. [Google Scholar] [CrossRef]
- Cheng, P.-Y.; Ting, Y.-C.; Cheng, C.-C.; Raja, D.S.; Lin, S.-H.; Yeh, Y.-X.; Su, J.-T.; Lu, S.-Y. Nitrogen-doped carbon armored Cobalt oxide hollow nanocubes electrochemically anchored on fluorine-doped tin oxide substrate for acidic oxygen evolution reaction. J. Colloid Interface Sci. 2022, 623, 327–336. [Google Scholar] [CrossRef]
- He, F.; Zhou, Y.; Hu, T.; Xu, Y.; Hou, M.; Zhu, F.; Liu, D.; Zhang, H.; Xu, K.; Liu, M.; et al. An Efficient High-Entropy Perovskite-Type Air Electrode for Reversible Oxygen Reduction and Water Splitting in Protonic Ceramic Cells. Adv. Mater. 2023, 35, 2209469. [Google Scholar] [CrossRef]
- Giesbrecht, P.K.; Freund, M.S. Investigation of Water Oxidation at IrO2 Electrodes in Nafion-Based Membrane Electrode Assemblies Using Impedance Spectroscopy and Distribution of Relaxation Times Analysis. J. Phys. Chem. C 2022, 126, 17844–17861. [Google Scholar] [CrossRef]
- Song, L.; Cai, Y.; Liu, Y.; Zhao, X.; Kuttiyiel, K.A.; Marinkovic, N.; Frenkel, A.I.; Kongkanand, A.; Choi, Y.; Adzic, R.R. One-Step Facile Synthesis of High-Activity Nitrogen-Doped PtNiN Oxygen Reduction Catalyst. ACS Appl. Energy Mater. 2022, 5, 5245–5255. [Google Scholar] [CrossRef]
- Felix, C.; Bladergroen, B.J.; Linkov, V.; Pollet, B.G.; Pasupathi, S. Ex-situ electrochemical characterization of IrO2 synthesized by a modified Adams fusion method for the oxygen evolution reaction. Catalysts 2019, 9, 318. [Google Scholar] [CrossRef]
- Puthiyapura, V.K.; Mamlouk, M.; Pasupathi, S.; Pollet, B.G.; Scott, K. Physical and electrochemical evaluation of ATO supported IrO2 catalyst for proton exchange membrane water electrolyser. J. Power Sources 2014, 269, 451–460. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Seo, D.H.; Baek, J.H.; Kang, M.J.; Kim, B.N.; Kim, S.K.; Zheng, X.; Cho, I.S. Crystal Reconstruction of Mo:BiVO4: Improved Charge Transport for Efficient Solar Water Splitting. Adv. Funct. Mater. 2022, 32, 2208196. [Google Scholar] [CrossRef]
- Bi, W.; Fuller, T.F. Modeling of PEM fuel cell Pt/C catalyst degradation. J. Power Sources 2008, 178, 188–196. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Du, X.; Zhang, X. Promoted electrocatalytic water splitting by regulating the concentration of oxygen vacancies. Int. J. Hydrogen Energy 2023, 48, 34783–34793. [Google Scholar] [CrossRef]
- Shetty, A.R.; Hegde, A.C. Effect of TiO2 on electrocatalytic behavior of Ni-Mo alloy coating for hydrogen energy. Mater. Sci. Energy Technol. 2018, 1, 97–105. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, M.J.; Kim, J.J. Impact of Surface Hydrophilicity on Electrochemical Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 11940–11947. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Ding, C.; Chen, Z.; Zhang, F.; Shi, J.; Li, C. Synergetic Effect of Conjugated Ni(OH)2/IrO2 Cocatalyst on Titanium-Doped Hematite Photoanode for Solar Water Splitting. J. Phys. Chem. C 2015, 119, 19607–19612. [Google Scholar] [CrossRef]
- Hyun Oh, J.; Ho Han, G.; Kim, J.; Eun Lee, J.; Kim, H.; Kyung Kang, S.; Kim, H.; Wooh, S.; Soo Lee, P.; Won Jang, H.; et al. Self-supported electrodes to enhance mass transfer for high-performance anion exchange membrane water electrolyzer. Chem. Eng. J. 2023, 460, 141727. [Google Scholar] [CrossRef]
- Wang, Y.; Xue, Y.; Zhang, C. Rational Surface and Interfacial Engineering of IrO2/TiO2 Nanosheet Arrays toward High-Performance Chlorine Evolution Electrocatalysis and Practical Environmental Remediation. Small 2021, 17, 2006587. [Google Scholar] [CrossRef]
- Li, H.; Pan, Y.; Wu, L.; He, R.; Qin, Z.; Luo, S.; Yang, L.; Zeng, J. Highly active and stable IrO2 and IrO2-Ta2O5 catalysts for oxygen evolution reaction. Int. J. Hydrogen Energy 2023, 48, 26021–26031. [Google Scholar] [CrossRef]
- Uzunoglu, A.; Ipekci, H.H. The use of CeO2-modified Pt/C catalyst inks for the construction of high-performance enzyme-free H2O2 sensors. J. Electroanal. Chem. 2019, 848, 113302. [Google Scholar] [CrossRef]
- Sriring, N.; Tantavichet, N.; Pruksathorn, K. Preparation of Pt/C catalysts by electroless deposition for proton exchange membrane fuel cells. Korean J. Chem. Eng. 2010, 27, 439–445. [Google Scholar] [CrossRef]
- Singh, A.; Miyabayashi, K. Novel continuous flow synthesis of Pt NPs with narrow size distribution for Pt@ carbon catalysts. RSC Adv. 2020, 10, 362–366. [Google Scholar] [CrossRef]
- Huy, H.A.; Van Man, T.; Tai, H.T. Preparation and characterization of high-dispersed Pt/C nano-electrocatalysts for fuel cell applications. Vietnam J. Sci. Technol. 2016, 54, 472–482. [Google Scholar] [CrossRef]
- Zheng, H.-B.; An, L.; Zheng, Y.; Qu, C.; Fang, Y.; Liu, Q.; Dang, D. Tuning the catalytic activity of Ir@Pt nanoparticles through controlling Ir core size on cathode performance for PEM fuel cell application. Front. Chem. 2018, 6, 299. [Google Scholar] [CrossRef]
- Qin, B.; Yu, H.; Chi, J.; Jia, J.; Gao, X.; Yao, D.; Yi, B.; Shao, Z. A novel Ir/CeO2-C nanoparticle electrocatalyst for the hydrogen oxidation reaction of alkaline anion exchange membrane fuel cells. RSC Adv. 2017, 7, 31574–31581. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, M.; Zhou, Y.; Zhang, H.; Hu, G. Fluorinated MXenes accelerate the hydrogen evolution activity of in situ induced snowflake-like nano-Pt. J. Mater. Chem. A 2023, 11, 5830–5840. [Google Scholar] [CrossRef]
- Zeng, Z.; Küspert, S.; Balaghi, S.E.; Hussein, H.E.; Ortlieb, N.; Knäbbeler-Buß, M.; Hügenell, P.; Pollitt, S.; Hug, N.; Melke, J. Ultrahigh Mass Activity Pt Entities Consisting of Pt Single atoms, Clusters, and Nanoparticles for Improved Hydrogen Evolution Reaction. Small 2023, 19, 2205885. [Google Scholar] [CrossRef]
- Mendoza-Pérez, R.; Guisbiers, G. Bimetallic Pt-Pd nano-catalyst: Size, shape and composition matter. Nanotechnology 2019, 30, 305702. [Google Scholar] [CrossRef]
- Saeed, U.; Jilani, A.; Iqbal, J.; Al-Turaif, H. Reduced graphene oxide-assisted graphitic carbon nitride@ZnO rods for enhanced physical and photocatalytic degradation. Inorg. Chem. Commun. 2022, 142, 109623. [Google Scholar] [CrossRef]
- Jilani, A.; Hussain, S.Z.; Ansari, M.O.; Kumar, R.; Dustgeer, M.R.; Othman, M.H.D.; Barakat, M.A.; Melaibari, A.A. Facile synthesis of silver decorated reduced graphene oxide@zinc oxide as ternary nanocomposite: An efficient photocatalyst for the enhanced degradation of organic dye under UV-visible light. J. Mater. Sci. 2021, 56, 7434–7450. [Google Scholar] [CrossRef]
- Yu, B.; Liu, J.-H.; Guo, S.; Huang, G.; Zhang, S.; Chen, S.; Li, X.; Wang, Y.; Lv, L.-P. Densely populated tiny RuO2 crystallites supported by hierarchically porous carbon for full acidic water splitting. Mater. Horizons 2023, 10, 4589–4596. [Google Scholar] [CrossRef]
- Shi, J.; Kao, C.-w.; Lan, J.; Jiang, K.; Peng, M.; Luo, M.; Lu, Y.-R.; Zhang, S.; Tan, Y. Nanoporous PdIr alloy for high-efficiency and durable water splitting in acidic media. J. Mater. Chem. A 2023, 11, 11526–11533. [Google Scholar] [CrossRef]
- Xie, Y.; Chang, C.; Luo, F.; Yang, Z. Modulation in the d Band of Ir by Core-Shell Construction for Robust Water Splitting Electrocatalysts in Acid. ACS Appl. Mater. Interfaces 2023, 15, 20081–20088. [Google Scholar] [CrossRef]
- Shao, X.; Liang, M.; Kim, M.G.; Ajmal, S.; Kumar, A.; Liu, X.; Jung, H.S.; Jin, H.; Cao, F.; Yu, J.; et al. Density-Controlled Metal Nanocluster with Modulated Surface for pH-Universal and Robust Water Splitting. Adv. Funct. Mater. 2023, 33, 2211192. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, Y.; He, H.; Shao, H.; Zhang, Y.; Li, J.; Cai, W. Valence regulation of Ru/Mo2C heterojunction for efficient acidic overall water splitting. Electrochim. Acta 2023, 443, 141920. [Google Scholar] [CrossRef]
- Khamgaonkar, S.; Chen, Q.; Musselman, K.; Maheshwari, V. Stable perovskite photocathodes for efficient hydrogen evolution in acidic and basic conditions. J. Mater. Chem. A 2023, 11, 20079–20088. [Google Scholar] [CrossRef]
- Kuang, P.; Wang, Y.; Zhu, B.; Xia, F.; Tung, C.-W.; Wu, J.; Chen, H.M.; Yu, J. Pt Single Atoms Supported on N-Doped Mesoporous Hollow Carbon Spheres with Enhanced Electrocatalytic H2-Evolution Activity. Adv. Mater. 2021, 33, 2008599. [Google Scholar] [CrossRef]
- Kaneva, M.V.; Gulina, L.B.; Tolstoy, V.P. Pt nanoparticles synthesized by successive ionic layers deposition method and their electrocatalytic properties in hydrogen evolution reaction during water splitting in the acidic medium. J. Alloys Compd. 2022, 901, 163640. [Google Scholar] [CrossRef]
- Ge, Y.; Wang, X.; Chen, B.; Huang, Z.; Shi, Z.; Huang, B.; Liu, J.; Wang, G.; Chen, Y.; Li, L.; et al. Preparation of fcc-2H-fcc Heterophase Pd@Ir Nanostructures for High-Performance Electrochemical Hydrogen Evolution. Adv. Mater. 2022, 34, 2107399. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, D.; Liu, W.; Xia, D.; Hu, L.; Yang, J.; Liao, P.; He, C. Enhanced Catalytic Ozonation for Eliminating CH3SH via Graphene-Supported Positively Charged Atomic Pt Undergoing Pt2+/Pt4+ Redox Cycle. Environ. Sci. Technol. 2021, 55, 16723–16734. [Google Scholar] [CrossRef] [PubMed]
- Prabhuram, J.; Wang, X.; Hui, C.L.; Hsing, I.M. Synthesis and Characterization of Surfactant-Stabilized Pt/C Nanocatalysts for Fuel Cell Applications. J. Phys. Chem. B 2003, 107, 11057–11064. [Google Scholar] [CrossRef]
- Zaman, W.Q.; Sun, W.; Tariq, M.; Zhou, Z.; Farooq, U.; Abbas, Z.; Cao, L.; Yang, J. Iridium substitution in nickel cobaltite renders high mass specific OER activity and durability in acidic media. Appl. Catal. B 2019, 244, 295–302. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Wang, X.; Huang, J.; Yin, L.; Zhu, W.; Zhuang, Z. Construction of Steady-Active Self-supported Porous Ir-based Electrocatalysts for Oxygen Evolution Reaction. Chem. Commun. 2023, 59, 1813–1816. [Google Scholar] [CrossRef]
- Zheng, Y.; Ye, Z.; Peng, X.; Zhuang, S.; Li, D.; Jin, Z. Cobalt vacancy-originated TiMnCoCN compounds with a self-adjusting ability for the high-efficiency acidic oxygen evolution reaction. J. Colloid Interface Sci. 2023, 652, 164–173. [Google Scholar] [CrossRef]
- Raja, D.S.; Cheng, P.-Y.; Cheng, C.-C.; Chang, S.-Q.; Huang, C.-L.; Lu, S.-Y. In-situ grown metal-organic framework-derived carbon-coated Fe-doped cobalt oxide nanocomposite on fluorine-doped tin oxide glass for acidic oxygen evolution reaction. Appl. Catal. B 2022, 303, 120899. [Google Scholar] [CrossRef]
- Dang, Q.; Lin, H.; Fan, Z.; Ma, L.; Shao, Q.; Ji, Y.; Zheng, F.; Geng, S.; Yang, S.-Z.; Kong, N.; et al. Iridium metallene oxide for acidic oxygen evolution catalysis. Nat. Commun. 2021, 12, 6007. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, J.; Xu, J.; Zhang, N.; Li, Y.; Liu, L.; Pan, D.; Wang, Z.; Deepak, F.L. Ultrafine-grained porous Ir-based catalysts for high-performance overall water splitting in acidic media. ACS Appl. Energy Mater. 2020, 3, 3736–3744. [Google Scholar] [CrossRef]
- Cai, C.; Han, S.; Tang, Y. Engineering oxygen vacancies on dendrite-like IrO2 for the oxygen evolution reaction in acidic solution. Sustain. Energy Fuels 2020, 4, 2462–2468. [Google Scholar] [CrossRef]
- Chen, X.; Xu, M.; Li, S.; Li, C.; Sun, X.; Mu, S.; Yu, J. Ultrafine IrNi Bimetals Encapsulated in Zeolitic Imidazolate Frameworks-Derived Porous N-Doped Carbon for Boosting Oxygen Evolution in Both Alkaline and Acidic Electrolytes. Adv. Mater. Interfaces 2020, 7, 2001145. [Google Scholar] [CrossRef]
- Yao, Q.; Huang, B.; Xu, Y.; Li, L.; Shao, Q.; Huang, X. A chemical etching strategy to improve and stabilize RuO2-based nanoassemblies for acidic oxygen evolution. Nano Energy 2021, 84, 105909. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Yin, K.; Zhang, J.; Gao, H.; Liu, N.; Peng, Z.; Zhang, Z. Nanoporous Iridium-Based Alloy Nanowires as Highly Efficient Electrocatalysts Toward Acidic Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 39728–39736. [Google Scholar] [CrossRef]
- Li, G.; Li, S.; Xiao, M.; Ge, J.; Liu, C.; Xing, W. Nanoporous IrO2 catalyst with enhanced activity and durability for water oxidation owing to its micro/mesoporous structure. Nanoscale 2017, 9, 9291–9298. [Google Scholar] [CrossRef]
- Jin, C.; Lu, F.; Cao, X.; Yang, Z.; Yang, R. Facile synthesis and excellent electrochemical properties of NiCo2O4 spinel nanowire arrays as a bifunctional catalyst for the oxygen reduction and evolution reaction. J. Mater. Chem. A 2013, 1, 12170–12177. [Google Scholar] [CrossRef]
- Bizzotto, F.; Quinson, J.; Zana, A.; Kirkensgaard, J.J.; Dworzak, A.; Oezaslan, M.; Arenz, M. Ir nanoparticles with ultrahigh dispersion as oxygen evolution reaction (OER) catalysts: Synthesis and activity benchmarking. Catal. Sci. Technol. 2019, 9, 6345–6356. [Google Scholar] [CrossRef]
- Bredar, A.R.C.; Chown, A.L.; Burton, A.R.; Farnum, B.H. Electrochemical Impedance Spectroscopy of Metal Oxide Electrodes for Energy Applications. ACS Appl. Energy Mater. 2020, 3, 66–98. [Google Scholar] [CrossRef]
- Lyons, M.E.; Brandon, M.P. The significance of electrochemical impedance spectra recorded during active oxygen evolution for oxide covered Ni, Co and Fe electrodes in alkaline solution. J. Electroanal. Chem. 2009, 631, 62–70. [Google Scholar] [CrossRef]
- García-Osorio, D.A.; Jaimes, R.; Vazquez-Arenas, J.; Lara, R.H.; Alvarez-Ramirez, J. The Kinetic Parameters of the Oxygen Evolution Reaction (OER) Calculated on Inactive Anodes via EIS Transfer Functions: •OH Formation. J. Electrochem. Soc. 2017, 164, E3321. [Google Scholar] [CrossRef]
- Negahdar, L.; Zeng, F.; Palkovits, S.; Broicher, C.; Palkovits, R. Mechanistic Aspects of the Electrocatalytic Oxygen Evolution Reaction over Ni-Co Oxides. ChemElectroChem 2019, 6, 5588–5595. [Google Scholar] [CrossRef]
- Amadelli, R.; Maldotti, A.; Molinari, A.; Danilov, F.I.; Velichenko, A.B. Influence of the electrode history and effects of the electrolyte composition and temperature on O2 evolution at β-PbO2 anodes in acid media. J. Electroanal. Chem. 2002, 534, 1–12. [Google Scholar] [CrossRef]
- Wang, C.; John Appleby, A.; Little, F.E. Electrochemical study on nano-Sn, Li4.4Sn and AlSi0.1 powders used as secondary lithium battery anodes. J. Power Sources 2001, 93, 174–185. [Google Scholar] [CrossRef]
- Guo, F.; Ye, K.; Du, M.; Huang, X.; Cheng, K.; Wang, G.; Cao, D. Electrochemical impedance analysis of urea electro-oxidation mechanism on nickel catalyst in alkaline medium. Electrochim. Acta 2016, 210, 474–482. [Google Scholar] [CrossRef]
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the Discretization Methods on the Distribution of Relaxation Times Deconvolution: Implementing Radial Basis Functions with DRT tools. Electrochim. Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Ciucci, F.; Chen, C. Analysis of Electrochemical Impedance Spectroscopy Data Using the Distribution of Relaxation Times: A Bayesian and Hierarchical Bayesian Approach. Electrochim. Acta 2015, 167, 439–454. [Google Scholar] [CrossRef]
- Effat, M.B.; Ciucci, F. Bayesian and Hierarchical Bayesian Based Regularization for Deconvolving the Distribution of Relaxation Times from Electrochemical Impedance Spectroscopy Data. Electrochim. Acta 2017, 247, 1117–1129. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Z.; Xu, L.; Li, J.; Gan, Q.; Du, X.; Ouyang, M. A comparative study of equivalent circuit model and distribution of relaxation times for fuel cell impedance diagnosis. Int. J. Energy Res. 2021, 45, 15948–15961. [Google Scholar] [CrossRef]
- Zhang, X.; Song, W.-L.; Wang, M.; Tu, J.; Jiao, H.; Jiao, S. Photo-electrochemical enhanced mechanism enables a fast-charging and high-energy aqueous Al/MnO2 battery. Energy Storage Mater. 2022, 45, 586–594. [Google Scholar] [CrossRef]
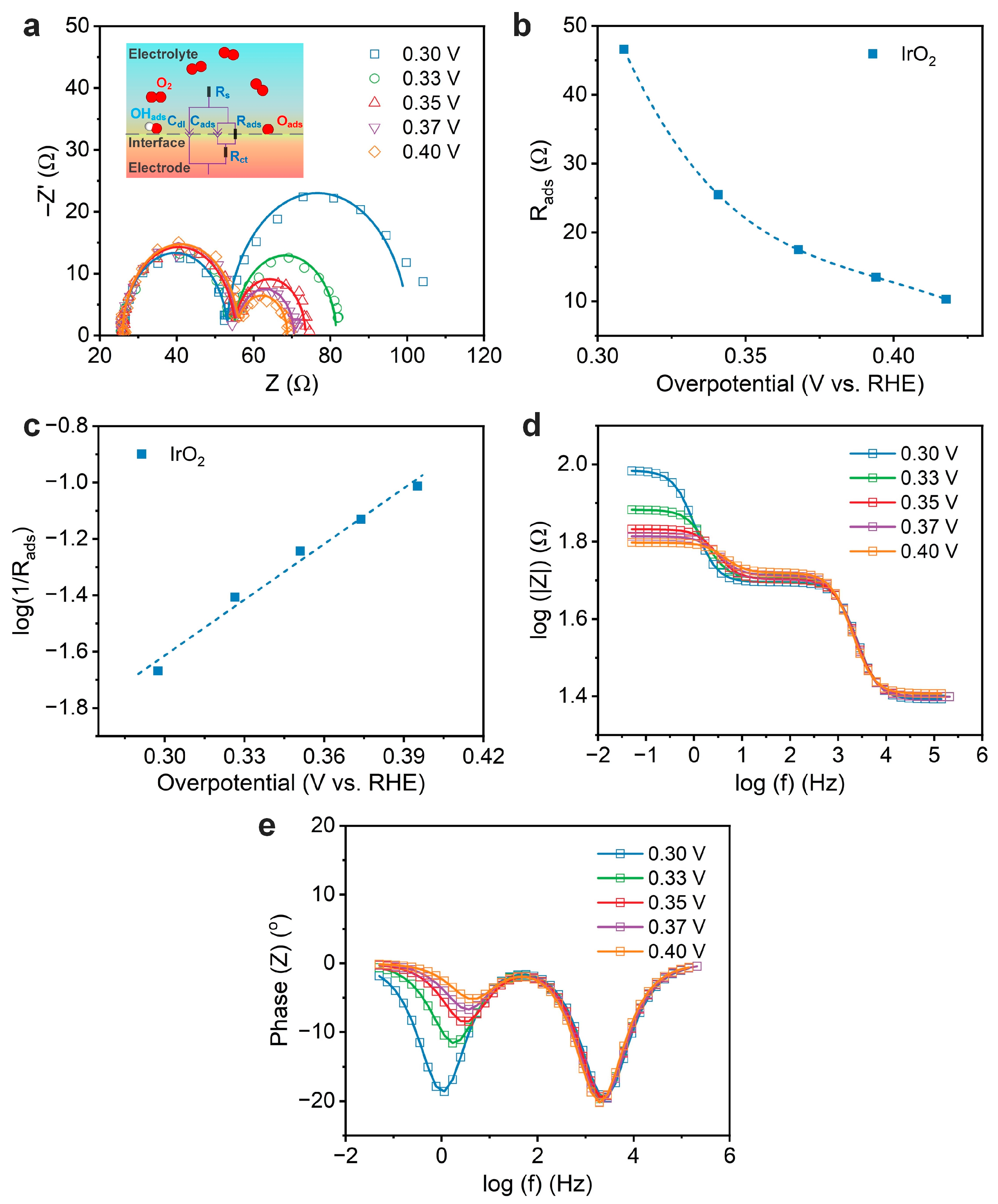
| Overpotential (V) 1 | Rs (Ω) | Cdl × 10−6 (F) | Rct (Ω) | Cads × 10−3 (F) | Rads (Ω) | log(1/Rads) |
|---|---|---|---|---|---|---|
| 0.30 | 24.7 | 4.14 | 25.0 | 4.53 | 46.6 | –1.67 |
| 0.33 | 25.1 | 4.02 | 25.7 | 4.18 | 25.5 | –1.41 |
| 0.35 | 25.2 | 3.75 | 25.3 | 3.41 | 17.5 | –1.24 |
| 0.37 | 25.4 | 4.19 | 25.7 | 3.77 | 13.5 | –1.13 |
| 0.40 | 25.5 | 4.28 | 26.9 | 3.86 | 10.3 | –1.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-W.; Li, Y.-S.; Chang, C.-W.; Huang, L.-C.; Yin, T.-H.; Liu, Y.-T.; Park, D.K.; Choi, C.; Choi, Y. Kinetic Analysis of Oxygen Evolution on Spin-Coated Thin-Film Electrodes via Electrochemical Impedance Spectroscopy. Coatings 2023, 13, 1957. https://doi.org/10.3390/coatings13111957
Lin Y-W, Li Y-S, Chang C-W, Huang L-C, Yin T-H, Liu Y-T, Park DK, Choi C, Choi Y. Kinetic Analysis of Oxygen Evolution on Spin-Coated Thin-Film Electrodes via Electrochemical Impedance Spectroscopy. Coatings. 2023; 13(11):1957. https://doi.org/10.3390/coatings13111957
Chicago/Turabian StyleLin, Yu-Wei, Yi-Syuan Li, Chun-Wei Chang, Li-Cheng Huang, Tai-Hsin Yin, Yu-Ting Liu, Dong Kyoo Park, Changsik Choi, and YongMan Choi. 2023. "Kinetic Analysis of Oxygen Evolution on Spin-Coated Thin-Film Electrodes via Electrochemical Impedance Spectroscopy" Coatings 13, no. 11: 1957. https://doi.org/10.3390/coatings13111957
APA StyleLin, Y.-W., Li, Y.-S., Chang, C.-W., Huang, L.-C., Yin, T.-H., Liu, Y.-T., Park, D. K., Choi, C., & Choi, Y. (2023). Kinetic Analysis of Oxygen Evolution on Spin-Coated Thin-Film Electrodes via Electrochemical Impedance Spectroscopy. Coatings, 13(11), 1957. https://doi.org/10.3390/coatings13111957







